Synthesis, Structural Characterization, Luminescent Properties, and Antibacterial and Anticancer Activities of Rare Earth-Caffeic Acid Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis the Content of Rare Earth Ions Ln3+ in the Complexes
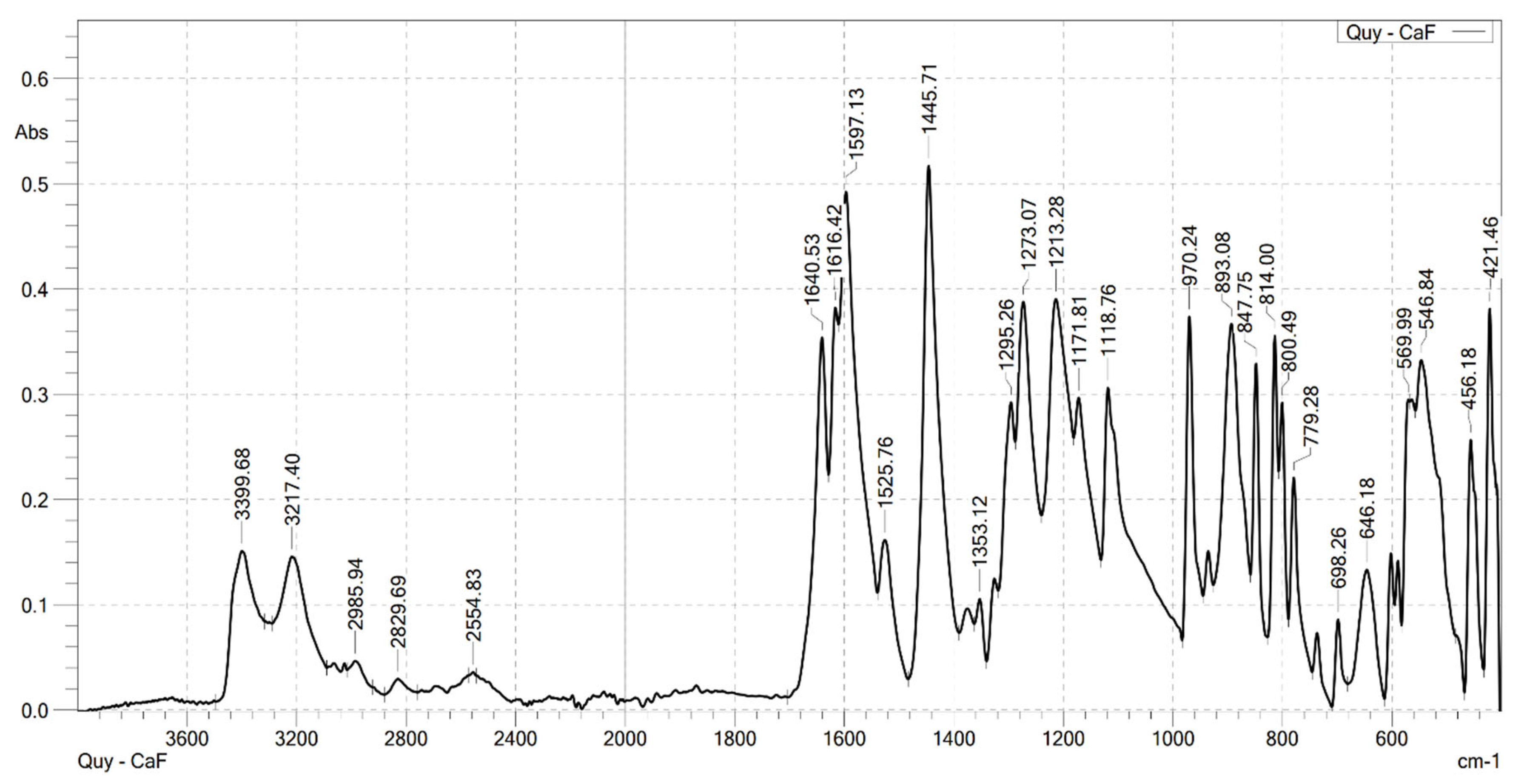
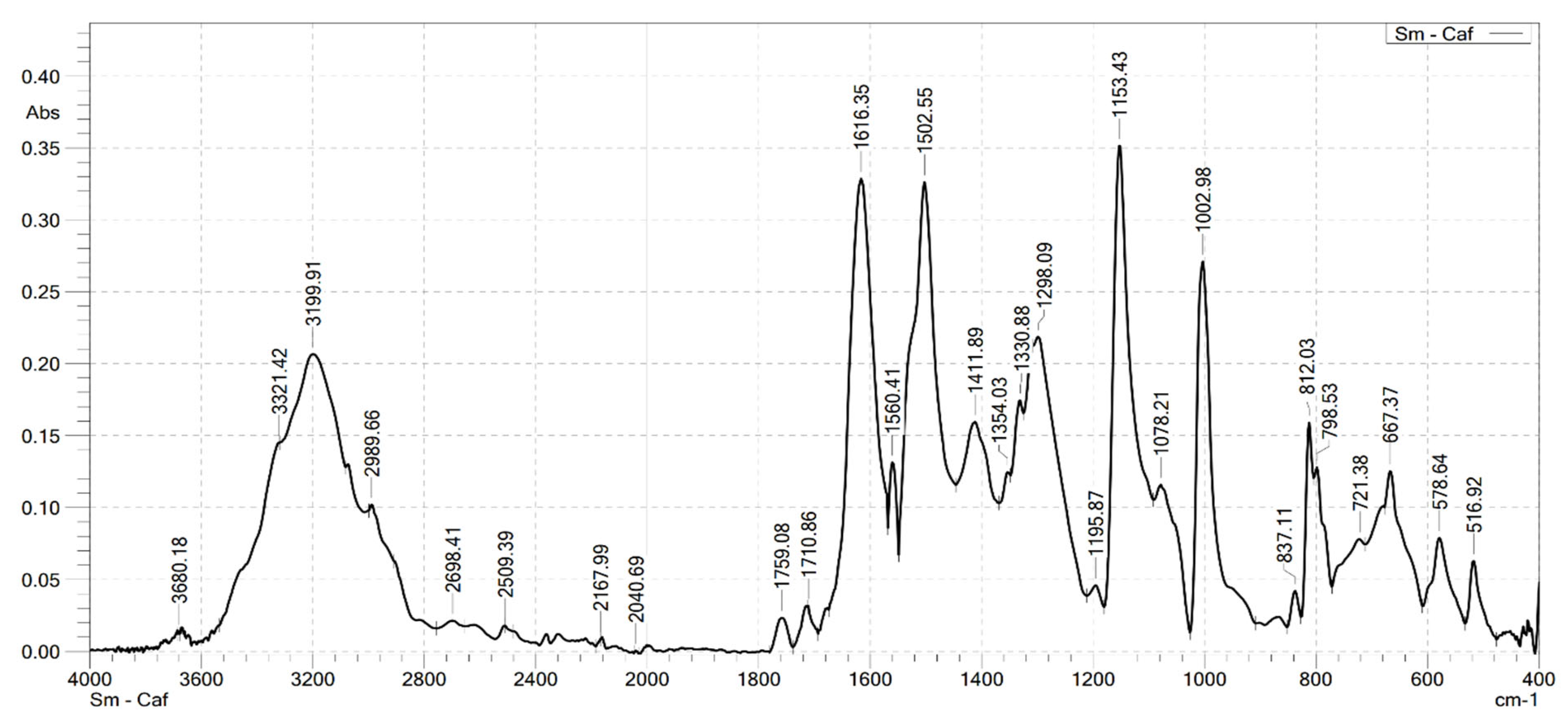
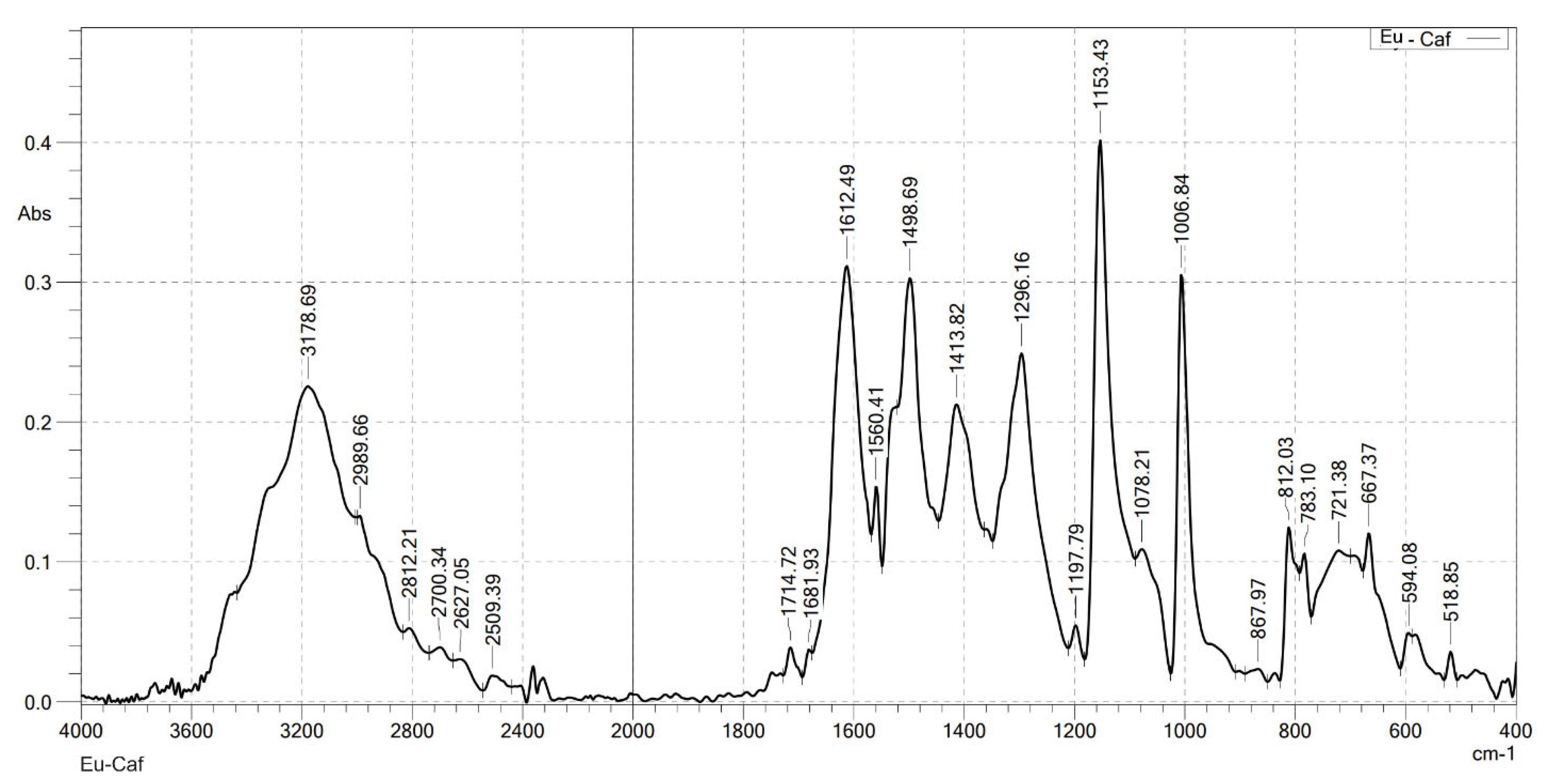
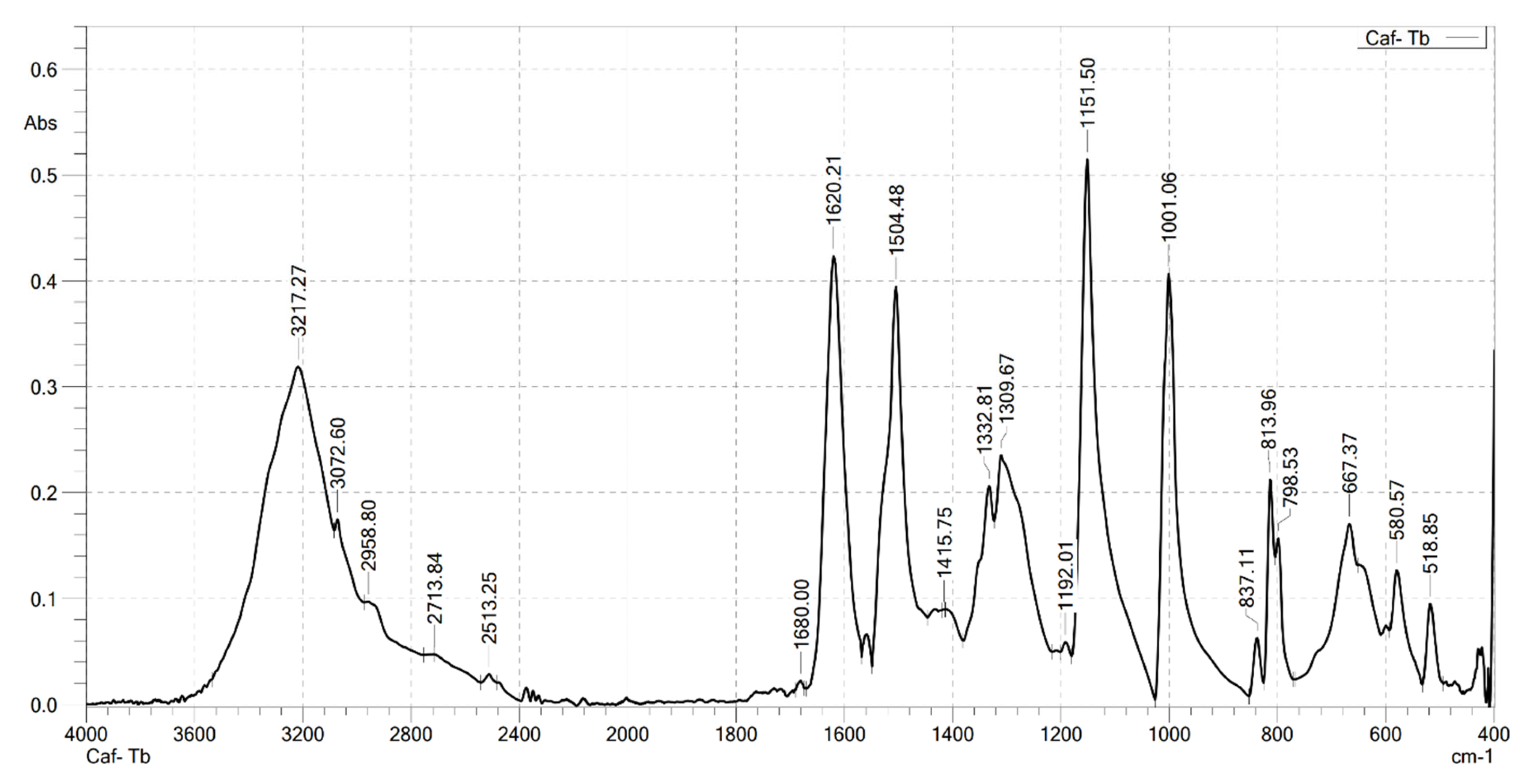
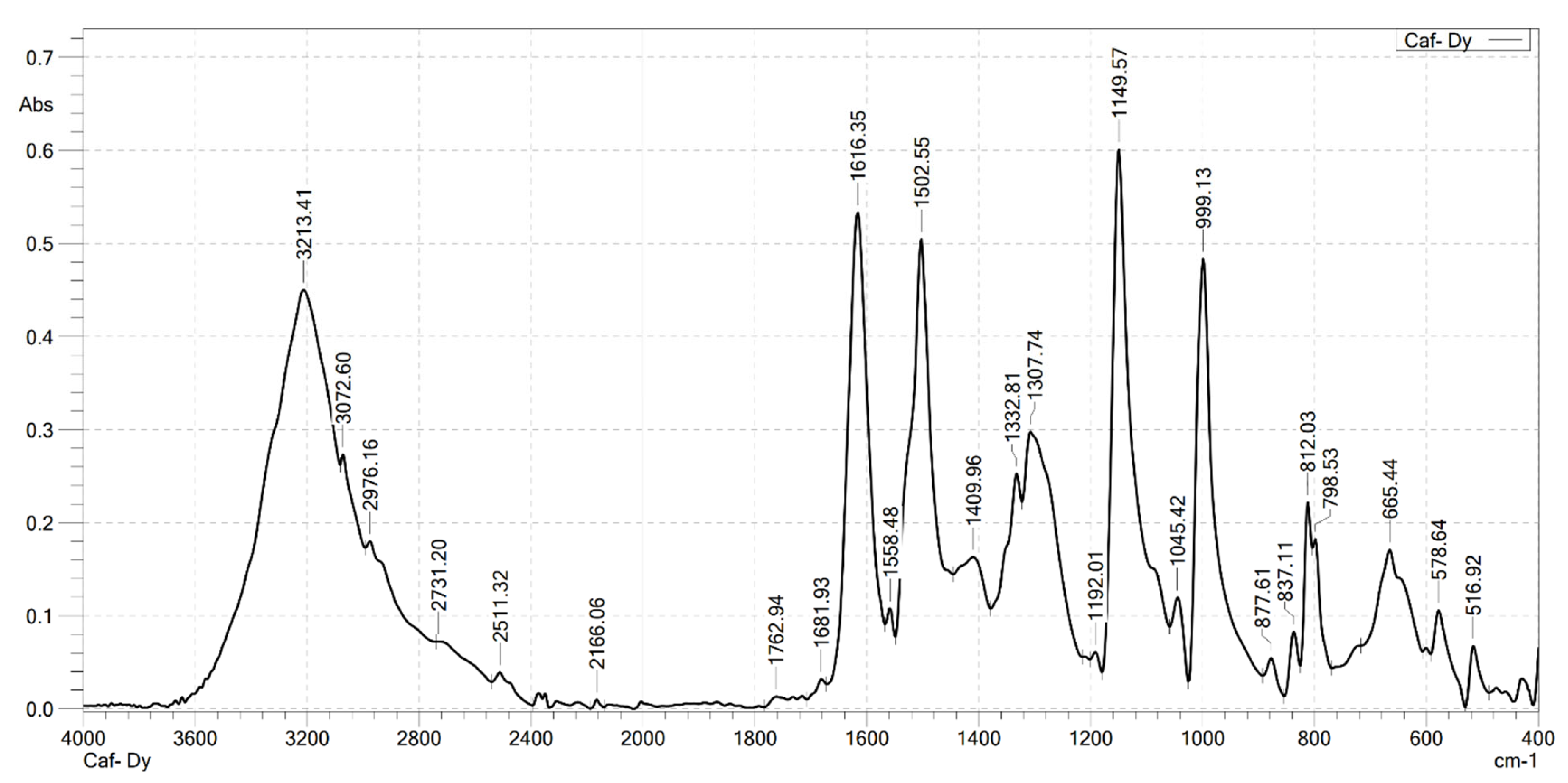
2.2. Analysis the FT-IR Spectroscopy of Complexes
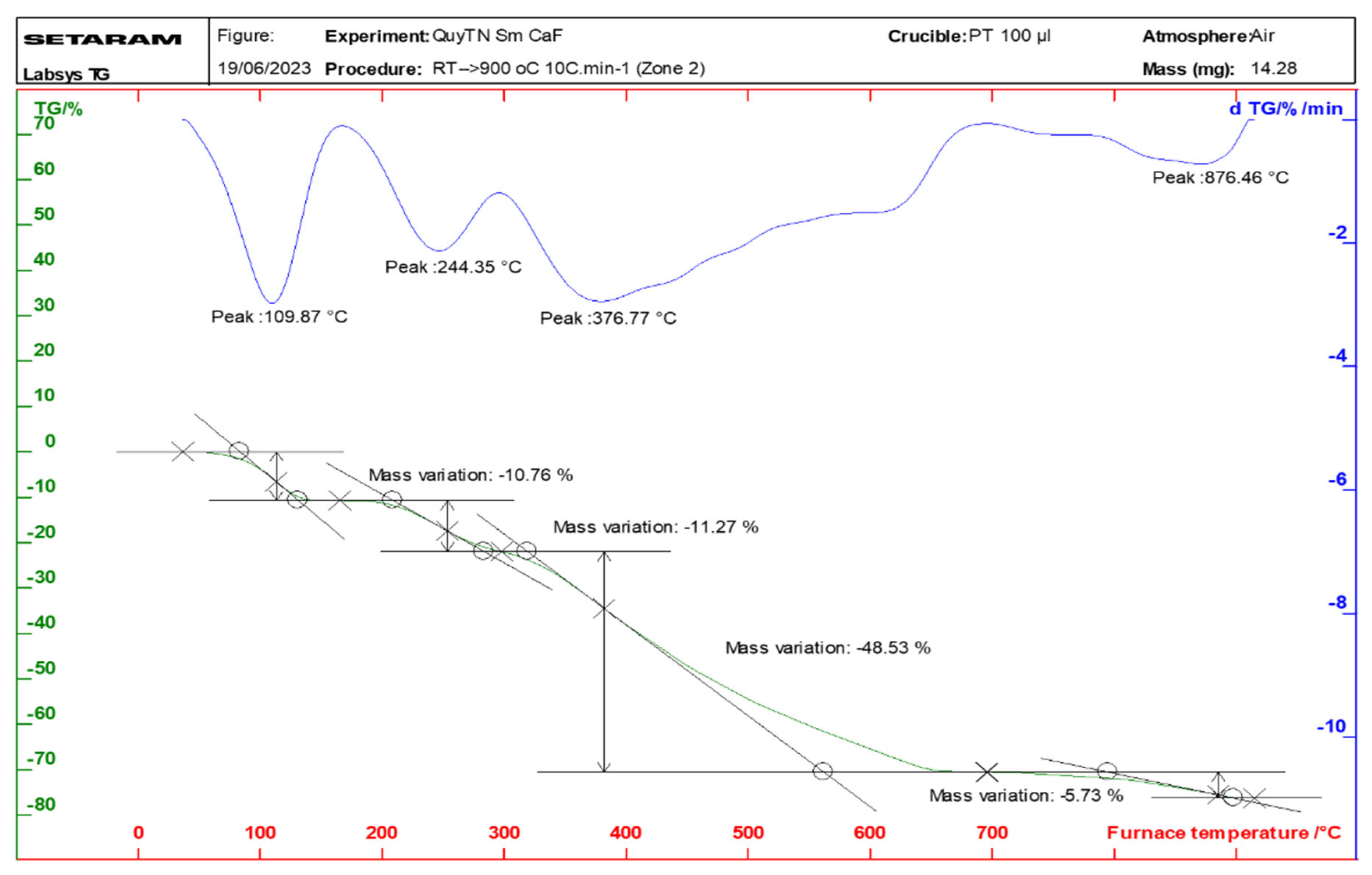
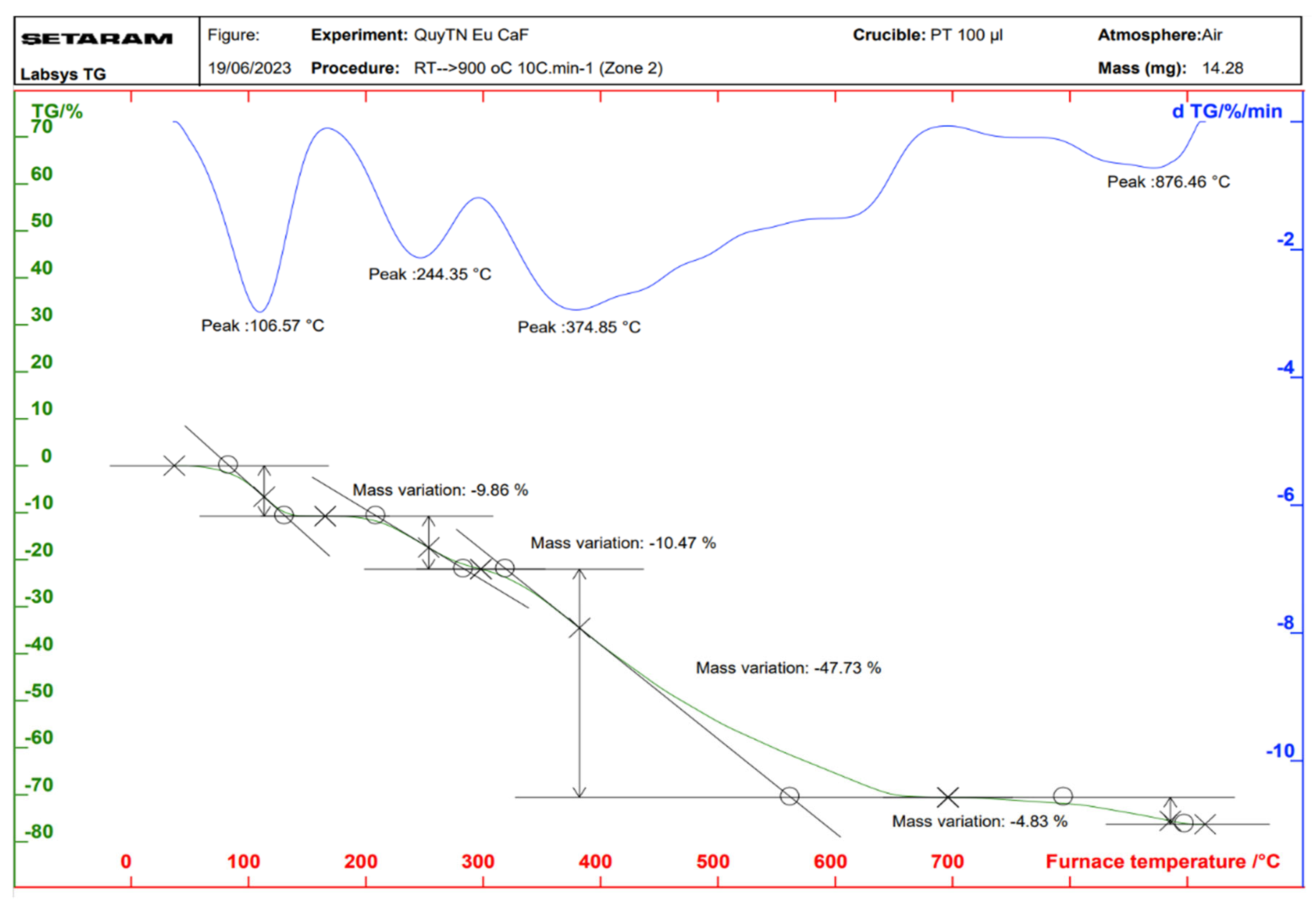
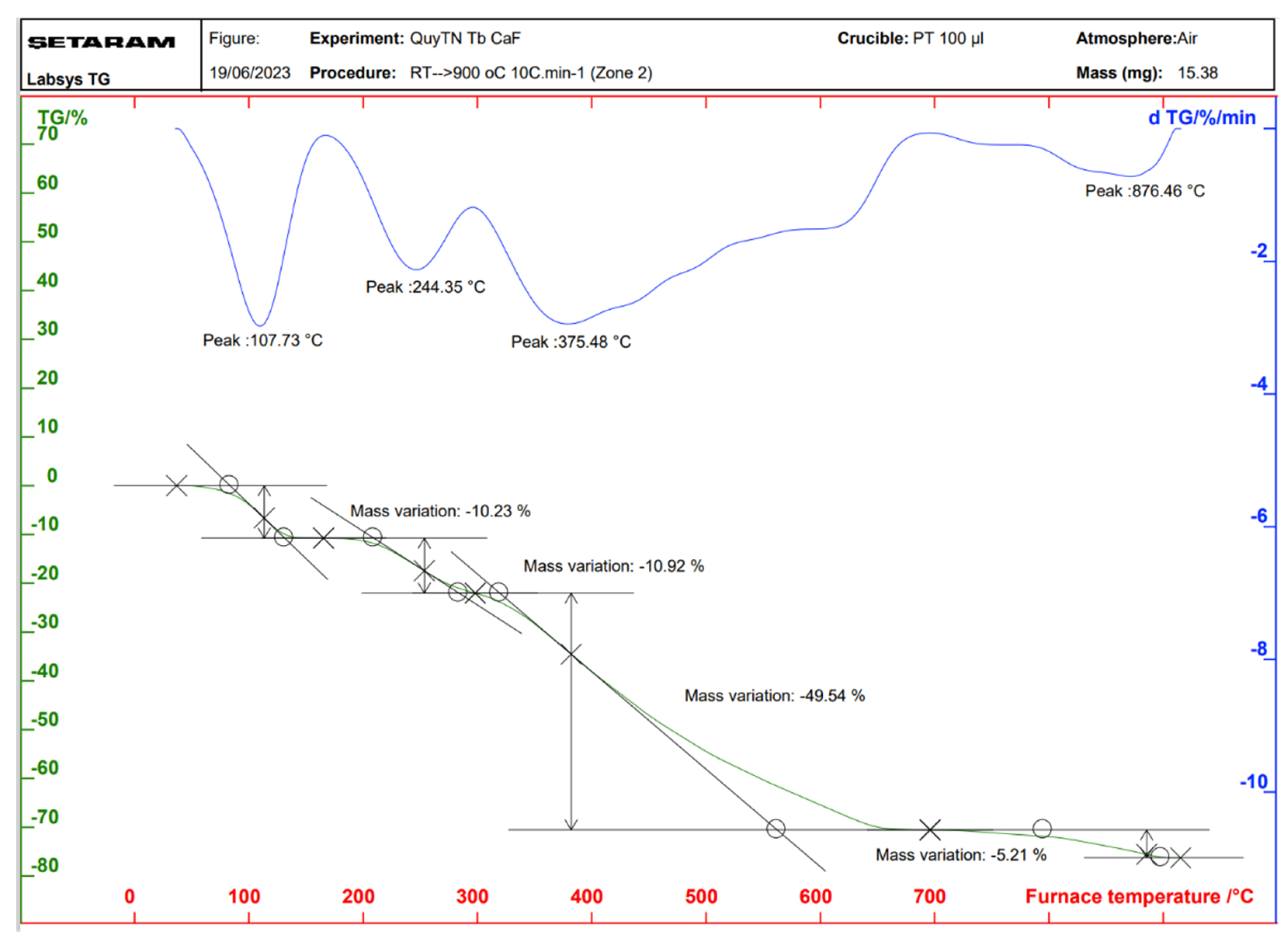
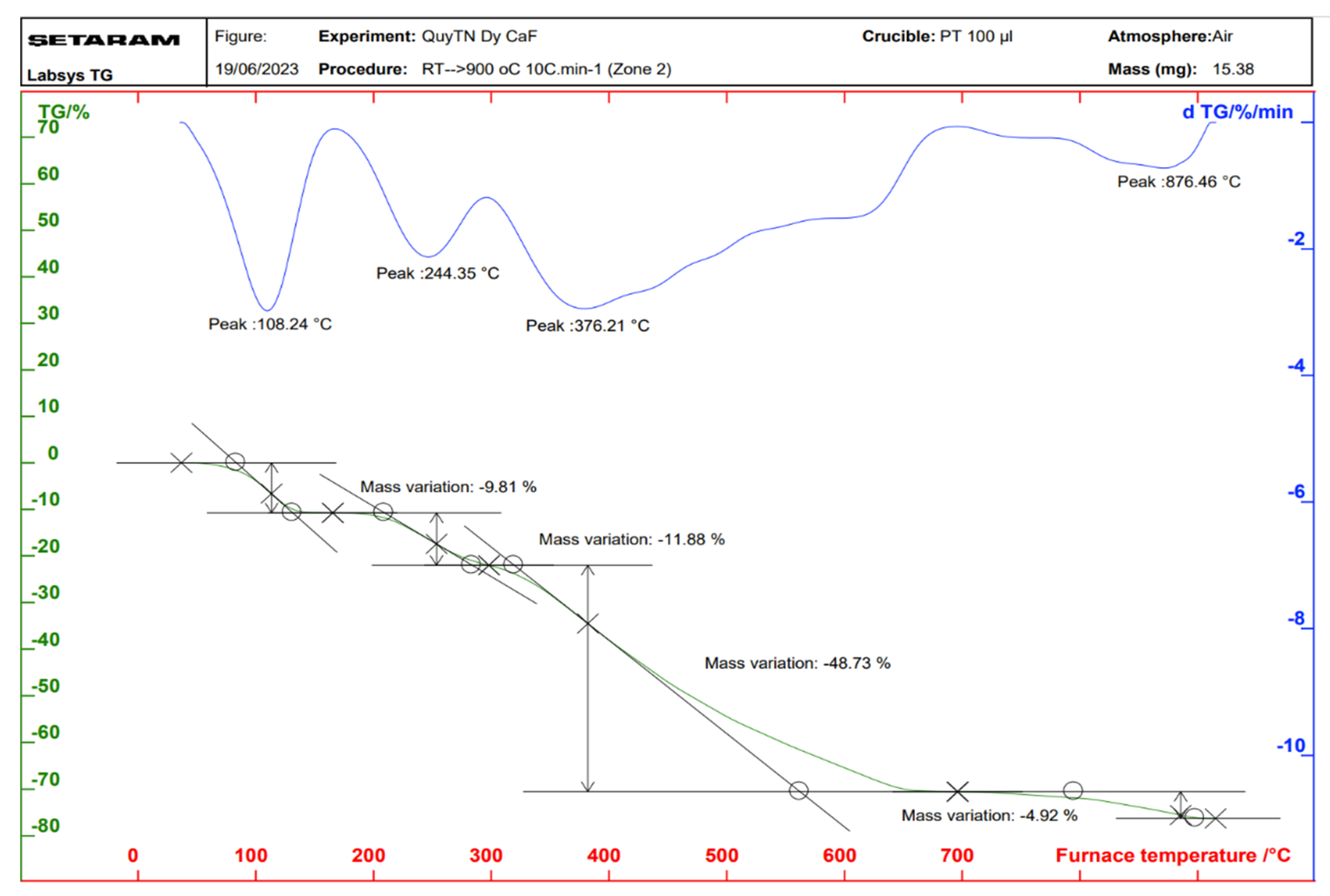
2.3. Properties of the Thermal Analysis of the Complexes
2.4. Mass Spectrum Analysis of the Complexes
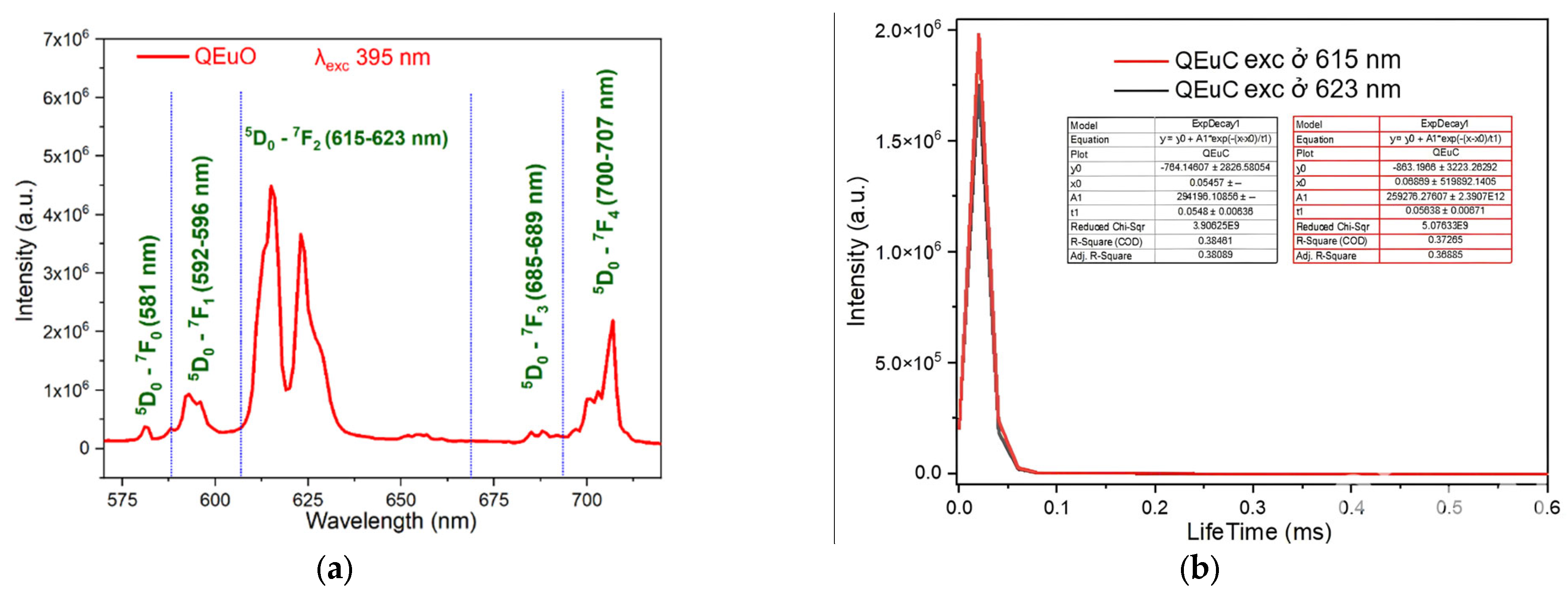
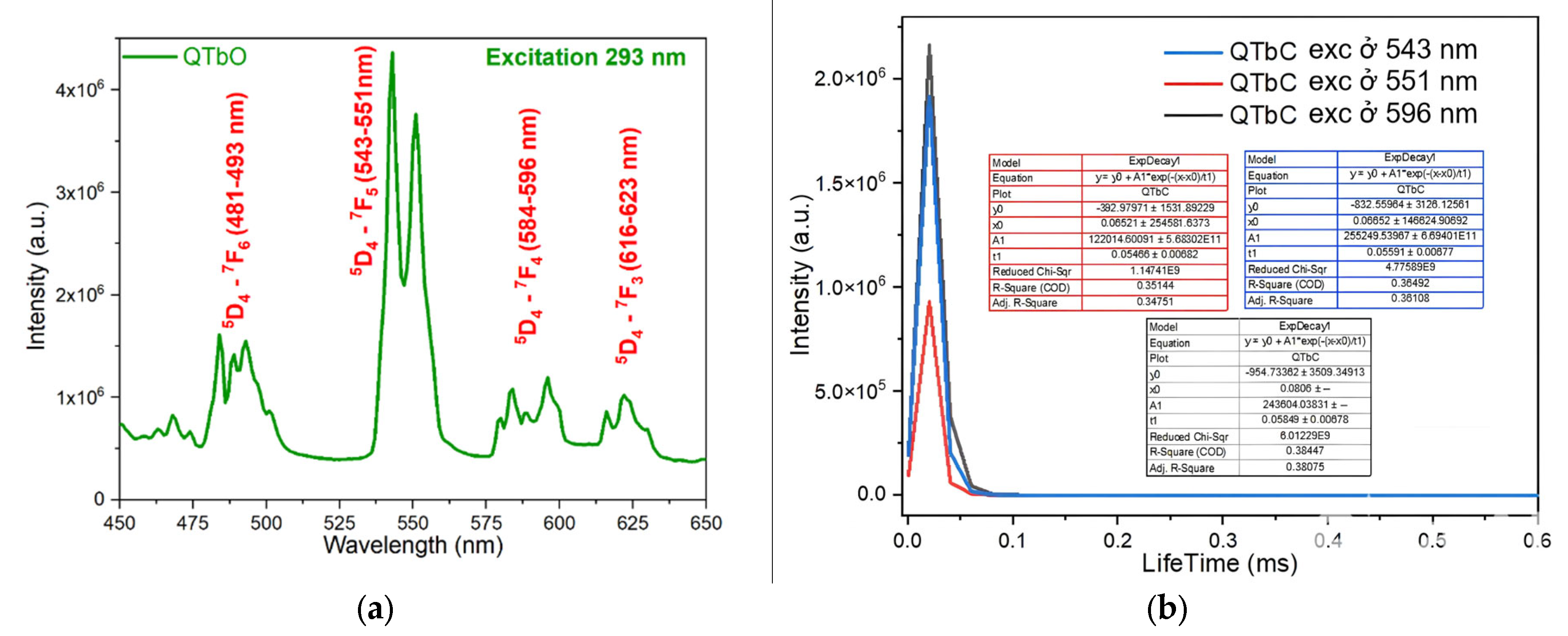
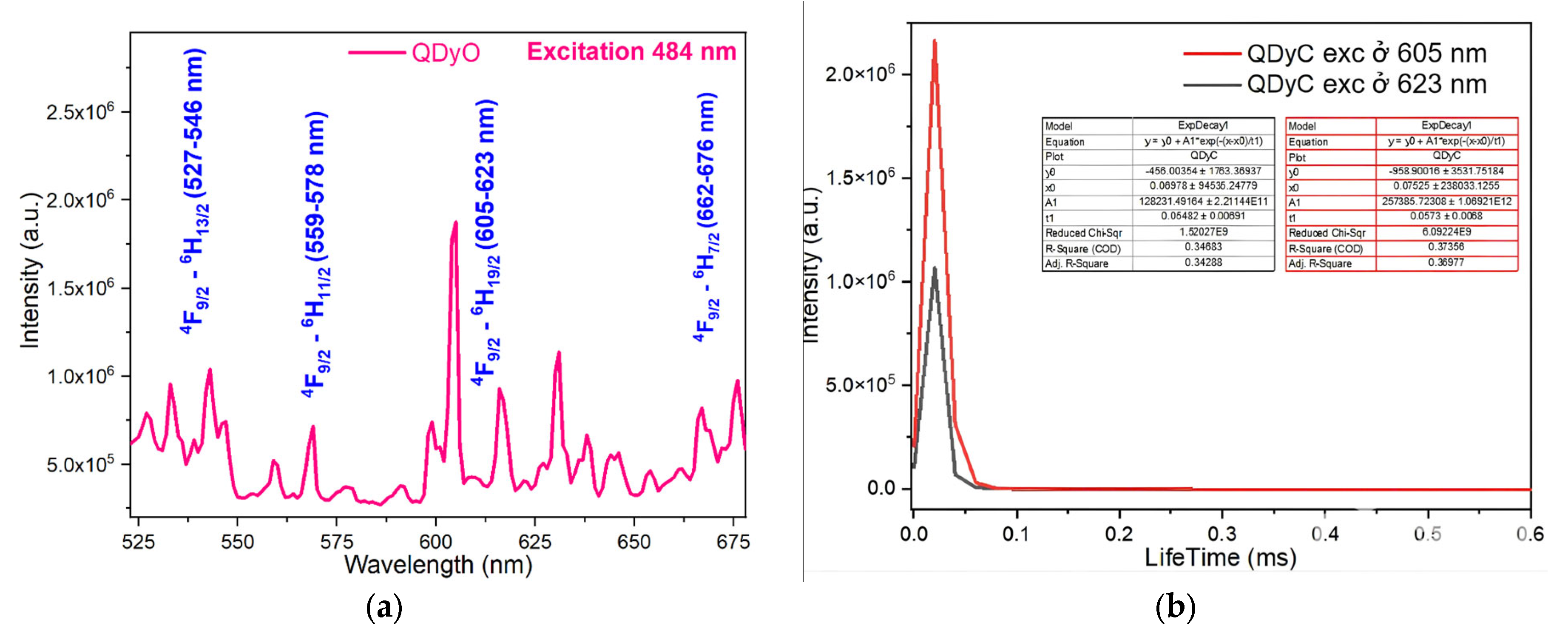
2.5. Study of Fluorescence Character of Complexes
2.6. The Antimicrobial Activity
2.7. Anticancer Activity
3. Materials and Methods
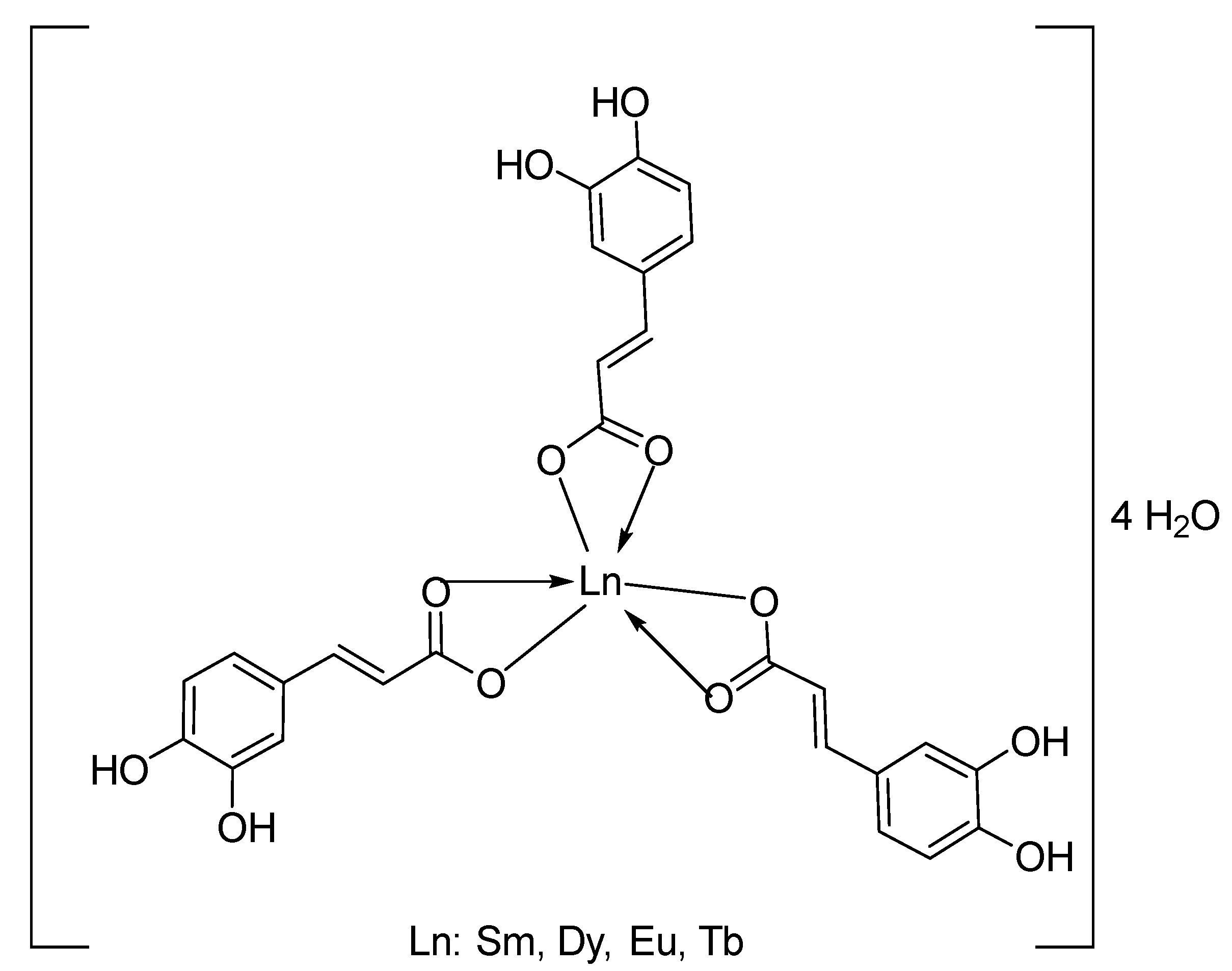
3.1. Synthesis of Ln(Caf)3·4H2O Complexes
3.2. Antibacterial Activity
3.3. Cytotoxicity Assay
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Li, B.; Yue, S.; Li, M.; Hong, Z.; Li, W. A terbium (III) complex with triphenylamine-functionalized ligand for organic electroluminescent device. J. Lumin. 2008, 128, 620–624. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; El-Sayed, M.M.; Al-Mohammadi, A.R. Some novel rare earth metal ions complexes: Synthesis, characterization, luminescence and biocidal efficiency. Anal. Biochem. 2020, 598, 113645. [Google Scholar] [CrossRef] [PubMed]
- Pichaimani, P.; Lo, K.M.; Elango, K.P. Synthesis, crystal structures, luminescence properties and catalytic application of lanthanide (III) piperidine dithiocarbamate complexes. Polyhedron 2015, 93, 8–16. [Google Scholar] [CrossRef]
