Probing the Interaction of Diester Internal Donors (ID) with AlEt3 on Ziegler-Natta Surfaces: A Comparison Between Binary (MgCl2/ID) and Ternary (MgCl2/ID/TiCl4) Formulations
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cecchin, G.; Morini, G.; Piemontesi, F.; Seidel, A. Ziegler-Natta Catalysts. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: Hoboken, NJ, USA, 2007; Volume 26. [Google Scholar]
- Antinucci, G.; Cipullo, R.; Busico, V. Imagine Polypropylene. Nat. Catal. 2023, 6, 456–457. [Google Scholar] [CrossRef]
- Pasquini, N. Polypropylene Handbook, 2nd ed.; Hanser Publishers: Munich, Germany, 2005. [Google Scholar]
- Soga, K.; Shiono, T. Ziegler-Natta Catalysts for Olefin Polymerizations. Prog. Polym. Sci. 1997, 22, 1503–1546. [Google Scholar] [CrossRef]
- Albizzati, E.; Giannini, U.; Morini, G.; Galimberti, M.; Barino, L.; Scordamaglia, R. Recent Advances in Propylene Polymerization with MgCl2 Supported Catalysts. Macromol. Symp. 1995, 89, 73–89. [Google Scholar] [CrossRef]
- Noristi, L.; Barbè, P.C.; Baruzzi, G. Effect of the Internal/External Donor Pair in High-Yield Catalysts for Propylene Polymerization, 1. Catalyst-Cocatalyst Interactions. Die Makromol. Chem. 1991, 192, 1115–1127. [Google Scholar] [CrossRef]
- Morini, G.; Albizzati, E.; Balbontin, G.; Mingozzi, I.; Sacchi, M.C.; Forlini, F.; Tritto, I. Microstructure Distribution of Polypropylenes Obtained in the Presence of Traditional Phthalate/Silane and Novel Diether Donors: A Tool for Understanding the Role of Electron Donors in MgCl2-Supported Ziegler-Natta Catalysts. Macromolecules 1996, 29, 5770–5776. [Google Scholar] [CrossRef]
