Mechanical and Thermal Performance of In-Situ Synthesized PDMS-SiO2 Composite as Electrical Insulating Coatings
Abstract
1. Introduction
2. Results
2.1. PDMS-%SiO2 Mechanical and Thermal Properties
2.1.1. Tensile Mechanical Analysis
2.1.2. Dynamic Mechanic Analysis
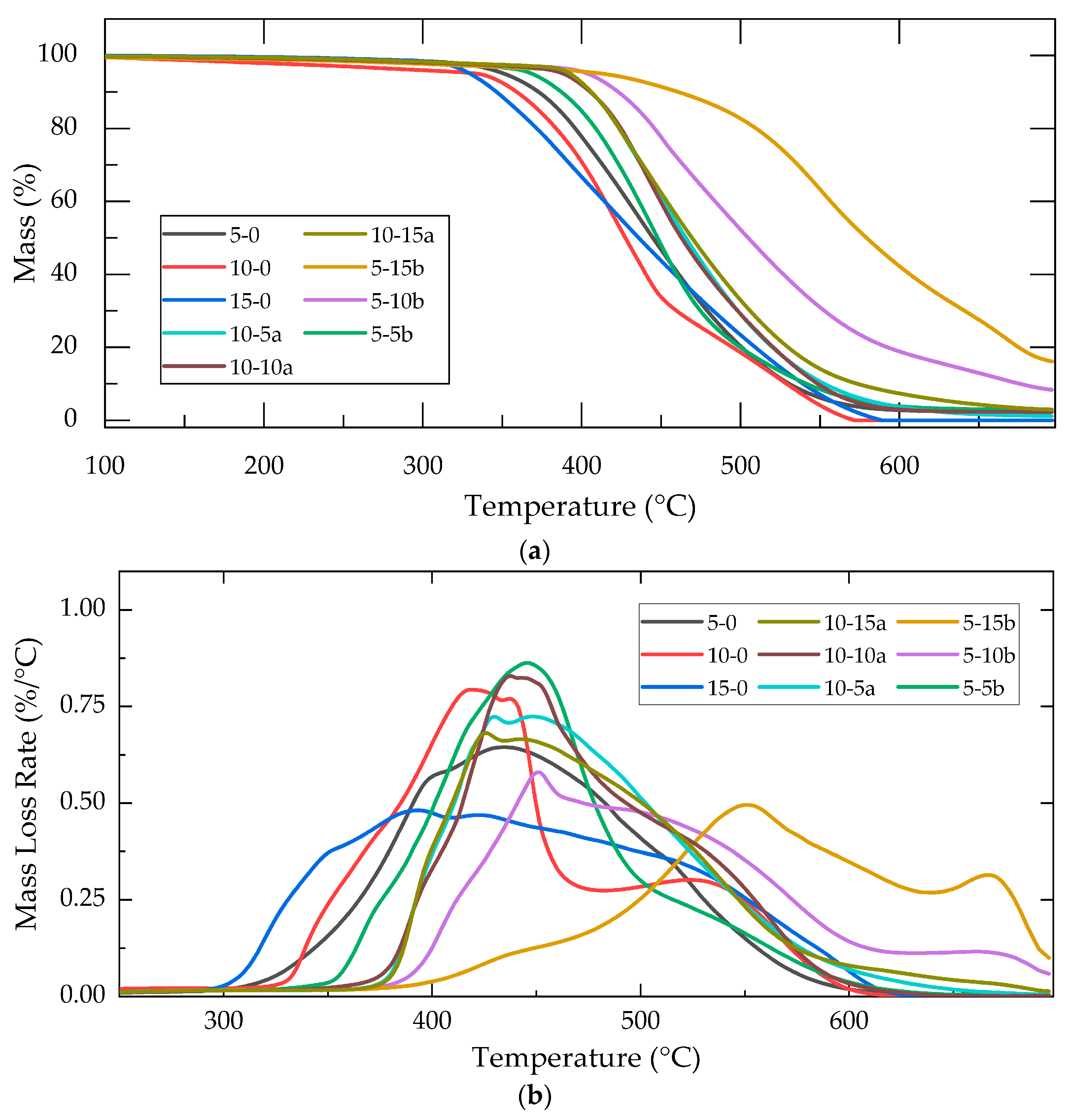
2.1.3. Thermogravimetric Analysis
2.2. PDMS-%SiO2 Coating Characterization
2.2.1. Crosslinking Efficiency
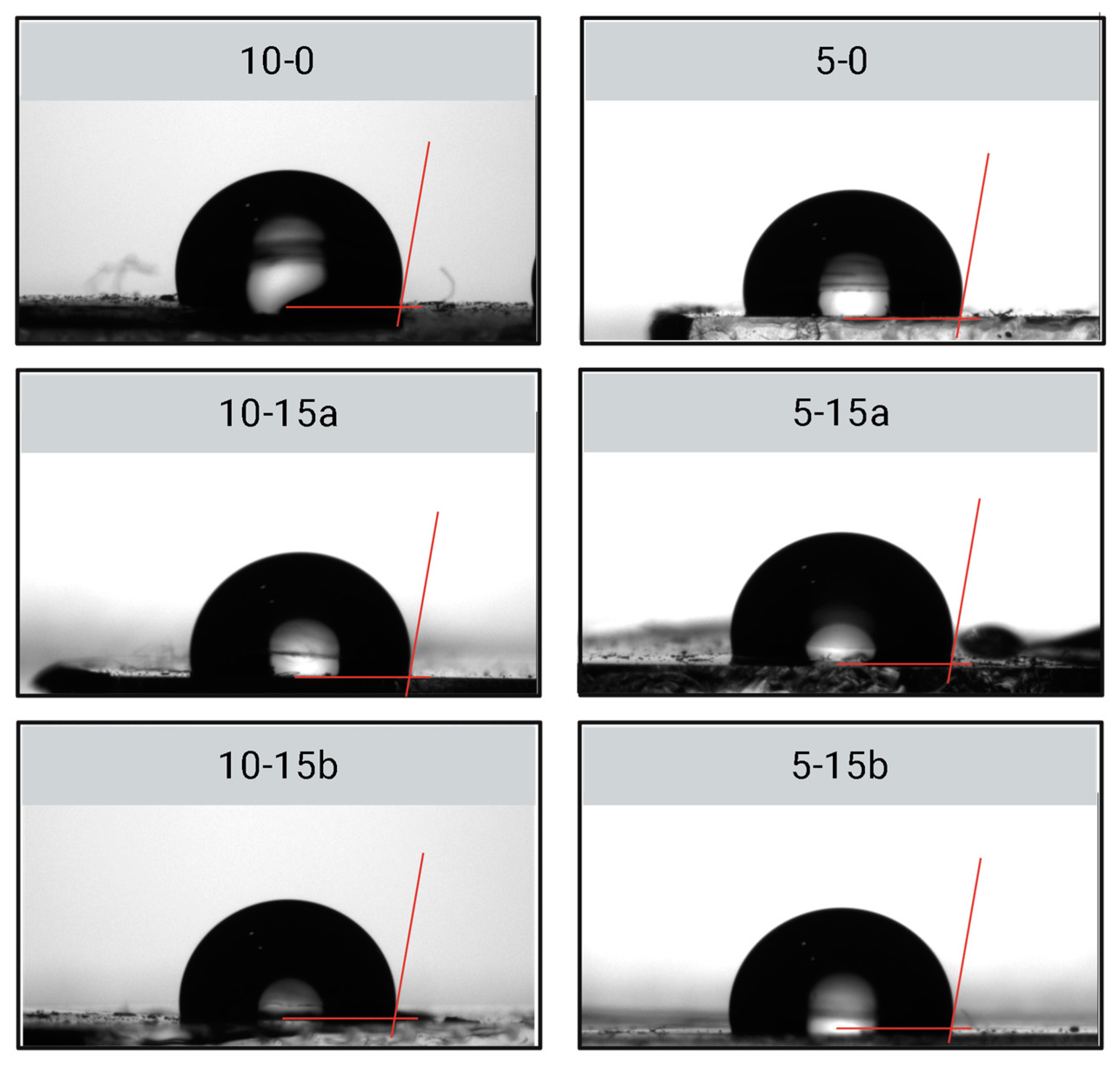
2.2.2. Hydrophobicity and Electric Insulation
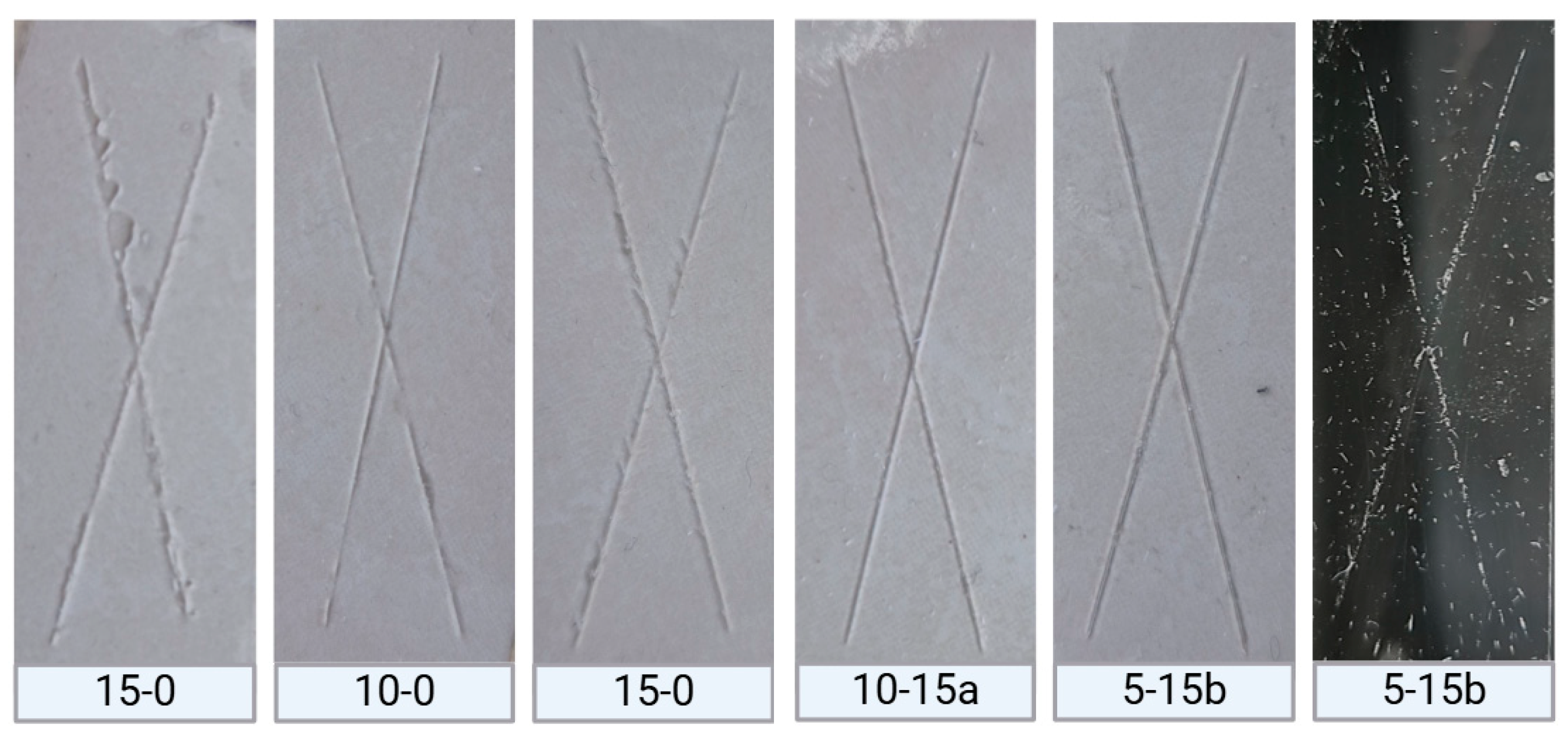
2.2.3. Superficial Adherence
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PDMS-SiO2 Nanocomposites
4.3. Characterization Techniques
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DBTDL | Dibutyltin dilaurate |
| DMA | Dynamic mechanical analysis |
| PDMS | Polydimethylsiloxane |
| TEOS | Tetraethyl orthosilicate |
| TGA | Thermogravimetric analysis |
References
- Teixeira, I.; Castro, I.; Carvalho, V.; Rodrigues, C.; Souza, A.; Lima, R.; Teixeira, S.; Ribeiro, J. Polydimethylsiloxane Mechanical Properties: A Systematic Review. AIMS Mater. Sci. 2021, 8, 952–973. [Google Scholar] [CrossRef]
- Ghosh, D.; Khastgir, D. Degradation and Stability of Polymeric High-Voltage Insulators and Prediction of Their Service Life through Environmental and Accelerated Aging Processes. ACS Omega 2018, 3, 11317–11330. [Google Scholar] [CrossRef] [PubMed]
- Weththimuni, M.L.; Chobba, M.B.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Cordoba, A.; Rivera-Muñoz, E.M.; Velázquez-Castillo, R.; Esquivel, K. PDMS/TiO2 and PDMS/SiO2 Nanocomposites: Mechanical Properties’ Evaluation for Improved Insulating Coatings. Nanomaterials 2023, 13, 1699. [Google Scholar] [CrossRef]
