One-Step Microwave Synthesis of NiSb/NiSe Nanomaterials for High Performance Supercapacitors
Abstract
1. Introduction
2. Results
2.1. Morphology and Microstructure
2.2. Electrochemical Performance Analysis of NiSb/NiSe Electrode Materials
2.2.1. Electrochemical Properties of the Three-Electrode System
2.2.2. Electrochemical Properties of the Two-Electrode System
3. Materials and Methods
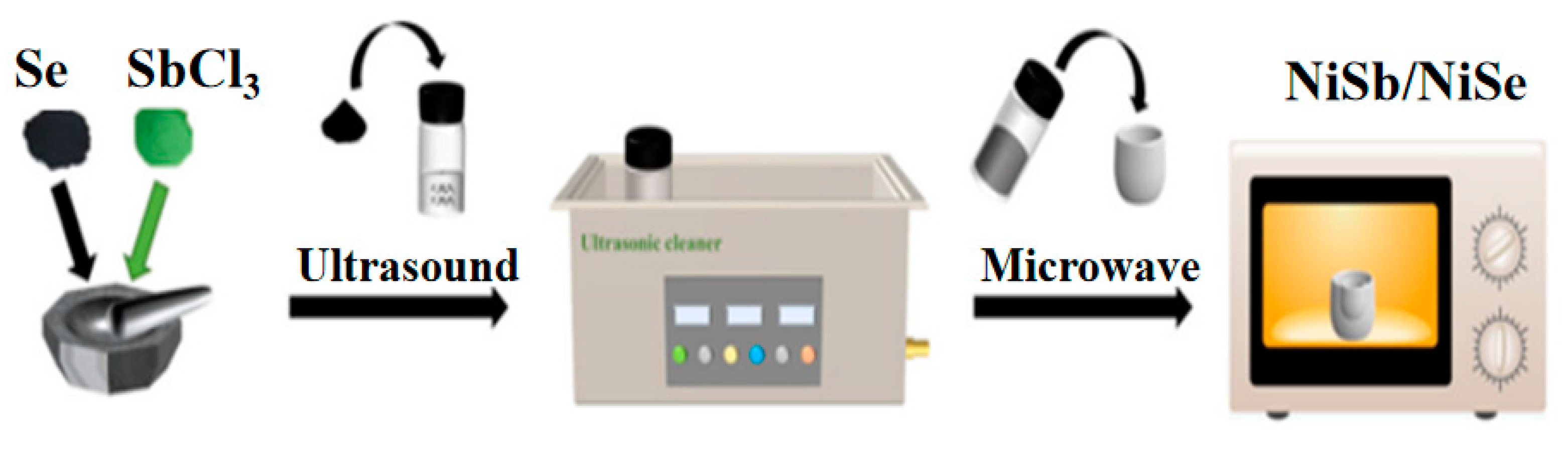
3.1. Material Preparation
3.2. Preparation of NiSb/NiSe
3.3. NiSb/NiSe/Ni//AC Assembly of Asymmetric Supercapacitors ASC
3.4. Characterization of NiSb/NiSe
3.5. Electrochemical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Liang, J.; Chen, Y. An Overview of the Applications of Graphene-Based Materials in Supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Z.; Xiao, K.; Li, N.; Liu, Z.-Q.; Qiao, S.-Z. Amorphous Ni(OH)2 @ three-dimensional Ni core–shell nanostructures for high capacitance pseudocapacitors and asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 13845–13853. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Han, J.; Wei, W.; Zhang, C.; Tao, Y.; Lv, W.; Ling, G.; Kang, F.; Yang, Q.-H. Engineering Graphenes from the Nano- to the Macroscale for Electrochemical Energy Storage. Electrochem. Energy Rev. 2018, 1, 139–168. