1. Introduction
The escalating global energy demand, traditionally met by conventional sources such as fossil fuels, coal, and natural gas, has raised pressing environmental concerns, including air and water pollution, greenhouse gas emissions, and threats to biodiversity [
1,
2,
3]. A substantial shift toward renewable energy sources, such as solar, wind, biomass, and tidal energy, has become imperative to mitigate these challenges. Among these alternatives, solar energy is particularly promising due to its abundance, sustainability, and potential as a clean and efficient substitute for fossil fuels [
3,
4,
5,
6].
Solar energy is primarily harnessed through photovoltaic (PV) technology, directly converting sunlight into electricity. Crystalline silicon (c-Si) technology, including monocrystalline (Mono-Si) and multi-crystalline (Multi-Si) silicon, dominates the market, accounting for approximately 95% of global solar installations. Despite the high efficiency of Mono-Si (~26.7%), the energy-intensive production processes of c-Si-based solar cells, including purification, reduction, and crystallization, present environmental and economic challenges [
7,
8,
9,
10]. While gallium arsenide (GaAs) solar cells achieve efficiencies exceeding 30%, their high cost limits large-scale to terrestrial applications [
11].
Second-generation thin-film solar cells, utilizing materials such as amorphous silicon, copper indium gallium selenide (CIGS), cadmium telluride (CdTe), and GaAs, offer a more economical approach but still face limitations [
12,
13]. The emergence of third-generation solar cells, including organic photovoltaics, dye-sensitized solar cells (DSSCs), and perovskite solar cells (PSCs), has marked a breakthrough in the field. Among these, PSCs have demonstrated remarkable records, achieving power conversion efficiencies (PCEs) of up to 27% over the past decade [
14,
15,
16]. This success is attributed to their exceptional material properties, such as their strong visible light absorption, long charge carrier diffusion lengths, and cost-effective fabrication methods [
15,
16,
17]. Despite these advancements, PSCs encounter critical challenges that hinder commercialization, including sensitivity to oxygen, moisture, temperature fluctuations, and ultraviolet (UV) light exposure. Additionally, the narrow absorption range of efficient low-bandgap PSCs limits their ability to fully utilize the solar spectrum, resulting in reduced efficiency and fast degradation [
14,
15,
16,
17]. Given that conventional solar cells primarily capture energy from the visible light spectrum (about 48% of total solar radiation), the substantial energy present in the near-infrared (NIR) range (~44%) remains largely untapped [
18,
19].
Upconversion technology offers a promising strategy to enhance PSC efficiency by converting low-energy NIR photons into higher-energy photons, such as UV and visible light, which can be effectively absorbed by the electron transport layer (TiO
2) [
20] and the photo-active perovskite layer in the PSC device [
21,
22,
23,
24]. This process generates additional charge carriers, improving the overall photovoltaic performance of PSCs. A previous study [
20] demonstrated this approach by incorporating β-NaYF
4:Yb
3+,Tm
3+@TiO
2 core–shell UCNPs into the mesoporous TiO
2 layer, resulting in a significant increase in the power conversion efficiency (PCE) from 13.98% to 16.27% (champion device) and from 13.53% to 16.07% for average fabricated devices. This corresponds to a 16.38% improvement in their champion device performance and an 18% improvement in the average performance across multiple tested devices. Although the results showed a consistent and good enhancement, the achieved efficiencies still remained slightly below the best performances reported for standard perovskite solar cells (PSCs) [
22,
23]. This limited improvement in the fabricated PSC devices can be attributed to two key factors. First, conventional UCNPs, typically doped with ytterbium (Yb
3+) and thulium (Tm
3+), primarily absorb NIR light at 980 nm, a wavelength highly susceptible to energy losses due to water vapor absorption [
25,
26,
27]. Second, the lack of precise control over the crystallinity and uniformity of the TiO
2 shell in previous designs has led to suboptimal charge transport and interfacial properties, thereby restricting the PSC efficiency and overall performance.
To address these limitations, two synergistic strategies are proposed. First, the NIR absorption of UCNPs will be precisely shifted from 980 nm to 808 nm by incorporating neodymium ions (Nd3+), which exhibit a strong absorption at 808 nm, a spectral region with minimal water vapor interference. This targeted spectral tuning is designed to enhance NIR light harvesting while simultaneously mitigating photothermal effects, thereby improving the operational stability of PSCs under ambient conditions. Second, a highly uniform, crystalline TiO2 shell will be meticulously engineered and coated onto thulium-doped (Tm3+) UCNPs. This core–shell structure ensures the efficient integration into the electron transport layer (ETL), where the upconverted ultraviolet and visible emissions from the UCNPs are effectively absorbed by the TiO2 shell. The resulting enhancement in the charge carrier generation and transport is expected to significantly boost the overall photovoltaic performance and long-term durability of the device.
While perovskite materials indeed possess the capability to absorb photons in the near-infrared (NIR) region around 800–810 nm, their absorption coefficient in this spectral range is considerably lower compared to their absorption in the visible spectrum [
28,
29,
30]. Consequently, a substantial portion of NIR photons remains insufficiently utilized, limiting the photovoltaic performance potential of perovskite solar cells. To address this, we strategically employ neodymium-doped upconversion nanoparticles (UCNPs) designed to absorb NIR photons specifically at 808 nm, a wavelength with minimal interference from atmospheric water vapor absorption. The rationale for using upconversion here, despite its lower intrinsic conversion efficiency relative to direct absorption, lies in its ability to transform these weakly absorbed NIR photons into more energetically favorable ultraviolet (UV) and blue photons [
31,
32]. These higher-energy photons are subsequently efficiently absorbed by the TiO
2 electron transport layer and the perovskite active layer, thereby significantly enhancing the generation of additional charge carriers. Furthermore, to mitigate potential stability concerns associated with UV-induced photocatalytic activity at the TiO
2–perovskite interface, our nanoparticles feature a carefully engineered core–shell structure. This design helps isolate the UV-excited TiO
2 from direct interaction with the perovskite layer, thereby substantially reducing the risk of degradation at the interface and contributing positively to device stability.