- Wondimagegn, T.; Ziegler, T. The Role of External Alkoxysilane Donors on Stereoselectivity and Molecular Weight in MgCl2-Supported Ziegler–Natta Propylene Polymerization: A Density Functional Theory Study. J. Phys. Chem. C 2012, 116, 1027–1033. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. The Use of Donors to Increase the Isotacticity of Polypropylene. In Polyolefins: 50 Years after Ziegler and Natta, I.; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–97. [Google Scholar]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler–Natta Catalysts: The Origin of Stereoselectivity. ACS Catal. 2017, 7, 4509–4518. [Google Scholar] [CrossRef]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key Elements in the Structure and Function Relationship of the MgCl2/TiCl4/Lewis Base Ziegler-Natta Catalytic System. Macromolecules 2007, 40, 9181–9189. [Google Scholar] [CrossRef]
- Busico, V.; Corradini, P.; De Martino, L.; Proto, A.; Savino, V.; Albizzati, E. Polymerization of Propene in the Presence of MgCl2-Supported Ziegler-Natta Catalysts, 1. The Role of Ethyl Benzoate as “Internal” and “External” Base. Die Makromol. Chem. 1985, 186, 1279–1288. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tritto, I.; Shan, C.; Mendichi, R.; Noristi, L. Role of the Pair of Internal and External Donors in MgCl2-Supported Ziegler-Natta Catalysts. Macromolecules 1991, 24, 6823–6826. [Google Scholar] [CrossRef]
- Scordamaglia, R.; Barino, L. Theoretical Predictive Evaluation of New Donor Classes in Ziegler-Natta Heterogeneous Catalysis for Propene Isospecific Polymerization. Macromol. Theory Simul. 1998, 7, 399–405. [Google Scholar] [CrossRef]
- Cannavacciuolo, F.D.; Falivene, L.; Zhang, Z.; Takasao, G.; De Canditiis, D.; Khoshsefat, M.; Chammingkwan, P.; Antinucci, G.; Taniike, T.; Cipullo, R.; et al. Data Driven Modeling of Ziegler–Natta Polypropylene Catalysts: Revisiting the Role of the Internal Donor. ACS Catal. 2025, 15, 5770–5780. [Google Scholar] [CrossRef]
- Zaccaria, F.; Vittoria, A.; Correa, A.; Ehm, C.; Budzelaar Peter, H.M.; Busico, V.; Cipullo, R. Internal Donors in Ziegler–Natta Systems: Is Reduction by AlR3 a Requirement for Donor Clean-Up? ChemCatChem 2018, 10, 984–988. [Google Scholar] [CrossRef]