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Zhang, Z.; Li, J.; Zhou, X.; Lei, Z. A novel aqueous asymmetric supercapacitor based on petal-like cobalt selenide nanosheets and nitrogen-doped porous carbon networks electrodes. J. Power Sources 2015, 297, 351–358. [Google Scholar] [CrossRef]
- Xu, L.; Duan, F.; He, K.; Ma, Y.-l.; Zhu, L.; Zheng, Y.; Huang, T.; Kimoto, T.; Ma, T.; Li, H.; et al. Characteristics of the secondary water-soluble ions in a typical autumn haze in Beijing. Environ. Pollut. 2017, 227, 296–305. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, X.; Wang, T.; Huang, X.; Dou, J.; Wu, D.; Yu, J.; Wu, S.; Chen, X. S/N-codoped carbon nanotubes and reduced graphene oxide aerogel based supercapacitors working in a wide temperature range. J. Colloid Interface Sci. 2023, 638, 709–718. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, M.; Li, X. Carbon nanomaterials and their composites for supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Ahmad, M.; Chen, Y.; Shuang, S.; Javed, M.S.; Yang, Y.; Zhang, K. High entropy alloys as electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103405. [Google Scholar] [CrossRef]
- Melkiyur, I.; Rathinam, Y.; Kumar, P.S.; Sankaiya, A.; Pitchaiya, S.; Ganesan, R.; Velauthapillai, D. A comprehensive review on novel quaternary metal oxide and sulphide electrode materials for supercapacitor: Origin, fundamentals, present perspectives and future aspects. Renew. Sustain. Energy Rev. 2023, 173, 113106. [Google Scholar] [CrossRef]