- Chen, Z.; Su, X.; Wu, W.; Chen, S.; Zhang, X.; Wu, Y.; Xie, H.; Li, K. Superhydrophobic PDMS@TiO2 Wood for Photocatalytic Degradation and Rapid Oil-Water Separation. Surf. Coat. Technol. 2022, 434, 128182. [Google Scholar] [CrossRef]
- Ain, Q.U.; Wani, M.F.; Sehgal, R.; Singh, M.K. Role of Al2O3 Reinforcements in Polymer-Based Nanocomposites for Enhanced Nanomechanical Properties: Time-Dependent Modeling of Creep and Stress Relaxation. Ceram. Int. 2024, 50, 33817–33838. [Google Scholar] [CrossRef]
- Cordoba, A.; Cauich-Rodríguez, J.V.; Vargas-Coronado, R.F.; Velázquez-Castillo, R.; Esquivel, K. A Novel In Situ Sol-Gel Synthesis Method for PDMS Composites Reinforced with Silica Nanoparticles. Polymers 2024, 16, 1125. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, B.; Dong, K.; Yan, Y.; Guan, Z. HD-SiO2/SiO2 Sol@PDMS Superhydrophobic Coating with Good Durability and Anti-Corrosion for Protection of Al Sheets. Materials 2023, 16, 3532. [Google Scholar] [CrossRef]
- Tamayo-Vegas, S.; Muhsan, A.; Liu, C.; Tarfaoui, M.; Lafdi, K. The Effect of Agglomeration on the Electrical and Mechanical Properties of Polymer Matrix Nanocomposites Reinforced with Carbon Nanotubes. Polymers 2022, 14, 1842. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ren, Y.J.; Guo, H.C.; Bai, S. Enhanced Thermal Properties of PDMS Composites Containing Vertically Aligned Graphene Tubes. Appl. Therm. Eng. 2019, 150, 840–848. [Google Scholar] [CrossRef]
- Katz, S.; Lachman, N.; Hafif, N.; Rosh, L.; Pevzner, A.; Lybman, A.; Amitay-Rosen, T.; Nir, I.; Rotter, H. Studying the Physical and Chemical Properties of Polydimethylsiloxane Matrix Reinforced by Nanostructured TiO2 Supported on Mesoporous Silica. Polymers 2022, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, L.; Shi, Y.; Zhang, F.; Hu, S.; Liu, P.; Li, A.; Chen, J. Optimized CNT-PDMS Flexible Composite for Attachable Health-Care Device. Sensors 2020, 20, 4523. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Jia, Q. Evaluation the Mechanical and Electrochemical Anti-Corrosion Properties of Polydimethylsiloxane/SiO2 Gel Coated Carbon Steel Rebar in Concrete Pore Solution. Int. J. Electrochem. Sci. 2023, 18, 100043. [Google Scholar] [CrossRef]
- Muhammed Shameem, M.; Sasikanth, S.M.; Annamalai, R.; Ganapathi Raman, R. A Brief Review on Polymer Nanocomposites and Its Applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- Kamal, A.; Ashmawy, M.; Sengottaiyan, S.; Algazzar, A.M.; Elsheikh, A.H. Fabrication Techniques of Polymeric Nanocomposites: A Comprehensive Review. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 236, 4843–4861. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, D.; Liu, Z.; Zhang, X.; Yu, K.; Ma, W. A Simple and Efficient in Situ Polymerization of Silica Xerogel-Acrylic Thermal Insulation Coatings. Prog. Org. Coat. 2024, 187, 108142. [Google Scholar] [CrossRef]
- Shibu, M.C.; Benoy, M.D.; Kumar, G.S.; Duraimurugan, J.; Vasudevan, V.; Shkir, M.; AL-Otaibi, O. Hydrothermal-Assisted Synthesis and Characterization of MWCNT/Copper Oxide Nanocomposite for the Photodegradation of Methyl Orange under Direct Sunlight. Diam. Relat. Mater. 2023, 134, 109778. [Google Scholar] [CrossRef]
- Florensa, M.; Llenas, M.; Medina-Gutiérrez, E.; Sandoval, S.; Tobías-Rossell, G. Key Parameters for the Rational Design, Synthesis, and Functionalization of Biocompatible Mesoporous Silica Nanoparticles. Pharmaceutics 2022, 14, 2703. [Google Scholar] [CrossRef]
