New Visible-Light-Sensitive Dicyanocoumarin- and COUPY-Based Caging Groups with Improved Photolytic Efficiency
Abstract
1. Introduction
2. Results and Discussion
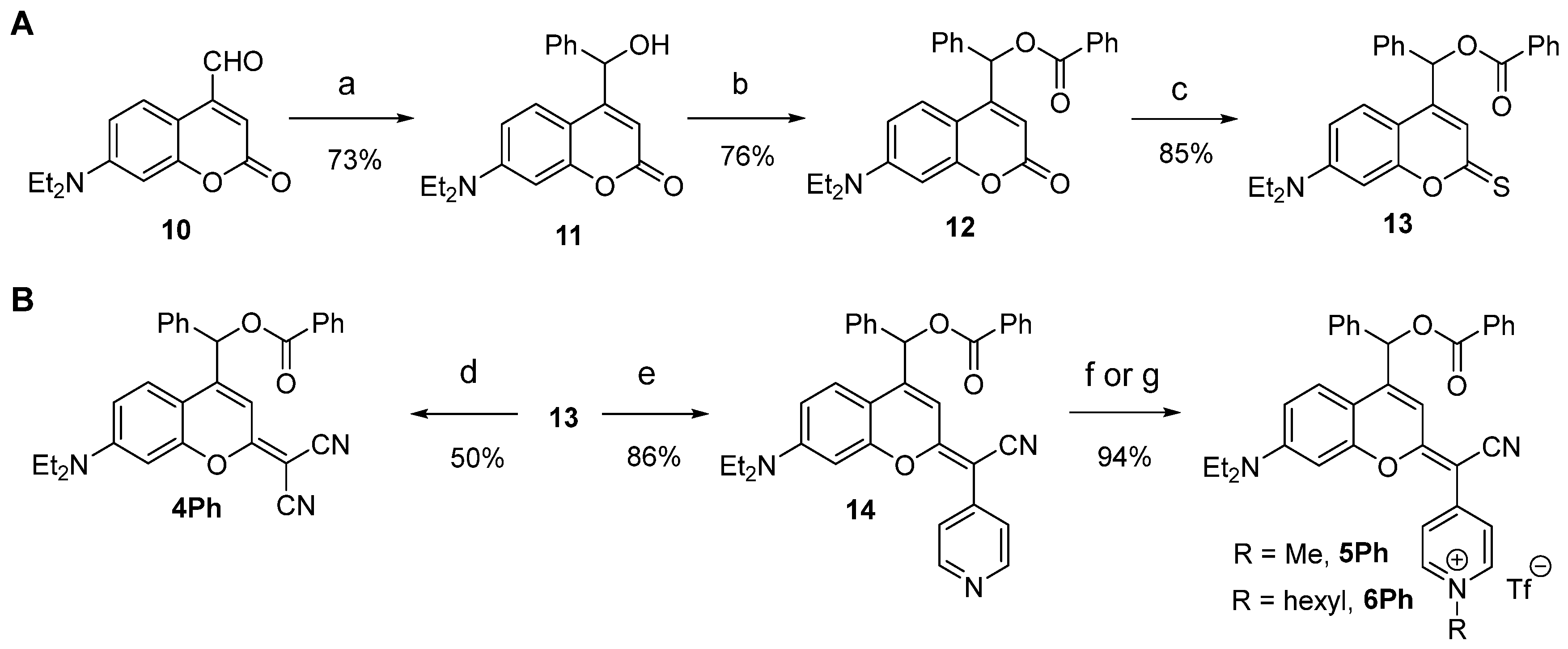
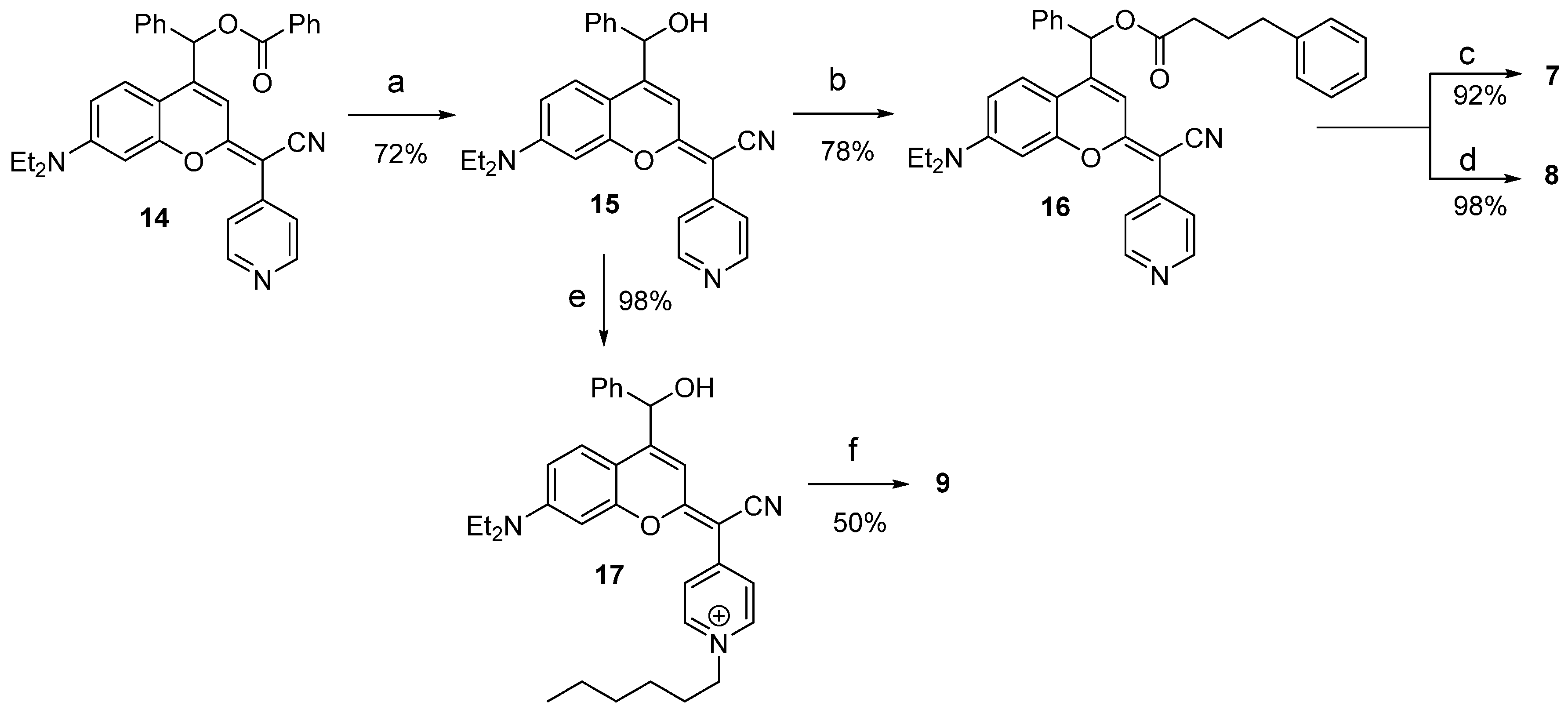
2.1. Synthesis and Characterization of Dicyanocoumarin (4Ph) and COUPY (5Ph, 6Ph, 7–9) Photocages
2.2. Photophysical Characterization of the Compounds
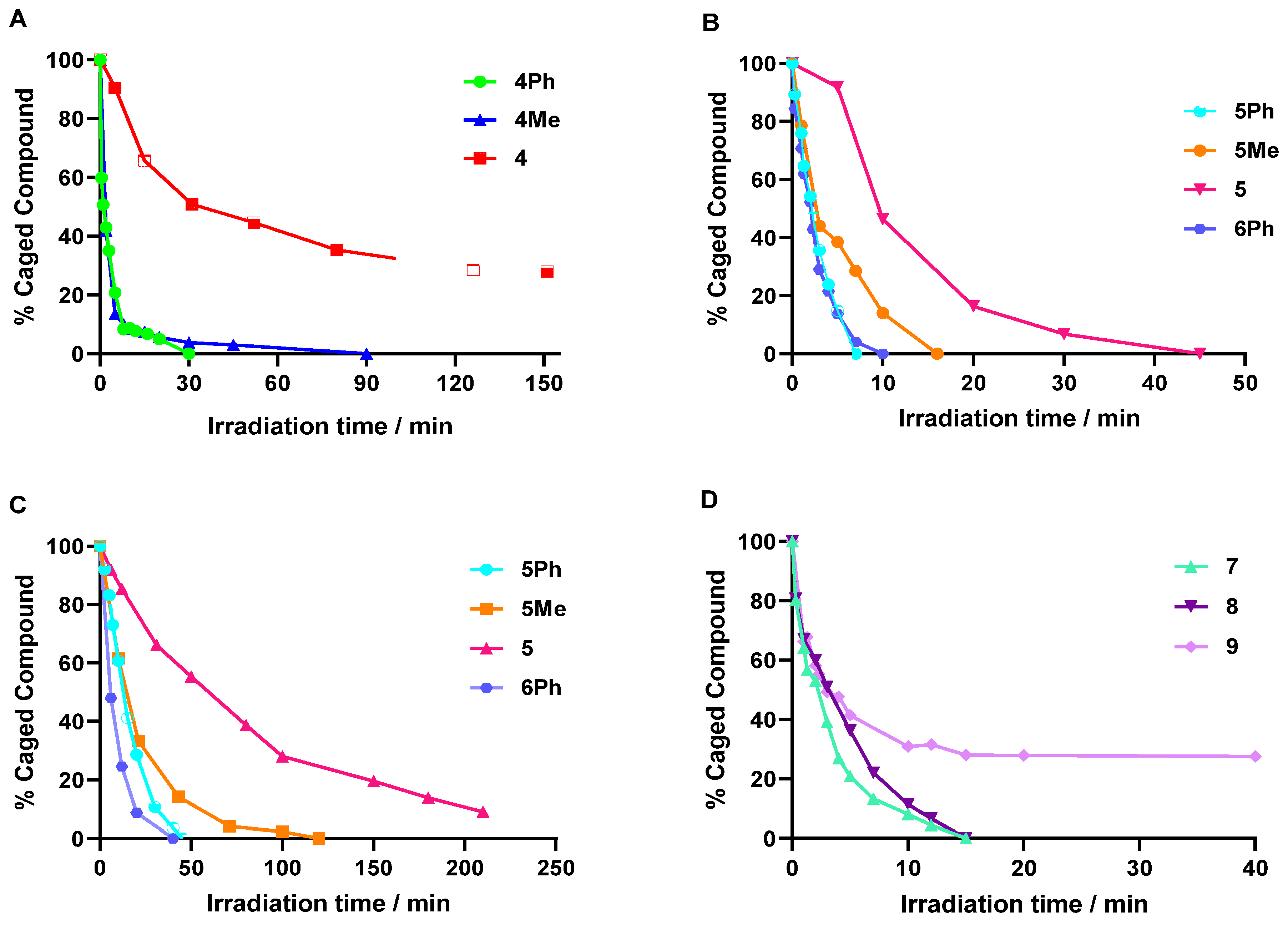
2.3. Photolysis Studies of Dicyanocoumarin and COUPY Photocages
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Coumarin Scaffolds (11–17)
- Compound 11
- Compound 12
- Compound 13
- Compound 14
- Compound 15
- Compound 16
- Compound 17
3.3. Synthesis of Photocages (4Ph, 5Ph, 7–9)
- Compound 4Ph
- Compound 5Ph
- Compound 6
- Compound 7
- Compound 8
- Compound 9
3.4. Photophysical Characterization of the Compounds
3.5. Irradiation Experiments
3.6. Uncaging Quantum Yield Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klan, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Wavelength-selective cleavage of photoprotecting groups: Strategies and applications in dynamic systems. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Empel, C.; Pelliccia, S.; Koenigs, R.M.; Proschak, E.; Hernandez-Olmos, V. Photochemistry in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2024, 67, 4322–4345. [Google Scholar] [CrossRef]
- Brieke, C.; Rohrbach, F.; Gottschalk, A.; Mayer, G.; Heckel, A. Light-controlled tools. Angew. Chem. Int. Ed. 2012, 51, 8446–8476. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 37–61. [Google Scholar] [CrossRef]
- Sabino, C.P.; Deana, A.M.; Yoshimura, T.M.; da Silva, D.F.T.; Franca, C.M.; Hamblin, M.R.; Ribeiro, M.S. The optical properties of mouse skin in the visible and near infrared spectral regions. J. Photochem. Photobiol. B 2016, 160, 72–78. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Josa-Culleré, L.; Llebaria, A. In the Search for Photocages Cleavable with Visible Light: An Overview of Recent Advances and Chemical Strategies. ChemPhotoChem 2021, 5, 296–314. [Google Scholar] [CrossRef]
