Highly Luminescent and Scintillating Hybrid Halide of (C13H25N)2[MnBr4] Enabled by Rigid Cation
Abstract
1. Introduction
2. Results and Discussions
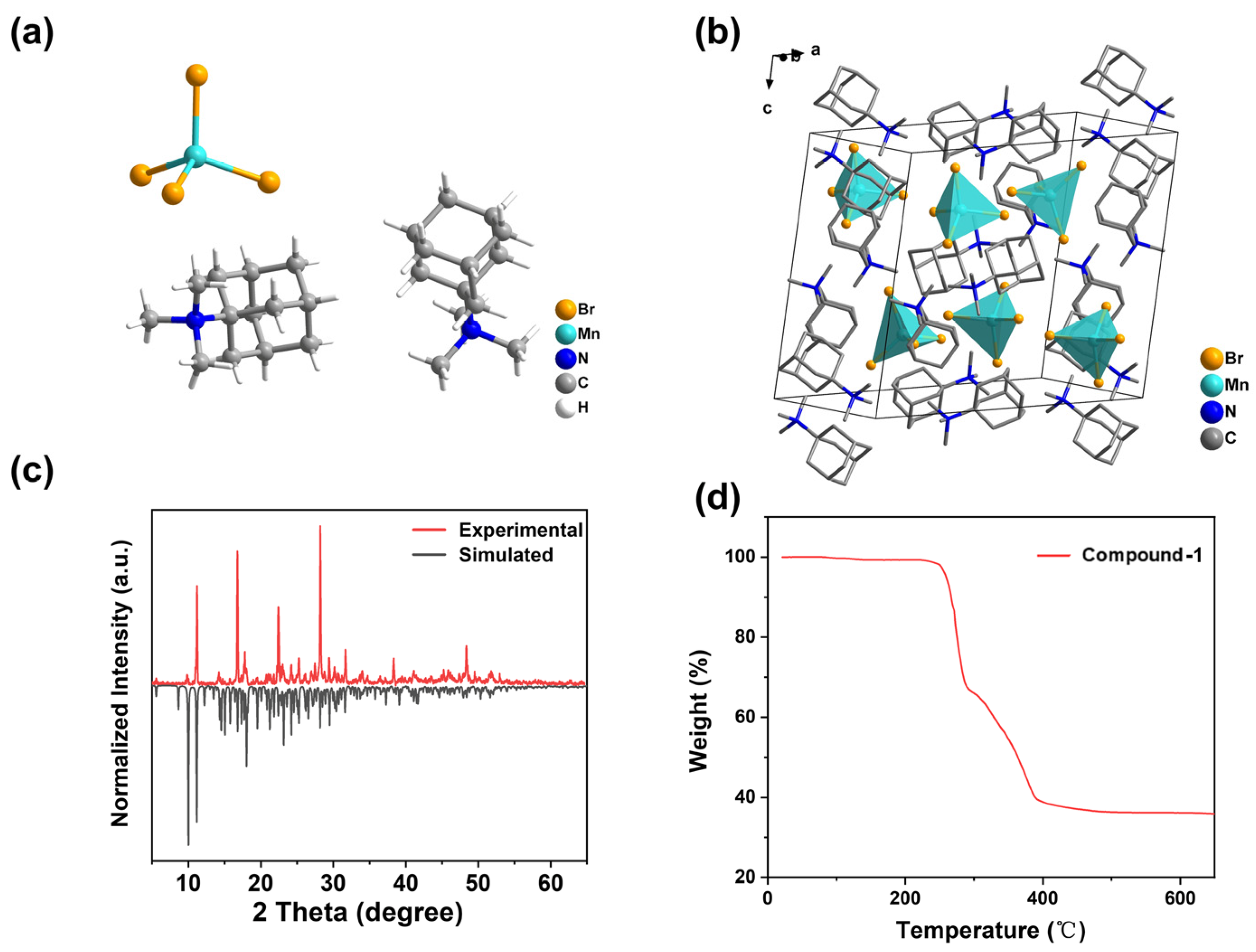
2.1. Crystal Structure Descriptions
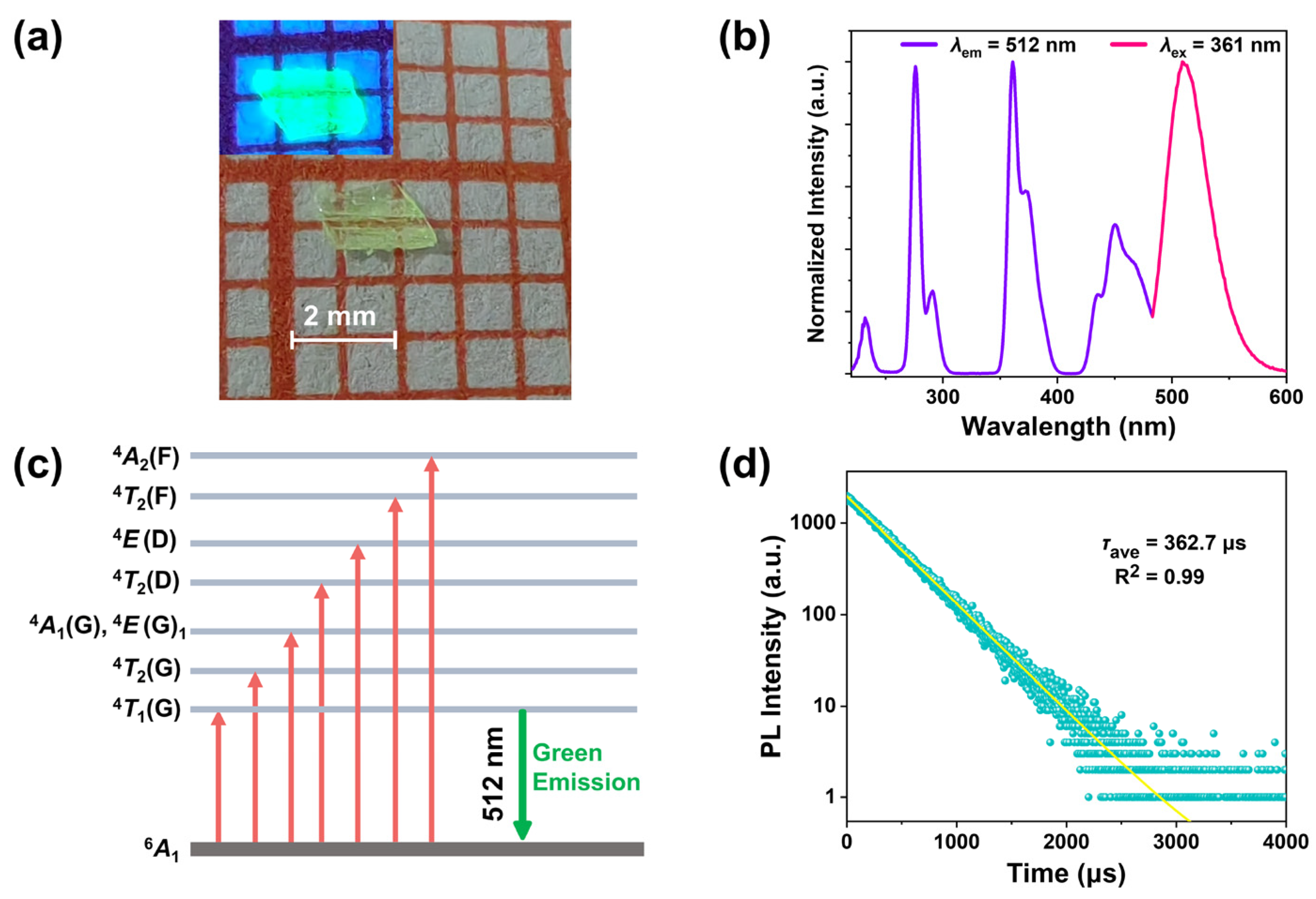
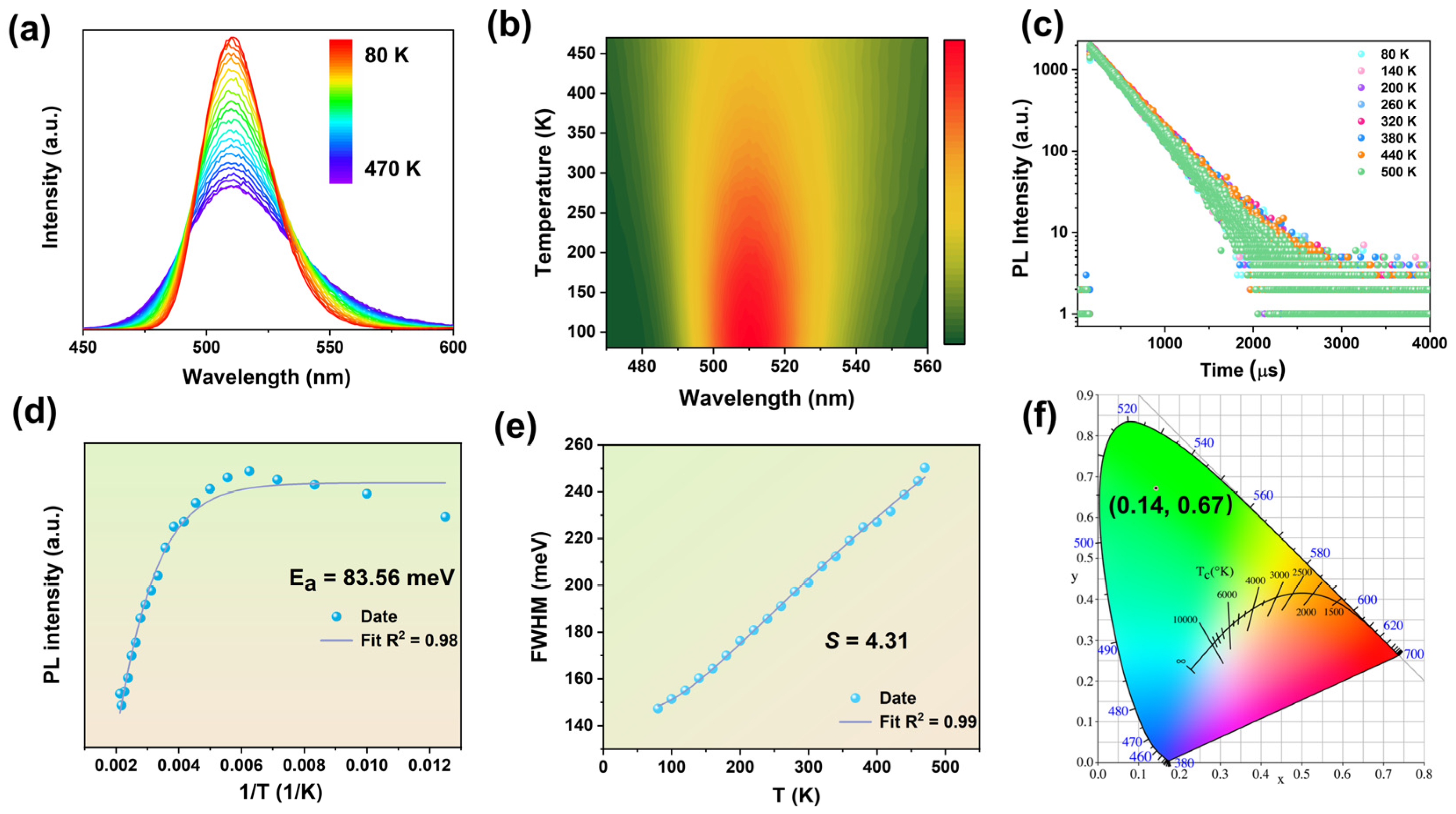
2.2. The Luminescence Characteristics of Compound 1
2.3. The Scintillation Characteristics of Compound 1
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.-J.; Lin, X.; He, Q.; Worku, M.; Ma, B. Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide. Nat. Commun. 2020, 11, 4329. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhang, J.; Deng, X.; Ren, M.P.; Wang, W.Q.; Wang, Y.J.; Cao, H.; Wang, L.; He, Y.C.; Lei, X.W. Near-unity broadband emissive hybrid manganese bromides as highly-efficient radiation scintillators. Aggregate 2024, 5, e574. [Google Scholar] [CrossRef]
- Zou, Q.; Yang, W.; Wu, L.; Jiang, L.; Wang, S.; Liu, L.; Li, R.; Ye, H.; Li, J. Ionothermal synthesis of a hybrid cuprous (I) iodide scintillator with efficient cyan emission and high antiwater stability. Chem. Eng. J. 2025, 506, 159971. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Zhang, F.; Niu, S.; Zhu, M.; Shi, Z.; Shen, G. Stable Organic-Inorganic Hybrid Sb (III) Halide Scintillator for Nonplanar Ultra-Flexible X-Ray Imaging. Adv. Funct. Mater. 2025, 35, 2412597. [Google Scholar] [CrossRef]
- Shonde, T.B.; Chaaban, M.; Liu, H.; Olasupo, O.J.; Ben-Akacha, A.; Gonzalez, F.G.; Julevich, K.; Lin, X.; Winfred, J.R.V.; Stand, L.M. Molecular sensitization enabled high performance organic metal halide hybrid scintillator. Adv. Mater. 2023, 35, 2301612. [Google Scholar] [CrossRef]