- Levy, D.; Zayat, M. The Sol-Gel Handbook: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Demir, E.C.; Benkaddour, A.; Aldrich, D.R.; McDermott, M.T.; Kim, C., II; Ayranci, C. A Predictive Model towards Understanding the Effect of Reinforcement Agglomeration on the Stiffness of Nanocomposites. J. Compos. Mater. 2022, 56, 1591–1604. [Google Scholar] [CrossRef]
- Szymoniak, P.; Pauw, B.R.; Qu, X.; Schönhals, A. Competition of Nanoparticle-Induced Mobilization and Immobilization Effects on Segmental Dynamics of an Epoxy-Based Nanocomposite. Soft Matter 2020, 16, 5406–5421. [Google Scholar] [CrossRef]
- Moreno-Mateos, M.A.; Steinmann, P. Crosslinking Degree Variations Enable Programming and Controlling Soft Fracture via Sideways Cracking. npj Comput. Mater. 2024, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Syafiq, A.; Vengadaesvaran, B.; Rahim, N.A.; Pandey, A.K.; Bushroa, A.R.; Ramesh, K.; Ramesh, S. Transparent Self-Cleaning Coating of Modified Polydimethylsiloxane (PDMS) for Real Outdoor Application. Prog. Org. Coat. 2019, 131, 232–239. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Mao, J.; Zhang, B.; Zhang, G.-X.; Dang, Z.-M. Polymeric Insulating Materials Characteristics for High-Voltage Applications. Nat. Rev. Electr. Eng. 2024, 1, 516–528. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk Composites, Surface Engineering, and Precision Fabrication. Adv. Sci. 2023, 10, 2304506. [Google Scholar] [CrossRef]
- Thongchom, C.; Refahati, N.; Roodgar Saffari, P.; Roudgar Saffari, P.; Niyaraki, M.N.; Sirimontree, S.; Keawsawasvong, S. An Experimental Study on the Effect of Nanomaterials and Fibers on the Mechanical Properties of Polymer Composites. Buildings 2021, 12, 7. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, X.; Su, W.; Zhao, J.; Zhou, C. Molecular Insights into Sequence Distributions and Conformation-Dependent Properties of High-Phenyl Polysiloxanes. Polymers 2019, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. Kinetic Aspects of the Thermal Degradation of Poly(Dimethyl Siloxane) and Poly(Dimethyl Diphenyl Siloxane). Polym. Degrad. Stab. 2002, 76, 17–24. [Google Scholar] [CrossRef]
- Bardelli, T.; Marano, C.; Briatico Vangosa, F. Polydimethylsiloxane Crosslinking Kinetics: A Systematic Study on Sylgard184 Comparing Rheological and Thermal Approaches. J. Appl. Polym. Sci. 2021, 138, 51013. [Google Scholar] [CrossRef]
- Rajawasam, C.W.H.; Dodo, O.J.; Weerasinghe, M.A.S.N.; Raji, I.O.; Wanasinghe, S.V.; Konkolewicz, D.; De Alwis Watuthanthrige, N. Educational Series: Characterizing Crosslinked Polymer Networks. Polym. Chem. 2024, 15, 219–247. [Google Scholar] [CrossRef]
- Sosnin, I.M.; Vlassov, S.; Dorogin, L.M. Application of Polydimethylsiloxane in Photocatalyst Composite Materials: A Review. React. Funct. Polym. 2021, 158, 104781. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, J.-G. Volume and Surface Resistivity Measurement of Insulating Materials Using Guard-Ring Terminal Electrodes. Energies 2020, 13, 2811. [Google Scholar] [CrossRef]
- Shivashankar, H.; Kevin, A.M.; Manohar, S.B.S.; Kulkarni, S.M. Investigation on Dielectric Properties of PDMS Based Nanocomposites. Phys. B Condens. Matter 2021, 602, 412357. [Google Scholar] [CrossRef]
- ASTM D3359-23; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2017.