- Buldurun, K.; Turan, N.; Savcı, A.; Çolak, N. Synthesis, structural characterization and biological activities of metal (II) complexes with Schiff bases derived from 5-bromosalicylaldehyde: Ru (II) complexes transfer hydrogenation. J. Saudi Chem. Soc. 2019, 23, 205–214. [Google Scholar] [CrossRef]
- Shaygan, S.; Pasdar, H.; Foroughifar, N.; Davallo, M.; Motiee, F. Cobalt (II) complexes with Schiff base ligands derived from terephthalaldehyde and ortho-substituted anilines: Synthesis, characterization and antibacterial activity. Appl. Sci. 2018, 8, 385. [Google Scholar] [CrossRef]
- Taha, Z.A.; Ajlouni, A.M.; Al Momani, W.; Al-Ghzawi, A.A. Syntheses, characterization, biological activities and photophysical properties of lanthanides complexes with a tetradentate Schiff base ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 81, 570–577. [Google Scholar] [CrossRef]
- Ajlouni, A.M.; Al Momani, W.; Al-Ghzawi, A.A.; Taha, Z.A. Synthesis, characterization, biological activities and luminescent properties of lanthanide complexes with [2-thiophenecarboxylic acid, 2-(2-pyridinylmethylene)hydrazide] Schiff bases ligand. J. Rare Earths 2016, 34, 986–993. [Google Scholar] [CrossRef]
- Kathiresan, S.; Annaraj, J.; Bhuvanesh, N.S. Cu (II) and Ni (II) Complexes of Anthracene-Affixed Schiff Base: A Conflict between Covalent and Stacking Interactions with DNA Bases. ChemistrySelect 2017, 2, 5475–5484. [Google Scholar] [CrossRef]
- Song, X.-Q.; Wang, Z.-G.; Wang, Y.; Huang, Y.-Y.; Sun, Y.-X.; Ouyang, Y.; Xie, C.-Z.; Xu, J.-Y. Syntheses, characterization, DNA/HSA binding ability and antitumor activities of a family of isostructural binuclear lanthanide complexes containing hydrazine Schiff base. J. Biomol. Struct. Dyn. 2020, 38, 733–743. [Google Scholar] [CrossRef]
- Kafi-Ahmadi, L.; Marjani, A.P. Mononuclear Schiff base complexes derived from 5-azophenylsalicylaldehyde with Co (ii), Ni (ii) ions: Synthesis, characterization, electrochemical study and antibacterial properties. S. Afr. J. Chem. 2019, 72, 101–107. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Structural characterization and biological activity of a new metal complexes based of Schiff base. J. Mol. Liq. 2021, 330, 115522. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization, antioxidant, and antibacterial studies of some metal (II) complexes of tetradentate schiff base ligand:(4E)-4-[(2-(E)-[1-(2, 4-dihydroxyphenyl) ethylidene] aminoethyl) imino] pentan-2-one. Bioinorg. Chem. Appl. 2015, 2015, 890734. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.T.C.; Silva-Caldeira, P.P.; Demarqui, F.M.; Lopes, C.D.; de Albuquerque, S.; Pavan, F.R.; Pereira-Maia, E.C.; Diniz, R.; de Oliveira, A.; de Oliveira Rezende Júnior, C.; et al. Crystal structure and in vitro biological studies of a Pt(II) complex based on gallic acid and triphenylphosphine. Inorganica Chim. Acta 2024, 569, 122124. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. An approach towards the quantitative structure-activity relationships of caffeic acid and its derivatives. ChemBioChem 2004, 5, 1188–1195. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, Y.; Yan, Y. Caffeic acid production enhancement by engineering a phenylalanine over-producing Escherichia coli strain. Biotechnol. Bioeng. 2013, 110, 3188–3196. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef]
- Secme, M.; Mutlu, D.; Elmas, L.; Arslan, S. Assessing effects of caffeic acid on cytotoxicity, apoptosis, invasion, GST enzyme activity, oxidant, antioxidant status and micro-RNA expressions in HCT116 colorectal cancer cells. S. Afr. J. Bot. 2023, 157, 19–26. [Google Scholar] [CrossRef]
- Tosovic, J. Spectroscopic features of caffeic acid: Theoretical study. Kragujev. J. Sci. 2017, 39, 99–108. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular antioxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure-activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hong, C.O.; Lee, G.P.; Kim, C.T.; Lee, K.W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef]