- Wibowo, A.; Sheikh, M.A.K.; Diguna, L.J.; Ananda, M.B.; Marsudi, M.A.; Arramel, A.; Zeng, S.; Wong, L.J.; Birowosuto, M.D. Development and challenges in perovskite scintillators for high-resolution imaging and timing applications. Commun. Mater. 2023, 4, 21. [Google Scholar] [CrossRef]
- He, X.; Wei, Q.; Peng, H.; Li, Y.; Wang, X.; Ke, B.; Zhao, J.; Zou, B. Large-Scale Room-Temperature Synthesis of the First Sb3+-Doped Organic Ge (IV)-Based Metal Halides with Efficient Yellow Emission for Solid-State Lighting and Latent Fingerprint Detection. Small Struct. 2024, 5, 2300472. [Google Scholar] [CrossRef]
- Meng, X.; Ji, S.J.; Wang, Q.J.; Wang, X.C.; Bai, T.X.; Zhang, R.L.; Yang, B.; Li, Y.M.; Shao, Z.P.; Jiang, J.K.; et al. Organic-Inorganic Hybrid Cuprous-Based Metal Halides for Warm White Light-Emitting Diodes. Adv. Sci. 2022, 9, 2203596. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, L.; Sun, H.; Yin, H.; Zou, Q.; Deng, J.; Li, R.; Ye, H.; Li, J. Antimony doping towards fast digital encryption and decryption and high-efficiency WLED in zero-dimensional hybrid indium chlorides. Chem. Eng. J. 2024, 494, 153060. [Google Scholar] [CrossRef]
- An, R.; Hao, J.Y.; Song, S.Y.; Zhang, H.J.; Feng, J.; Wang, X.Y.; Sun, H.Z. Multi-Responsive 0D Organic-Inorganic Hybrid Cuprous Bromides Constructed via Ultra-Facile Approaches for Multimodal Luminescent Anti-Counterfeiting. Adv. Opt. Mater. 2023, 11, 2202596. [Google Scholar] [CrossRef]