- Weinstain, R.; Slanina, T.; Kand, D.; Klan, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef]
- Shrestha, P.; Kand, D.; Weinstain, R.; Winter, A.H. meso-Methyl BODIPY Photocages: Mechanisms, Photochemical Properties, and Applications. J. Am. Chem. Soc. 2023, 145, 17497–17514. [Google Scholar] [CrossRef] [PubMed]
- Poryvai, A.; Galkin, M.; Shvadchak, V.; Slanina, T. Red-Shifted Water-Soluble BODIPY Photocages for Visualisation and Controllable Cellular Delivery of Signaling Lipids. Angew. Chem. Int. Ed. 2022, 61, e202205855. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Chok, K.; Junek, S.; Glaubitz, C.; Heckel, A. Rhodamine-Sensitized Two-Photon Activation of a Red Light-Absorbing BODIPY Photocage. Chem.-Eur. J. 2023, 29, e202300149. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; Aujard, I.; Le Saux, T.; Maurin, S.; Beaupierre, S.; Baudin, J.-B.; Jullien, L. Coumarinylmethyl caging groups with redshifted absorption. Chem.-Eur. J. 2013, 19, 17494–17507. [Google Scholar] [CrossRef]
- Gandioso, A.; Palau, M.; Nin-Hill, A.; Melnyk, I.; Rovira, C.; Nonell, S.; Velasco, D.; Garcia-Amoros, J.; Marchán, V. Sequential Uncaging with Green Light can be Achieved by Fine-Tuning the Structure of a Dicyanocoumarin Chromophore. ChemistryOpen 2017, 6, 375–384. [Google Scholar] [CrossRef]
- Sansalone, L.; Zhao, J.; Nguyen, L.; Gupta, S.; Benson, D.; Abe, M.; Ellis-Davies, G. Bidirectional Neuronal Actuation by Uncaging with Violet and Green Light. Angew. Chem. Int. Ed. 2024, 63, e202315726. [Google Scholar] [CrossRef]