- Nagaoka, T.; Banskota, A.H.; Tezuka, Y.; Saiki, I.; Kadota, S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg. Med. Chem. 2002, 10, 3351–3359. [Google Scholar] [CrossRef]
- Kabat, M.; Popiół, J.; Gunia-Krzyżak, A. Cinnamic Acid Derivatives as Potential Multifunctional Agents in Cosmetic Formulations Used for Supporting the Treatment of Selected Dermatoses. Molecules 2024, 29, 5806. [Google Scholar] [CrossRef]
- Selka, A.; Abidli, A.; Schiavo, L.; Jeanmart, L.; Hanquet, G.; Lubell, W. Recent Advances in Sustainable Total Synthesis and Chiral Pool Strategies with Emphasis on (−)-Sclareol in Natural Products Synthesis. Eur. J. Org. Chem. 2025, 28, e202400983. [Google Scholar] [CrossRef]
- Peng, X.; Hu, T.; Zhang, Y.; Zhao, A.; Natarajan, B.; Wei, J.; Yan, H.; Chen, H.; Lin, C. Synthesis of caffeic acid sulfonamide derivatives and their protective effect against H2O2 induced oxidative damage in A549 cells. RSC Adv. 2020, 10, 9924. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation—A chemical approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]
- Ju, Y.; Cui, J.; Sun, H.; Müllner, M.; Dai, Y.; Guo, J.; Bertleff-Zieschang, N.; Suma, T.; Richardson, J.J.; Caruso, F. Engineered Metal-Phenolic Capsules Show Tunable Targeted Delivery to Cancer Cells. Biomacromolecules 2016, 17, 2268–2276. [Google Scholar] [CrossRef]
- Matowane, G.R.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Novel Caffeic Acid—Zinc Acetate Complex: Studies on Promising Antidiabetic and Antioxidative Synergism Through Complexation. Med. Chem. 2023, 19, 147–162. [Google Scholar]
- Bastianini, M.; Faffa, C.; Sisani, M.; Petracci, A. Caffeic Acid-layered Double Hydroxide Hybrid: A New Raw Material for Cosmetic Applications. Cosmetics 2018, 5, 51. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Manabe, R.; Kimoto, M.; Fukuda, M.; Mase, N.; Miyazawa, M.; Hosokawa, K.; Kamei, J. Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions. Antioxidants 2022, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołębiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu(III) Complexes: Solution Equilibrium Studies, Structure Characterization and Biological Activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef]