- Niu, X.; Li, Y.; Lu, H.; Wang, Z.; Zhang, Y.; Shao, T.; Wang, H.; Gull, S.; Sun, B.; Zhang, H.-L.; et al. Chiral europium halides with high-performance magnetic field tunable red circularly polarized luminescence at room temperature. Nat. Commun. 2025, 16, 2525. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Dai, Y.R.; Wei, Q.L.; Xu, X.; Cao, S.; Zou, B.S.; Zhang, Q.L.; Zeng, R.S. Temperature-Dependent Reversible Optical Properties of Mn-Based Organic-Inorganic Hybrid (C8H20N)2MnCl4 Metal Halides. ACS Appl. Mater. Interfaces 2023, 15, 5487–5494. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.Y.; Shi, Y.P.; Fang, C.L.; Zhao, S.; Shen, H.Y.; Chen, M.X.; Wang, Z.J.; Ma, Y.; Liu, Y.; et al. Anti-perovskites with long carrier lifetime for ultralow dose and stable X-ray detection. Nat. Photon. 2024, 18, 990–997. [Google Scholar] [CrossRef]
- Chen, S.C.; Guo, C.L.; Chen, S.C.; Di, Y.M.; Fang, X.; Lin, M.J.; Yang, H.H. Enhanced Stability of Melt-Processable Organic-Inorganic Hybrid Manganese Halides for X-Ray Imaging. Small 2024, 20, 2406032. [Google Scholar] [CrossRef]
- Haruta, Y.; Ikenoue, T.; Miyake, M.; Hirato, T. Fabrication of CsPbBr3 thick films by using a mist deposition method for highly sensitive X-ray detection. MRS Adv. 2020, 5, 395–401. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Liao, J.F.; Xing, G.C. Regulating the coordination geometry of polyhedra in zero-dimensional metal halides for tunable emission. Nanoscale 2023, 15, 5241–5248. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Shin, D.H.; Park, J.K.; Kim, D.H.; Lee, S.J.; Im, S.H. High-Performance Next-Generation Perovskite Nanocrystal Scintillator for Nondestructive X-Ray Imaging. Adv. Mater. 2018, 30, 1801743. [Google Scholar] [CrossRef]
- Fan, J.L.; Li, H.B.; Liu, W.; Ouyang, G.F. Fabrication Strategies of Mn2+-Based Scintillation Screens for X-Ray Detection and Imaging. Angew. Chem. Int. Ed. 2025, 137, e202425661. [Google Scholar] [CrossRef]