- Langer, A.W.; Burkhardt, T.J.; Steger, J.J. Supported Catalysts for Polypropylene: Aluminum Alkyl-Ester Chemistry. In Coordination Polymerization; Price, C.C., Vandenberg, E.J., Eds.; Springer: Boston, MA, USA, 1983; pp. 225–248. [Google Scholar]
- Chien, J.C.W.; Wu, J.-C. Magnesium-Chloride-Supported High-Mileage Catalysts for Olefin Polymerization. II. Reactions between Aluminum Alkyl and Promoters. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 2445–2460. [Google Scholar] [CrossRef]
- Yu, Y.; Busico, V.; Budzelaar, P.H.M.; Vittoria, A.; Cipullo, R. Of Poisons and Antidotes in Polypropylene Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8590–8594. [Google Scholar] [CrossRef]
- Terano, M.; Kataoka, T.; Keii, T. Stopped Flow Polymerization of Propene with Typical MgCl2-Supported High-Yield Catalysts. J. Mol. Catal. 1989, 56, 203–210. [Google Scholar] [CrossRef]
- Taniike, T.; Sano, S.; Ikeya, M.; Thang, V.Q.; Terano, M. Development of a Large-Scale Stopped-Flow System for Heterogeneous Olefin Polymerization Kinetics. Macromol. React. Eng. 2012, 6, 275–279. [Google Scholar] [CrossRef]
- Thakur, A.; Wada, T.; Chammingkwan, P.; Terano, M.; Taniike, T. Development of Large-Scale Stopped-Flow Technique and Its Application in Elucidation of Initial Ziegler-Natta Olefin Polymerization Kinetics. Polymers 2019, 11, 1012. [Google Scholar] [CrossRef]
- Taniike, T.; Cannavacciuolo, F.D.; Khoshsefat, M.; De Canditiis, D.; Antinucci, G.; Chammingkwan, P.; Cipullo, R.; Busico, V. End-to-End High-Throughput Approach for Data-Driven Internal Donor Development in Heterogeneous Ziegler-Natta Propylene Polymerization. ACS Catal. 2024, 14, 7589–7599. [Google Scholar] [CrossRef]