- Love, A.C.; Caldwell, D.R.; Kolbaba-Kartchner, B.; Townsend, K.M.; Halbers, L.P.; Yao, Z.; Brennan, C.K.; Ivanic, J.; Hadjian, T.; Mills, J.H.; et al. Red-Shifted Coumarin Luciferins for Improved Bioluminescence Imaging. J. Am. Chem. Soc. 2023, 145, 3335–3345. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Shi, X.; Liu, W.; Liang, L.; Xia, S.; Yan, J.; Sun, X. In Search of Visible Light Activatable Photocages: Structure-Activity Relationship Study on C-8 Substituted Indene-Fused-Coumarinyl Photoremovable Protecting Groups. ChemPhotoChem 2024, 8, e202300309. [Google Scholar] [CrossRef]
- Gorka, A.P.; Yamamoto, T.; Schnermann, J.M. Cyanine Photocages Enable Spatial Control of Inducible Cre-Mediated Recombination. ChemBioChem 2018, 19, 1239–1243. [Google Scholar] [CrossRef]
- Janekova, H.; Russo, M.; Ziegler, U.; Štacko, P. Photouncaging of Carboxylic Acids from Cyanine Dyes with Near-Infrared Light. Angew. Chem. Int. Ed. 2022, 61, e202204391. [Google Scholar] [CrossRef]
- Singh, A.K.; Banerjee, S.; Nair, A.V.; Ray, S.; Ojha, M.; Mondal, A.; Singh, N.D.P. Green Light-Activated Single-Component Organic Fluorescence-Based Nano-Drug Delivery System for Dual Uncaging of Anticancer Drugs. ACS Appl. Bio Mater. 2022, 5, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Mengji, R.; Roy, S.; Pal, B.; Jana, A.; Singh, N.D.P. NIR-Responsive Lysosomotropic Phototrigger: An “AIE + ESIPT” Active Naphthalene-Based Single-Component Photoresponsive Nanocarrier with Two-Photon Uncaging and Real-Time Monitoring Ability. ACS Appl. Mater. Interfaces 2022, 14, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Egyed, A.; Nemeth, K.; Molnar, T.A.; Kallay, M.; Kele, P.; Bojtar, M. Turning Red without Feeling Embarrassed-Xanthenium-Based Photocages for Red-Light-Activated Phototherapeutics. J. Am. Chem. Soc. 2023, 145, 4026–4034. [Google Scholar] [CrossRef]
- Lu, D.; Yang, S.; Yu, Q.; Zhu, T.; Ji, L.; Wang, C.; Deng, T.; Liu, S.; Lv, W.; Zhao, Q. Red/near-infrared light triggered photorelease via sensitized photolysis. Coord. Chem. Rev. 2024, 518, 216117. [Google Scholar] [CrossRef]