This paper presents a simple yet efficient solvothermal method for synthesizing neodymium (Nd)-doped UCNPs with a core–shell structure, followed by the continuous deposition of a TiO2 layer. The structural and optical properties of the TiO2-coated Nd-doped UCNPs were thoroughly characterized. These engineered UCNPs were then systematically integrated into PSC architectures, with their photovoltaic performance rigorously evaluated and benchmarked against control PSCs. By enhancing the NIR absorption and optimizing the ETL design, this research aims to advance PSC efficiency, contributing to developing more efficient and sustainable solar energy technologies.
2. Results and Discussion
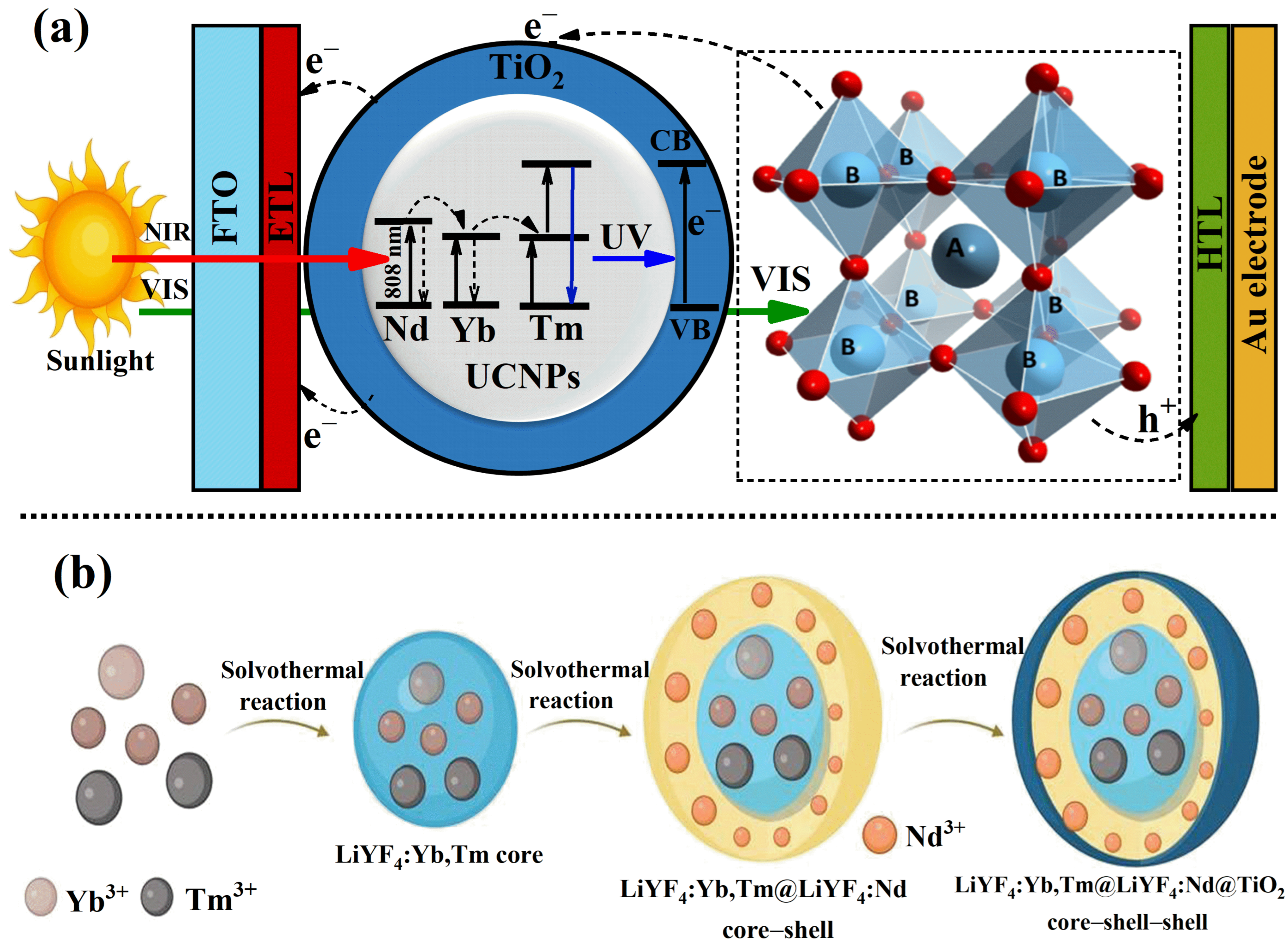
The proof of concept for this study is illustrated in
Figure 1a. It proposes the integration of UCNPs coated with a uniform layer of TiO
2 into the mesoporous electron transport layer of PSC devices. In this approach, the absorbed NIR light is upconverted into UV and blue light by Tm ions, which is then reabsorbed by the TiO
2 layer. This process generates additional electrons, ultimately enhancing the overall photovoltaic performance of the PSCs. Experimentally, the UCNPs were synthesized with a core–shell structure using a solvothermal method, as previously reported [
22,
27,
33] and detailed in
Section 3. The incorporation of Nd
3+ into the core–shell structure of the UCNPs introduces a new sensitizer and an additional NIR absorber at the 808 nm band. This specific wavelength minimizes water vapor absorption in the sunlight spectrum, improving light utilization. As shown in
Figure 1b, the Nd
3+ sensitizer is incorporated into a separate shell during synthesis to maintain a sufficient spatial separation from the upconverting ion, Tm