- Yakimov, A.; Xu, J.; Searles, K.; Liao, W.C.; Antinucci, G.; Friederichs, N.; Busico, V.; Copéret, C. DNP-SENS Formulation Protocols to Study Surface Sites in Ziegler-Natta Catalyst MgCl2 Supports Modified with Internal Donors. J. Phys. Chem. C 2021, 125, 15994–16003. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Gaffet, E.; Louison, C.; Harmelin, M.; Faudot, F. Metastable Phase Transformations Induced by Ball-Milling in the Cu-W System. Mater. Sci. Eng. A 1991, 134, 1380–1384. [Google Scholar] [CrossRef]
- Mori, H.; Sawada, M.; Higuchi, T.; Hasebe, K.; Otsuka, N.; Terano, M. Direct Observation of MgCl2-Supported Ziegler Catalysts by High Resolution Transmission Electron Microscopy. Macromol. Rapid Commun. 1999, 20, 245–250. [Google Scholar] [CrossRef]
- McKenna, T.F.L.; Di Martino, A.; Weickert, G.; Soares, J.B.P. Particle Growth During the Polymerisation of Olefins on Supported Catalysts, 1—Nascent Polymer Structures. Macromol. React. Eng. 2010, 4, 40–64. [Google Scholar] [CrossRef]
- Andoni, A.; Chadwick, J.C.; Niemantsverdriet, H.J.W.; Thune, P.C. A Flat Model Approach to Ziegler-Natta Catalysts for Propylene Polymerization and a Preparation Method of Well-Defined Crystallites of MgCl2-Supported Catalysts. Macromol. Symp. 2007, 260, 140–146. [Google Scholar] [CrossRef]
- Partin, D.E.; O’Keeffe, M. The Structures and Crystal Chemistry of Magnesium Chloride and Cadmium Chloride. J. Solid State Chem. 1991, 95, 176–183. [Google Scholar] [CrossRef]
- Malizia, F.; Fait, A.; Cruciani, G. Crystal Structures of Ziegler–Natta Catalyst Supports. Chem. A Eur. J. 2011, 17, 13892–13897. [Google Scholar] [CrossRef]
- Zannetti, R.; Marega, C.; Marigo, A.; Martorana, A. Layer Lattices in Ziegler–Natta Catalysts. J. Polym. Sci. Part B Polym. Phys. 1988, 26, 2399. [Google Scholar] [CrossRef]
- Wada, T.; Takasao, G.; Piovano, A.; D’Amore, M.; Thakur, A.; Chammingkwan, P.; Bruzzese, P.C.; Terano, M.; Civalleri, B.; Bordiga, S.; et al. Revisiting the Identity of δ-MgCl2: Part, I. Structural Disorder Studied by Synchrotron X-Ray Total Scattering. J. Catal. 2020, 385, 76–86. [Google Scholar] [CrossRef]
