1H Nuclear Magnetic Resonance-Based Targeted and Untargeted Metabolomics Profiling of Retail Samples of Cuachalalate (Amphipterygium adstringens)
Abstract
1. Introduction
2. Results and Discussion
2.1. Hexane Extracts as Criterion to Autentify the Samples Studied
2.2. Metabolomic Profiling of the Aqueous Extracts of Amphipterygium adstringens
2.3. Multivariate Statistical Analyses
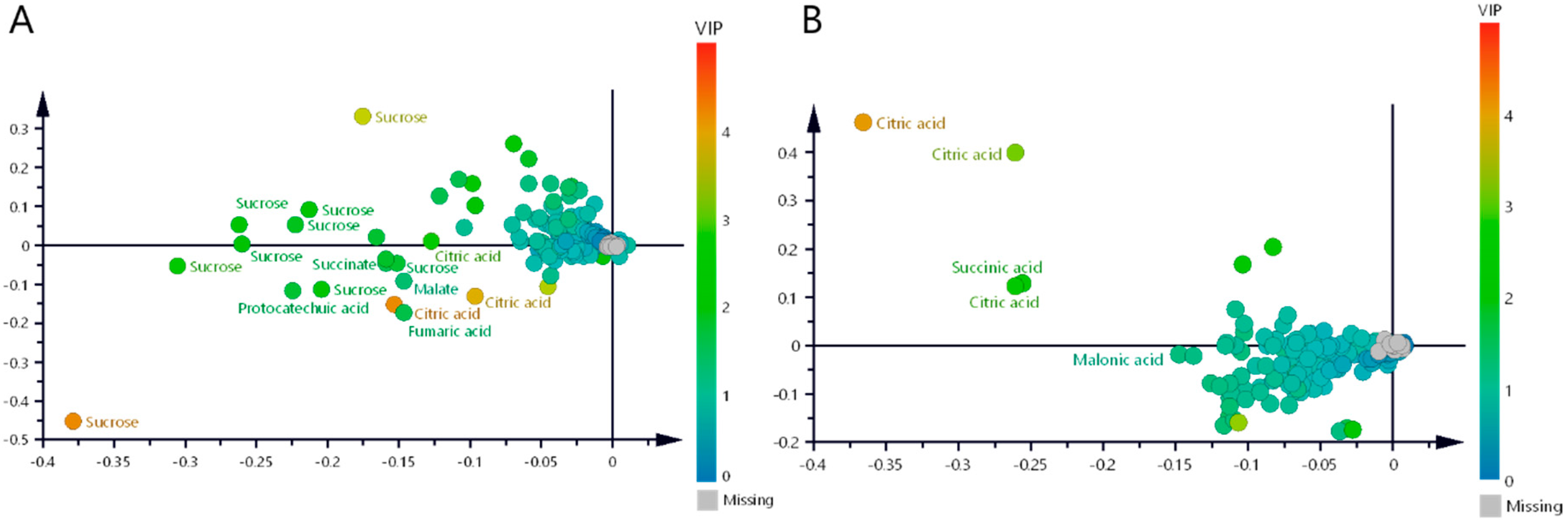
2.3.1. Aqueous Extracts—Untargeted Metabolomic Profiles of Cuachalalate Samples
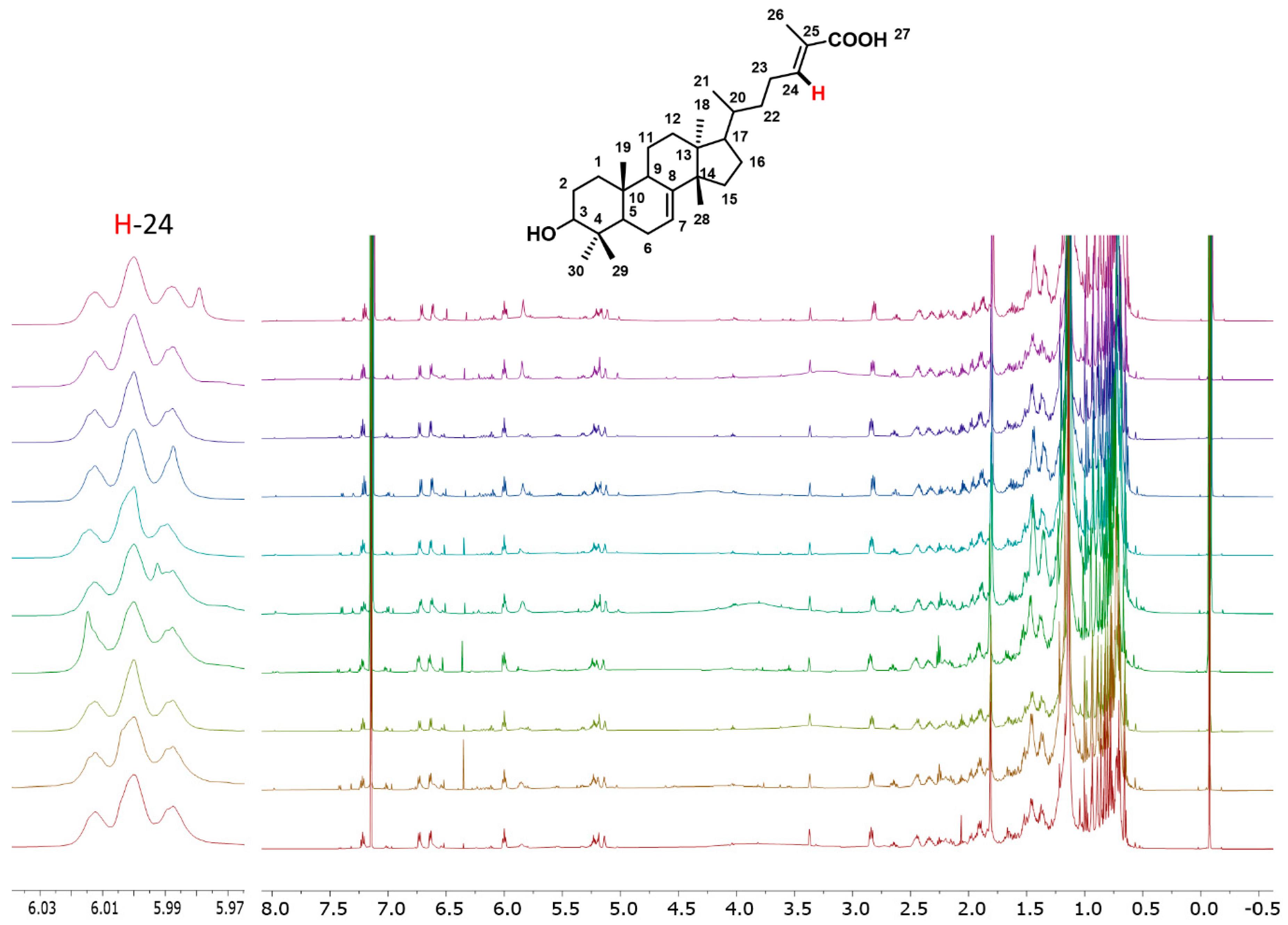
2.3.2. Organic Extract—Targeting the Analysis Through 3α-HMDA and Anacardic Acids
3. Materials and Methods
3.1. Collection and Preparation of Botanical Material
3.2. Obtaining Aqueous Extract from Cuachalalate Samples
3.3. Obtaining Organic Extracts from Cuachalalate
3.4. Acquisition of the 1H NMR Spectra
3.4.1. 1H NMR of Aqueous Samples
3.4.2. 1H NMR of Organic Samples
3.5. Metabolites Identification on the 1H NMR Spectra
3.6. 1H NMR Data Processing
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Chemistry and Biology of Selected Mexican Medicinal Plants. In Progress in the Chemistry of Organic Natural Products 108; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2019; Volume 108. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domínguez, F.; Maldonado-Miranda, J.J.; Castillo-Pérez, L.J.; Carranza-Álvarez, C.; Solano, E.; Orozco-Castellanos, L.M. Use of medicinal plants by health professionals in Mexico. J. Ethnopharmacol. 2017, 198, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Piñón, Y.; Mejía, A.; Díaz-Ruiz, G.; Aguilar, M.I.; Sánchez-Nieto, S.; Rivero-Cruz, J.F. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. J. Ethnopharmacol. 