3+. The energy losses associated with water vapor absorption arise because conventional UCNPs, typically doped with ytterbium (Yb
3+) and thulium (Tm
3+), have absorption bands centered at approximately 980 nm, a spectral region strongly absorbed by atmospheric water vapor, with a first window peak around 1000 nm. This overlap significantly diminishes the intensity of NIR photons reaching the UCNPs, thereby reducing the overall photon conversion efficiency and limiting photovoltaic performance improvements. By shifting the absorption band to 808 nm, where atmospheric water vapor absorption is minimal, our (Nd
3+) doped UCNPs mitigate these energy losses, resulting in improved light utilization and higher device efficiency. This strategic design minimizes detrimental cross-talk interactions, which are known to decrease the upconversion efficiency of UCNPs significantly [
25,
26]. Moreover, the addition of the shell to the UCNPs plays a critical role in preventing hydroxyl (OH) groups in water from interacting with the upconverting ions. The presence of OH groups and surface defects can resonate with the intermediate states of the rare-earth ions, leading to poor upconversion efficiency. The shell effectively shields the active ions, preserving their efficiency and contributing to the overall enhancement of the device’s performance [
27,
34].
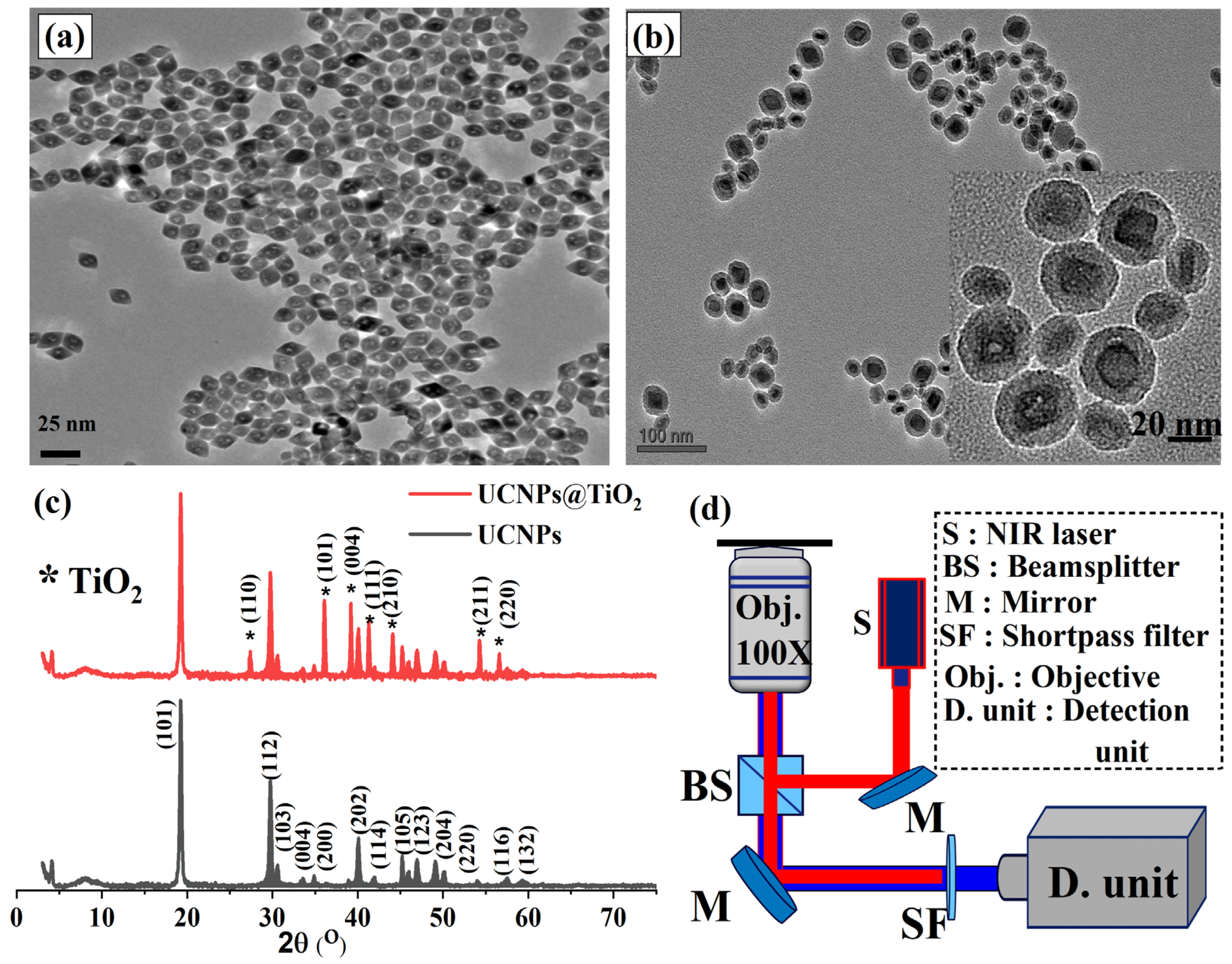
The morphology and structural properties of the synthesized UCNPs and UCNPs@TiO
2 nanoparticles were characterized using transmission electron microscopy (TEM) and X-ray diffraction (XRD) techniques. For TEM imaging, a few drops of each sample suspension were carefully deposited onto carbon grids and allowed to dry before analysis.
