Nitroxides: Chemistry, Antioxidant Properties, and Biomedical Applications
Abstract
1. Introduction
2. Chemical Properties of Nitroxides
3. Application of Nitroxides in Biology and Medicine
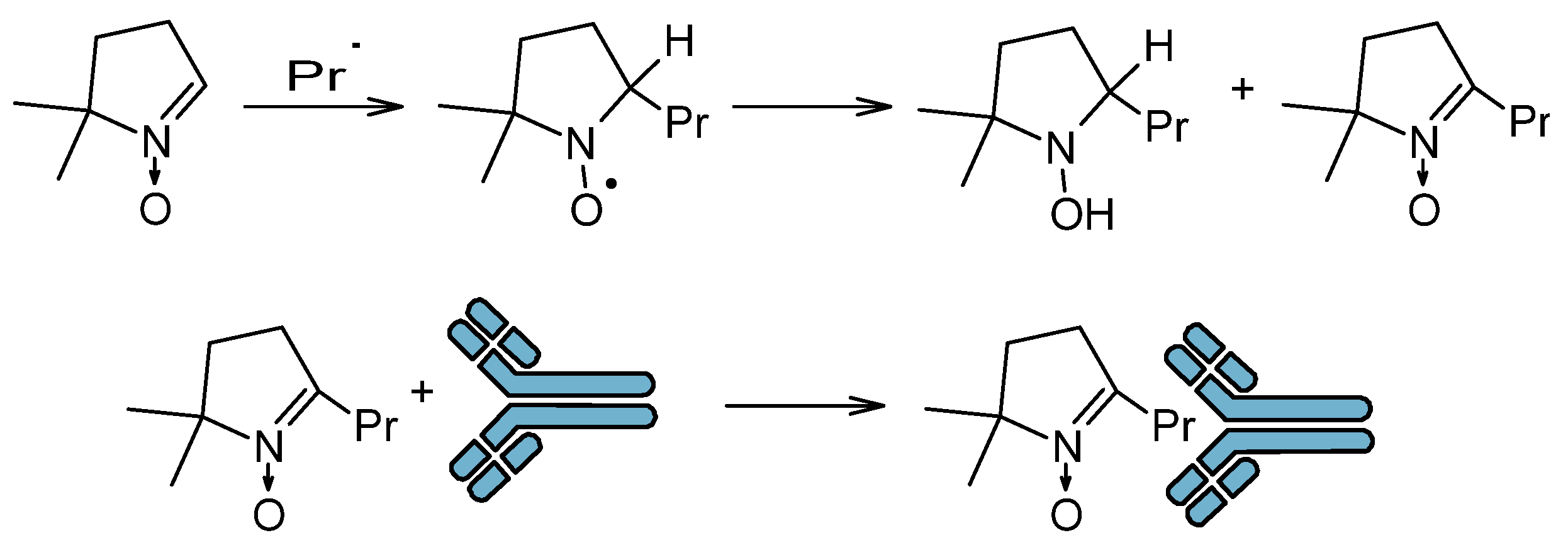
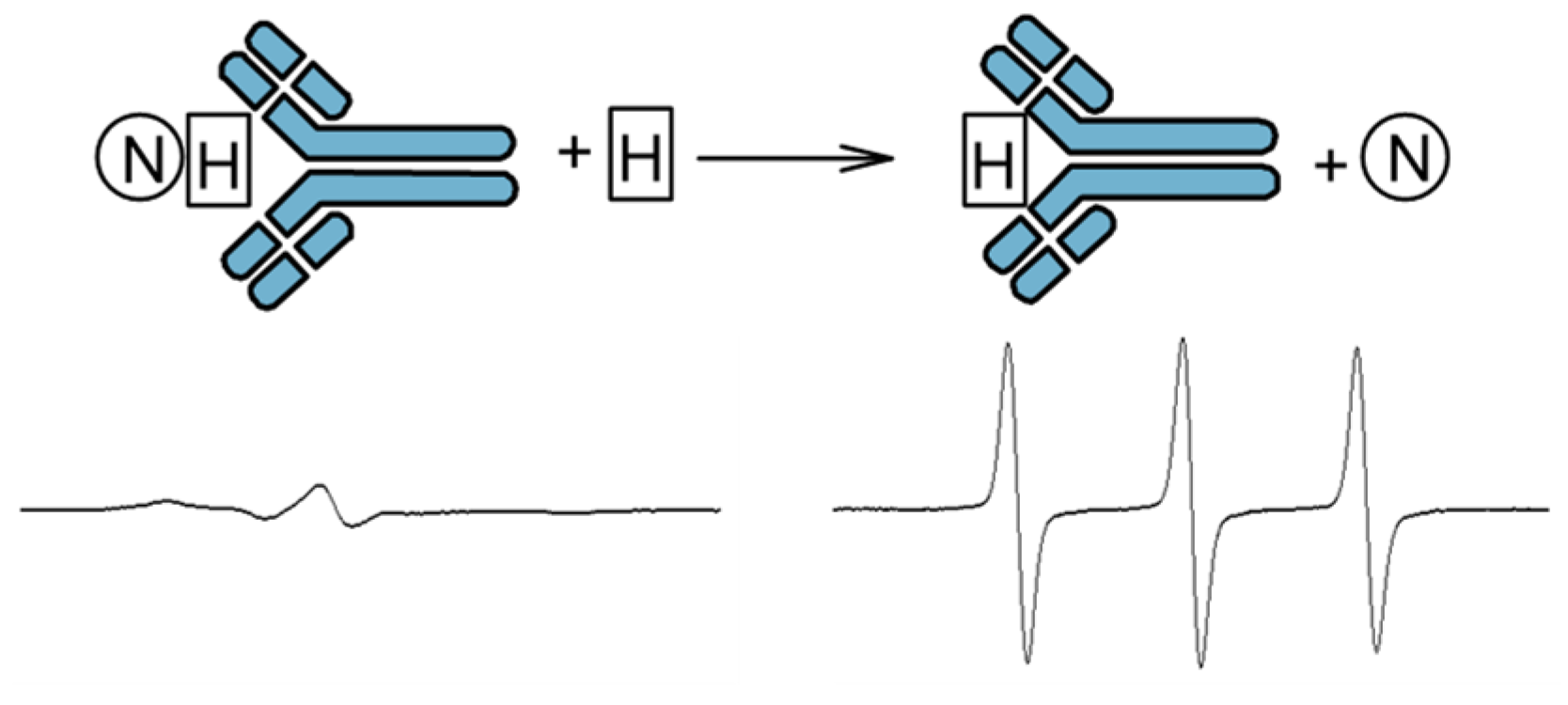
3.1. Immuno-Spin-Trapping
3.2. Antioxidant Properties of Nitroxides
3.2.1. Pseudodismutase Properties of Nitroxides
3.2.2. Catalase Properties of Nitroxides
3.2.3. Nitroxides as Inhibitors of Haber–Weiss and Fenton-like Reactions
3.2.4. Reactions of Nitroxides with Radicals
3.2.5. Reactions of Nitroxides with Singlet Oxygen
3.2.6. Reactions of Nitroxides with Peroxynitrite
3.2.7. Reactions of Nitroxides with Hypochlorous Acid
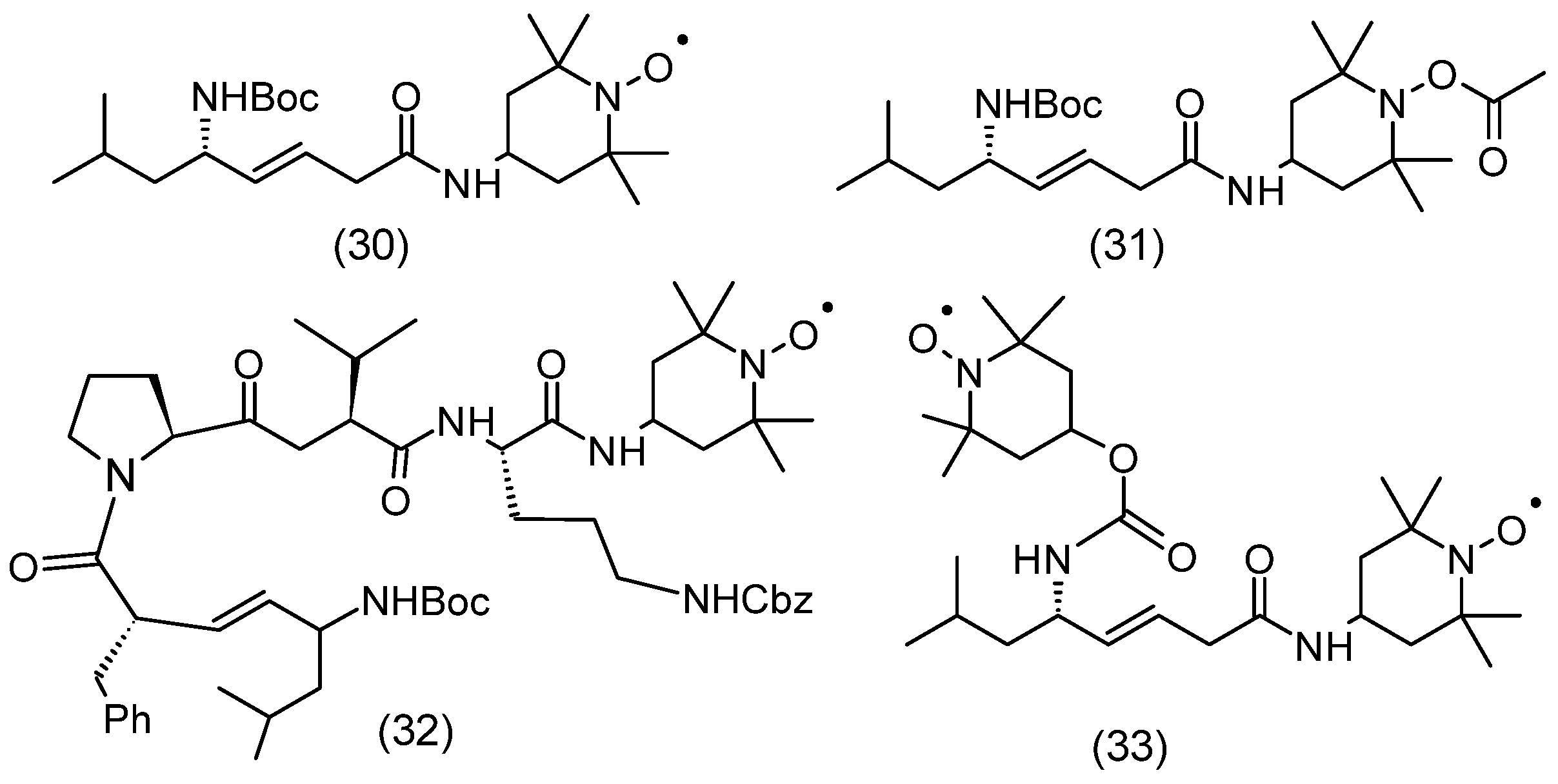
3.3. Radioprotective Effect of Nitroxides
3.4. Nitroxides in the Protection of Cells and Organs by Hypoxia/Reperfusion
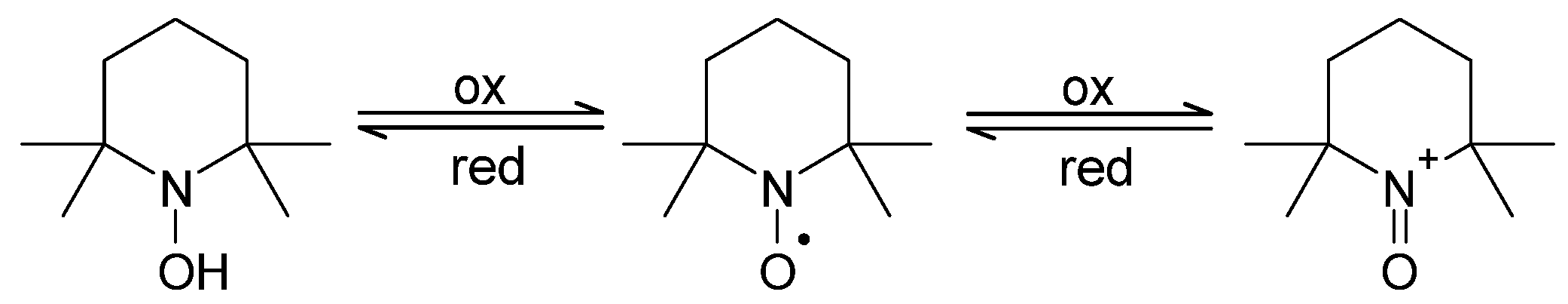
3.5. Nitroxide Reduction/Bioreduction
3.6. Nitroxide Actions in Cells and Tissues
3.7. Nitroxides in Erythrocytes
3.8. Nitroxide in Other Cells
3.9. Applications of Nitroxides in Experimental Animals
3.10. Nitroxides in Neurodegenerative Diseases
3.11. Spin Immunoassay
3.12. Nitroxide in Cancer Cells and Tissues
3.13. Application of Nitroxides in Protein Research
3.14. Site-Directed Spin Labeling Inside Cells
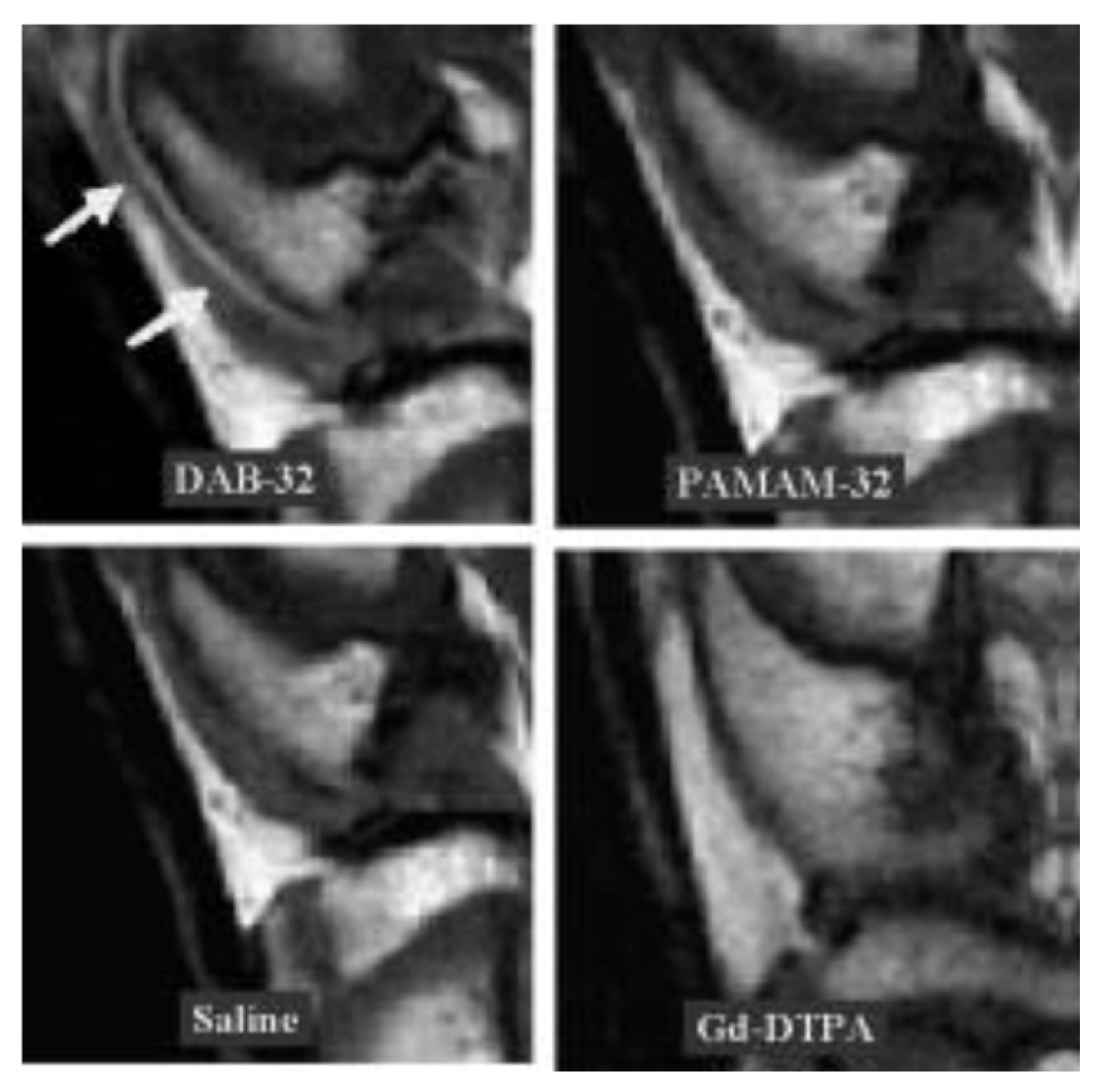
3.15. Application of Nitroxides in EPR/MRI
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swartz, H.M. Principles of the metabolism of nitroxides and their implications for spin trapping. Free Radic. Res. Commun. 1990, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Leifert, D.; Studer, A. Organic Synthesis Using Nitroxides. Chem. Rev. 2023, 123, 10302–10380. [Google Scholar] [CrossRef] [PubMed]
- Fichman, G.; Schneider, J.P. Utilizing Frémy’s Salt to Increase the Mechanical Rigidity of Supramolecular Peptide-Based Gel Networks. Front. Bioeng. Biotechnol. 2020, 8, 594258. [Google Scholar] [CrossRef]
- Haugland, M.M.; Lovett, J.E.; Anderson, E.A. Advances in the synthesis of nitroxide radicals for use in biomolecule spin labelling. Chem. Soc. Rev. 2018, 47, 668–680. [Google Scholar] [CrossRef]
- Hansen, K.-A.; Blinco, J.P. Nitroxide radical polymers—A versatile material class for high-tech applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- García-Rubio, I. EPR of site-directed spin-labeled proteins: A powerful tool to study structural flexibility. Arch. Biochem. Biophys. 2020, 684, 108323. [Google Scholar] [CrossRef]
- Nguyen, H.V.-T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef]
- Samuni, A.; Krishna, C.M.; Riesz, P.; Finkelstein, E.; Russo, A. A novel metal-free low molecular weight superoxide dismutase mimic. J. Biol. Chem. 1988, 263, 17921–17924. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-Mediated Polymerization. In Polymer Science; Elsevier Science: Burlington, NY, USA, 2012; pp. 277–350. ISBN 9780080878621. [Google Scholar]
- Peyrot, F.; Lajnef, S.; Versace, D.-L. Electron Paramagnetic Resonance Spin Trapping (EPR–ST) Technique in Photopolymerization Processes. Catalysts 2022, 12, 772. [Google Scholar] [CrossRef]
- Rozantsev, E.G.; Ulrich, H. Free Nitroxyl Radicals; Springer: Boston, MA, USA, 1970; ISBN 978-1-4757-0712-0. [Google Scholar]
- Rozantsev, E.G.; Sholle, V.D. Synthesis and Reactions of Stable Nitroxyl Radicals II. Reactions 1. Synthesis 1971, 1971, 401–414. [Google Scholar] [CrossRef]
- Volodarsky, L.B.; Kutikova, G.A.; Sagdeev, R.Z.; Molin, Y.N. A route to stable nitroxide radicals of imidazoline N-oxide. Tetrahedron Lett. 1968, 9, 1065–1068. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Keana, S.B.; Beetham, D. New versatile ketone spin label. J. Am. Chem. Soc. 1967, 89, 3055–3056. [Google Scholar] [CrossRef]
- Grigor’eva, L.N.; Tikhonov, A.Y.; Lomanovich, K.A.; Mazhukin, D.G. Stable Bicyclic Functionalized Nitroxides: The Synthesis of Derivatives of Aza-nortropinone-5-Methyl-3-oxo-6,8-diazabicyclo3.2.1-6-octene 8-oxyls. Molecules 2021, 26, 3050. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.F.; Lee, T.D.; Bernard, E.M. Letter: Side-chain substituted 2,2,5,5-tetramethylpyrrolidone-N-oxyl (proxyl) nitroxides. A new series of lipid spin labels showing improved properties for the study of biological membranes. J. Am. Chem. Soc. 1976, 98, 3052–3053. [Google Scholar] [CrossRef]
- Lee, T.D.; Birrell, G.B.; Keana, J.F.W. A new series of minimum steric perturbation nitroxide lipid spin labels. J. Am. Chem. Soc. 1978, 100, 1618–1619. [Google Scholar] [CrossRef]
- Moser, W.; Howie, R.A. Nitrosodisulphonates. Part I. Fremy’s salt (potassium nitrosodisulphonate). J. Chem. Soc. A 1968, 3039–3043. [Google Scholar] [CrossRef]
- Wieland, H.; Offenbächer, M. Diphenylstickstoffoxyd, ein neues organisches Radikal mit vierwertigem Stickstoff. Ber. Dtsch. Chem. Ges. 1914, 47, 2111–2115. [Google Scholar] [CrossRef]
- Wieland, H.; Kögl, F. Über organische Radikale mit vierwertigem Stickstoff (III). Ber. Dtsch. Chem. Ges. A/B 1922, 55, 1798–1803. [Google Scholar] [CrossRef]
- Amar, M.; Bar, S.; Iron, M.A.; Toledo, H.; Tumanskii, B.; Shimon, L.J.W.; Botoshansky, M.; Fridman, N.; Szpilman, A.M. Design concept for α-hydrogen-substituted nitroxides. Nat. Commun. 2015, 6, 6070. [Google Scholar] [CrossRef]
- Forrester, A.R.; Hay, J.M.; Thomson, R.H. Organic Chemistry of Stable Free Radicals; Academic Press: London, UK, 1968. [Google Scholar]
- Sosnovsky, G.; Konieczny, M. Preparation of 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl A Reinvestigation of Methods Using Hydrogen Peroxide in the Presence of Catalysts. Z. Für Naturforschung B 1976, 31, 1376–1378. [Google Scholar] [CrossRef]
- Dupeyre, R.M.; Rassat, A. Nitroxydes—LXXVI. Tetrahedron 1978, 34, 1501–1507. [Google Scholar] [CrossRef]
- Janzen, E.G. Spin trapping. Acc. Chem. Res. 1971, 4, 31–40. [Google Scholar] [CrossRef]
- Haywood, R. Spin-Trapping: Theory and Applications. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2447–2453. ISBN 978-3-642-16711-9. [Google Scholar]
- Zielonka, J.; Hardy, M.; Kalyanaraman, B. Chapter 13. Spin Trapping. In Nitroxides; Ouari, O., Gigmes, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 482–518. ISBN 978-1-78801-752-7. [Google Scholar]