- Piovano, A.; D’Amore, M.; Wada, T.; Cleto Bruzzese, P.; Takasao, G.; Thakur, A.; Chammingkwan, P.; Terano, M.; Civalleri, B.; Bordiga, S.; et al. Revisiting the Identity of δ-MgCl2: Part II. Morphology and Exposed Surfaces Studied by Vibrational Spectroscopies and DFT Calculation. J. Catal. 2020, 387, 1–11. [Google Scholar] [CrossRef]
- Giunchi, G.; Allegra, G. Structural Disorder in Microcrystalline MgCl2. J. Appl. Crystallogr. 1983, 17, 172–178. [Google Scholar] [CrossRef]
- D’Amore, M.; Thushara, K.S.; Piovano, A.; Causà, M.; Bordiga, S.; Groppo, E. Surface Investigation and Morphological Analysis of Structurally Disordered MgCl2 and MgCl2/TiCl4 Ziegler–Natta Catalysts. ACS Catal. 2016, 6, 5786–5796. [Google Scholar] [CrossRef]
- Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T.; Piovano, A.; Groppo, E. Structural Disorder of Mechanically Activated δ-MgCl2 Studied by Synchrotron X-Ray Total Scattering and Vibrational Spectroscopy. Catalysts 2020, 10, 1089. [Google Scholar] [CrossRef]
- Wada, T.; Takasao, G.; Terano, M.; Chammingkwan, P.; Taniike, T. Structure Determination of the δ-MgCl2 Support in Ziegler-Natta Catalysts. J. Japan Pet. Inst. 2022, 65, 88–96. [Google Scholar] [CrossRef]
- Correa, A.; Talarico, G.; Cavallo, L. Regiochemistry of Propene Insertion with Group 4 Polymerization Catalysts from a Theoretical Perspective. J. Organomet. Chem. 2007, 692, 4519–4527. [Google Scholar] [CrossRef]
- Capone, F.; Rongo, L.; D’Amore, M.; Budzelaar, P.H.M.; Busico, V. Periodic Hybrid DFT Approach (Including Dispersion) to MgCl2-Supported Ziegler–Natta Catalysts. 2. Model Electron Donor Adsorption on MgCl2 Crystal Surfaces. J. Phys. Chem. C 2013, 117, 24345–24353. [Google Scholar] [CrossRef]