Figure 2a displays the TEM images of the well-dispersed YLiF
4:Yb,Tm@YLiF
4:Nd core–shell UCNPs, exhibiting a uniform octahedral shape with an average particle size of approximately 20 nm. The high dispersion quality and uniformity of the synthesized UCNPs are critical for achieving a consistent and homogeneous coating of the TiO
2 layer. Subsequently, a uniform TiO
2 layer with a thickness of approximately 7 nm was successfully coated onto the core–shell UCNPs, as illustrated in
Figure 2b. The TEM images of the synthesized UCNPs@TiO
2 nanoparticles align well with previous studies that employed similar particles for biological applications [
35]. The even distribution and conformal coverage of the TiO
2 shell are essential to maintain the optical and electronic properties of the nanoparticles while enhancing their stability and performance in photovoltaic applications.
The successful deposition and crystallinity of the TiO
2 coating were further validated by an XRD analysis, presented in
Figure 2c. The XRD pattern of the synthesized core–shell UCNPs (black curve) revealed well-defined crystalline peaks that corresponded to the standard pattern of the highly crystalline octahedral phase of YLiF
4 (JCPDS 01-081-2254). After the TiO
2 coating, the XRD pattern of UCNPs@TiO
2 (shown in the red curve) maintained the characteristic peaks of the core UCNPs while also displaying new peaks that aligned with the standard crystalline phase of TiO
2 (JCPDS card no. 21-1272). The retention of the core UCNPs’ crystalline structure, along with the appearance of distinct TiO
2 peaks, indicates that the TiO
2 shell is not only uniformly coated but also well crystallized. This high degree of crystallinity is crucial for optimizing the electron transport properties and enhancing the overall performance of the PSC devices.
The optical properties of the synthesized UCNPs and UCNPs@TiO
2 were investigated using a custom-built confocal scanning microscope. This setup included an 808 nm laser excitation source, a high-resolution microscope objective, and a custom-built spectrometer, as illustrated in
Figure 2d. To prepare the samples for optical analysis, a few drops of each sample were deposited onto a quartz substrate and spin-coated to achieve a uniform, thin film suitable for detailed optical study.
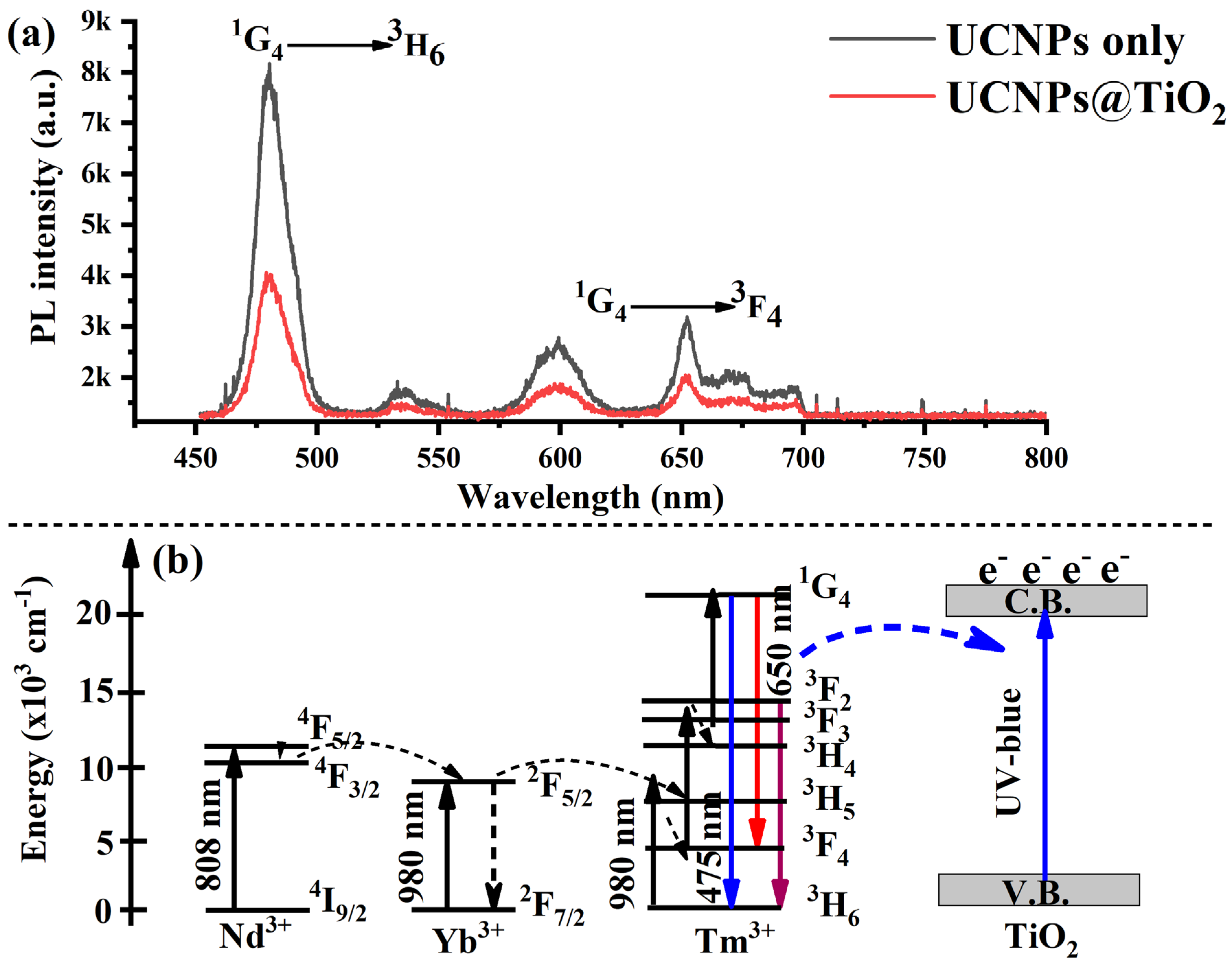
The uncoated core–shell UCNPs exhibited a strong upconversion (UC) emission from Tm
3+ ions, with a prominent blue emission peak at 475 nm under 808 nm excitation at a low laser intensity of 10 W/cm
2. This emission corresponds to the radiative transition from the excited
1G
4 state to the
3H
6 ground state. Additionally, weaker emission peaks at 600 nm and 650 nm were observed, attributed to less efficient higher-order transitions, as shown in
Figure 3a. Upon coating the core–shell UCNPs with a TiO