- Meng, H.X.; Zhu, W.J.; Zhou, Z.J.; Zhou, R.Y.; Yan, D.; Zhao, Q.; Liu, S.J. High-efficiency luminescent organic-inorganic hybrid manganese(II) halides applied to X-ray imaging. J. Mater. Chem. C 2022, 10, 12286–12291. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Wei, J.H.; Luo, J.B.; Wang, X.D.; He, Z.L.; Kuang, D.B. Large-Area Laminar TEA2MnI4 Single-Crystal Scintillator for X-ray Imaging with Impressive High Resolution. ACS Appl. Mater. Interfaces 2022, 14, 47913–47921. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, W.Q.; Deng, X.Y.; Yue, C.Y.; Lei, X.W.; Gong, Z.L. Near-Unity Green Luminescent Hybrid Manganese Halides as X-ray Scintillators. Inorg. Chem. 2024, 63, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.M.; Ma, W.B.; Zhang, H.; Tian, Y.; Lin, G.; Xiao, W.G.; Yu, X.; Qiu, J.B.; Xu, X.H.; Yang, Y.; et al. Highly Efficient and Tunable Emission of Lead-Free Manganese Halides toward White Light-Emitting Diode and X-Ray Scintillation Applications. Adv. Funct. Mater. 2021, 31, 2009973. [Google Scholar] [CrossRef]
- Hu, G.C.; Xu, B.; Wang, A.F.; Guo, Y.; Wu, J.J.; Muhammad, F.; Meng, W.; Wang, C.Y.; Sui, S.Q.; Liu, Y.; et al. Stable and Bright Pyridine Manganese Halides for Efficient White Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2011191. [Google Scholar] [CrossRef]
- Mao, L.L.; Guo, P.J.; Wang, S.X.; Cheetham, A.K.; Seshadri, R. Design Principles for Enhancing Photoluminescence Quantum Yield in Hybrid Manganese Bromides. J. Am. Chem. Soc. 2020, 142, 13582–13589. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, K.K.; Zhao, Q.Y.; Tang, Z.B.; Su, B.B.; Du, J.R.; Liu, H.; Xie, H.D. Triple-mode Luminescence and Versatile Applications of 0D Manganese-based Hybrid Halides. Adv. Opt. Mater. 2025, 13, 2403234. [Google Scholar] [CrossRef]