- Kuklin, M.S.; Bazhenov, A.S.; Denifl, P.; Leinonen, T.; Linnolahti, M.; Pakkanen, T.A. Stabilization of Magnesium Dichloride Surface Defects by Mono- and Bidentate Donors. Surf. Sci. 2015, 635, 5–10. [Google Scholar] [CrossRef]
- da Silveira, J.M.; Chikuma, H.; Takasao, G.; Wada, T.; Chammingkwan, P.; Taniike, T. Deciphering the Role of Internal Donors in Shaping Heterogeneous Ziegler–Natta Catalysts Based on Nonempirical Structural Determination. ACS Catal. 2024, 14, 2300–2312. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Antinucci, G.; Cannavacciuolo, F.D.; Ehm, C.; Budzelaar, P.H.M.; Cipullo, R.; Busico, V. MgCl2-Supported Ziegler-Natta Catalysts for Propene Polymerization: Before Activation. Macromolecules 2024, 57, 5712–5719. [Google Scholar] [CrossRef]
- Monrabal, B.; Romero, L.; Mayo, N.; Sancho-Tello, J. Advances in Crystallization Elution Fractionation. Macromol. Symp. 2009, 282, 14–24. [Google Scholar] [CrossRef]
- Monrabal, B.; Romero, L. Separation of Polypropylene Polymers by Crystallization and Adsorption Techniques. Macromol. Chem. Phys. 2014, 215, 1818–1828. [Google Scholar] [CrossRef]
- Antinucci, G.; Pucciarelli, A.; Vittoria, A.; Zaccaria, F.; Urciuoli, G.; Ehm, C.; Cannavacciuolo, F.D.; Cipullo, R.; Busico, V. Fast Analytics of High-Impact Polypropylene (HIPP). ACS Appl. Polym. Mater. 2023, 5, 3894–3897. [Google Scholar] [CrossRef]
- Corradini, P.; Barone, V.; Fusco, R.; Guerra, G. Analysis of Models for the Ziegler-Natta Stereospecific Polymerization on the Basis of Non-Bonded Interactions at the Catalytic Site—I. The Cossee Model. Eur. Polym. J. 1979, 15, 1133–1141. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Fusco, R.; Barone, V. Analysis of Models for the Ziegler-Natta Stereospecific Polymerization on the Basis of Non-Bonded Interactions at the Catalytic Site—II: Edges, Steps and Reliefs on the Surface of Layered Modifications of TiCl3. Eur. Polym. J. 1980, 16, 835–842. [Google Scholar] [CrossRef]