2 layer, a significant quenching of the blue emission from Tm
3+ ions was observed. This reduction is primarily due to the strong absorption of TiO
2 in the ultraviolet and blue regions of the spectrum, as depicted by the red curve in
Figure 3a. Furthermore, although Tm
3+-doped UCNPs typically exhibit UV emissions in the 360–375 nm range, this band was not detected in our measurements due to spectrometer limitations.
To explain the underlying photophysical process, the upon 808 nm excitation, YLiF
4:Tm,Yb@YLiF
4:Nd UCNPs absorb NIR light and emit both upconverted ultraviolet (UV) and blue light. These emissions excite electrons from the valence band (VB) to the conduction band (CB) of the TiO
2 shell, generating photoinduced electron–hole pairs [
35], as illustrated in
Figure 3b. This charge carrier generation plays a crucial role in enhancing photocatalytic and photovoltaic applications. For instance, in the context of perovskite solar cells, the excited electrons in the TiO
2 conduction band can efficiently transfer to the ETL, typically composed of TiO
2, enhancing the charge collection and improving the overall photovoltaic performance. Simultaneously, the holes left behind in the TiO
2 valence band may either recombine or participate in interfacial charge transfer processes, potentially influencing carrier dynamics in the perovskite absorber layer.
We measured the optical transmittance of FTO/UCNP@TiO
2 films at various UCNP concentrations (0%, 10%, 20%, 30%, 40%, 50%, and 60%), as shown in
Figure S1. The results indicate a progressive decrease in the visible light transmittance (400–800 nm) with an increasing UCNP content, which is attributed to enhanced scattering and absorption within the ETL. The UCNPs used are doped with Tm
3+ ions, which emit UV and blue photons under NIR excitation. These emissions align well with the absorption spectrum of TiO
2, allowing the upconverted photons to be efficiently utilized for charge generation. These findings suggest that the photocurrent enhancement observed is not solely due to light trapping or optical losses, but rather due to actual upconversion-mediated photon harvesting facilitated by the spectral overlap between the UCNP emission and TiO
2 absorption.
The synthesized UCNPs@TiO2 nanoparticles were incorporated into the fabricated PSC devices to systematically assess their photovoltaic performance. The mesoporous layer of the PSCs was modified with UCNPs@TiO2 at varying weight ratios (10%, 20%, 30%, 40%, 50%, and 60%) alongside a control device without UCNPs@TiO2. The photovoltaic performance parameters, including the PCE, open-circuit voltage (Voc), short-circuit current density (Jsc), and fill factor (FF), were evaluated through photocurrent density–voltage (J–V) measurements under a standard one-sun illumination (AM 1.5 G).