2012, 141, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ruiz, M.G.; Gómez-Velasco, A.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Exploring the biological activities of Echeveria leucotricha. Nat. Prod. Res. 2013, 27, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.E. Helicobacter pylori and its role in gastric cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef]
- Castillo-Juárez, I.; González, V.; Jaime-Aguilar, H.; Martínez, G.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J. Ethnopharmacol. 2009, 122, 402–405. [Google Scholar] [CrossRef]
- Palacios-Espinosa, J.F.; Núñez-Aragón, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of Artemisia ludoviciana subsp. mexicana and two of its bioactive components, estafiatin and eupatilin. Molecules 2021, 26, 3654. [Google Scholar] [CrossRef]
- Solares, F.; Vázquez-Alvarado, J.; Gálvez-Cortés, M. Commercialization channels of cuachalalate (Amphipterygium adstringens Schiede ex Schlecht.) bark in Mexico. Rev. Mex. Cienc. For. 2012, 3, 29–42. [Google Scholar]
- Galván, M.F.V. El Tronco “Mágico” del Cuachalalate: Regalo de la Medicina Tradicional Mexicana a la Química de Productos Naturales; Universidad de Guanajuato: Guanajuato, Mexico, 2019. [Google Scholar]
- Pérez-Contreras, C.V.; Alvarado-Flores, J.; Orona-Ortiz, A.; Balderas-López, J.L.; Salgado, R.M.; Zacaula-Juárez, N.; Navarrete, A. Wound healing activity of the hydroalcoholic extract and the main metabolites of Amphipterygium adstringens (cuachalalate) in a rat excision model. J. Ethnopharmacol. 2022, 293, 115313. [Google Scholar] [CrossRef]
- Castillo-Juárez, I.; Rivero-Cruz, F.; Celis, H.; Romero, I. Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J. Ethnopharmacol. 2007, 114, 72–77. [Google Scholar] [CrossRef]