- Antinucci, G.; Vittoria, A.; Cipullo, R.; Busico, V. Regioirregular Monomeric Units in Ziegler–Natta Polypropylene: A Sensitive Probe of the Catalytic Sites. Macromolecules 2020, 53, 3789–3795. [Google Scholar] [CrossRef]
- Vittoria, A.; Antinucci, G.; Zaccaria, F.; Cipullo, R.; Busico, V. Monitoring the Kinetics of Internal Donor Clean-up from Ziegler–Natta Catalytic Surfaces: An Integrated Experimental and Computational Study. J. Phys. Chem. C 2020, 124, 14245–14252. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Khoshsefat, M.; Terano, M.; Taniike, T. Parallel Catalyst Synthesis Protocol for Accelerating Heterogeneous Olefin Polymerization Research. Polymers 2023, 15, 4729. [Google Scholar] [CrossRef]
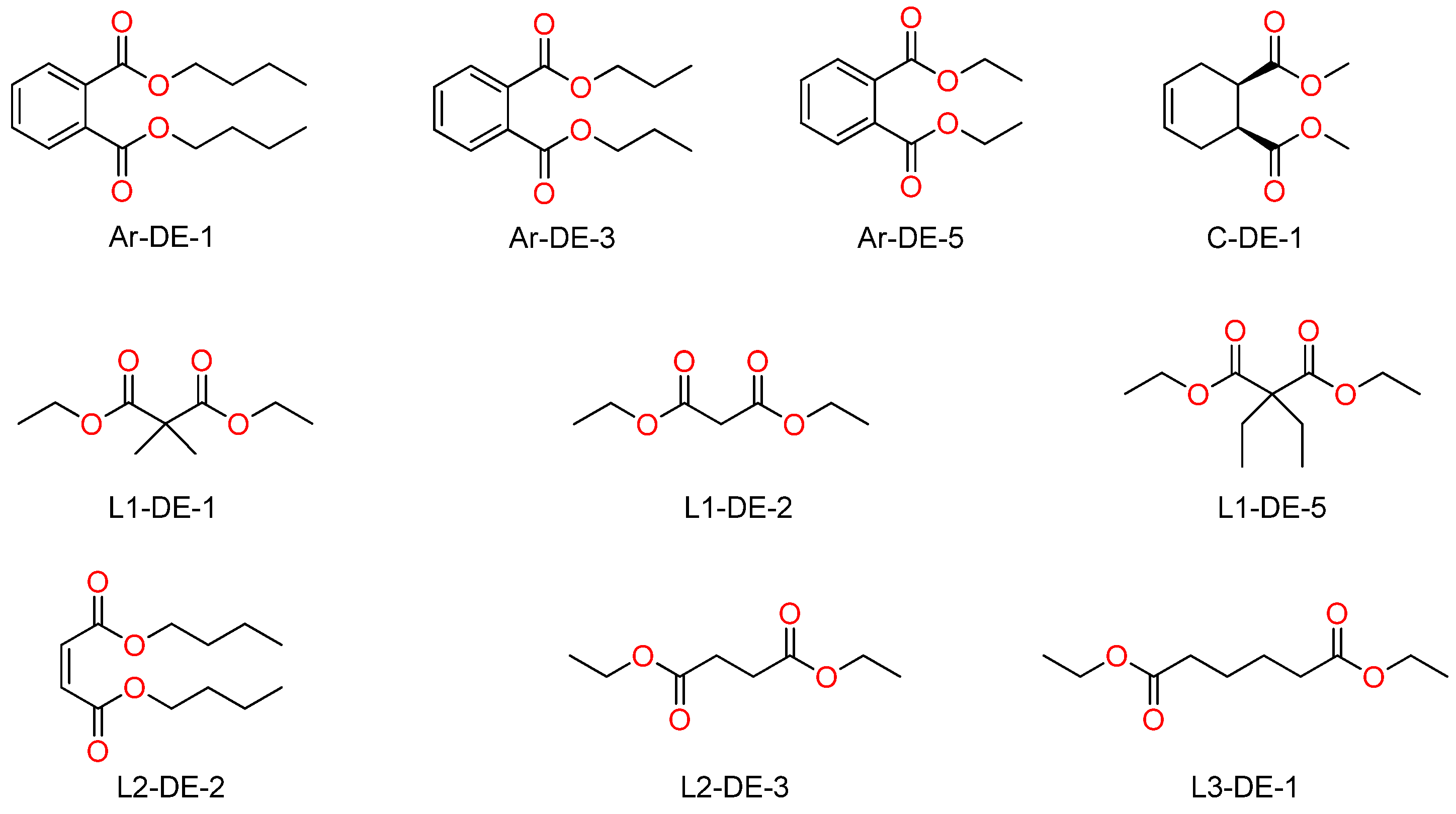
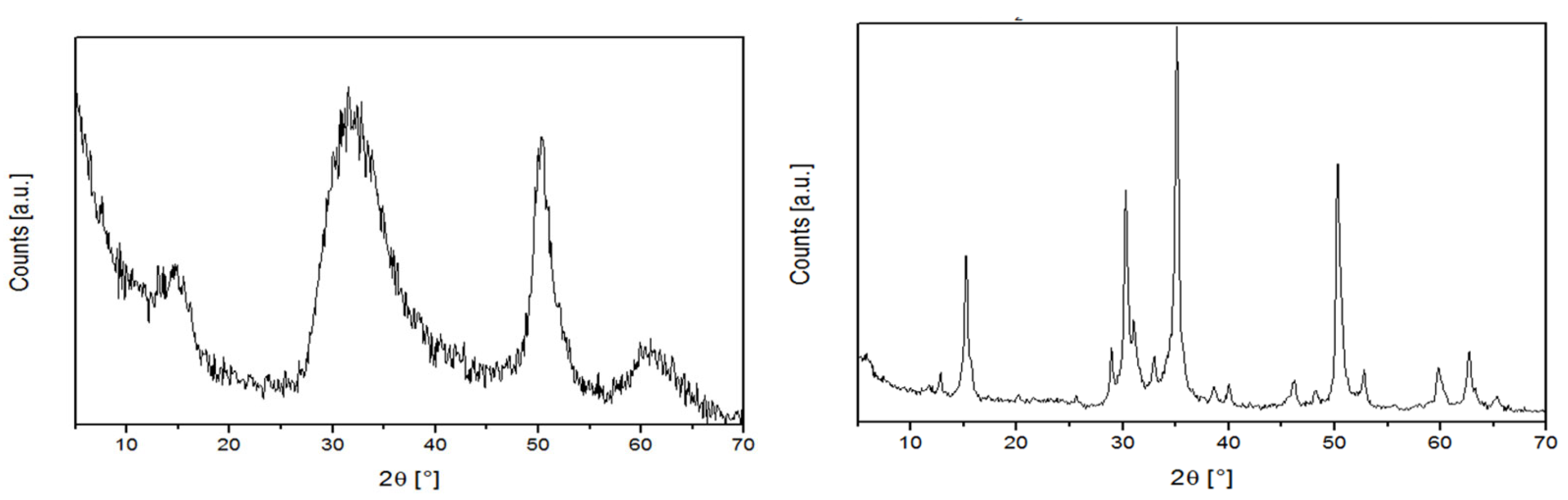
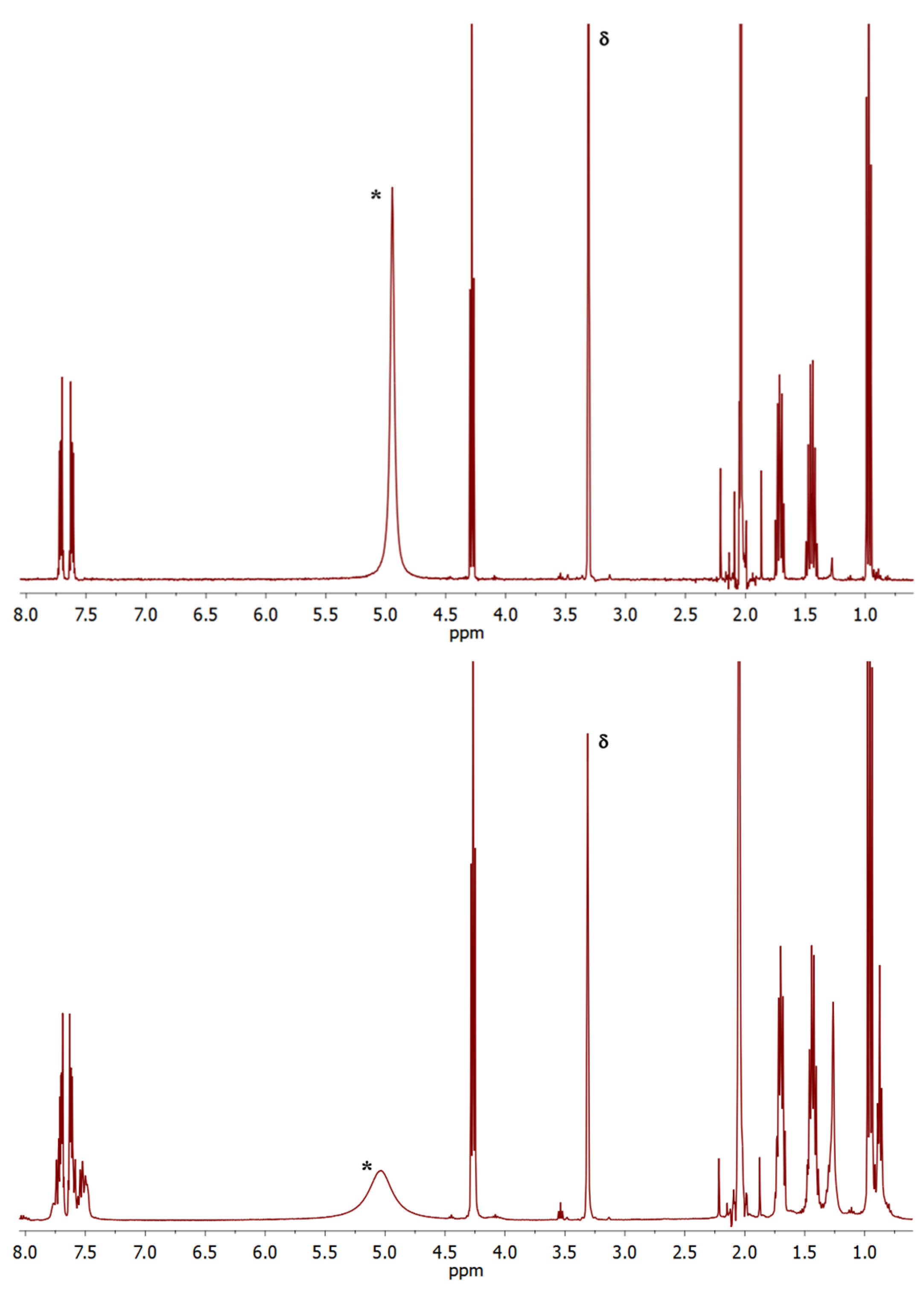
| Sample | Grinding Protocol | <Lc> (nm) | <La> (nm) | nAr-DE-1,total (% wrt Mg) | nAr-DE-1,degraded (% wrt Mg) |
|---|---|---|---|---|---|
| 1 | Harsh-Dry (hD) | 3.0 | 5.2 | 5.9 | 1.8 |
| 2 | Mild-Dry (mD) | 2.4 | 5.6 | 6.0 | 0.8 |
| 3 | Harsh-Wet (hW) | 32.1 | 26.3 | 5.6 | Not detected |
| 4 | Mild-Wet (mW) | 51.0 | 30.3 | 5.7 | Not detected |
| MgCl2/ID | MgCl2/ID/TiCl4 | ||||||
|---|---|---|---|---|---|---|---|
| ID | ID (wt%) | nID (% wrt Mg) | Ti (wt%) | nTi (% wrt Mg) | ID (wt%) | nID (% wrt Mg) | n(ID)/n(Ti) |
| Ar-DE-1 | 12.5 | 5.7 | 2.2 | 5.9 | 13.9 | 6.4 | 1.1 |
| Ar-DE-3 | 11.8 | 6.0 | 1.7 | 4.5 | 12.4 | 6.3 | 1.4 |
| Ar-DE-5 | 13.0 | 7.5 | 1.8 | 4.8 | 13.6 | 7.8 | 1.6 |
| C-DE-1 | 9.8 | 6.5 | 2.4 | 6.4 | 8.2 | 5.3 | 0.8 |
| L1-DE-1 | 7.2 | 4.9 | 2.0 | 5.3 | 9.6 | 6.5 | 1.2 |
| L1-DE-2 | 10.1 | 7.8 | 2.4 | 6.4 | 12.6 | 10.0 | 1.6 |
| L1-DE-5 | 9.2 | 5.5 | 2.7 | 7.2 | 10.2 | 6.0 | 0.8 |
| L2-DE-2 | 8.9 | 6.0 | 2.1 | 5.6 | 15.9 | 10.8 | 1.9 |
| L2-DE-3 | 9.1 | 6.8 | 2.5 | 6.7 | 7.6 | 5.6 | 0.8 |