- Cota, I.; Marturano, V.; Tylkowski, B. Ln complexes as double faced agents: Study of antibacterial and antifungal activity. Coord. Chem. Rev. 2019, 396, 49–71. [Google Scholar] [CrossRef]
- Overton, E. Studien über die Narkose Zugleich ein Beitrag zur Allgemeinen Pharmakologie; Gustav Fisher: Jena, Germany, 1901; p. 195. [Google Scholar]
- Tweedy, B.G. Plant Extracts with Metal Ions as Potential Antimicrobial Agents. Phytopatology 1964, 55, 910–918. [Google Scholar]
- Zong, G.-C.; Huo, J.-X.; Ren, N.; Zhang, J.-J.; Qi, X.-X.; Gao, J.; Geng, L.-N.; Wang, S.-P.; Shi, S.-K. Preparation, characterization and properties of four new trivalent lanthanide complexes constructed using 2-bromine-5-methoxybenzoic acid and 1,10-phenanthroline. Dalton Trans. 2015, 44, 14877–14886. [Google Scholar] [CrossRef]
- Mohanan, K.; Devi, S.N. Synthesis, characterization, thermal stability, reactivity, and antimicrobial properties of some novel lanthanide(III) complexes of 2-(N-salicylideneamino)-3-carboxyethyl-4,5,6,7-tetrahydrobenzo[b]thiophene. Russ. J. Coord. Chem. 2006, 32, 600–609. [Google Scholar] [CrossRef]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, Y.; Chen, K.; Li, X. Synthesis of Tb4O7 complexed with reduced graphene oxide for Rhodamine-B absorption. Mater. Res. Bull. 2016, 77, 111–114. [Google Scholar] [CrossRef]
- Esra, Ö.; Nilgun, K. Synthesis and characterization of terbium oxide (III–IV) doped bismuth trioxide polymorphs. J. Chin. Adv. Mater. Soc. 2013, 2, 90–96. [Google Scholar]
- Şen, P.; Hirel, C.; Andraud, C.; Aronica, C.; Bretonnière, Y.; Mohammed, A.; Ågren, H.; Minaev, B.; Minaeva, V.; Baryshnikov, G.; et al. Fluorescence and FTIR Spectra Analysis of Trans-A2B2-Substituted Di- and Tetra-Phenyl Porphyrins. Materials 2010, 3, 4446–4475. [Google Scholar] [CrossRef] [PubMed]