- Dahiya, Y.; Hariram, M.; Kumar, M.; Jain, A.; Sarkar, D. Modified transition metal chalcogenides for high performance supercapacitors: Current trends and emerging opportunities. Coord. Chem. Rev. 2022, 451, 214265. [Google Scholar] [CrossRef]
- Tian, Y.; Ruan, Y.; Zhang, J.; Yang, Z.; Jiang, J.; Wang, C. Controllable growth of NiSe nanorod arrays via one-pot hydrothermal method for high areal-capacitance supercapacitors. Electrochim. Acta 2017, 250, 327–334. [Google Scholar] [CrossRef]
- Khan, B.A.; Hussain, R.; Shah, A.; Mahmood, A.; Shah, M.Z.U.; Ismail, J.; Rahman, S.U.; Sajjad, M.; Assiri, M.A.; Imran, M.; et al. NiSe2 nanocrystals intercalated rGO sheets as a high-performance asymmetric supercapacitor electrode. Ceram. Int. 2022, 48, 5509–5517. [Google Scholar] [CrossRef]
- Nagaraju, G.; Cha, S.M.; Sekhar, S.C.; Yu, J.S. Metallic Layered Polyester Fabric Enabled Nickel Selenide Nanostructures as Highly Conductive and Binderless Electrode with Superior Energy Storage Performance. Adv. Energy Mater. 2017, 7, 1601362. [Google Scholar] [CrossRef]