- Štacko, P.; Šolomek, T. Photoremovable Protecting Groups: Across the Light Spectrum to Near- Infrared Absorbing Photocages. Chimia 2021, 75, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Clotworthy, M.R.; Dawson, J.J.; Johnstone, M.D.; Fleming, C.L. Coumarin-Derived Caging Groups in the Spotlight:Tailoring Physiochemical and Photophysical Properties. ChemPlusChem 2024, 89, e202400377. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Mechanism of Photocleavage of (Coumarin-4-yl)methyl Esters. J. Phys. Chem. A 2007, 111, 5768–5774. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Abe, M. Crucial Roles of Leaving Group and Open-Shell Cation in Photoreaction of (Coumarin-4-yl)methyl Derivatives. J. Am. Chem. Soc. 2024, 146, 10993–11001. [Google Scholar] [CrossRef]
- Klimek, R.; Asido, M.; Hermanns, V.; Junek, S.; Wachtveitl, J.; Heckel, A. Inactivation of Competitive Decay Channels Leads to Enhanced Coumarin Photochemistry. Chem.-Eur. J. 2022, 28, e202200647. [Google Scholar] [CrossRef]
- Schulte, A.M.; Alachouzos, G.; Szymanski, W.; Feringa, B.L. Strategy for Engineering High Photolysis Efficiency of Photocleavable Protecting Groups through Cation Stabilization. J. Am. Chem. Soc. 2022, 144, 12421–12430. [Google Scholar] [CrossRef]
- Schulte, A.M.; Alachouzos, G.; Szymanski, W.; Feringa, B.L. The fate of the contact ion pair determines the photochemistry of coumarin-based photocleavable protecting groups. Chem. Sci. 2024, 15, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- López-Corrales, M.; Rovira, A.; Gandioso, A.; Bosch, M.; Nonell, S.; Marchán, V. Transformation of COUPY Fluorophores into a Novel Class of Visible-Light-Cleavable Photolabile Protecting Groups. Chem.-Eur. J. 2020, 26, 16222–16227. [Google Scholar] [CrossRef] [PubMed]
- López-Corrales, M.; Rovira, A.; Gandioso, A.; Nonell, S.; Bosch, M.; Marchán, V. Mitochondria-Targeted COUPY Photocages: Synthesis and Visible-Light Photoactivation in Living Cells. J. Org. Chem. 2023, 88, 7128–7140. [Google Scholar] [CrossRef]