- Wang, M.Z.; Wang, X.M.; Zhang, B.T.; Li, F.Y.; Meng, H.X.; Liu, S.J.; Zhao, Q. Chiral hybrid manganese(II) halide clusters with circularly polarized luminescence for X-ray imaging. J. Mater. Chem. C 2023, 11, 3206–3212. [Google Scholar] [CrossRef]
- Abdelhadi, A.B.; Gutiérrez, M.; Cohen, B.; Lezama, L.; Lachkar, M.; Douhal, A. A new eco-friendly and highly emitting Mn-based hybrid perovskite toward high-performance green down-converted LEDs. J. Mater. Chem. C 2024, 12, 286–295. [Google Scholar] [CrossRef]
- Yan, S.Y.; Tian, W.L.; Chen, H.; Tang, K.X.; Lin, T.T.; Zhong, G.Y.; Qiu, L.Z.; Pan, X.Y.; Wang, W.Z. Synthesis of 0D Manganese-Based Organic-Inorganic Hybrid Perovskite and Its Application in Lead-Free Red Light-Emitting Diode. Adv. Funct. Mater. 2021, 31, 2100855. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Fan, W.B.; Gao, Z.R.; Tang, Z.; Lei, L.; Sun, X.F.; Li, Y.L.; Cai, H.L.; Wu, X.S. New photoluminescence hybrid perovskites with ultrahigh photoluminescence quantum yield and ultrahigh thermostability temperature up to 600 K. Nano Energy 2020, 77, 105170. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Gerasimova, M.A.; Ostapenko, I.A.; Zolotov, A.O.; Molokeev, M.S. Two organic-inorganic manganese(II) halide hybrids containing protonated N, N′-dialkylthioureas with efficient green-emission. J. Mol. Struct. 2023, 1277, 134851. [Google Scholar] [CrossRef]