- White, T.A.; Arachchige, S.M.; Sedai, B.; Brewer, K.J. Emission Spectroscopy as a Probe into Photoinduced Intramolecular Electron Transfer in Polyazine Bridged Ru(II),Rh(III) Supramolecular Complexes. Materials 2010, 3, 4328–4354. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, C.H.; Baker, A.D.; Strekas, T.C.; Gafney, H.D. Spectroscopic and electrochemical properties of the dimer tetrakis(2,2′-bipyridine)(mu-2,3-bis(2-pyridyl)pyrazine)diruthenium(II) and its monomeric analog. Inorg. Chem. 1984, 23, 857–864. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Nazeeruddin, M.K. Photophysics and photoredox reactions of ligand-bridged binuclear polypyridyl complexes of ruthenium(II) and of their monomeric analogs. Inorg. Chem. 1990, 29, 1888–1897. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomed. 2019, 9, 574–586. [Google Scholar]
- Serafim, T.L.; Carvalho, F.S.; Marques, M.P.; Calheiros, R.; Silva, T.; Garrido, J.; Milhazes, N.; Borges, F.; Roleira, F.; Silva, E.T.; et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Lin, Y.; Xu, J. Synthesis of Samarium-Based Metal Organic Compound Nanoparticles with Polychromatic-Photoluminescence for Bio-Tissue Fluorescence Imaging. Molecules 2019, 24, 3657. [Google Scholar] [CrossRef]
- Solov’ev, V.; Varnek, A. Thermodynamic Radii of Lanthanide Ions Derived from Metal–Ligand Complexes Stability Constants. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 69–78. [Google Scholar] [CrossRef]
- Duan, T.-W.; Yan, B. Lanthanide Ions (Eu3+, Tb3+, Sm3+, Dy3+) Activated ZnO Embedded Zinc 2,5-Pyridinedicarboxylic Metal–Organic Frameworks for Luminescence Application. J. Mater. Chem. 2015, 3, 9924–9931. [Google Scholar] [CrossRef]
- Raymond, K.N.; Xu, J.; Moore, E.G. Stable Lanthanide Luminescence Agents Highly Emissive in Aqueous Solution. J. Am. Chem. Soc. 2009, 131, 14240–14241. [Google Scholar]
- Trung, T.Q.; Hiep, H.P.; Khang, P. Chemical compositions of Litsea umbellata and inhibition activities. Open Chem. 2023, 21, 20220294. [Google Scholar] [CrossRef]