- Gomez-Mejiba, S.E.; Zhai, Z.; Della-Vedova, M.C.; Muñoz, M.D.; Chatterjee, S.; Towner, R.A.; Hensley, K.; Floyd, R.A.; Mason, R.P.; Ramirez, D.C. Immuno-spin trapping from biochemistry to medicine: Advances, challenges, and pitfalls. Focus on protein-centered radicals. Biochim. Biophys. Acta 2014, 1840, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.-I.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Samuni, A.; Mitchell, J.B.; DeGraff, W.; Krishna, C.M.; Samuni, U.; Russo, A. Nitroxide SOD-mimics: Modes of action. Free Radic. Res. Commun. 1991, 12–13 Pt 1, 187–194. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Samuni, A.; Krishna, M.C.; DeGraff, W.G.; Ahn, M.S.; Samuni, U.; Russo, A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 1990, 29, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Russo, A.; Mitchell, J.B.; Goldstein, S.; Dafni, H.; Samuni, A. Do nitroxide antioxidants act as scavengers of O2− or as SOD mimics? J. Biol. Chem. 1996, 271, 26026–26031. [Google Scholar] [CrossRef]
- Goldstein, S.; Merenyi, G.; Russo, A.; Samuni, A. The role of oxoammonium cation in the SOD-mimic activity of cyclic nitroxides. J. Am. Chem. Soc. 2003, 125, 789–795. [Google Scholar] [CrossRef]
- Aronovitch, Y.; Godinger, D.; Israeli, A.; Krishna, M.C.; Samuni, A.; Goldstein, S. Dual activity of nitroxides as pro- and antioxidants: Catalysis of copper-mediated DNA breakage and H2O2 dismutation. Free Radic. Biol. Med. 2007, 42, 1317–1325. [Google Scholar] [CrossRef]
- Trnka, J.; Blaikie, F.H.; Logan, A.; Smith, R.A.J.; Murphy, M.P. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic. Res. 2009, 43, 4–12. [Google Scholar] [CrossRef]
- Krishna, M.C.; Grahame, D.A.; Samuni, A.; Mitchell, J.B.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef]
- Trnka, J.; Blaikie, F.H.; Smith, R.A.J.; Murphy, M.P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Biol. Med. 2008, 44, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Kajer, T.B.; Fairfull-Smith, K.E.; Yamasaki, T.; Yamada, K.-i.; Fu, S.; Bottle, S.E.; Hawkins, C.L.; Davies, M.J. Inhibition of myeloperoxidase- and neutrophil-mediated oxidant production by tetraethyl and tetramethyl nitroxides. Free Radic. Biol. Med. 2014, 70, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Faivre, B.; Menu, P.; Labrude, P.; Vigneron, C. Hemoglobin autooxidation/oxidation mechanisms and methemoglobin prevention or reduction processes in the bloodstream. Literature review and outline of autooxidation reaction. Artif. Cells Blood Substit. Immobil. Biotechnol. 1998, 26, 17–26. [Google Scholar] [CrossRef]
- Krishna, M.C.; Samuni, A.; Taira, J.; Goldstein, S.; Mitchell, J.B.; Russo, A. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J. Biol. Chem. 1996, 271, 26018–26025. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, R.J.; Swanson, C.E. Nitroxide-stimulated H2O2 decomposition by peroxidases and pseudoperoxidases. Free Radic. Res. Commun. 1992, 17, 157–175. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Głębska, J.; Gwoździński, K. Nitroxides as protectors against oxidative damage induced by the Fenton system. Curr. Top. Biophys. 2000, 57–63. [Google Scholar]
- Deffner, U.; Schimmack, W. Letter: Radiation effects on aqueous solutions of the nitroxyl free radical TMPN (2,2,6,6-tetramethyl-4-piperidinol-N-oxyl). Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 29, 71–75. [Google Scholar] [CrossRef]
- Gadjeva, V.; Kuchukova, D.; Tolekova, A.; Tanchev, S. Beneficial effects of spin-labelled nitrosourea on CCNU-induced oxidative stress in rat blood compared with vitamin E. Pharmazie 2005, 60, 530–532. [Google Scholar]
- Offer, T.; Samuni, A. Nitroxides inhibit peroxyl radical-mediated DNA scission and enzyme inactivation. Free Radic. Biol. Med. 2002, 32, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.T.; Galatsis, P.; Borosky, S.; Kopec, K.K.; Kumar, V.; Althaus, J.S.; Hall, E.D. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol. 2000, 13, 294–300. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Filipe, H.M.; Centorrino, T.; Lepore, V.; Terranova, M.L.; Ciccolo, A.; Caputi, A.P.; Thiemermann, C. Beneficial effects of tempol, a membrane-permeable radical scavenger, on the multiple organ failure induced by zymosan in the rat. Crit. Care Med. 2001, 29, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.C.; Medinas, D.B.; Alves, M.J.M.; Augusto, O. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic. Biol. Med. 2005, 38, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Samuni, A. Kinetics and mechanism of peroxyl radical reactions with nitroxides. J. Phys. Chem. A 2007, 111, 1066–1072. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef]
- Samuni, A.; Goldstein, S.; Russo, A.; Mitchell, J.B.; Krishna, M.C.; Neta, P. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J. Am. Chem. Soc. 2002, 124, 8719–8724. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ishigaki, H.; Okada, H.; Suyama, S. New Method in Free Radical Chemistry Using 2,4-Diphenyl-4-methyl-1-pentene as Radical Trapping Agent. Polym. J. 1997, 29, 366–369. [Google Scholar] [CrossRef]
- Genovese, D.; Baschieri, A.; Vona, D.; Baboi, R.E.; Mollica, F.; Prodi, L.; Amorati, R.; Zaccheroni, N. Nitroxides as Building Blocks for Nanoantioxidants. ACS Appl. Mater. Interfaces 2021, 13, 31996–32004. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Maltby, D.A.; Medzihradszky, K.F.; de Montellano, P.R.O.; Tomer, K.B.; Mason, R.P.; Deterding, L.J. Spin scavenging analysis of myoglobin protein-centered radicals using stable nitroxide radicals: Characterization of oxoammonium cation-induced modifications. Chem. Res. Toxicol. 2009, 22, 1034–1049. [Google Scholar] [CrossRef] [PubMed]
- Lion, Y.; Gandin, E.; van de Vorst, A. On the production of nitroxide radicals by singlet oxygen reaction: An EPR study. Photochem. Photobiol. 1980, 31, 305–309. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Pezerat, H. Singlet oxygen-trapping reaction as a method of (1)O2 detection: Role of some reducing agents. Free Radic. Res. 1995, 23, 103–115. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Merenyi, G. Reactions of nitric oxide, peroxynitrite, and carbonate radicals with nitroxides and their corresponding oxoammonium cations. Chem. Res. Toxicol. 2004, 17, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Samuni, A.; Hideg, K.; Merenyi, G. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J. Phys. Chem. A 2006, 110, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.A.; Pattison, D.I.; Bottle, S.E.; Keddie, D.J.; Davies, M.J. Nitric oxide and nitroxides can act as efficient scavengers of protein-derived free radicals. Chem. Res. Toxicol. 2008, 21, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Samuni, A.; Merenyi, G. Kinetics of the reaction between nitroxide and thiyl radicals: Nitroxides as antioxidants in the presence of thiols. J. Phys. Chem. A 2008, 112, 8600–8605. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Gajewska, A.; Skolimowski, J.; Szewczyk, R.; Bartosz, G. Nitroxides protect against peroxynitrite-induced nitration and oxidation. Free Radic. Biol. Med. 2015, 89, 1165–1175. [Google Scholar] [CrossRef]