- Kirubasankar, B.; Murugadoss, V.; Lin, J.; Ding, T.; Dong, M.; Liu, H.; Zhang, J.; Li, T.; Wang, N.; Guo, Z.; et al. In situ grown nickel selenide on graphene nanohybrid electrodes for high energy density asymmetric supercapacitors. Nanoscale 2018, 10, 20414–20425. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Gou, J.; Zhu, Y.; Li, Y. Mixed Ni-Co selenides as advanced electrode materials for high-performance supercapacitor. Ionics 2023, 29, 3365–3371. [Google Scholar] [CrossRef]
- Saranya, S.; Dhanapandian, S.; Suthakaran, S.; Nagarajan, S.; Krishnakumar, N.; Dinesh, S.; Muthukrishnaraj, A.; Manikandan, A. Nickel-Manganese bimetallic Selenide as an electrode for supercapcitor applications. Sustain. Energy Technol. Assess. 2023, 59, 103376. [Google Scholar] [CrossRef]
- Begum, M.S.; Ahamed, A.J. Synthesis and characterization of NiSe and Doped NiSe: Mn. J. Chem. Pharm. Res. 2015, 7, 2031–2039. [Google Scholar]
- Ji, J.; Song, X.; Liu, J.; Yan, Z.; Huo, C.; Zhang, S.; Su, M.; Liao, L.; Wang, W.; Ni, Z.; et al. Two-dimensional antimonene single crystals grown by van der Waals epitaxy. Nat. Commun. 2016, 7, 13352. [Google Scholar] [CrossRef]
- Zheng, K.; Li, G.; Xu, C. Advanced battery-supercapacitor hybrid device based on Co/Ni-ZIFs-derived NiCo2S4 ultrathin nanosheets electrode with high performance. Appl. Surf. Sci. 2019, 490, 137–144. [Google Scholar] [CrossRef]
- Su, W.-w.; Wang, W.; Li, Y.-l.; Xu, L.; Wang, R. Fabrication of antimony-doped tin oxide/carbon black composite with oxygen plasma treatment for lithium-air batteries. Mater. Lett. 2016, 180, 203–206. [Google Scholar] [CrossRef]