- Khang, P.; Hiep, H.; Trung, T. Two new compounds from leaves of Capparis dongvanensis (Sy, B.H. Quang & D. V. Hai) and inhibition activities. Open Chem. 2023, 21, 20220317. [Google Scholar] [CrossRef]


| Complex | Theoretical Ln3+ Content (%) | Experimental Ln3+ Content (%) |
|---|---|---|
| Sm(Caf)3·4H2O | 19.78 | 19.75 |
| Eu(Caf)3·4H2O | 19.95 | 19.94 |
| Tb(Caf)3·4H2O | 20.68 | 20.64 |
| Dy(Caf)3·4H2O | 21.04 | 21.05 |
| Compounds | v(COOH) | νas(COO–) | νs(COO–) | v(Ln–O) | v(OH) | v(CH) |
|---|---|---|---|---|---|---|
| HCaf | 1640 | – | 1445 | – | 3217 3399 | 2985 |
| Sm(Caf)3.4H2O | – | 1616 | 1502 | 578 | 3199 | 2989 |
| Eu(Caf)3.4H2O | – | 1612 | 1498 | 594 | 3178 | 2812 |
| Tb(Caf)3.4H2O | – | 1620 | 1504 | 580 | 3217 | 2958 |
| Dy(Caf)3.4H2O | – | 1616 | 1502 | 578 | 3213 | 2976 |
| Complex | Temperature at Which the Mass Loss Effect Occurs (°C) | Process | The Remaining Structure | Loss Weight (%) | |
|---|---|---|---|---|---|
| Theory | Experiment | ||||
| Sm(Caf)3·4H2O | 109 | Remove water | Sm(Caf)3 | 9.47 | 10.76 |
| 244 | Decomposition | Sm2O3 | 67.63 | 65.53 | |
| 376 | |||||
| 876 | |||||
| Eu(Caf)3·4H2O | 106 | Remove water | Eu(Caf)3 | 9.45 | 9.86 |
| 244 | Decomposition | Eu2O3 | 67.43 | 63.03 | |
| 374 | |||||
| 876 | |||||
| Tb(Caf)3·4H2O | 107 | Remove water | Tb(Caf)3 | 9.36 | 10.23 |
| 244 | Decomposition | Tb4O7 | 66.31 | 65.67 | |
| 375 | |||||
| 876 | |||||
| Dy(Caf)3·4H2O | 108 | Remove water | Dy(Caf)3 | 9.32 | 9.81 |
| 244 | Decomposition | Dy2O3 | 66.62 | 65.53 | |
| 376 | |||||
| 876 | |||||
| Complex | Ion Fragment | m/z | (%) |
|---|---|---|---|
| Sm(Caf)3·4H2O | [Sm(Caf)3]+ | 687 | 100 |
| [Sm(Caf)2]+ | 508 | 75.71 | |
| Eu(Caf)3·4H2O | [Eu(Caf)3]+ | 689 | 100 |
| [Eu(Caf)2]+ | 510 | 73.91 | |
| Tb(Caf)3·4H2O | [Tb(Caf)3]+ | 696 | 100 |
| [Tb(Caf)2]+ | 517 | 88.40 | |
| Dy(Caf)3·4H2O | [Dy(Caf)3]+ | 699 | 100 |
| [Dy(Caf)2]+ | 520 | 47.14 |