- Gandioso, A.; Bresolí-Obach, R.; Nin-Hill, A.; Bosch, M.; Palau, M.; Galindo, A.; Contreras, S.; Rovira, A.; Rovira, C.; Nonell, S.; et al. Redesigning the Coumarin Scaffold into Small Bright Fluorophores with Far-Red to Near-Infrared Emission and Large Stokes Shifts Useful for Cell Imaging. J. Org. Chem. 2018, 83, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.; Gandioso, A.; Goñalons, M.; Galindo, A.; Massaguer, A.; Bosch, M.; Marchán, V. Solid-Phase Approaches for Labeling Targeting Peptides with Far-Red Emitting Coumarin Fluorophores. J. Org. Chem. 2019, 84, 1808–1817. [Google Scholar] [CrossRef]
- Rovira, A.; Pujals, M.; Gandioso, A.; López-Corrales, M.; Bosch, M.; Marchán, V. Modulating Photostability and Mitochondria Selectivity in Far-Red/NIR Emitting Coumarin Fluorophores through Replacement of Pyridinium by Pyrimidinium. J. Org. Chem. 2020, 85, 6086–6097. [Google Scholar] [CrossRef]
- Josa-Culleré, L.; Llebaria, A. Visible-Light-Controlled Histone Deacetylase Inhibitors for Targeted Cancer Therapy. J. Med. Chem. 2023, 66, 1909–1927. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journey of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef]
- Roibu, A.; Fransen, S.; Leblebici, M.E.; Meir, G.; Gerven, T.V.; Kuhn, S. An accessible visible-light actinometer for the determination of photon flux and optical pathlength in flow photo microreactors. Sci. Rep. 2018, 8, 5421. [Google Scholar] [CrossRef]
- Maafi, M. The potential of AB(1Φ) systems for direct actinometry. Diarylethenes as successful actinometers for the visible range. Phys. Chem. Chem. Phys. 2010, 12, 13248–13254. [Google Scholar] [CrossRef]
- Maafi, M.; Brown, R.G. The kinetic model for AB(1Φ) systems A closed-form integration of the differential equation with a variable photokinetic factor. J. Photochem. Photobiol. A 2007, 187, 319–324. [Google Scholar] [CrossRef]