- Lee, J.-H.; Lee, T.-H.; Shim, K.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y.; Jung, S. Effect of Crosslinking Density on Adhesion Performance and Flexibility Properties of Acrylic Pressure Sensitive Adhesives for Flexible Display Applications. Int. J. Adhes. Adhes. 2017, 74, 137–143. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Shen, J.; Zheng, Z.; Liu, J. Role of a Nanoparticle Network in Polymer Mechanical Reinforcement: Insights from Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2021, 23, 21797–21807. [Google Scholar] [CrossRef]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking Effect on Polydimethylsiloxane Elastic Modulus Measured by Custom-Built Compression Instrument. J. Appl. Polym. Sci. 2014, 131, 1–4. [Google Scholar] [CrossRef]
- Gupta, N.S.; Lee, K.; Labouriau, A. Tuning Thermal and Mechanical Properties of Polydimethylsiloxane with Carbon Fibers. Polymers 2021, 13, 1141. [Google Scholar] [CrossRef]
- Musa, A.A.; Bello, A.; Adams, S.M.; Onwualu, A.P.; Anye, V.C.; Bello, K.A.; Obianyo, I.I. Nano-Enhanced Polymer Composite Materials: A Review of Current Advancements and Challenges. Polymers 2025, 17, 893. [Google Scholar] [CrossRef]
- Tsige, M.; Soddemann, T.; Rempe, S.B.; Grest, G.S.; Kress, J.D.; Robbins, M.O.; Sides, S.W.; Stevens, M.J.; Webb, E. Interactions and Structure of Poly(Dimethylsiloxane) at Silicon Dioxide Surfaces: Electronic Structure and Molecular Dynamics Studies. J. Chem. Phys. 2003, 118, 5132–5142. [Google Scholar] [CrossRef]
- Ichim, M.; Muresan, E.I.; Codau, E. Natural-Fiber-Reinforced Polymer Composites for Furniture Applications. Polymers 2024, 16, 3113. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, J.; Chen, X.; Zhao, X.; Deng, J.; Zhang, G. Molecular-Dynamics Study on the Thermodynamic Properties of Nano-SiO2 Particle-Doped Silicone Rubber Composites. Comput. Mater. Sci. 2022, 212, 111571. [Google Scholar] [CrossRef]