| Sample | Concentration | Escherichia coli (E.) | Staphylococcus aureus (SA) | Pseudomonas aeruginosa (PA) |
|---|---|---|---|---|
| Amoxicillin | 50 µg/mL | 30 | 23 | 25 |
| Dy(Caf)3·4H2O | 50 µg/mL | - | - | - |
| 100 µg/mL | 35 | 37 | 35 | |
| 200 µg/mL | 38 | 40 | 38 | |
| Tb(Caf)3·4H2O | 50 µg/mL | - | - | - |
| 100 µg/mL | 25 | 28 | 26 | |
| 200 µg/mL | 35 | 36 | 36 | |
| Eu(Caf)3·4H2O | 50 µg/mL | 25 | 29 | 26 |
| 100 µg/mL | 36 | 43 | 40 | |
| 200 µg/mL | 40 | 44 | 42 | |
| Sm(Caf)3·4H2O | 50 µg/mL | - | - | - |
| 100 µg/mL | 18 | 18 | 25 | |
| 200 µg/mL | 29 | 30 | 31 |
| Complex | IC50 (µM) |
|---|---|
| Dy(Caf)3·4H2O | 23.2 ± 0.5 |
| Eu(Caf)3·4H2O | 20.4 ± 0.4 |
| Sm(Caf)3·4H2O | 15.5 ± 0.3 |
| Tb(Caf)3·4H2O | 16.5 ± 0.6 |
| Caffeic acid | 27.2 ± 0.7 |
| Elippticine (control) | 0.23 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, N.T.H.; Hiep, H.P.; Van Quy, T.; Van Khang, P. Synthesis, Structural Characterization, Luminescent Properties, and Antibacterial and Anticancer Activities of Rare Earth-Caffeic Acid Complexes. Molecules 2025, 30, 2162. https://doi.org/10.3390/molecules30102162
Lan NTH, Hiep HP, Van Quy T, Van Khang P. Synthesis, Structural Characterization, Luminescent Properties, and Antibacterial and Anticancer Activities of Rare Earth-Caffeic Acid Complexes. Molecules. 2025; 30(10):2162. https://doi.org/10.3390/molecules30102162
Chicago/Turabian StyleLan, Nguyen Thi Hien, Hoang Phu Hiep, Tran Van Quy, and Pham Van Khang. 2025. "Synthesis, Structural Characterization, Luminescent Properties, and Antibacterial and Anticancer Activities of Rare Earth-Caffeic Acid Complexes" Molecules 30, no. 10: 2162. https://doi.org/10.3390/molecules30102162
APA StyleLan, N. T. H., Hiep, H. P., Van Quy, T., & Van Khang, P. (2025). Synthesis, Structural Characterization, Luminescent Properties, and Antibacterial and Anticancer Activities of Rare Earth-Caffeic Acid Complexes. Molecules, 30(10), 2162. https://doi.org/10.3390/molecules30102162