- Wang, Y.; Djerdj, I.; Smarsly, B.M.; Antonietti, M. Antimony-Doped SnO2 Nanopowders with High Crystallinity for Lithium-Ion Battery Electrode. Chem. Mater. 2009, 21, 3202–3209. [Google Scholar] [CrossRef]
- Ali, F.; Khalid, N.R.; Tahir, M.B.; Nabi, G.; Shahzad, K.; Ali, A.M.; Kabli, M.R. Capacitive properties of novel Sb-doped Co3O4 electrode material synthesized by hydrothermal method. Ceram. Int. 2021, 47, 32210–32217. [Google Scholar] [CrossRef]
- Mariappan, V.K.; Krishnamoorthy, K.; Pazhamalai, P.; Natarajan, S.; Sahoo, S.; Nardekar, S.S.; Kim, S.-J. Antimonene dendritic nanostructures: Dual-functional material for high-performance energy storage and harvesting devices. Nano Energy 2020, 77, 105248. [Google Scholar] [CrossRef]
- Lv, W.; He, W.; Wang, X.; Niu, Y.; Cao, H.; Dickerson, J.H.; Wang, Z. Understanding the oriented-attachment growth of nanocrystals from an energy point of view: A review. Nanoscale 2014, 6, 2531–2547. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhou, J.; Sun, K.; Ma, G.; Lei, Z. High-Performance Asymmetric Supercapacitor Designed with a Novel NiSe@MoSe2 Nanosheet Array and Nitrogen-Doped Carbon Nanosheet. ACS Sustain. Chem. Eng. 2017, 5, 5951–5963. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Peng, W.; Chang, J.; Wang, H. Morphological modulation of NiCo2Se4 nanostructured arrays on carbon cloth and application to asymmetric supercapacitors. Solid State Ion. 2022, 386, 116046. [Google Scholar] [CrossRef]