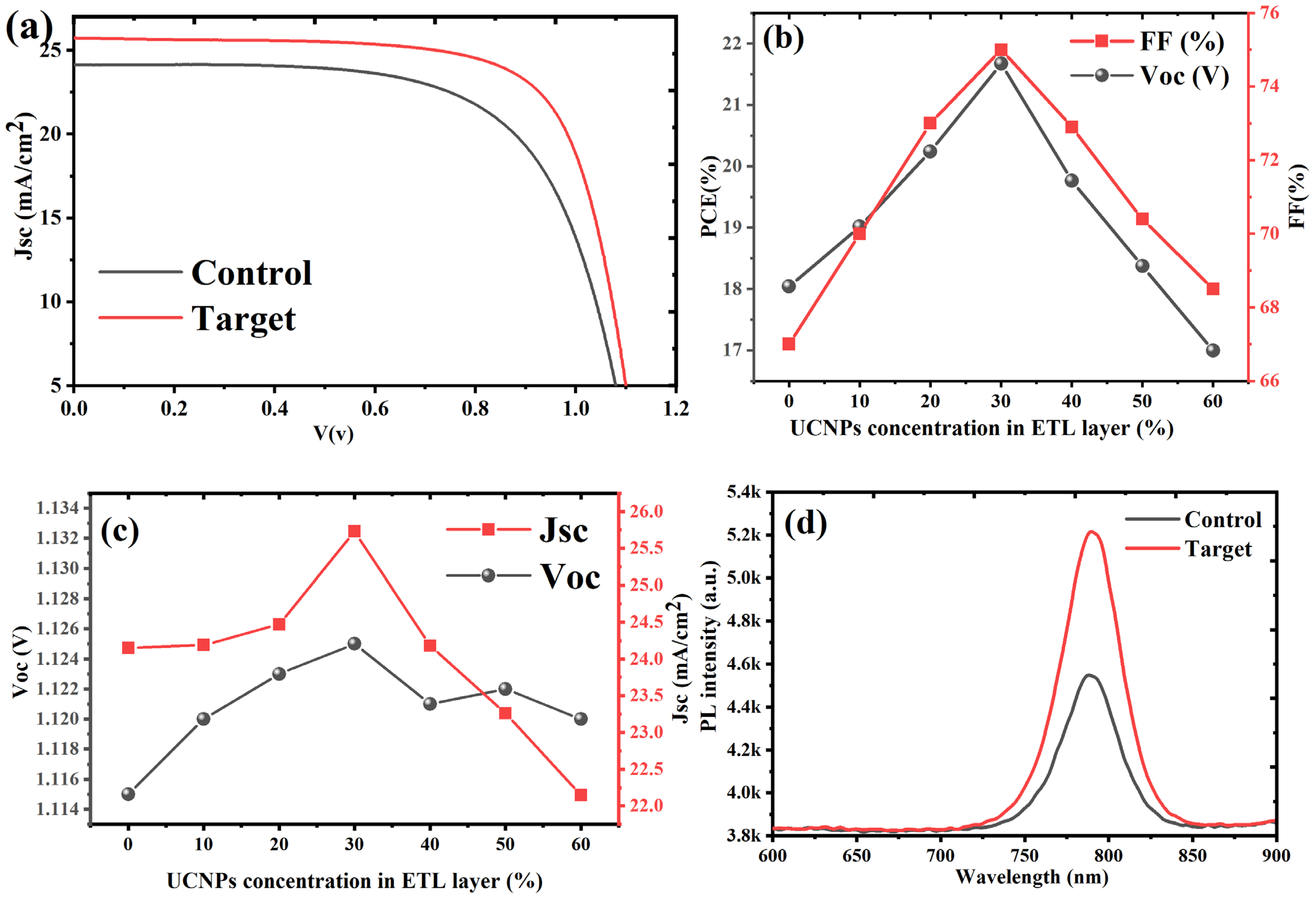
From
Table 1 and
Figure 4a–c, the control device exhibited a baseline PCE of 18.0%, with a
Voc of 1.115 V, a
Jsc of 24.15 mA/cm
2, and an FF of 67%. With the incorporation of UCNPs@TiO
2 there was a consistent increase in photovoltaic performance up to a 30% loading (target device). The statistical data of all conditions are illustrated in
Figure S2. The PCE increased by 20.4% in the target device, achieving a peak efficiency of 21.7% at 30% UCNPs@TiO
2, as shown in
Figure 4b. This enhancement was accompanied by an increase of 0.9% in
Voc, 6.6% in
Jsc, and 11.9% in FF, as illustrated in
Figure 4c. The data indicate that a moderate UCNPs@TiO
2 loading significantly improves the light harvesting and charge transfer within the device. This was mainly due to an improved
Jsc and FF, indicating an enhanced light absorption benefiting from the extra carriers generated with upconversion nanoparticles. Beyond the 30% loading, the performance began to decline. At 40% UCNPs@TiO
2, the PCE dropped to 19.8%, a 9.5% gain relative to the control but a 9.0% decrease from the optimal 30% loading. The 50% and 60% loadings showed even more pronounced reductions in the PCE to 18.4% (+1.8% from control) and 17.0% (−5.8% from control), respectively. The decrease in the
Jsc by 3.7% and 8.3% at 50% and 60% loadings further supports the hypothesis of the increased charge recombination and hindered charge mobility due to nanoparticle aggregation at higher concentrations.
The substantial enhancement observed in the fill factor (FF) and overall photovoltaic performance arises primarily from the improved interfacial energetics, reduced charge recombination, and optimized charge extraction facilitated by the integration of UCNP@TiO
2 nanoparticles. Specifically, the incorporation of these nanoparticles leads to the effective passivation of defect states at the ETL–perovskite interface. This passivation reduces trap-mediated recombination pathways, significantly diminishing non-radiative losses. Additionally, as confirmed by the ultraviolet photoelectron spectroscopy (UPS) analysis (vide infra) and explained in
Figure S3, the presence of UCNP@TiO
2 nanoparticles optimizes the energy-level alignment at the interface, enhancing the electron extraction efficiency from the perovskite to the ETL. This energetically favorable alignment mitigates the accumulation of charge carriers at the interface, thereby improving the FF significantly. Moreover, the uniform distribution and controlled morphology of the TiO
2-coated nanoparticles within the mesoporous ETL further promote efficient charge transport, minimizing the series resistance and enhancing the overall device performance. Thus, the observed improvements in electrical characteristics are consistently supported by the comprehensive interface analysis, energy-level characterizations, and optical assessments, substantiating the mechanistic explanation provided.
