D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterization
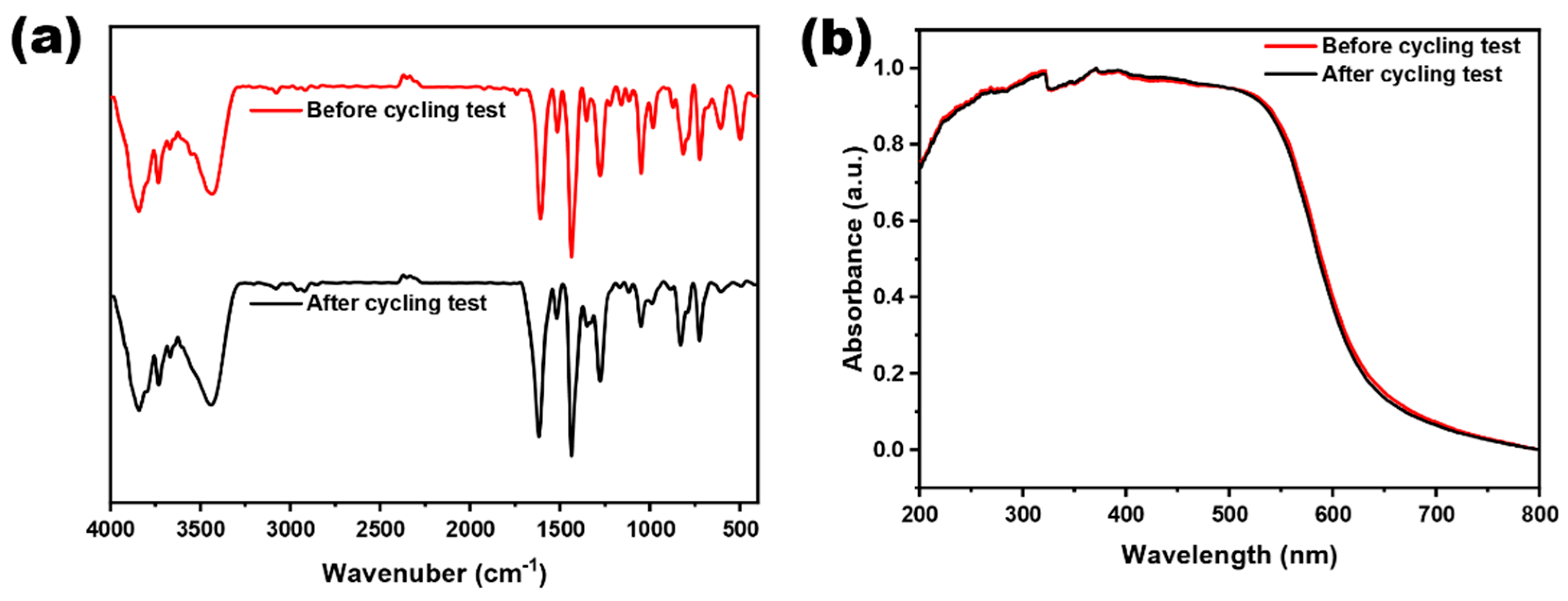
2.2. Optical and Electrochemical Properties
2.3. Photocatalytic Hydrogen Production Performance
3. Materials and Methods
3.1. Methods
3.2. Synthesis of bis(2-thiophene)ketone, Py-BKh0, Py-BKh1, Py-BKh2, and Py-BKh3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tadé, M.O.; Shao, Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Liu, S.; Li, Y.; Fan, J.; Lv, K. Effects of fluorine on photocatalysis. Chin. J. Catal. 2020, 41, 1451–1467. [Google Scholar] [CrossRef]
- Yanagida, S.; Kabumoto, A.; Mizumoto, K.; Pac, C.; Yoshino, K. Poly (p-phenylene)-catalysed photoreduction of water to hydrogen. Chem. Commun. 1985, 8, 474–475. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Cheng, R.; Wang, M.; Li, M.; Zhou, Y.; Shi, J. Construction of graphitic C3N4-based intramolecular donor–acceptor conjugated copolymers for photocatalytic hydrogen evolution. ACS Catal. 2015, 5, 5008–5015. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, B.; Xu, J.; Yu, J.; Cao, S. Donor-acceptor-based conjugated polymers for photocatalytic energy conversion. EnergyChem. 2024, 6, 100116. [Google Scholar] [CrossRef]
- Qiao, S.; Di, M.; Jiang, J.-X.; Han, B.-H. Conjugated porous polymers for photocatalysis: The road from catalytic mechanism, molecular structure to advanced applications. EnergyChem. 2022, 4, 100094. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Zhang, K.A. Designing conjugated porous polymers for visible light-driven photocatalytic chemical transformations. Mater. Horiz. 2020, 7, 15–31. [Google Scholar] [CrossRef]
- Yang, C.; Ma, B.C.; Zhang, L.; Lin, S.; Ghasimi, S.; Landfester, K.; Zhang, K.A.; Wang, X. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew. Chem. Int. Ed. 2016, 55, 9202–9206. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, W.; Xu, Y.; Zhang, C.; Wang, Q.; Yang, T.; Gao, X.; Wang, F.; Yan, C.; Jiang, J.-X. Effect of linking pattern of dibenzothiophene-S, S-dioxide-containing conjugated microporous polymers on the photocatalytic performance. Macromolecules 2018, 51, 9502–9508. [Google Scholar] [CrossRef]
- Tan, Z.R.; Xing, Y.Q.; Cheng, J.Z.; Zhang, G.; Shen, Z.Q.; Zhang, Y.J.; Liao, G.; Chen, L.; Liu, S.Y. EDOT-based conjugated polymers accessed via C-H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Zhang, X.; Tang, H.; Liu, X.; Huang, F.; Cao, Y. Conjugated polymers with oligoethylene glycol side chains for improved photocatalytic hydrogen evolution. iScience 2019, 13, 33–42. [Google Scholar] [CrossRef]
- Woods, D.J.; Hillman, S.A.J.; Pearce, D.; Wilbraham, L.; Flagg, L.Q.; Duffy, W.; McCulloch, I.; Durrant, J.R.; Guilbert, A.A.Y.; Zwijnenburg, M.A.; et al. Side-chain tuning in conjugated polymer photocatalysts for improved hydrogen production from water. Energy Environ. Sci. 2020, 13, 1843–1855. [Google Scholar] [CrossRef]