- Rees, M.D.; Bottle, S.E.; Fairfull-Smith, K.E.; Malle, E.; Whitelock, J.M.; Davies, M.J. Inhibition of myeloperoxidase-mediated hypochlorous acid production by nitroxides. Biochem. J. 2009, 421, 79–86. [Google Scholar] [CrossRef]
- Vaz, S.M.; Augusto, O. Inhibition of myeloperoxidase-mediated protein nitration by tempol: Kinetics, mechanism, and implications. Proc. Natl. Acad. Sci. USA 2008, 105, 8191–8196. [Google Scholar] [CrossRef]
- Herbert, J.M.; Coons, M.P. The Hydrated Electron. Annu. Rev. Phys. Chem. 2017, 68, 447–472. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.M.; Barenholz, Y. Use of nitroxides to protect liposomes against oxidative damage. Methods Enzymol. 2004, 387, 299–314. [Google Scholar] [CrossRef]
- Patyar, R.R.; Patyar, S. Role of drugs in the prevention and amelioration of radiation induced toxic effects. Eur. J. Pharmacol. 2018, 819, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jiang, J.; Belikova, N.A.; Stoyanovsky, D.A.; Kagan, V.E.; Mintz, A.H. Protection of normal brain cells from γ-irradiation-induced apoptosis by a mitochondria-targeted triphenyl-phosphonium-nitroxide: A possible utility in glioblastoma therapy. J. Neurooncol. 2010, 100, 1–8. [Google Scholar] [CrossRef]
- Greenberger, J.S.; Berhane, H.; Shinde, A.; Rhieu, B.H.; Bernard, M.; Wipf, P.; Skoda, E.M.; Epperly, M.W. Can Radiosensitivity Associated with Defects in DNA Repair be Overcome by Mitochondrial-Targeted Antioxidant Radioprotectors. Front. Oncol. 2014, 4, 24. [Google Scholar] [CrossRef]
- Guo, X.; Mittelstaedt, R.A.; Guo, L.; Shaddock, J.G.; Heflich, R.H.; Bigger, A.H.; Moore, M.M.; Mei, N. Nitroxide TEMPO: A genotoxic and oxidative stress inducer in cultured cells. Toxicol. In Vitr. 2013, 27, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Goff, J.P.; Li, S.; Gao, X.; Wipf, P.; Dixon, T.; Wang, H.; Franicola, D.; Shen, H.; Rwigema, J.-C.M.; et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo 2010, 24, 811–819. [Google Scholar] [PubMed]
- Epperly, M.W.; Sacher, J.R.; Krainz, T.; Zhang, X.; Wipf, P.; Liang, M.; Fisher, R.; Li, S.; Wang, H.; Greenberger, J.S. Effectiveness of Analogs of the GS-Nitroxide, JP4-039, as Total Body Irradiation Mitigators. In Vivo 2017, 31, 39–43. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Vitolo, J.M.; Cotrim, A.P.; Sowers, A.L.; Russo, A.; Wellner, R.B.; Pillemer, S.R.; Mitchell, J.B.; Baum, B.J. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin. Cancer Res. 2004, 10, 1807–1812. [Google Scholar] [CrossRef]
- Davis, R.M.; Sowers, A.L.; DeGraff, W.; Bernardo, M.; Thetford, A.; Krishna, M.C.; Mitchell, J.B. A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radic. Biol. Med. 2011, 51, 780–790. [Google Scholar] [CrossRef]
- Cuscela, D.; Coffin, D.; Lupton, G.P.; Cook, J.A.; Krishna, M.C.; Bonner, R.F.; Mitchell, J.B. Protection from radiation-induced alopecia with topical application of nitroxides: Fractionated studies. Cancer J. Sci. Am. 1996, 2, 273–278. [Google Scholar] [PubMed]
- Metz, J.M.; Smith, D.; Mick, R.; Lustig, R.; Mitchell, J.; Cherakuri, M.; Glatstein, E.; Hahn, S.M. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 2004, 10, 6411–6417. [Google Scholar] [CrossRef]
- Hahn, S.M.; Krishna, M.C.; DeLuca, A.M.; Coffin, D.; Mitchell, J.B. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic. Biol. Med. 2000, 28, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; DeLuca, A.M.; Coffin, D.; Krishna, C.M.; Mitchell, J.B. In vivo radioprotection and effects on blood pressure of the stable free radical nitroxides. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 839–842. [Google Scholar] [CrossRef]
- Frantz, M.-C.; Skoda, E.M.; Sacher, J.R.; Epperly, M.W.; Goff, J.P.; Greenberger, J.S.; Wipf, P. Synthesis of analogs of the radiation mitigator JP4-039 and visualization of BODIPY derivatives in mitochondria. Org. Biomol. Chem. 2013, 11, 4147–4153. [Google Scholar] [CrossRef] [PubMed]
- Rwigema, J.-C.M.; Beck, B.; Wang, W.; Doemling, A.; Epperly, M.W.; Shields, D.; Goff, J.P.; Franicola, D.; Dixon, T.; Frantz, M.-C.; et al. Two strategies for the development of mitochondrion-targeted small molecule radiation damage mitigators. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 860–868. [Google Scholar] [CrossRef]
- Jiang, J.; Belikova, N.A.; Hoye, A.T.; Zhao, Q.; Epperly, M.W.; Greenberger, J.S.; Wipf, P.; Kagan, V.E. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 816–825. [Google Scholar] [CrossRef]
- Brand, R.M.; Epperly, M.W.; Stottlemyer, J.M.; Skoda, E.M.; Gao, X.; Li, S.; Huq, S.; Wipf, P.; Kagan, V.E.; Greenberger, J.S.; et al. A Topical Mitochondria-Targeted Redox-Cycling Nitroxide Mitigates Oxidative Stress-Induced Skin Damage. J. Investig. Dermatol. 2017, 137, 576–586. [Google Scholar] [CrossRef]
- Adeghate, J.O.; Epperly, M.W.; Davoli, K.A.; Lathrop, K.L.; Wipf, P.; Hou, W.; Fisher, R.; Thermozier, S.; Huq, M.S.; Sahel, J.-A.; et al. JP4-039, a Mitochondria-Targeted Nitroxide, Mitigates the Effect of Apoptosis and Inflammatory Cell Migration in the Irradiated Mouse Retina. Int. J. Mol. Sci. 2024, 25, 6515. [Google Scholar] [CrossRef]
- Shinde, A.; Berhane, H.; Rhieu, B.H.; Kalash, R.; Xu, K.; Goff, J.; Epperly, M.W.; Franicola, D.; Zhang, X.; Dixon, T.; et al. Intraoral Mitochondrial-Targeted GS-Nitroxide, JP4-039, Radioprotects Normal Tissue in Tumor-Bearing Radiosensitive Fancd2(−/−) (C57BL/6) Mice. Radiat. Res. 2016, 185, 134–150. [Google Scholar] [CrossRef]
- Kim, H.; Bernard, M.E.; Epperly, M.W.; Shen, H.; Amoscato, A.; Dixon, T.M.; Doemling, A.S.; Li, S.; Gao, X.; Wipf, P.; et al. Amelioration of radiation esophagitis by orally administered p53/Mdm2/Mdm4 inhibitor (BEB55) or GS-nitroxide. In Vivo 2011, 25, 841–848. [Google Scholar]
- Berhane, H.; Shinde, A.; Kalash, R.; Xu, K.; Epperly, M.W.; Goff, J.; Franicola, D.; Zhang, X.; Dixon, T.; Shields, D.; et al. Amelioration of radiation-induced oral cavity mucositis and distant bone marrow suppression in fanconi anemia Fancd2−/− (FVB/N) mice by intraoral GS-nitroxide JP4-039. Radiat. Res. 2014, 182, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.E.; Kim, H.; Berhane, H.; Epperly, M.W.; Franicola, D.; Zhang, X.; Houghton, F.; Shields, D.; Wang, H.; Bakkenist, C.J.; et al. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi anemia cell lines. Radiat. Res. 2011, 176, 603–612. [Google Scholar] [CrossRef]