- Zhang, G.L.; Yang, C.Z.; Wei, Q.L.; Long, J.J.; Shen, X.D.; Chen, Y.J.; Ke, B.; Liang, W.Z.; Zhong, X.C.; Zou, B.S. Sb3+-Doped Indium-Based Metal Halide (Gua)3InCl6 with Efficient Yellow Emission. ACS Appl. Mater. Interfaces 2024, 16, 3841–3852. [Google Scholar] [CrossRef]
- Li, D.Y.; Wu, J.H.; Wang, X.Y.; Zhang, X.Y.; Yue, C.Y.; Lei, X.W. Reversible Triple-Mode Photo- and Radioluminescence and Nonlinear Optical Switching in Highly Efficient 0D Hybrid Cuprous Halides. Chem. Mater. 2023, 35, 6598–6611. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Qi, X.F.; Liang, W.Q.; Wang, M.; Ma, Z.Z.; Ji, X.Z.; Yang, D.W.; Jia, M.C.; Wu, D.; et al. Regulating the Singlet and Triplet Emission of Sb3+ Ions to Achieve Single-Component White-Light Emitter with Record High Color-Rendering Index and Stability. Nano Lett. 2022, 22, 5046–5054. [Google Scholar] [CrossRef]
- Hu, S.Y.; Jiang, Y.; Hu, B.Y.; Liu, S.S.; Li, X.R.; Li, X.L.; Su, J.L.; Zheng, W.X.; Zhou, W.Y.; Ni, C.L. Crystal structures, Hirshfeld surface analysis, antimicrobial activity, and optical properties based on DFT calculations of two new organic-inorganic hybrids: nClBzPy2MnCl4 (n = 2, 4). J. Mol. Struct. 2024, 1313, 138694. [Google Scholar] [CrossRef]
- Chen, D.; Hao, S.Q.; Zhou, G.J.; Deng, C.K.; Liu, Q.L.; Ma, S.L.; Wolverton, C.; Zhao, J.; Xia, Z.G. Lead-Free Broadband Orange-Emitting Zero-Dimensional Hybrid (PMA)3InBr6 with Direct Band Gap. Inorg. Chem. 2019, 58, 15602–15609. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Chen, B.K.; Xie, L.L.; Li, X.T.; Chen, X.Y.; Lv, N.; Zheng, K.; Liu, Z.; Pi, H.H.; Lin, Z.G.; et al. Organic-Inorganic Copper Halide Compound with a Near-Unity Emission: Large-Scale Synthesis and Diverse Light-Emitting Applications. Adv. Funct. Mater. 