- Cho, S.; Seo, J.H.; Kim, S.H.; Song, S.; Jin, Y.; Lee, K.; Suh, H.; Heeger, A.J. Effect of substituted side chain on donor-acceptor conjugated copolymers. Appl. Phys. Lett. 2008, 93, 263301. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W. Organic donor-acceptor systems for photocatalysis. Adv. Sci. 2024, 11, 2307227. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Mondal, S.; Ghosh, A.; Banerjee, S.; Das, A.K.; Bhaumik, A. Rational design of highly porous donor–acceptor based conjugated microporous polymer for photocatalytic benzylamine coupling reaction. Small 2024, 20, 2406723. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, X.; Xu, X.; Zhang, C.; He, X. Donor-acceptor type triazine-based conjugated porous polymer for visible-light-driven photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 257, 117935. [Google Scholar] [CrossRef]
- Li, Z.; Fang, H.; Chen, Z.; Zou, W.; Zhao, C.; Yang, X. Regulating donor-acceptor interactions in triazine-based conjugated polymers for boosted photocatalytic hydrogen production. Appl. Catal. B Environ. 2022, 312, 121374. [Google Scholar] [CrossRef]
- Yan, Y.; Yu, X.; Shao, C.; Hu, Y.; Huang, W.; Li, Y. Atomistic structural engineering of conjugated microporous polymers promotes photocatalytic biomass valorization. Adv. Funct. Mater. 2023, 33, 2304604. [Google Scholar] [CrossRef]
- Yang, C.; Xiang, Y.; Wang, W.; Cheng, B.; Yang, K.; Yu, J.; Cao, S. Enhancing photocatalytic H2O2 production of donor− acceptor polymers by modulation of polymerization modes. Appl. Catal. B Environ. 2025, 365, 124856. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, W.; Wang, H.; He, Q.; Zhou, Y.; Yang, P.; Li, Y.; Li, Y. Metal-free photocatalytic hydrogenation using covalent triazine polymers. Angew. Chem. Int. Ed. 2020, 59, 14378–14382. [Google Scholar] [CrossRef]
- Hayat, A.; Rahman, M.U.; Khan, I.; Khan, J.; Sohail, M.; Yasmeen, H.; Liu, S.Y.; Qi, K.; Lv, W. Conjugated electron donor-acceptor hybrid polymeric carbon nitride as a photocatalyst for CO2 reduction. Molecules 2019, 24, 1779. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Zhang, Y.; Qiu, J.; Tan, Z.; Xing, Y.; Liu, S. Tunable donor-acceptor linear conjugated polymers involving cyanostyrylthiophene linkages for visible-light-driven hydrogen production. Molecules 2023, 28, 2203. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Long, X.; Jin, X.; Zhu, Y.; Duan, S.; Zhao, J. Triazine and fused thiophene-based donor-acceptor type semiconducting conjugated polymer for enhanced visible-light-induced H2 production. Molecules 2024, 29, 2807. [Google Scholar] [CrossRef]
- Szuromi, P. Tuning band gaps with three halides. Science 2020, 367, 1086–1088. [Google Scholar]