- Ji, J.; Baart, S.; Vikulina, A.S.; Clark, R.S.; Anthonymuthu, T.S.; Tyurin, V.A.; Du, L.; St Croix, C.M.; Tyurina, Y.Y.; Lewis, J.; et al. Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2015, 35, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Azhar, G.; Gao, W.; Liu, L.; Wei, J.Y. Ischemia-reperfusion in the adult mouse heart influence of age. Exp. Gerontol. 1999, 34, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Ou, R.; Bailey, M.; Rowland, M.; Nagley, P.; Rosenfeldt, F.; Pepe, S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J. Thorac. Cardiovasc. Surg. 2000, 120, 660–667. [Google Scholar] [CrossRef]
- Escobales, N.; Nuñez, R.E.; Jang, S.; Parodi-Rullan, R.; Ayala-Peña, S.; Sacher, J.R.; Skoda, E.M.; Wipf, P.; Frontera, W.; Javadov, S. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J. Mol. Cell. Cardiol. 2014, 77, 136–146. [Google Scholar] [CrossRef]
- Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Nitroxide antioxidant as a potential strategy to attenuate the oxidative/nitrosative stress induced by hydrogen peroxide plus nitric oxide in cultured neurons. Nitric Oxide 2016, 54, 38–50. [Google Scholar] [CrossRef]
- Hogg, N.; Zielonka, J.; Kalyanaraman, B. Detection of Nitric Oxide and Peroxynitrite in Biological Systems: A State-of-the-Art Review. In Nitric Oxide; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–44. ISBN 9780128042731. [Google Scholar]
- Bi, W.; Cai, J.; Xue, P.; Zhang, Y.; Liu, S.; Gao, X.; Li, M.; Wang, Z.; Baudy-Floc’h, M.; Green, S.A.; et al. Protective effect of nitronyl nitroxide-amino acid conjugates on liver ischemia-reperfusion induced injury in rats. Bioorg. Med. Chem. Lett. 2008, 18, 1788–1794. [Google Scholar] [CrossRef]
- McDonald, M.C.; Zacharowski, K.; Bowes, J.; Cuzzocrea, S.; Thiemermann, C. Tempol reduces infarct size in rodent models of regional myocardial ischemia and reperfusion. Free Radic. Biol. Med. 1999, 27, 493–503. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Ravizza, R.; Petterino, C.; Castagnaro, M.; Finocchiaro, G.; Monti, E. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol--a potential new therapeutic agent for gliomas. Eur. J. Cancer 2003, 39, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, J.; Xu, H.; Zhou, C.; Han, B.; Zhu, H.; Hu, Z.; Ma, Z.; Ming, Z.; Yao, Y.; et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 2020, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Marx, L.; Chiarelli, R.; Guiberteau, T.; Rassat, A. A comparative study of the reduction by ascorbate of 1,1,3,3-tetraethylisoindolin-2-yloxyl and of 1,1,3,3-tetramethylisoindolin-2-yloxyl. J. Chem. Soc. Perkin Trans. 1 2000, 1181–1182. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Bobko, A.A.; Grigor’ev, I.A.; Khramtsov, V.V. Synthesis of the tetraethyl substituted pH-sensitive nitroxides of imidazole series with enhanced stability towards reduction. Org. Biomol. Chem. 2004, 2, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Birrell, G.B.; Bjorkman, P.J.; Keana, J.F. Azethoxyl nitroxide spin labels. ESR studies involving thiourea crystals, model membrane systems and chromatophores, and chemical reduction with ascorbate and dithiothreitol. Biochim. Biophys. Acta 1979, 550, 369–383. [Google Scholar] [CrossRef]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, W.; Ohno, H.; Ogata, T. Determination of Ascorbate Concentration in a Raw Leaf with Electron Spin Resonance Spectroscopy. Anal. Sci. 1999, 15, 973–977. [Google Scholar] [CrossRef]
- Keana, J.F.; van Nice, F.L. Influence of structure on the reduction of nitroxide MRI contrast-enhancing agents by ascorbate. Physiol. Chem. Phys. Med. NMR 1984, 16, 477–480. [Google Scholar]
- Kocherginsky, N. Nitroxide Spin Labels: Reactions in Biology and Chemistry; CRC Press: Boca Raton, FL, USA, 1995; ISBN 084934204X. [Google Scholar]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef]
- Paletta, J.T.; Pink, M.; Foley, B.; Rajca, S.; Rajca, A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org. Lett. 2012, 14, 5322–5325. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Yamada, K.-i.; Yamasaki, T.; Mito, F.; Yamato, M.; Kosem, N.; Deguchi, H.; Shirahama, C.; Ito, Y.; Kitagawa, K.; et al. In vivo evaluation of novel nitroxyl radicals with reduction stability. Free Radic. Biol. Med. 2010, 49, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, S.A.; Usatov, M.S.; Zhurko, I.F.; Morozov, D.A.; Polienko, Y.F.; Glazachev, Y.I.; Parkhomenko, D.A.; Tyumentsev, M.A.; Gatilov, Y.V.; Chernyak, E.I.; et al. A Simple Method of Synthesis of 3-Carboxy-2,2,5,5-Tetraethylpyrrolidine-1-oxyl and Preparation of Reduction-Resistant Spin Labels and Probes of Pyrrolidine Series. Molecules 2021, 26, 5761. [Google Scholar] [CrossRef]
- Jagtap, A.P.; Krstic, I.; Kunjir, N.C.; Hänsel, R.; Prisner, T.F.; Sigurdsson, S.T. Sterically shielded spin labels for in-cell EPR spectroscopy: Analysis of stability in reducing environment. Free Radic. Res. 2015, 49, 78–85. [Google Scholar] [CrossRef]
- Bobko, A.A.; Dhimitruka, I.; Zweier, J.L.; Khramtsov, V.V. Trityl radicals as persistent dual function pH and oxygen probes for in vivo electron paramagnetic resonance spectroscopy and imaging: Concept and experiment. J. Am. Chem. Soc. 2007, 129, 7240–7241. [Google Scholar] [CrossRef] [PubMed]
- Iwama, M.; Amano, A.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J. Nutr. Sci. Vitaminol. 2012, 58, 169–174. [Google Scholar] [CrossRef]
- Babić, N.; Orio, M.; Peyrot, F. Unexpected rapid aerobic transformation of 2,2,6,6-tetraethyl-4-oxo(piperidin-1-yloxyl) radical by cytochrome P450 in the presence of NADPH: Evidence against a simple reduction of the nitroxide moiety to the hydroxylamine. Free Radic. Biol. Med. 2020, 156, 144–156. [Google Scholar] [CrossRef]
- Hedlund, E.; Gustafsson, J.A.; Warner, M. Cytochrome P450 in the brain; A review. Curr. Drug Metab. 2001, 2, 245–263. [Google Scholar] [CrossRef]
- Knights, K.M.; Rowland, A.; Miners, J.O. Renal drug metabolism in humans: The potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT). Br. J. Clin. Pharmacol. 2013, 76, 587–602. [Google Scholar] [CrossRef]
- Omura, T. Mitochondrial P450s. Chem. Biol. Interact. 2006, 163, 86–93. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Kotamraju, S.; Karunakaran, C.; Kalivendi, S.V.; Thomas, S.; Joseph, J.; Kalyanaraman, B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: Role of mitochondrial superoxide. Free Radic. Biol. Med. 2005, 39, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Krainz, T.; Gaschler, M.M.; Lim, C.; Sacher, J.R.; Stockwell, B.R.; WIPF, P. A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Cent. Sci. 2016, 2, 653–659. [Google Scholar] [CrossRef]
- Black, H.D.; Xu, W.; Hortle, E.; Robertson, S.I.; Britton, W.J.; Kaur, A.; New, E.J.; Witting, P.K.; Chami, B.; Oehlers, S.H. The cyclic nitroxide antioxidant 4-methoxy-TEMPO decreases mycobacterial burden in vivo through host and bacterial targets. Free Radic. Biol. Med. 2019, 135, 157–166. [Google Scholar] [CrossRef]