- Sumi, T.; Takagi, Y.; Yagi, A.; Morimoto, M.; Irie, M. Photoirradiation wavelength dependence of cycloreversion quantum yields of diarylethenes. Chem. Commun. 2014, 50, 3928–3930. [Google Scholar] [CrossRef] [PubMed]


| Absorption | Emission | ||||
|---|---|---|---|---|---|
| Compound | λmax (nm) a | ε(λmax) (mM−1 cm−1) b | λem (nm) c | Stokes’ Shift (nm) d | ΦF e |
| 4Ph | 509 | 30.0 | 556 | 47 | 0.10 |
| 4Me | 498 | 30.7 | 555 | 57 | 0.08 |
| 4 | 486 | 30.0 | 560 | 74 | 0.09 |
| 5Ph | 561 | 47.2 | 619 | 58 | 0.20 |
| 5Me | 558 | 46.6 | 616 | 58 | 0.13 |
| 5 | 557 | 44.2 | 627 | 70 | 0.12 |
| Compound | Source (nm) | ku (min−1) b | ΦPhot [×10−5] c | ε(λirr) [mM−1 cm−1] d | ΦPhot × ε(λirr) [M−1cm−1] e |
|---|---|---|---|---|---|
| 4Ph | 505 | 0.184 | 22.0 | 29.7 | 4.60 |
| 4Me | 505 | 0.069 | 18.2 | 29.6 | 4.12 |
| 4 | 505 | 0.009 | 0.13 | 12.3 | 0.03 |
| 5Ph | 530 | 0.384 | 11.0 | 30.3 | 3.57 |
| 620 | 0.086 | 19.4 | 2.79 | 0.54 | |
| 5Me | 530 | 0.177 | 8.68 | 29.8 | 2.59 |
| 620 | 0.037 | 14.7 | 1.53 | 0.23 | |
| 5 | 530 | 0.099 | 3.91 | 31.9 | 1.25 |
| 620 | 0.011 | 1.57 | 2.3 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Corrales, M.; Marchán, V. New Visible-Light-Sensitive Dicyanocoumarin- and COUPY-Based Caging Groups with Improved Photolytic Efficiency. Molecules 2025, 30, 2158. https://doi.org/10.3390/molecules30102158
López-Corrales M, Marchán V. New Visible-Light-Sensitive Dicyanocoumarin- and COUPY-Based Caging Groups with Improved Photolytic Efficiency. Molecules. 2025; 30(10):2158. https://doi.org/10.3390/molecules30102158
Chicago/Turabian StyleLópez-Corrales, Marta, and Vicente Marchán. 2025. "New Visible-Light-Sensitive Dicyanocoumarin- and COUPY-Based Caging Groups with Improved Photolytic Efficiency" Molecules 30, no. 10: 2158. https://doi.org/10.3390/molecules30102158
APA StyleLópez-Corrales, M., & Marchán, V. (2025). New Visible-Light-Sensitive Dicyanocoumarin- and COUPY-Based Caging Groups with Improved Photolytic Efficiency. Molecules, 30(10), 2158. https://doi.org/10.3390/molecules30102158