- Niu, W.; Ma, J.; Feng, X. Precise structural regulation and band-gap engineering of curved graphene nanoribbons. Acc. Chem. Res. 2022, 55, 3322–3333. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, W.; Ke, X.; Cai, X.; Chen, X.; Wang, S.; Lin, W.; Wang, X. A heptazine-based polymer photocatalyst with donor-acceptor configuration to promote exciton dissociation and charge separation. Appl. Catal. B Environ. 2023, 325, 122312. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, N.; Zhang, C.; Wang, X.; Zeng, J.; Chen, Y.; Wang, F.; Jiang, J.-X. Rational design of donor-π-acceptor conjugated microporous polymers for photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Shu, C.; Han, C.; Yang, X.; Zhang, C.; Chen, Y.; Ren, S.; Wang, F.; Huang, F.; Jiang, J.X. Boosting the photocatalytic hydrogen evolution activity for D–π–A conjugated microporous polymers by statistical copolymerization. Adv. Mater. 2021, 33, 2008498. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Shen, Z.-Q.; Cheng, J.-Z.; Liu, L.-L.; Yang, K.; Chen, X.; Wen, H.-R.; Liu, S.-Y. C–H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent. J. Mater. Chem. A 2019, 7, 24222–24230. [Google Scholar] [CrossRef]
- Gong, H.; Li, J.; Xie, Z.-H.; Lang, C.; Liu, S.-Y. Accessing poly (DA-ran-Dπ) ternary copolymers via direct C–H arylation for ultrahigh photocatalytic hydrogen production. Macromolecules 2024, 57, 7208–7218. [Google Scholar] [CrossRef]
- Lang, C.; Gong, H.; Ye, G.; Murugan, P.; Xie, Z.-H.; Dai, Y.-F.; Yang, K.; Yu, C.; Liu, S.-Y. D1-D2-Aternary conjugated microporous polymers synthesized via direct CH arylation for enhancing photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2025, 688, 818–829. [Google Scholar] [CrossRef]
- Wu, K.; Liu, X.-Y.; Xie, M.; Cheng, P.-W.; Zheng, J.; Lu, W.; Li, D. Rational design of D−π−A−π−D porous organic polymer with polarized π for photocatalytic aerobic oxidation. Appl. Catal. B Environ. 2023, 334, 122847. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, Z.; Wang, S.; Wang, J.; Xu, Z.; Song, F.; Dong, Z.; Zhang, Z. Novel donor-acceptor-acceptor ternary conjugated microporous polymers with boosting forward charge separation and suppressing backward charge recombination for photocatalytic reduction of uranium (VI). Appl. Catal. B Environ. 2022, 301, 120819. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, C.; Zhao, D.; Wang, Z.; Mao, D. Adsorption behavior of a ternary covalent organic polymer anchored with SO3H for ciprofloxacin. Molecules 2023, 28, 6941. [Google Scholar] [CrossRef]
- Sprick, R.S.; Jiang, J.X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Niu, Y.; Razzaque, S.; Tan, B.; Jin, S. Design of D–A1–A2 covalent triazine frameworks via copolymerization for photocatalytic hydrogen evolution. ACS Catal. 2019, 9, 9438–9445. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.-Y.; Huang, X.-M.; Yang, L.-J.; Liu, P.; Chen, N.; Li, C.-Z.; Liu, S.-Y. Synthesis of Long Chain Oligomeric Donor and Acceptors via Direct Arylation for Organic Solar Cells. Chin. J. Chem. 2024, 42, 523–532. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Chen, N.; Liu, S.-Y. Stepwise Extended π−Conjugation Lengths of Chlorinated Oligomeric Non-fullerene Acceptors Accessed via Direct C−H Arylation. Acta Polym. Sin. 2023, 54, 1122–1130. [Google Scholar]