- Wipf, P.; Xiao, J.; Jiang, J.; Belikova, N.A.; Tyurin, V.A.; Fink, M.P.; Kagan, V.E. Mitochondrial targeting of selective electron scavengers: Synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J. Am. Chem. Soc. 2005, 127, 12460–12461. [Google Scholar] [CrossRef]
- Xun, Z.; Rivera-Sánchez, S.; Ayala-Peña, S.; Lim, J.; Budworth, H.; Skoda, E.M.; Robbins, P.D.; Niedernhofer, L.J.; Wipf, P.; McMurray, C.T. Targeting of XJB-5-131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington’s disease. Cell Rep. 2012, 2, 1137–1142. [Google Scholar] [CrossRef]
- Macias, C.A.; Chiao, J.W.; Xiao, J.; Arora, D.S.; Tyurina, Y.Y.; Delude, R.L.; Wipf, P.; Kagan, V.E.; Fink, M.P. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann. Surg. 2007, 245, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Gwoździński, K.; Bartosz, G.; Leyko, W. Effect of thiol reagents and ionizing radiation on the permeability of erythrocyte membrane for spin-labeled non-electrolytes. Radiat. Environ. Biophys. 1983, 22, 53–59. [Google Scholar] [CrossRef]
- Gwoździński, K.; Bartosz, G. Nitroxide reduction in human red blood cells. Curr. Top. Biophys. 1996, 60–65. [Google Scholar]
- Gwoździński, K.; Bartosz, G.; Leyko, W. Effect of gamma radiation on the transport of spin-labeled compounds across the erythrocyte membrane. Radiat. Environ. Biophys. 1981, 19, 275–285. [Google Scholar] [CrossRef]
- Glebska, J.; Gwozdzinski, K. Oxygen-dependent reduction of nitroxides by ascorbic acid and glutathione. EPR investigations. Curr. Top. Biophys. 1998, 75–82. [Google Scholar]
- Hiramoto, K.; Ojima, N.; Kikugawa, K. Conversion of nitroxide radicals by phenolic and thiol antioxidants. Free Radic. Res. 1997, 27, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Nothig-Laslo, V.; Bobst, A.M. Reinvestigation of the Oxidation Properties of Nitroxides. Croat. Chem. Acta 1991, 64, 1–8. [Google Scholar]
- Bujak, S.; Gwozdzinski, K. Nitroxides lead to reduced level of glutathione in red blood cells. Medimond Int. Proc. 2003, 105–108. [Google Scholar]
- Balcerczyk, A.; Łuczak, K.; Soszyński, M.; Bartosz, G. Prooxidative effects of TEMPO on human erythrocytes. Cell Biol. Int. 2004, 28, 585–591. [Google Scholar] [CrossRef]
- Bujak, S.; Gwozdzinski, K. The effect of stable nitroxide radicals on the activity of glutathione-dependent enzymes: Reductase, peroxidase and trans-ferase in human erythrocytes. Medimond Int. Proc. 2006. [Google Scholar]
- Krishna, M.C.; Samuni, A. Nitroxides as antioxidants. Methods Enzymol. 1994, 234, 580–589. [Google Scholar] [CrossRef]
- Falcioni, G.; Gabbianelli, R.; Damiani, E.; Santroni, A.M.; Fedeli, D.; Wozniak, M.; Greci, L. The effect of indolinic and quinolinic nitroxide radicals on trout erythrocytes exposed to oxidative stress. Free Radic. Res. 1998, 28, 507–516. [Google Scholar] [CrossRef]
- Gwozdzinski, K.; Bujak-Pietrek, S.; Pieniazek, A.; Gwozdzinski, L. Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides. Molecules 2024, 29, 2941. [Google Scholar] [CrossRef] [PubMed]
- Czepas, J.; Koceva-Chyła, A.; Gwoździński, K.; Jóźwiak, Z. Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide. Cell Biol. Toxicol. 2008, 24, 101–112. [Google Scholar] [CrossRef]
- Mołoń, M.; Szlachcikowska, D.; Stępień, K.; Kielar, P.; Galiniak, S. Two faces of TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxyl)—An antioxidant or a toxin? Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119412. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Zou, Z.; Li, L.; Zhu, L.; Wang, Q.; Gao, W.; Wang, Y.; Huang, W.; Liu, R.; et al. Piperidine nitroxide Tempol enhances cisplatin-induced apoptosis in ovarian cancer cells. Oncol. Lett. 2018, 16, 4847–4854. [Google Scholar] [CrossRef] [PubMed]
- Głebska, J.; Skolimowski, J.; Kudzin, Z.; Gwoździński, K.; Grzelak, A.; Bartosz, G. Pro-oxidative activity of nitroxides in their reactions with glutathione. Free Radic. Biol. Med. 2003, 35, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lv, Z.; Li, W.; Lu, J.; Xie, Y.; Wang, P.; Jiang, R.; Dong, J.; Guo, H.; Liu, Z.; et al. XJB-5-131 protects chondrocytes from ferroptosis to alleviate osteoarthritis progression via restoring Pebp1 expression. J. Orthop. Translat. 2024, 44, 114–124. [Google Scholar] [CrossRef]
- Amankwa, C.E.; Kodati, B.; Donkor, N.; Acharya, S. Therapeutic Potential of Antioxidants and Hybrid TEMPOL Derivatives in Ocular Neurodegenerative Diseases: A Glimpse into the Future. Biomedicines 2023, 11, 2959. [Google Scholar] [CrossRef]
- Larin, A.C.R.; Pfrunder, M.C.; Mullen, K.M.; Wiedbrauk, S.; Boase, N.R.; Fairfull-Smith, K.E. Synergistic or antagonistic antioxidant combinations—A case study exploring flavonoid-nitroxide hybrids. Org. Biomol. Chem. 2023, 21, 1780–1792. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.-J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (−)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, W.; Wang, Z.; Xie, T.; Yi, C.; Xu, Z. Design and engineering of heterogeneous nitroxide-mediated catalytic systems for selective oxidation: Efficiency and sustainability. Mater. Today Chem. 2022, 24, 100872. [Google Scholar] [CrossRef]
- Beit-Yannai, E.; Zhang, R.; Trembovler, V.; Samuni, A.; Shohami, E. Cerebroprotective effect of stable nitroxide radicals in closed head injury in the rat. Brain Res. 1996, 717, 22–28. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X. Caspase-independent component of retinal ganglion cell death, in vitro. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4049–4059. [Google Scholar] [CrossRef]
- Wang, M.; Lam, T.T.; Fu, J.; Tso, M.O. TEMPOL, a superoxide dismutase mimic, ameliorates light-induced retinal degeneration. Res. Commun. Mol. Pathol. Pharmacol. 1995, 89, 291–305. [Google Scholar]
- Rosales, M.A.B.; Silva, K.C.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Exogenous SOD mimetic tempol ameliorates the early retinal changes reestablishing the redox status in diabetic hypertensive rats. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4327–4336. [Google Scholar] [CrossRef]
- Wright, M.V.; Kuhn, T.B. CNS neurons express two distinct plasma membrane electron transport systems implicated in neuronal viability. J. Neurochem. 2002, 83, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Thaler, S.; Fiedorowicz, M.; Grieb, P.; Wypych, Z.; Knap, N.; Borowik, T.; Zawada, K.; Kaminski, J.; Wozniak, M.; Rejdak, R.; et al. Neuroprotective effects of tempol acyl esters against retinal ganglion cell death in a rat partial optic nerve crush model. Acta Ophthalmol. 2011, 89, e555–e560. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Rejdak, R.; Schuettauf, F.; Wozniak, M.; Grieb, P.; Thaler, S. Age-dependent neuroprotection of retinal ganglion cells by tempol-C8 acyl ester in a rat NMDA toxicity model. Folia Neuropathol. 2014, 3, 291–297. [Google Scholar] [CrossRef]