- Lazareva, S.V.; Shikina, N.V.; Tatarova, L.E.; Ismagilov, Z.R. Synthesis of High-Purity Silica Nanoparticles by Sol-Gel Method. Eurasian Chem. J. 2017, 19, 295–302. [Google Scholar] [CrossRef]
- He, H.; Fan, G.; Saba, F.; Tan, Z.; Su, Z.; Xiong, D.; Li, Z. Enhanced Distribution and Mechanical Properties of High Content Nanoparticles Reinforced Metal Matrix Composite Prepared by Flake Dispersion. Compos. Part B Eng. 2023, 252, 110514. [Google Scholar] [CrossRef]
- Park, J.; Ha, H.; Yoon, H.W.; Noh, J.; Park, H.B.; Paul, D.R.; Ellison, C.J.; Freeman, B.D. Gas Sorption and Diffusion in Poly(Dimethylsiloxane) (PDMS)/Graphene Oxide (GO) Nanocomposite Membranes. Polymer 2021, 212, 123185. [Google Scholar] [CrossRef]
- Abu Bakar, N.H.; Wan Ismail, W.N.; Mohd Yusop, H.; Mohd Zulkifli, N.F. Synthesis of a Water-Based TEOS–PDMS Sol–Gel Coating for Hydrophobic Cotton and Polyester Fabrics. New J. Chem. 2024, 48, 933–950. [Google Scholar] [CrossRef]
- Sakasegawa, D.; Tsuzuki, T.; Sugizaki, Y.; Goto, M.; Suzuki, A. Effects of Degree of Cross-Links on Adhesion Curves of Cross-Linked Polymers Observed by a Point-Contact Method. Langmuir 2010, 26, 5856–5863. [Google Scholar] [CrossRef]
- Alarifi, I.M. A Comprehensive Review on Advancements of Elastomers for Engineering Applications. Adv. Ind. Eng. Polym. Res. 2023, 6, 451–464. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Guan, J.; Fan, W.; Li, H.; Mai, Z.; Jing, Y.; Chen, J.; Zhang, M.; Tang, B.; Yang, Y.; Shen, X. Soft and Stretchable Protective Substrates for Wearable Thermal Managements: Polydimethylsiloxane (PDMS) Composites Containing Paraffin Microcapsules with Silica Nanoshells. Colloids Surf. A Physicochem. Eng. Asp. 2024, 690, 133809. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- ISO 527-1; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019.