- Xie, Z.H.; Ye, G.; Gong, H.; Pachaiyappan, M.; Lang, C.; Dai, Y.F.; Liu, S.Y. Ultrahigh photocatalytic hydrogen evolution of linear conjugated terpolymer enabled by an ultra-low ratio of benzothiadiazole monomer. Chem. Sci. 2025. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Ding, C.; Xu, J. Boosting charge separation and photocatalytic CO2 reduction of CsPbBr3 perovskite quantum dots by hybridizing with P3HT. Chem. Eng. J. 2021, 419, 129543. [Google Scholar] [CrossRef]
- Biswal, B.P.; Vignolo-González, H.A.; Banerjee, T.; Grunenberg, L.; Savasci, G.; Gottschling, K.; Nuss, J.; Ochsenfeld, C.; Lotsch, B.V. Sustained solar H2 evolution from a thiazolo, [.5.; 4-d] thiazole-bridged covalent organic framework nickel-thiolate cluster in water. J. Am. Chem. Soc. 2019, 141, 11082–11092. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Bi, S.; Wang, W.; He, Y.; Wu, D.; Liang, Q.; Wang, X.; Zhang, F. Vinylene-linked covalent organic frameworks (COFs) with symmetry-tuned polarity and photocatalytic activity. Angew. Chem. 2020, 132, 24053–24061. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Cong, M.; Liu, X.; Zhang, Z.; Bahri, M.; Li, B.; Yang, J.; Yu, M.; Liu, L.; et al. Nanoscale covalent organic frameworks for enhanced photocatalytic hydrogen production. Nat. Commun. 2024, 15, 6482. [Google Scholar] [CrossRef]
- Li, L.; Cai, Z.; Wu, Q.; Lo, W.Y.; Zhang, N.; Chen, L.X.; Yu, L. Rational design of porous conjugated polymers roles of residual palladium for photocatalytic hydrogen production. J. Am. Chem. Soc. 2016, 138, 7681–7686. [Google Scholar] [CrossRef]
- Cervo, R.; Brandl, C.A.; Bortolotto, T.; Cechin, C.N.; Daudt, N.D.F.; Iglesias, B.A.; Lang, E.S.; Tirloni, B.; Cargnelutti, R. Structural Analysis of Selenium Coordination Compounds and Mesoporous TiO2-Based Photocatalysts for Hydrogen Generation. Inorg. Chem. 2025, 64, 7902–7919. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Y.; Yu, C.; Wang, Z.; Jin, C.; Li, Z.; Yin, S. Impact of the Donor–Acceptor Structure on Photocatalytic Hydrogen Generation by Polyfluorene Polymer Dots. ACS Appl. Polym. Mater. 2025, 7, 3399–3408. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Wang, K.; Wang, J.; Wu, X.; Wang, G. Sponge-like inorganic–organic S-scheme heterojunction for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2025, 692, 137475. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, L.; Sheng, Y.; Song, L.; Zhou, H.; Jian, J.; Huang, T.; Liu, B.; Li, X. Defects enriched carbon nitride sponge with high surface area for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2025, 688, 59–66. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W.; Zhang, Y.; Jia, X.; Sun, J.; Zhang, C.; Liu, M. Apost-modified donor–acceptor covalent organic framework for enhanced photocatalytic H2 production high proton transport. J. Mater. Chem. A 2024, 12, 28161–28169. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, X.; Zhang, C.; Yang, Y.; Ding, X.; Ding, D.; Chai, B.; Dai, K.; Chen, H. Thienyl-fused dibenzothiophene-S, S-dioxide based conjugated polymer toward highly efficient photocatalytic hydrogen production. Int. J. Hydrog. Energy 2024, 80, 115–124. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.E.; Wang, W.R.; Li, Q.; Liu, L.N.; Xu, Z.W.; Gao, T.; Wang, Y.; Li, W.S. Conjugated diacetylene polymers with intrachain DA block heterostructure for efficient photocatalytic hydrogen production. Polymer 2024, 308, 127334. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, W.; Zhang, G.; Chen, Y. Interface molecular wires induce electron transfer from COFs to Pt for enhanced photocatalytic H2 evolution. J. Mater. Chem. A 2023, 11, 26052–26062. [Google Scholar] [CrossRef]
- Zhang, G.; Elewa, A.M.; Rashad, M.; Helali, S.; Chou, H.H.; EL-Mahdy, A.F. Triazine-and porphyrin-based donor-acceptor microporous conjugated polymers for enhanced photocatalytic hydrogen production activity. Microporous Mesoporous Mater. 2023, 363, 112824. [Google Scholar] [CrossRef]



| Material | Pd2(dba)3 a (mol%) | W (Pd) b (wt%) | H2 Evolution Rate (mmol h−1 g−1) |
|---|---|---|---|
| Py-BKh1-2 | 2.0 | 0.6636 | 8.8 |
| Py-BKh1-4 | 4.0 | 0.6154 | 10.2 |
| Py-BKh1-6 | 6.0 | 0.6347 | 12.5 |
| Pub Date | Materials | H2 Yield (mmol h−1 g−1) | AQY | Ref. |
|---|---|---|---|---|
| This Work | Py-BKh1 | 10.2 | 9.5% (500 nm) | - |
| 2025.4 | M-TiO2-7 | 1.3 | - | [51] |
| 2025.3 | ST@BTTA-120 | 3.69 | - | [52] |
| 2025.3 | PFBT-Pdots | 2.23 | - | [53] |
| 2025.2 | SCN-0.5 | 1.67 | 5.8% (420 nm) | [54] |
| 2024.9 | PyBT-COF-COOH | 8.15 | 5.1% (420 nm) | [55] |
| 2024.7 | Py-T-BTDO-3 | 8.66 | 4.42% (420 nm) | [56] |
| 2024.6 | PDTPDPA-BP | 2.16 | - | [57] |
| 2023.11 | NPs-TpPa-1 | 1.13 | 13.5% (450 nm) | [58] |
| 2023.9 | Zn-Por-TPT | 11.45 | - | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.-H.; Zhang, Y.-J.; Li, J.; Liu, S.-Y. D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production. Molecules 2025, 30, 2190. https://doi.org/10.3390/molecules30102190
Xie Z-H, Zhang Y-J, Li J, Liu S-Y. D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production. Molecules. 2025; 30(10):2190. https://doi.org/10.3390/molecules30102190
Chicago/Turabian StyleXie, Zheng-Hui, Yu-Jie Zhang, Jinhua Li, and Shi-Yong Liu. 2025. "D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production" Molecules 30, no. 10: 2190. https://doi.org/10.3390/molecules30102190
APA StyleXie, Z.-H., Zhang, Y.-J., Li, J., & Liu, S.-Y. (2025). D1-A-D2 Conjugated Porous Polymers Provide Additional Electron Transfer Pathways for Efficient Photocatalytic Hydrogen Production. Molecules, 30(10), 2190. https://doi.org/10.3390/molecules30102190