- Vidhya, M.S.; Yuvakkumar, R.; Ravi, G.; Pannipara, M.; Al-Sehemi, A.G.; Velauthapillai, D. PVP-assisted grass-like NiSe@ZnSe composite for environmental energy applications. J. Mater. Sci. Mater. Electron. 2022, 33, 8409–8416. [Google Scholar] [CrossRef]
- Mei, H.; Zhang, L.; Zhang, K.; Gao, J.; Zhang, H.; Huang, Z.; Xu, B.; Sun, D. Conversion of MOF into carbon-coated NiSe2 yolk-shell microspheres as advanced battery-type electrodes. Electrochim. Acta 2020, 357, 136866. [Google Scholar] [CrossRef]
- Li, J.; Bi, R.; Zhang, T.; Du, L.; Hou, Y.; Wang, F.; Zhang, Z. Microwave one-step controllable synthesis of NiSb materials for high-performance energy storage. J. Alloys Compd. 2022, 909, 164770. [Google Scholar] [CrossRef]
- Li, G.; Song, M.; Zhang, X.; Sun, Y.; Guo, J. Carbon coated heterojunction CoSe2/Sb2Se3 nanospheres for high-efficiency sodium storage. Dalton Trans. 2023, 52, 4973–4979. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Li, X.; Cao, S.-a.; Li, T.; Luo, W.; Xu, F. Ni0.85Se hexagonal nanosheets as an advanced conversion cathode for Mg secondary batteries. J. Energy Chem. 2020, 48, 226–232. [Google Scholar] [CrossRef]