- Sotelo-Barrera, M.; Cília-García, M.; Luna-Cavazos, M.; Díaz-Núñez, J.L.; Romero-Manzanares, A.; Soto-Hernández, R.M.; Castillo-Juárez, I. Amphipterygium adstringens (Schltdl.) Schiede Ex Standl (Anacardiaceae): An Endemic Plant with Relevant Pharmacological Properties. Plants 2022, 11, 1766. [Google Scholar] [CrossRef]
- Esquivel-García, R.; Ayiania, M.; Abu-Lail, N.; López-Meza, J.E.; del Río, R.E.; García-Pérez, M.; García-Pérez, M.E. Pyrolytic oils from Amphipterygium adstringens bark inhibit IL-8 production of IL-17-stimulated HaCaT keratinocytes. J. Anal. Appl. Pyrolysis 2020, 145, 104749. [Google Scholar] [CrossRef]
- Gonzalez, E.E.; Delgado, J.N. Phytochemical investigation of Amphipterygium adstringens. J. Pharm. Sci. 1962, 51, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Canales, M.; Jiménez-Rivas, R.; Canales-Martínez, M.M.; García-López, A.J.; Rivera-Yañez, N.; Nieto-Yañez, O.; Rodríguez-Monroy, M.A. Protective effect of Amphipterygium adstringens extract on dextran sulphate sodium-induced ulcerative colitis in mice. Mediators Inflamm. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Makino, M.; Motegi, T.; Fujimoto, Y. Tirucallane-type triterpenes from Juliania adstringens. Phytochemistry 2004, 65, 891–896. [Google Scholar] [CrossRef]
- Díaz-Sánchez, L.; Zentella-Dehesa, A.; Castro-Torres, V.A.; Silva-Jiménez, N.; Jacobo-Herrera, N.J.; Martínez-Vázquez, M. Evaluations of anticancer effects of combinations of cisplatin and tirucallane-type triterpenes isolated from Amphipterygium adstringens (Schltdl). Chem. Biodivers. 2023, 20, e202300893. [Google Scholar] [CrossRef] [PubMed]
- Cofepris. RCC7-Herbolarios. 2022. Available online: https://www.gob.mx/cms/uploads/attachment/file/777503/RCC7-Herbolarios.PDF (accessed on 15 March 2025).
- Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos. Farmacopea Herbolaria de los Estados Unidos Mexicanos 3.0; Secretaría de Salud: Mexico City, Mexico, 2021; Available online: https://www.farmacopea.org.mx/publicaciones-detalle.php?m=3&pid=11 (accessed on 15 March 2025).
- Shaheen, S.; Ramzan, S.; Khan, F.; Ahmad, M. Adulteration in Herbal Drugs: A Burning Issue; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Rainha, N.; Medeiros, V.P.; Ferreira, C.; Raposo, A.; Leite, J.P.; Cruz, C.; Pacheco, C.A.; Ponte, D.; Silva, A.B. Leaf malate and succinate accumulation are out of phase throughout the development of the CAM plant Ananas comosus. Plant Physiol. Biochem. 2016, 100, 47–51. [Google Scholar] [CrossRef]
- Zhuoma, Y.; Yang, M.; Chen, Y.; Zhang, X.; Duan, X.; Cui, H.; Hu, X. NMR-Based Metabolomics Analysis of Metabolite Profiles in Two Species of Boletes Subjected to Different Drying Methods. Metabolites 2025, 15, 152. [Google Scholar] [CrossRef]
- Ochoa-Jiménez, V.A.; Berumen-Varela, G.; Pérez-Ramírez, I.F.; Balois-Morales, R.; Rubio-Melgarejo, A.; Bautista-Rosales, P.U. Metabolomics approach for phenolic compounds profiling of soursop (Annona muricata L.) fruit during postharvest storage. Metabolomics 2024, 20, 26. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, W.; Xiong, M.; Gao, J.; Zhou, H.; Chen, D.; Li, Y. Metabolomics investigation of the chemical variations in white teas with different producing areas and storage durations. Food Chem. X 2024, 21, 101127. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Salgado, M.D.R.H.; Beltrán-Gómez, J.Á.; Díaz-Núñez, J.L.; Rivera-Chávez, J.A.; García-Contreras, R.; Estrada-Velasco, Á.Y.; Castillo-Juárez, I. Efficacy of a Mexican folk remedy containing cuachalalate (Amphipterygium adstringens (Schltdl.) Schiede ex Standl) for the treatment of burn wounds infected with Pseudomonas aeruginosa. J. Ethnopharmacol. 2024, 319, 117305. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Quevedo, M.G.; Gracia-Fadrique, J. Amphipterygium adstringens (cuachalalate) extract by supercritical CO2. Chem. Thermodyn. Therm. Anal. 2024, 13, 100128. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Jurado, E.A.; Terán-Sánchez, E.D.J.; Serrano-Contreras, J.I.; Zepeda-Vallejo, L.G. 1H Nuclear Magnetic Resonance-Based Targeted and Untargeted Metabolomics Profiling of Retail Samples of Cuachalalate (Amphipterygium adstringens). Molecules 2025, 30, 2185. https://doi.org/10.3390/molecules30102185
Herrera-Jurado EA, Terán-Sánchez EDJ, Serrano-Contreras JI, Zepeda-Vallejo LG. 1H Nuclear Magnetic Resonance-Based Targeted and Untargeted Metabolomics Profiling of Retail Samples of Cuachalalate (Amphipterygium adstringens). Molecules. 2025; 30(10):2185. https://doi.org/10.3390/molecules30102185
Chicago/Turabian StyleHerrera-Jurado, Erick Alejandro, Estefanía De Jesús Terán-Sánchez, José Iván Serrano-Contreras, and L. Gerardo Zepeda-Vallejo. 2025. "1H Nuclear Magnetic Resonance-Based Targeted and Untargeted Metabolomics Profiling of Retail Samples of Cuachalalate (Amphipterygium adstringens)" Molecules 30, no. 10: 2185. https://doi.org/10.3390/molecules30102185
APA StyleHerrera-Jurado, E. A., Terán-Sánchez, E. D. J., Serrano-Contreras, J. I., & Zepeda-Vallejo, L. G. (2025). 1H Nuclear Magnetic Resonance-Based Targeted and Untargeted Metabolomics Profiling of Retail Samples of Cuachalalate (Amphipterygium adstringens). Molecules, 30(10), 2185. https://doi.org/10.3390/molecules30102185