- Gelvan, D.; Saltman, P.; Powell, S.R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc. Natl. Acad. Sci. USA 1991, 88, 4680–4684. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Siriwardena, D.; Costantino, G.; Fulia, F.; Cucinotta, G.; Gitto, E.; Cordaro, S.; Barberi, I.; et al. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 2000, 875, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rak, R.; Chao, D.L.; Pluta, R.M.; Mitchell, J.B.; Oldfield, E.H.; Watson, J.C. Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. J. Neurosurg. 2000, 92, 646–651. [Google Scholar] [CrossRef]
- Kato, N.; Yanaka, K.; Hyodo, K.; Homma, K.; Nagase, S.; Nose, T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003, 979, 188–193. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Chao, D.L.; Malloy, K.; Sun, D.; Alessandri, B.; Bullock, M.R. Tempol, a novel stable nitroxide, reduces brain damage and free radical production, after acute subdural hematoma in the rat. J. Neurotrauma 2003, 20, 337–345. [Google Scholar] [CrossRef]
- Leach, M.; Frank, S.; Olbrich, A.; Pfeilschifter, J.; Thiemermann, C. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: Effects of the superoxide anion radical scavenger, tempol, on organ injury. Br. J. Pharmacol. 1998, 125, 817–825. [Google Scholar] [CrossRef]
- Cate, J.; Clarkson, M.; Strickland, J.; D’Amato, N.A. Spin immunoassay for opiates in urine--results of screening military personnel. Clin. Toxicol. 1976, 9, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L.; Griffith, O.H. A spin-labeled hapten. Proc. Natl. Acad. Sci. USA 1965, 54, 1785–1791. [Google Scholar] [CrossRef]
- Leute, R.K.; Ullman, E.F.; Goldstein, A.; Herzenberg, L.A. Spin immunoassay technique for determination of morphine. Nat. New Biol. 1972, 236, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Janzen, E.G. Electron spin resonance. Anal. Chem. 1972, 44, 113–121. [Google Scholar] [CrossRef]
- Montgomery, M.R.; Holtzman, J.L.; Leute, R.K. Application of electron spin resonance to determination of serum drug concentrations. Clin. Chem. 1975, 21, 1323–1328. [Google Scholar] [CrossRef]
- Yang, G.C.; Copeland, E.S. Spin immunoassay. Methods Enzymol. 1981, 74 Pt C, 140–151. [Google Scholar] [CrossRef]
- Singh, R.; Manna, P.P. Reactive oxygen species in cancer progression and its role in therapeutics. Explor. Med. 2022, 3, 43–57. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as Antioxidants and Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Hermetter, A. Mediation of apoptosis by oxidized phospholipids. Subcell. Biochem. 2008, 49, 351–367. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Tabaczar, S.; Koceva-Chyla, A.; Czepas, J.; Pieniazek, A.; Piasecka-Zelga, J.; Gwozdzinski, K. Nitroxide pirolin reduces oxidative stress generated by doxorubicin and docetaxel in blood plasma of rats bearing mammary tumor. J. Physiol. Pharmacol. 2012, 63, 153–163. [Google Scholar]
- Tabaczar, S.; Domeradzka, K.; Czepas, J.; Piasecka-Zelga, J.; Stetkiewicz, J.; Gwoździński, K.; Koceva-Chyła, A. Anti-tumor potential of nitroxyl derivative Pirolin in the DMBA-induced rat mammary carcinoma model: A comparison with quercetin. Pharmacol. Rep. 2015, 67, 527–534. [Google Scholar] [CrossRef]
- Tabaczar, S.; Czepas, J.; Koceva-Chyla, A.; Kilanczyk, E.; Piasecka-Zelga, J.; Gwozdzinski, K. The effect of the nitroxide pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J. Physiol. Pharmacol. 2017, 68, 295–308. [Google Scholar]
- Czepas, J.; Matczak, K.; Koceva-Chyła, A.; Grobelski, B.; Jóźwiak, Z.; Gwoździński, K. Doxyl Nitroxide Spin Probes Can Modify Toxicity of Doxorubicin towards Fibroblast Cells. Molecules 2020, 25, 5138. [Google Scholar] [CrossRef] [PubMed]
- Bagryanskaya, E.G.; Krumkacheva, O.A.; Fedin, M.V.; Marque, S.R.A. Development and Application of Spin Traps, Spin Probes, and Spin Labels. Methods Enzymol. 2015, 563, 365–396. [Google Scholar] [CrossRef]
- DeGraff, W.G.; Krishna, M.C.; Kaufman, D.; Mitchell, J.B. Nitroxide-mediated protection against X-ray- and neocarzinostatin-induced DNA damage. Free Radic. Biol. Med. 1992, 13, 479–487. [Google Scholar] [CrossRef]
- Ankel, E.G.; Lai, C.S.; Hopwood, L.E.; Zivkovic, Z. Cytotoxicity of commonly used nitroxide radical spin probes. Life Sci. 1987, 40, 495–498. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Lucchi, S.; Caserini, C.; Supino, R.; Oliva, C.; Monti, E. Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic. Biol. Med. 1998, 24, 913–923. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Rimoldi, V.; Supino, R.; Favini, E.; Monti, E. The nitroxide tempol induces oxidative stress, p21(WAF1/CIP1), and cell death in HL60 cells. Free Radic. Biol. Med. 2000, 29, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Monti, E.; Supino, R.; Colleoni, M.; Costa, B.; Ravizza, R.; Gariboldi, M.B. Nitroxide TEMPOL impairs mitochondrial function and induces apoptosis in HL60 cells. J. Cell. Biochem. 2001, 82, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Möbius, K.; Savitsky, A.; Wegener, C.; Plato, M.; Fuchs, M.; Schnegg, A.; Dubinskii, A.A.; Grishin, Y.A.; Grigor’ev, I.A.; Kühn, M.; et al. Combining high-field EPR with site-directed spin labeling reveals unique information on proteins in action. Magn. Reson. Chem. 2005, 43, S4–S19. [Google Scholar] [CrossRef]
- Voinov, M.A.; Kirilyuk, I.A.; Smirnov, A.I. Spin-labeled pH-sensitive phospholipids for interfacial pKa determination: Synthesis and characterization in aqueous and micellar solutions. J. Phys. Chem. B 2009, 113, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Samouilov, A.; Efimova, O.V.; Bobko, A.A.; Sun, Z.; Petryakov, S.; Eubank, T.D.; Trofimov, D.G.; Kirilyuk, I.A.; Grigor’ev, I.A.; Takahashi, W.; et al. In vivo proton-electron double-resonance imaging of extracellular tumor pH using an advanced nitroxide probe. Anal. Chem. 2014, 86, 1045–1052. [Google Scholar] [CrossRef]
- Milov, A.D.; Maryasov, A.G.; Tsvetkov, Y.D. Pulsed electron double resonance (PELDOR) and its applications in free-radicals research. Appl. Magn. Reson. 1998, 15, 107–143. [Google Scholar] [CrossRef]
- Jeschke, G. The contribution of modern EPR to structural biology. Emerg. Top. Life Sci. 2018, 2, 9–18. [Google Scholar] [CrossRef]