- Amin, B.G.; Masud, J.; Nath, M. Facile one-pot synthesis of NiCo2Se4-rGO on Ni foam for high performance hybrid supercapacitors. RSC Adv. 2019, 9, 37939–37946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.Y.; Rajput, S.; Gilks, D.; Lari, L.; Galindo, P.L.; Weinert, M.; Lazarov, V.K.; Li, L. Tuning Dirac states by strain in the topological insulator Bi2Se3. Nat. Phys. 2014, 10, 294–299. [Google Scholar] [CrossRef]
- Moradi, Z.; Vaezzadeh, M.; Saeidi, M. Thermoelectric, spin-dependent optical and quantum transport properties of 2D half-metallic Co2Se3. Phys. Chem. Chem. Phys. 2022, 24, 22016–22027. [Google Scholar] [CrossRef]
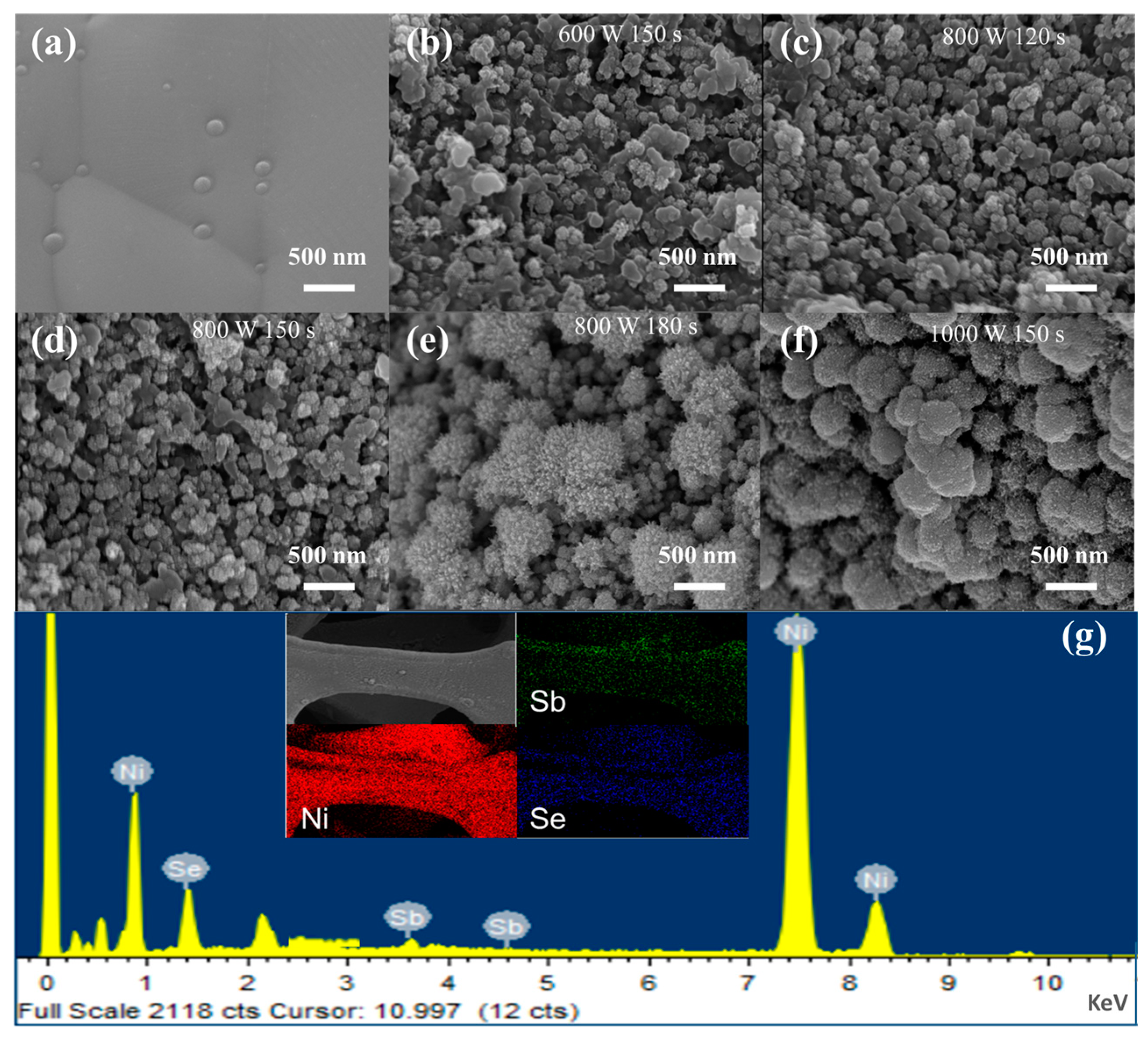
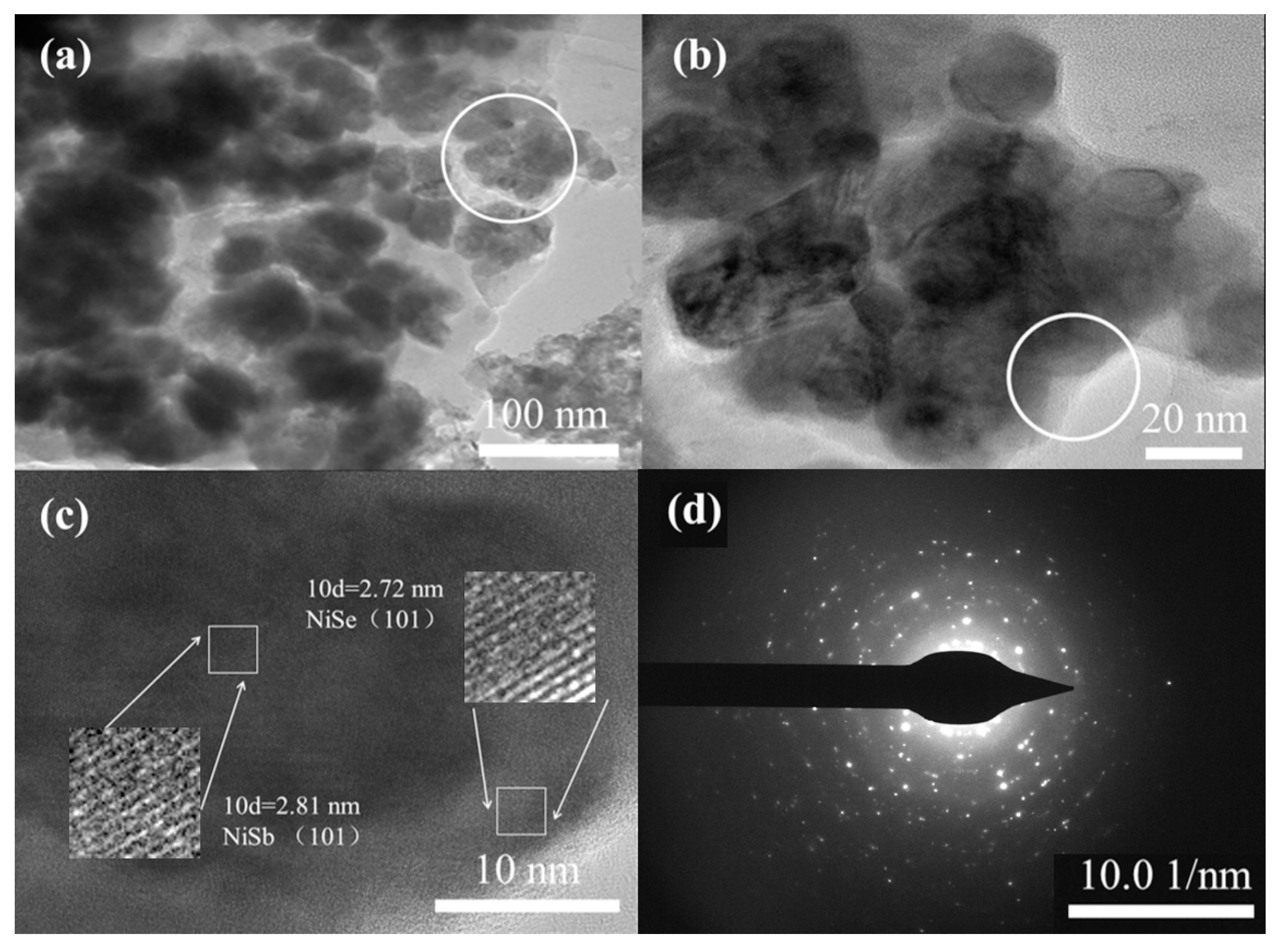

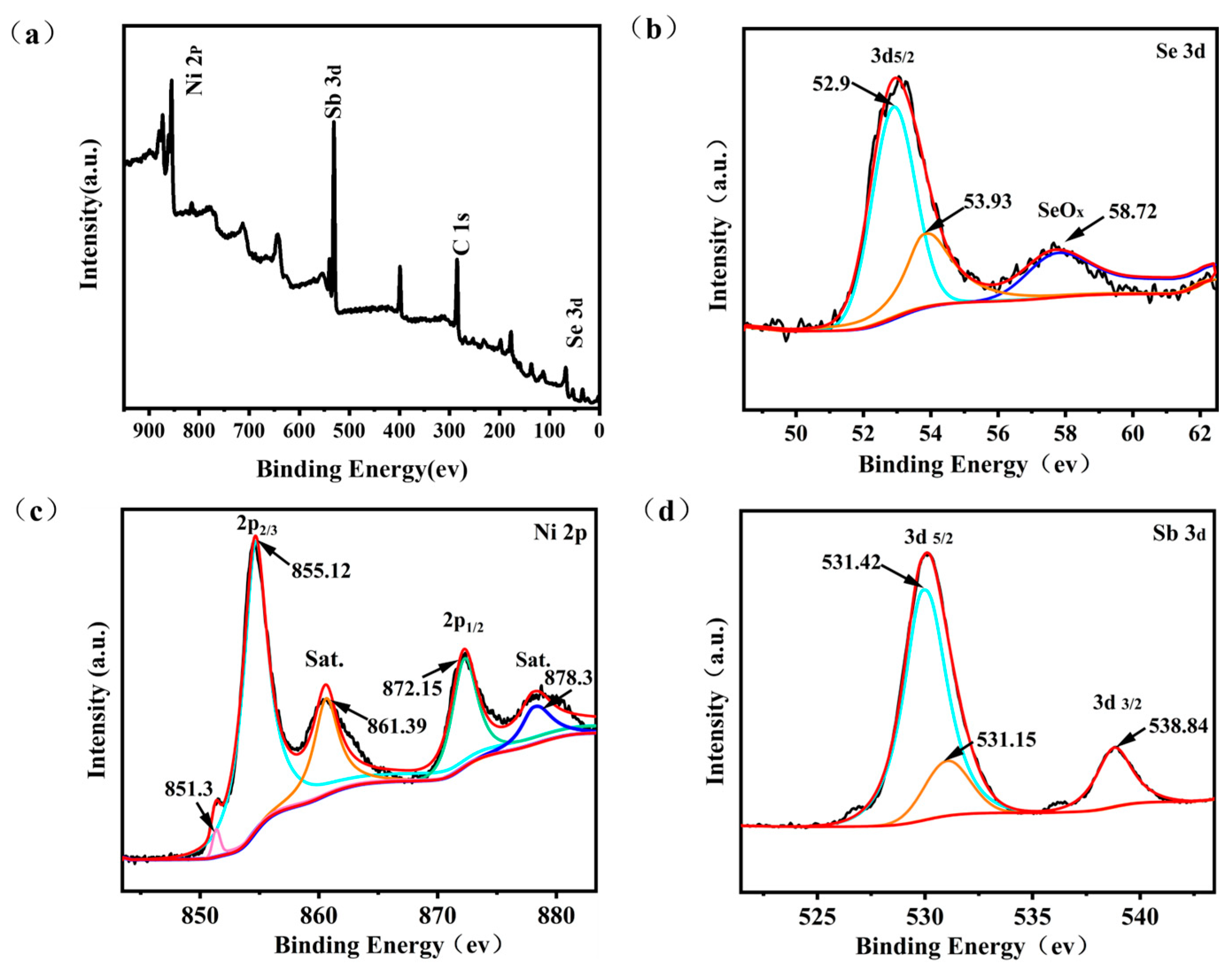

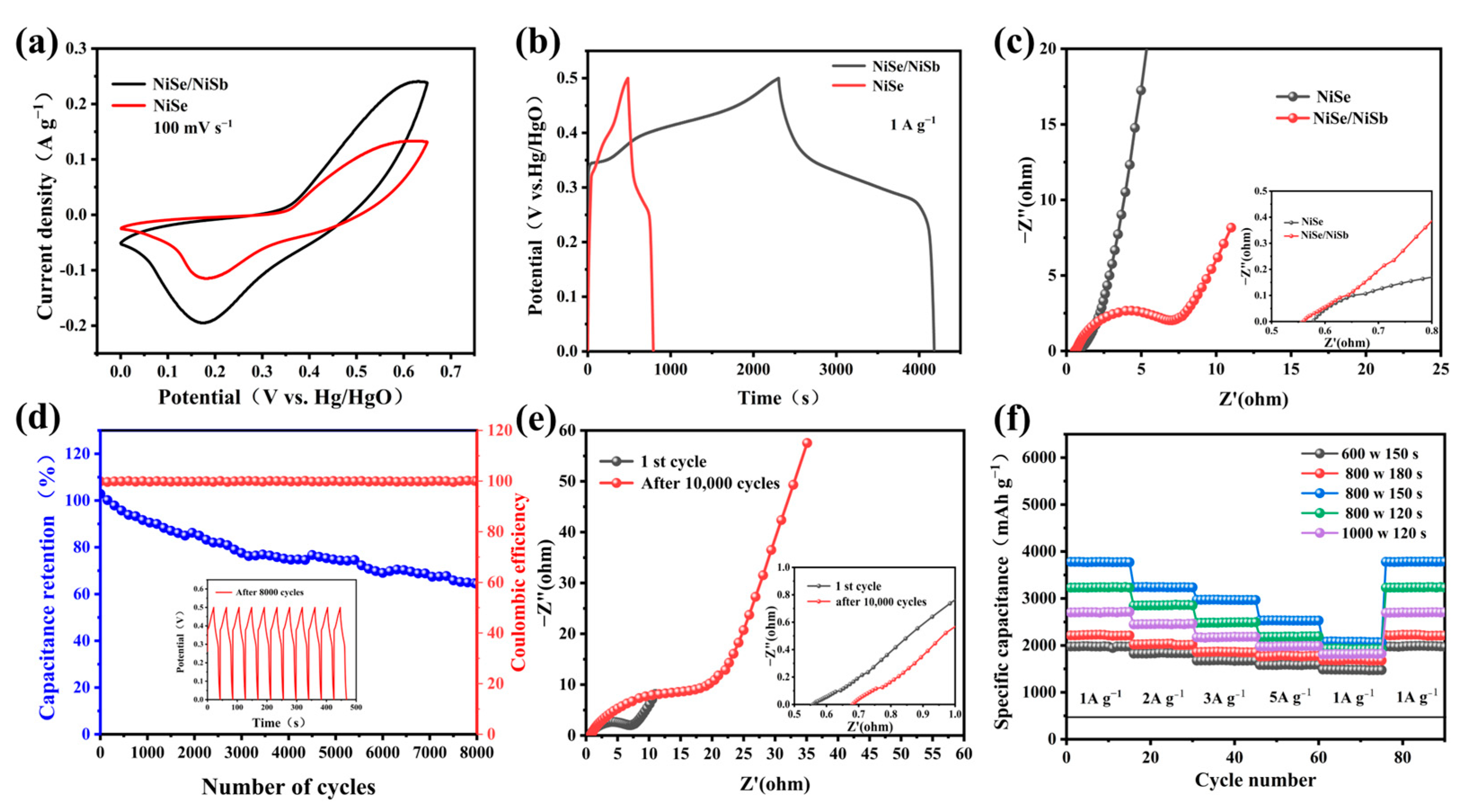

| Sample | Microwave Power | Reaction Time/s | Capacity Value/mAh g−1 (1 A g−1) |
|---|---|---|---|
| A1 | 600 W | 150 s | 264 mAh g−1 |
| A2 | 800 W | 120 s | 450 mAh g−1 |
| A3 | 800 W | 150 s | 525 mAh g−1 |
| A4 | 800 W | 180 s | 306 mAh g−1 |
| A5 | 1000 W | 150 s | 378 mAh g−1 |
| Materia | Method | Specific Capacity/mAh g−1 | Cyclic Stability/Cycles | Ref. |
|---|---|---|---|---|
| NiSe-G | Hydrothermal | 142.2 mAh g−1 | 98%/2500 | [15] |
| NiSe@Mn | Hydrothermal | 80.1 mAh g−1 | 14%/1400 | [18] |
| NiSe@MoSe2 | Hydrothermal | 128.2 mAh g−1 | 65%/1000 | [26] |