Furthermore, an additional critical aspect of this study was the utilization of Nd-doped UCNPs to shift the NIR absorption band to 808 nm, strategically minimizing water absorption interference. Traditional UCNPs that absorb at 980 nm are prone to significant energy losses due to the strong absorption of water at this wavelength, leading to up conversion quenching effects for the UCNPs. By shifting the absorption to 808 nm, where water absorption is minimized, the Nd-doped UCNPs enhance the light-harvesting capability of the PSCs, particularly under natural sunlight where NIR wavelengths contribute substantially to the solar spectrum. The spectral shift not only improved the photon conversion efficiency but also contributed to the observed increases in the Jsc and PCE at optimal UCNP loadings. The results indicate that this strategic shift in absorption properties enhances the utilization of sub-bandgap light, contributing to a better overall device performance.
The optical properties of the control and target devices (with 30% UCNPs@TiO
2) were thoroughly evaluated by analyzing their photoluminescence (PL) and internal photon conversion efficiency (IPCE) spectra. The PL emissions of perovskite films, both with and without UCNPs@TiO
2, were examined to assess the influence of the UCNP incorporation on the luminescence behavior. As depicted in
Figure 4d, the steady-state PL spectra of the perovskite films exhibited a distinct and prominent luminescence peak, precisely aligning with the absorption edges of the perovskite photoactive layer. The target device, containing 30% UCNPs coated with TiO
2, demonstrated a significantly higher PL intensity compared to the control device. This enhancement indicates that the optimal incorporation of UCNPs@TiO
2 effectively mitigates trap-mediated and non-radiative recombination within the perovskite layer. The reduced recombination losses contribute to an increase in the overall luminescence efficiency, highlighting the functional role of UCNPs in boosting the photoactive layer’s optical performance.
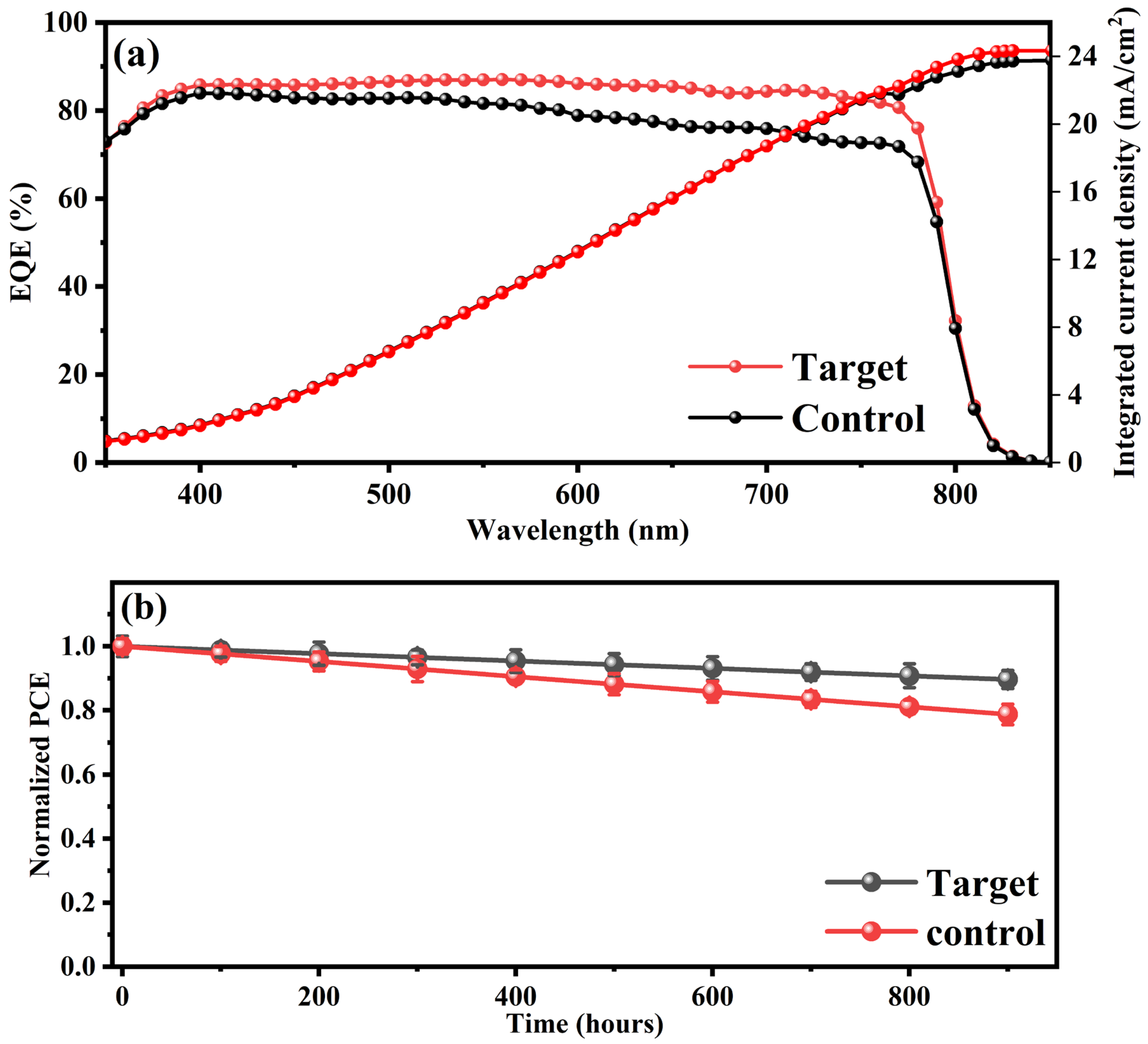
In addition to improved luminescence, the target device with 30% UCNPs@TiO
2 also exhibited a superior IPCE across a broad wavelength range of 300–800 nm, as shown in
Figure 5a. The integrated current density shows an improvement in agreement with the J-V data. The improved quantum efficiency spectrum observed in the device incorporating UCNP@TiO
2 nanoparticles, characterized by enhanced performance in the near-infrared (NIR) spectral region, highlights the significant impact of defect states located near the perovskite band edges on the device’s photovoltaic behavior. These defect states typically act as recombination centers, capturing photogenerated carriers and reducing the effective charge extraction efficiency. The incorporation of UCNP@TiO
2 nanoparticles mitigates this effect by effectively passivating interface and bulk defects, thus reducing the density of these recombination centers. Consequently, this defect passivation enhances the charge carrier lifetime and mobility, resulting in an increased quantum efficiency across a broader spectral range, notably around the band edges. Thus, the dual NIR absorption bands not only reflect the upconversion activity but also underscore a critical improvement in defect passivation and charge extraction processes within the perovskite solar cells. Furthermore, defects trap charge carriers, promoting non-radiative recombination and reducing both the open-circuit voltage (
Voc) and overall efficiency. Sub-bandgap photons (e.g., 850 nm) primarily interact with these defect states, causing trapped carriers and increased recombination losses, while above-bandgap photons (e.g., 620 nm) generate free charge carriers more effectively, enhancing the photocurrent [
36]. The incorporation of TiO
2-coated UCNPs embedded within the ETL further mitigates these limitations by converting two low-energy photons (e.g., 808 nm) into a higher-energy photon in the visible range, which is efficiently absorbed by the perovskite layer. The TiO