- Peter, M.F.; Gebhardt, C.; Mächtel, R.; Muñoz, G.G.M.; Glaenzer, J.; Narducci, A.; Thomas, G.H.; Cordes, T.; Hagelueken, G. Cross-validation of distance measurements in proteins by PELDOR/DEER and single-molecule FRET. Nat. Commun. 2022, 13, 4396. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, A.; Ouari, O.; Guigliarelli, B.; Belle, V.; Mileo, E. In-Cell EPR: Progress towards Structural Studies Inside Cells. Chembiochem 2020, 21, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Widder, P.; Schuck, J.; Summerer, D.; Drescher, M. Combining site-directed spin labeling in vivo and in-cell EPR distance determination. Phys. Chem. Chem. Phys. 2020, 22, 4875–4879. [Google Scholar] [CrossRef] [PubMed]
- Jugniot, N.; Duttagupta, I.; Rivot, A.; Massot, P.; Cardiet, C.; Pizzoccaro, A.; Jean, M.; Vanthuyne, N.; Franconi, J.-M.; Voisin, P.; et al. An elastase activity reporter for Electronic Paramagnetic Resonance (EPR) and Overhauser-enhanced Magnetic Resonance Imaging (OMRI) as a line-shifting nitroxide. Free Radic. Biol. Med. 2018, 126, 101–112. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Bonucci, A.; Casano, G.; Gerbaud, G.; Abel, S.; Thomé, V.; Kodjabachian, L.; Magalon, A.; Guigliarelli, B.; Belle, V.; et al. A Bioresistant Nitroxide Spin Label for In-Cell EPR Spectroscopy: In Vitro and in Oocytes Protein Structural Dynamics Studies. Angew. Chem. Int. Ed Engl. 2018, 57, 1366–1370. [Google Scholar] [CrossRef]
- Bleicken, S.; Assafa, T.E.; Zhang, H.; Elsner, C.; Ritsch, I.; Pink, M.; Rajca, S.; Jeschke, G.; Rajca, A.; Bordignon, E. gem-Diethyl Pyrroline Nitroxide Spin Labels: Synthesis, EPR Characterization, Rotamer Libraries and Biocompatibility. ChemistryOpen 2019, 8, 1057–1065. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Polienko, Y.F.; Krumkacheva, O.A.; Strizhakov, R.K.; Gatilov, Y.V.; Grigor’ev, I.A.; Bagryanskaya, E.G. Synthesis of 2,5-bis(spirocyclohexane)-substituted nitroxides of pyrroline and pyrrolidine series, including thiol-specific spin label: An analogue of MTSSL with long relaxation time. J. Org. Chem. 2012, 77, 8016–8027. [Google Scholar] [CrossRef]
- Pierro, A.; Drescher, M. Dance with spins: Site-directed spin labeling coupled to electron paramagnetic resonance spectroscopy directly inside cells. Chem. Commun. 2023, 59, 1274–1284. [Google Scholar] [CrossRef]
- Klug, C.S.; Feix, J.B. Methods and applications of site-directed spin labeling EPR spectroscopy. Methods Cell Biol. 2008, 84, 617–658. [Google Scholar] [CrossRef]
- Klare, J.P. Site-directed spin labeling EPR spectroscopy in protein research. Biol. Chem. 2013, 394, 1281–1300. [Google Scholar] [CrossRef] [PubMed]
- Sowa, G.Z.; Qin, P.Z. Site-directed spin labeling studies on nucleic acid structure and dynamics. Prog. Nucleic Acid Res. Mol. Biol. 2008, 82, 147–197. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Raguz, M.; Widomska, J. Studying lipid organization in biological membranes using liposomes and EPR spin labeling. Methods Mol. Biol. 2010, 606, 247–269. [Google Scholar] [CrossRef]
- Hubbell, W.L.; López, C.J.; Altenbach, C.; Yang, Z. Technological advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 2013, 23, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef]
- Torricella, F.; Pierro, A.; Mileo, E.; Belle, V.; Bonucci, A. Nitroxide spin labels and EPR spectroscopy: A powerful association for protein dynamics studies. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140653. [Google Scholar] [CrossRef] [PubMed]
- Krstić, I.; Hänsel, R.; Romainczyk, O.; Engels, J.W.; Dötsch, V.; Prisner, T.F. Long-range distance measurements on nucleic acids in cells by pulsed EPR spectroscopy. Angew. Chem. Int. Ed Engl. 2011, 50, 5070–5074. [Google Scholar] [CrossRef]
- Azarkh, M.; Okle, O.; Singh, V.; Seemann, I.T.; Hartig, J.S.; Dietrich, D.R.; Drescher, M. Long-range distance determination in a DNA model system inside Xenopus laevis oocytes by in-cell spin-label EPR. Chembiochem 2011, 12, 1992–1995. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Borbas, J.; Drescher, M.; Summerer, D. A genetically encoded spin label for electron paramagnetic resonance distance measurements. J. Am. Chem. Soc. 2014, 136, 1238–1241. [Google Scholar] [CrossRef]
- Wang, Y.; Paletta, J.T.; Berg, K.; Reinhart, E.; Rajca, S.; Rajca, A. Synthesis of unnatural amino acids functionalized with sterically shielded pyrroline nitroxides. Org. Lett. 2014, 16, 5298–5300. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Cortes, D.M.; Perozo, E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 2004, 306, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.D.; Lorigan, G.A. Site-Directed Spin Labeling EPR for Studying Membrane Proteins. Biomed Res. Int. 2018, 2018, 3248289. [Google Scholar] [CrossRef]
- Roser, P.; Schmidt, M.J.; Drescher, M.; Summerer, D. Site-directed spin labeling of proteins for distance measurements in vitro and in cells. Org. Biomol. Chem. 2016, 14, 5468–5476. [Google Scholar] [CrossRef]
- Kim, S.S.; Upshur, M.A.; Saotome, K.; Sahu, I.D.; McCarrick, R.M.; Feix, J.B.; Lorigan, G.A.; Howard, K.P. Cholesterol-Dependent Conformational Exchange of the C-Terminal Domain of the Influenza A M2 Protein. Biochemistry 2015, 54, 7157–7167. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.; Ling, S.; Liu, S.; Xiao, L.; Xin, Y.; Lai, C.; Xiong, Y.; Zhang, L.; Tian, C. CW-EPR studies revealed different motional properties and oligomeric states of the integrin β1a transmembrane domain in detergent micelles or liposomes. Sci. Rep. 2015, 5, 7848. [Google Scholar] [CrossRef]
- Columbus, L.; Hubbell, W.L. A new spin on protein dynamics. Trends Biochem. Sci. 2002, 27, 288–295. [Google Scholar] [CrossRef]
- Bordignon, E.; Steinhoff, H.-J. Membrane Protein Structure and Dynamics Studied by Site-Directed Spin-Labeling ESR; Springer: Boston, MA, USA, 2007; Volume 27, pp. 129–164. [Google Scholar] [CrossRef]
- Baker, M. Structural biology: The gatekeepers revealed. Nature 2010, 465, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.M.; Ulloa-Aguirre, A.; Ito, J.; Janovick, J.A. G protein-coupled receptor trafficking in health and disease: Lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol. Rev. 2007, 59, 225–250. [Google Scholar] [CrossRef]
- Cheung, J.C.; Deber, C.M. Misfolding of the cystic fibrosis transmembrane conductance regulator and disease. Biochemistry 2008, 47, 1465–1473. [Google Scholar] [CrossRef]
- Igarashi, R.; Sakai, T.; Hara, H.; Tenno, T.; Tanaka, T.; Tochio, H.; Shirakawa, M. Distance determination in proteins inside Xenopus laevis oocytes by double electron-electron resonance experiments. J. Am. Chem. Soc. 2010, 132, 8228–8229. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sikora, A.; Bordignon, E.; Jeschke, G.; Cafiso, D.S.; Prisner, T.F. Distance Measurement on an Endogenous Membrane Transporter in E. coli Cells and Native Membranes Using EPR Spectroscopy. Angew. Chem. Int. Ed Engl. 2015, 54, 6196–6199. [Google Scholar] [CrossRef]