| NiCo2Se | Hydrothermal | 60.1 mAh g−1 | 90%/1000 | [27] |
| NiSe@ZnSe | Hydrothermal | 61.4 mAh g−1 | 99%/2000 | [28] |
| NiSe2@C | High temperature calcination | 111.5 mAh g−1 | 94%/1000 | [29] |
| NiSb/NiSe | Microwave | 525 mAh g−1 | 65%/8000 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Q.; Kang, H.; Guan, C.; Zhao, X.; Zhao, H.; Jing, B.; Lu, Z.; Feng, F. One-Step Microwave Synthesis of NiSb/NiSe Nanomaterials for High Performance Supercapacitors. Molecules 2025, 30, 2168. https://doi.org/10.3390/molecules30102168
Duan Q, Kang H, Guan C, Zhao X, Zhao H, Jing B, Lu Z, Feng F. One-Step Microwave Synthesis of NiSb/NiSe Nanomaterials for High Performance Supercapacitors. Molecules. 2025; 30(10):2168. https://doi.org/10.3390/molecules30102168
Chicago/Turabian StyleDuan, Qianwen, Hongjie Kang, Cuiling Guan, Xueming Zhao, Haidong Zhao, Buqin Jing, Zhen Lu, and Feng Feng. 2025. "One-Step Microwave Synthesis of NiSb/NiSe Nanomaterials for High Performance Supercapacitors" Molecules 30, no. 10: 2168. https://doi.org/10.3390/molecules30102168
APA StyleDuan, Q., Kang, H., Guan, C., Zhao, X., Zhao, H., Jing, B., Lu, Z., & Feng, F. (2025). One-Step Microwave Synthesis of NiSb/NiSe Nanomaterials for High Performance Supercapacitors. Molecules, 30(10), 2168. https://doi.org/10.3390/molecules30102168