2 coating enhances the charge extraction and transport by facilitating a better energy alignment between the UCNPs and the ETL, thereby improving carrier mobility and minimizing interfacial recombination. This process reduces the population of low-energy carriers prone to defect trapping, leading to a significant reduction in non-radiative recombination losses and a notable improvement in the device performance through the enhanced generation and extraction of high-energy, mobile carriers [
36,
37,
38]. The observed enhancement in the incident photon-to-current conversion efficiency (IPCE) across the entire spectral range, rather than exclusively at the wavelength around 475 nm, highlights the multifunctional role of UCNPs integrated into the ETL. While the upconversion mechanism indeed contributes by converting NIR photons into UV and blue photons, thus theoretically enhancing the IPCE significantly around 475 nm, experimental results indicate a broader and more uniform improvement. This broader spectral enhancement arises because the integration of UCNP@TiO
2 nanoparticles also substantially modifies interfacial energetics, passivates surface defects, and improves the overall charge transport efficiency at the ETL–perovskite interface. Consequently, the reduction in defect-mediated recombination and improvement in the carrier extraction efficiency have a pronounced positive effect throughout the absorption spectrum, overshadowing any specific narrowband enhancements exclusively attributable to upconversion at 475 nm. Therefore, the data strongly suggest that the comprehensive improvement in the IPCE is predominantly driven by the combined effects of the optimized charge transport, defect passivation, and effective interfacial engineering, in addition to optical conversion phenomena facilitated by UCNPs.
Ultraviolet photoelectron spectroscopy (UPS) was employed to investigate the electronic structures and energy levels of TiO
2 and perovskite layers with and without 30% UCNPs incorporation, as shown in
Figure S3. The UPS spectra revealed clear shifts in both the secondary electron cut-off (SECO) and valence band (VB) regions upon the integration of UCNP@TiO
2 nanoparticles. Specifically, the SECO edge shifted towards lower binding energies after the UCNP inclusion, which is indicative of a reduced work function and improved electron extraction capabilities. Additionally, the analysis of the VB edges demonstrated beneficial adjustments to the energy alignment between TiO
2 and the perovskite layer, which facilitated efficient charge carrier transfer at their interface. The resulting optimized energy-level alignment contributed significantly to the enhanced photovoltaic performance via the improved
Voc observed in devices incorporating 30% UCNP@TiO
2 nanoparticles.
We performed comprehensive long-term stability assessments of the optimized perovskite solar cells incorporating UCNP@TiO
2 nanoparticles under ambient conditions, including humidity-controlled and accelerated aging tests,
Figure 5b. The enhanced stability observed, retaining over 90% of the initial PCE after 900 h of exposure, can be attributed primarily to the effective passivation of defect states and the interfacial stabilization provided by the UCNP@TiO
2 nanoparticles. Specifically, the engineered TiO
2 shell surrounding the upconversion nanoparticles acts as a protective barrier, significantly mitigating moisture ingress and preventing the direct interaction of moisture with sensitive perovskite layers. Moreover, the optimized energy-level alignment and reduced recombination at the ETL–perovskite interface significantly slow degradation processes, such as ion migration and chemical decomposition, which are commonly accelerated in the presence of humidity and UV radiation.
The enhanced stability of the target device is attributed to the incorporation of Nd3+-doped UCNPs, which effectively convert near-infrared light into visible light, thereby enhancing the electron transport within the perovskite layer. Additionally, the UCNPs contribute to passivating grain boundaries and reducing defect densities in the perovskite film, which mitigates degradation under the prolonged exposure to ambient conditions. This dual functionality of light conversion and defect passivation underscores the potential of UCNPs to not only boost device efficiency but also prolong operational stability, making them a valuable addition to PSC technology.