| L3-DE-1 | 11.7 | 7.9 | 1.8 | 4.8 | 13.5 | 9.2 | 1.9 |
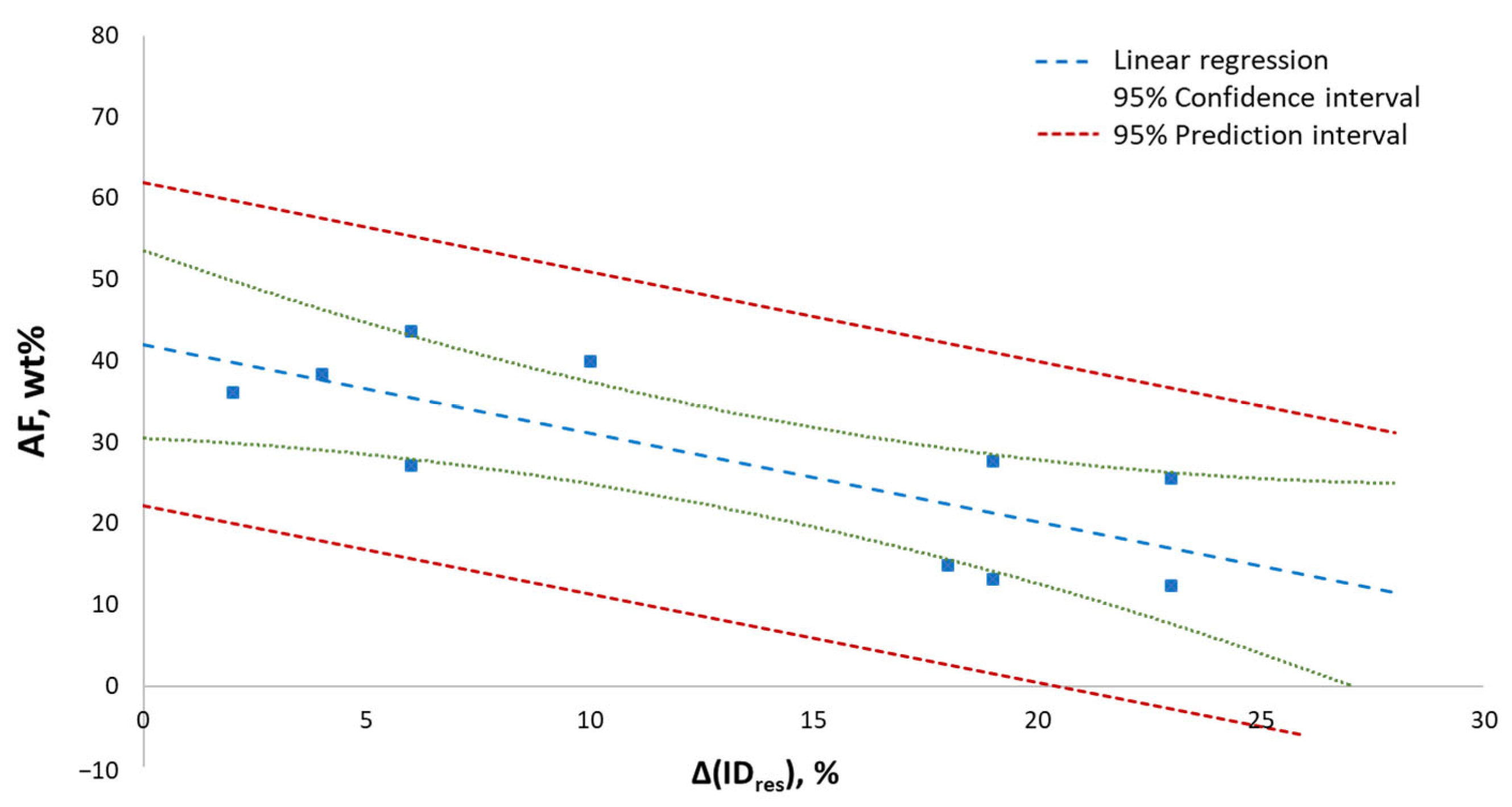
| ID | IDres/binary (%) | IDres/ternary (%) | Δ(IDres) (a) (%) | AF (b) (wt%) |
|---|---|---|---|---|
| Ar-DE-1 | 59 | 78 | 19 | 13.1 |
| Ar-DE-3 | 72 | 90 | 18 | 14.8 |
| Ar-DE-5 | 65 | 88 | 23 | 12.3 |
| C-DE-1 | 68 | 70 | 2 | 36.1 |
| L1-DE-1 | 62 | 81 | 19 | 27.7 |
| L1-DE-2 | 76 | 80 | 4 | 38.4 |
| L1-DE-5 | 67 | 90 | 23 | 25.5 |
| L2-DE-2 | 54 | 60 | 6 | 43.6 |
| L2-DE-3 | 64 | 74 | 10 | 40.0 |
| L3-DE-1 | 59 | 65 | 6 | 27.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavacciuolo, F.D.; Antinucci, G.; Cipullo, R.; Busico, V. Probing the Interaction of Diester Internal Donors (ID) with AlEt3 on Ziegler-Natta Surfaces: A Comparison Between Binary (MgCl2/ID) and Ternary (MgCl2/ID/TiCl4) Formulations. Molecules 2025, 30, 2176. https://doi.org/10.3390/molecules30102176
Cannavacciuolo FD, Antinucci G, Cipullo R, Busico V. Probing the Interaction of Diester Internal Donors (ID) with AlEt3 on Ziegler-Natta Surfaces: A Comparison Between Binary (MgCl2/ID) and Ternary (MgCl2/ID/TiCl4) Formulations. Molecules. 2025; 30(10):2176. https://doi.org/10.3390/molecules30102176
Chicago/Turabian StyleCannavacciuolo, Felicia Daniela, Giuseppe Antinucci, Roberta Cipullo, and Vincenzo Busico. 2025. "Probing the Interaction of Diester Internal Donors (ID) with AlEt3 on Ziegler-Natta Surfaces: A Comparison Between Binary (MgCl2/ID) and Ternary (MgCl2/ID/TiCl4) Formulations" Molecules 30, no. 10: 2176. https://doi.org/10.3390/molecules30102176
APA StyleCannavacciuolo, F. D., Antinucci, G., Cipullo, R., & Busico, V. (2025). Probing the Interaction of Diester Internal Donors (ID) with AlEt3 on Ziegler-Natta Surfaces: A Comparison Between Binary (MgCl2/ID) and Ternary (MgCl2/ID/TiCl4) Formulations. Molecules, 30(10), 2176. https://doi.org/10.3390/molecules30102176