- Joseph, B.; Jaumann, E.A.; Sikora, A.; Barth, K.; Prisner, T.F.; Cafiso, D.S. In situ observation of conformational dynamics and protein ligand-substrate interactions in outer-membrane proteins with DEER/PELDOR spectroscopy. Nat. Protoc. 2019, 14, 2344–2369. [Google Scholar] [CrossRef]
- Cafiso, D.S. Identifying and quantitating conformational exchange in membrane proteins using site-directed spin labeling. Acc. Chem. Res. 2014, 47, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.G.; Emoto, M.C.; Sato-Akaba, H. Brain Redox Imaging Using In Vivo Electron Paramagnetic Resonance Imaging and Nitroxide Imaging Probes. Magnetochemistry 2019, 5, 11. [Google Scholar] [CrossRef]
- Emoto, M.C.; Sato, S.; Fujii, H.G. Development of nitroxide-based theranostic compounds that act both as anti-inflammatory drugs and brain redox imaging probes in MRI. Magn. Reson. Chem. 2016, 54, 705–711. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Q.; Garcia-Hernandez, J.D.; Watanabe, L.K.; Rawson, J.M.; Rao, J.; Manners, I. Uniform and Length-Tunable, Paramagnetic Self-Assembled Nitroxide-Based Nanofibers for Magnetic Resonance Imaging. Macromolecules 2023, 56, 263–270. [Google Scholar] [CrossRef]
- Lee, H.; Shahrivarkevishahi, A.; Lumata, J.L.; Luzuriaga, M.A.; Hagge, L.M.; Benjamin, C.E.; Brohlin, O.R.; Parish, C.R.; Firouzi, H.R.; Nielsen, S.O.; et al. Supramolecular and biomacromolecular enhancement of metal-free magnetic resonance imaging contrast agents. Chem. Sci. 2020, 11, 2045–2050. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, S.; Lloveras, V.; Gancedo, J.V. Application of radical dendrimers as organic radical contrast agents for magnetic resonance imaging. Adv. Ind. Eng. Polym. Res. 2024, 7, 255–261. [Google Scholar] [CrossRef]
- Villaraza, A.J.L.; Bumb, A.; Brechbiel, M.W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The interplay between size, function, and pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef]
- Badetti, E.; Lloveras, V.; Muñoz-Gómez, J.L.; Sebastián, R.M.; Caminade, A.M.; Majoral, J.P.; Veciana, J.; Vidal-Gancedo, J. Radical Dendrimers: A Family of Five Generations of Phosphorus Dendrimers Functionalized with TEMPO Radicals. Macromolecules 2014, 47, 7717–7724. [Google Scholar] [CrossRef]
- Winalski, C.S.; Shortkroff, S.; Mulkern, R.V.; Schneider, E.; Rosen, G.M. Magnetic resonance relaxivity of dendrimer-linked nitroxides. Magn. Reson. Med. 2002, 48, 965–972. [Google Scholar] [CrossRef]
- Lloveras, V.; Vidal-Gancedo, J. Polyphosphorhydrazone-Based Radical Dendrimers. Molecules 2021, 26, 1230. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Boothapandi, M.; Sultan Nasar, A. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data Brief 2020, 28, 104972. [Google Scholar] [CrossRef]
- Mohamad Ali, B.; Velavan, B.; Sudhandiran, G.; Sridevi, J.; Sultan Nasar, A. Radical dendrimers: Synthesis, anti-tumor activity and enhanced cytoprotective performance of TEMPO free radical functionalized polyurethane dendrimers. Eur. Polym. J. 2020, 122, 109354. [Google Scholar] [CrossRef]
- Zhang, S.; Lloveras, V.; Pulido, D.; Liko, F.; Pinto, L.F.; Albericio, F.; Royo, M.; Vidal-Gancedo, J. Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics 2020, 12, 772. [Google Scholar] [CrossRef]
- Wu, Y.; Lloveras, V.; Lope-Piedrafita, S.; Mulero-Acevedo, M.; Candiota, A.P.; Vidal-Gancedo, J. Synthesis and Relaxivity study of amino acid-branched radical dendrimers as MRI contrast agents for potential brain tumor imaging. Acta Biomater. 2025, 192, 461–472. [Google Scholar] [CrossRef]
- Carloni, R.; Sanz Del Olmo, N.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de La Mata, F.J. Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR. Biomolecules 2019, 9, 540. [Google Scholar] [CrossRef]
- Zhang, S.; Lloveras, V.; Lope-Piedrafita, S.; Calero-Pérez, P.; Wu, S.; Candiota, A.P.; Vidal-Gancedo, J. Metal-Free Radical Dendrimers as MRI Contrast Agents for Glioblastoma Diagnosis: Ex Vivo and In Vivo Approaches. Biomacromolecules 2022, 23, 2767–2777. [Google Scholar] [CrossRef]
- Zhang, S.; Lloveras, V.; Wu, Y.; Tolosa, J.; García-Martínez, J.C.; Vidal-Gancedo, J. Fluorescent and Magnetic Radical Dendrimers as Potential Bimodal Imaging Probes. Pharmaceutics 2023, 15, 1776. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Shortkroff, S.; Winalski, C.S.; Mulkern, R.V.; Chatha, A.; Schneider, E.; Rosen, G.M. Dendrimer-linked nitroxides as MRI contrast agents for cartilage. In Proceedings of the 49th Annual Meeting of the Orthopaedic Research Society, Tampa, FL, USA, 4–8 February 2022. [Google Scholar]
- Winalski, C.S.; Shortkroff, S.; Schneider, E.; Yoshioka, H.; Mulkern, R.V.; Rosen, G.M. Targeted dendrimer-based contrast agents for articular cartilage assessment by MR imaging. Osteoarthr. Cartil. 2008, 16, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Chinnagounder Periyasamy, P.; Leijten, J.C.H.; Dijkstra, P.J.; Karperien, M.; Post, J.N. Nanomaterials for the Local and Targeted Delivery of Osteoarthritis Drugs. J. Nanomater. 2012, 2012, 673968. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, T.; Wang, F.; Feng, L.; Deng, G.; Wu, T.; Yin, L.; Hu, Y. T1/T2 Proportional Magnetic Resonance Nanoprobe Monitoring Tumor Autophagy during Chemotherapy. Nanomaterials 2024, 14, 1673. [Google Scholar] [CrossRef]
- Mitin, D.; Bullinger, F.; Dobrynin, S.; Engelmann, J.; Scheffler, K.; Kolokolov, M.; Krumkacheva, O.; Buckenmaier, K.; Kirilyuk, I.; Chubarov, A. Contrast Agents Based on Human Serum Albumin and Nitroxides for 1H-MRI and Overhauser-Enhanced MRI. Int. J. Mol. Sci. 2024, 25, 4041. [Google Scholar] [CrossRef]
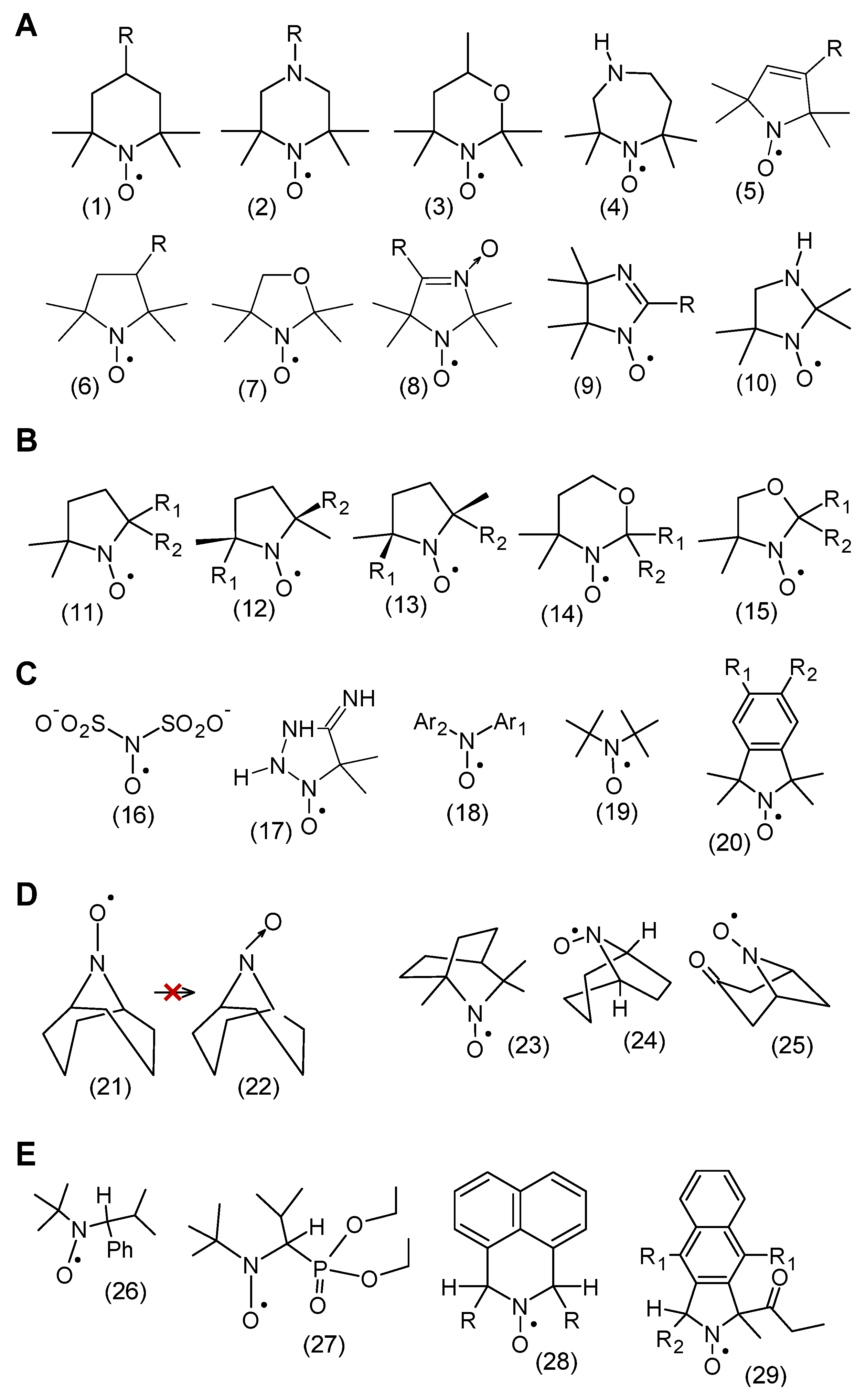



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Nitroxides: Chemistry, Antioxidant Properties, and Biomedical Applications. Molecules 2025, 30, 2159. https://doi.org/10.3390/molecules30102159
Gwozdzinski K, Pieniazek A, Gwozdzinski L. Nitroxides: Chemistry, Antioxidant Properties, and Biomedical Applications. Molecules. 2025; 30(10):2159. https://doi.org/10.3390/molecules30102159
Chicago/Turabian StyleGwozdzinski, Krzysztof, Anna Pieniazek, and Lukasz Gwozdzinski. 2025. "Nitroxides: Chemistry, Antioxidant Properties, and Biomedical Applications" Molecules 30, no. 10: 2159. https://doi.org/10.3390/molecules30102159
APA StyleGwozdzinski, K., Pieniazek, A., & Gwozdzinski, L. (2025). Nitroxides: Chemistry, Antioxidant Properties, and Biomedical Applications. Molecules, 30(10), 2159. https://doi.org/10.3390/molecules30102159