2024, 34, 2310561. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, Y.Y.; Nikl, M.; Kucerkova, R.; Zhou, Z.Y.; Chen, J.; Sun, Y.Y.; Niu, G.D.; Tang, J.; Wang, Q.; et al. Lead-Free Zero-Dimensional Organic-Copper(I) Halides as Stable and Sensitive X-ray Scintillators. ACS Appl. Mater. Interfaces 2022, 14, 14157–14164. [Google Scholar] [CrossRef]
- Zhou, Y.; He, T.Y.; Yuan, P.; Yin, J.; Chen, S.L.; Gutiérrez-Arzaluz, L.; Wang, L.J.; Bakr, O.M.; Mohammed, O.F. Colloidal Cu4I4 Clusters for High-Resolution X-ray Imaging Scintillation Screens. ACS Mater. Lett. 2023, 5, 2002–2008. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystalstructure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Jiang, L.; Zou, Q.; Bai, J.; Wu, L.; Li, J.; Liao, J. Highly Luminescent and Scintillating Hybrid Halide of (C13H25N)2[MnBr4] Enabled by Rigid Cation. Molecules 2025, 30, 2157. https://doi.org/10.3390/molecules30102157
Li R, Jiang L, Zou Q, Bai J, Wu L, Li J, Liao J. Highly Luminescent and Scintillating Hybrid Halide of (C13H25N)2[MnBr4] Enabled by Rigid Cation. Molecules. 2025; 30(10):2157. https://doi.org/10.3390/molecules30102157
Chicago/Turabian StyleLi, Renfu, Lulu Jiang, Qinghua Zou, Jianlong Bai, Lingkun Wu, Jianrong Li, and Jinsheng Liao. 2025. "Highly Luminescent and Scintillating Hybrid Halide of (C13H25N)2[MnBr4] Enabled by Rigid Cation" Molecules 30, no. 10: 2157. https://doi.org/10.3390/molecules30102157
APA StyleLi, R., Jiang, L., Zou, Q., Bai, J., Wu, L., Li, J., & Liao, J. (2025). Highly Luminescent and Scintillating Hybrid Halide of (C13H25N)2[MnBr4] Enabled by Rigid Cation. Molecules, 30(10), 2157. https://doi.org/10.3390/molecules30102157