- Hirschl, C.; Biebl-Rydlo, M.; Debiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the Degree of Crosslinking of Ethylene Vinyl Acetate Photovoltaic Module Encapsulants—A Comparative Study. Sol. Energy Mater. Sol. Cells 2013, 116, 203–218. [Google Scholar] [CrossRef]
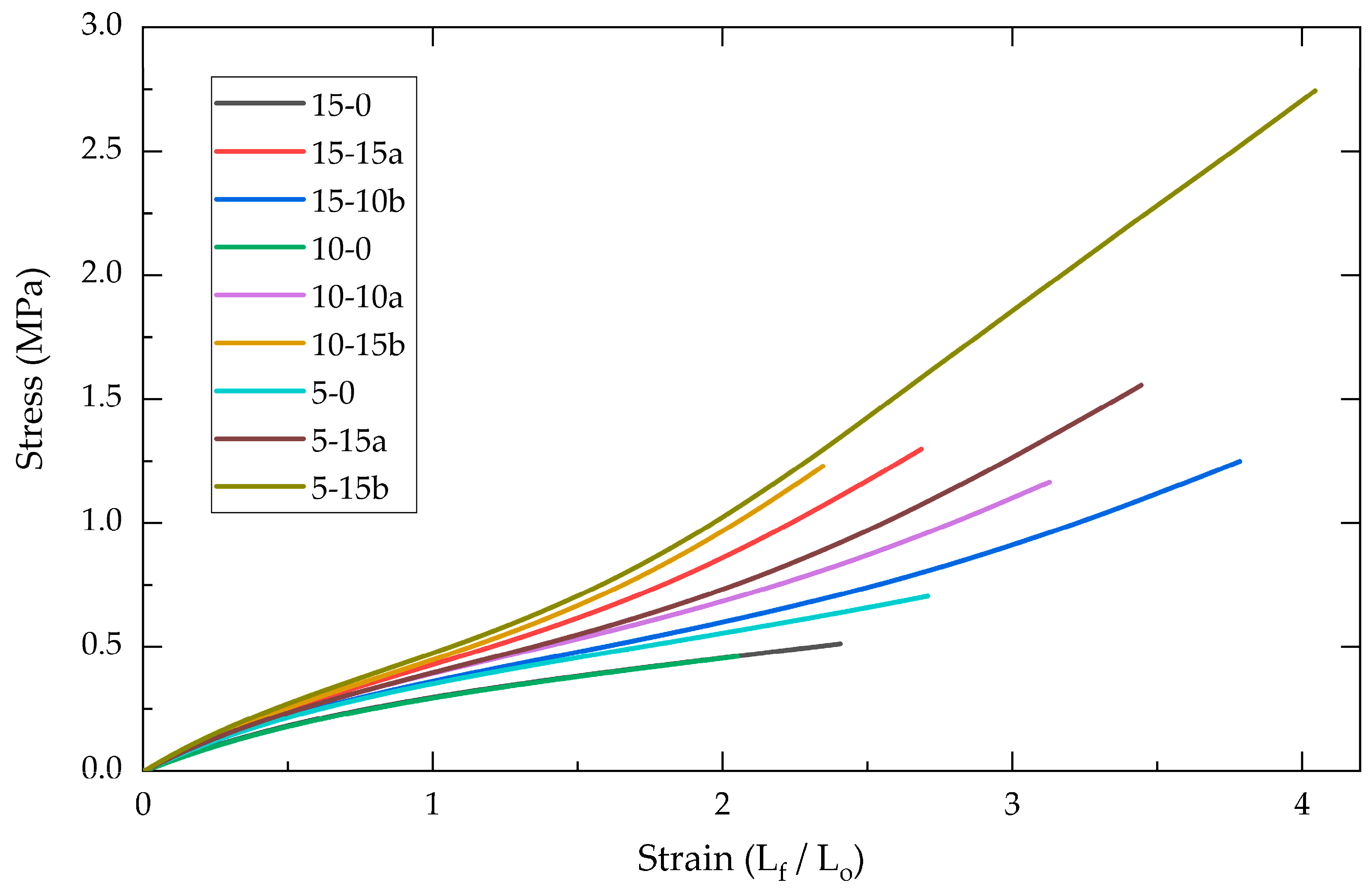
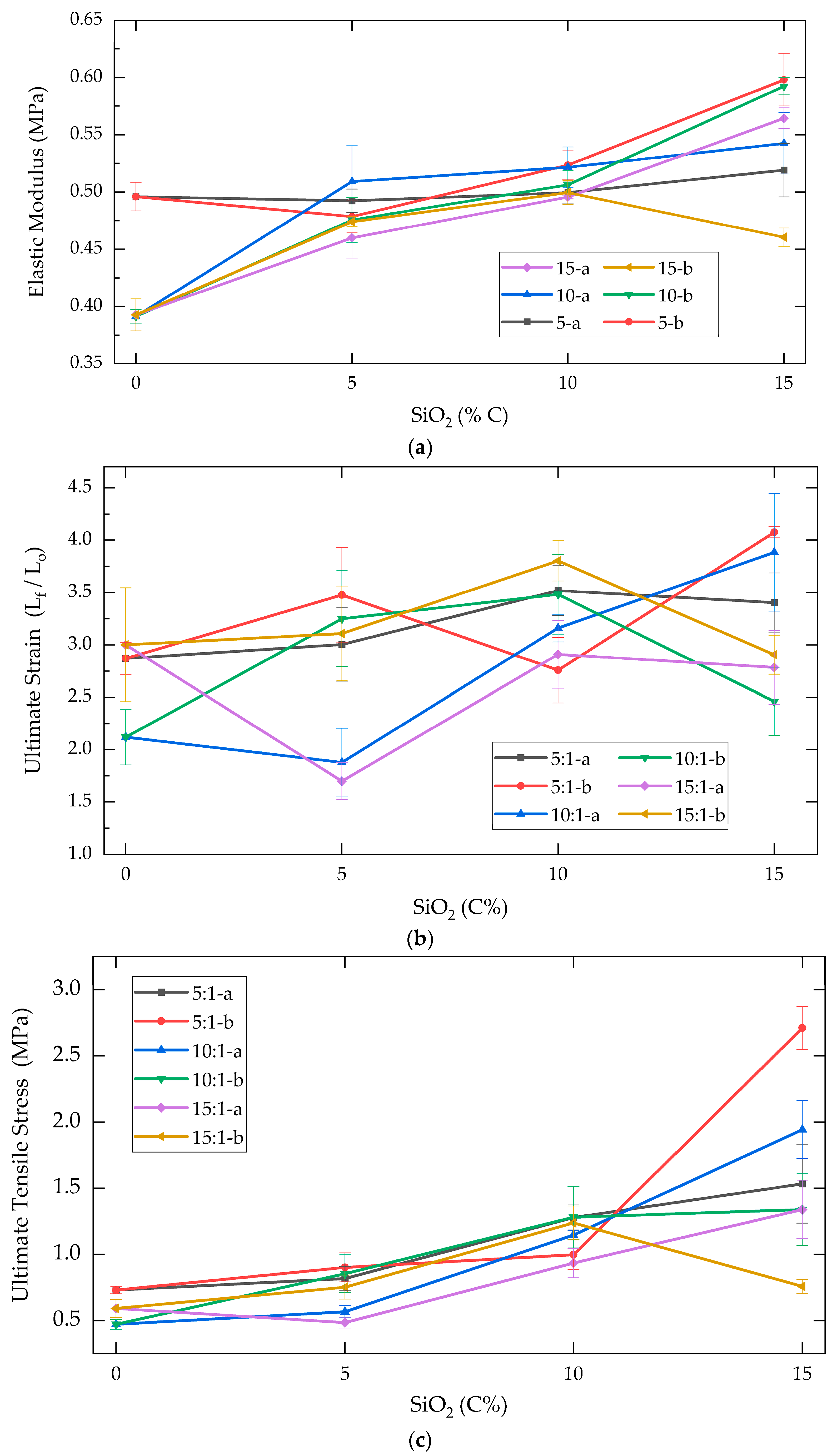
| Elastic Modulus (MPa) | ||||||
|---|---|---|---|---|---|---|
| PDMS:TEOS | 15 | 10 | 5 | |||
| SiO2 1 (%w) | a | b | a | b | a | b |
| 0 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.50 ± 0.013 | 0.50 ± 0.01 |
| 5 | 0.46 ± 0.02 | 0.47 ± 0.004 | 0.51 ± 0.03 | 0.48 ± 0.02 | 0.49 ± 0.010 | 0.48 ± 0.01 |
| 10 | 0.50 ± 0.001 | 0.50 ± 0.01 | 0.52 ± 0.02 | 0.51 ± 0.01 | 0.50 ± 0.009 | 0.52 ± 0.01 |
| 15 | 0.56 ± 0.01 | 0.46 ± 0.01 | 0.54 ± 0.03 | 0.59 ± 0.01 | 0.52 ± 0.023 | 0.60 ± 0.02 |
| Ultimate Strain (MPa) | ||||||
| PDMS:TEOS | 15 | 10 | 5 | |||
| SiO2 1 (%w) | a | b | a | b | a | b |
| 0 | 3.00 ± 0.15 | 3.00 ± 0.15 | 2.12 ± 0.26 | 2.12 ± 0.26 | 2.87 ± 0.54 | 2.87 ± 0.54 |
| 5 | 1.70 ± 0.35 | 3.11 ± 0.45 | 1.88 ± 0.32 | 3.25 ± 0.46 | 3.00 ± 0.18 | 3.48 ± 0.45 |
| 10 | 2.91 ± 0.24 | 3.80 ± 0.31 | 3.16 ± 0.13 | 3.48 ± 0.38 | 3.52 ± 0.32 | 2.76 ± 0.19 |
| 15 | 2.79 ± 0.28 | 2.91 ± 0.05 | 3.88 ± 0.56 | 2.46 ± 0.33 | 3.40 ± 0.35 | 4.08 ± 0.19 |
| SiO2 1 (%w) | Ultimate Tensile Stress (MPa) | |||||
| PDMS:TEOS | 15 | 10 | 5 | |||
| [C] SiO2 1 | a | b | a | b | a | b |
| 0 | 0.59 ± 0.07 | 0.59 ± 0.07 | 0.47 ± 0.04 | 0.47 ± 0.04 | 0.73 ± 0.02 | 0.73 ± 0.03 |
| 5 | 0.48 ± 0.04 | 0.75 ± 0.09 | 0.57 ± 0.05 | 0.85 ± 0.14 | 0.82 ± 0.09 | 0.90 ± 0.11 |
| 10 | 0.93 ± 0.11 | 1.24 ± 0.13 | 1.15 ± 0.03 | 1.28 ± 0.23 | 1.28 ± 0.10 | 1.00 ± 0.11 |
| 15 | 1.34 ± 0.22 | 0.76 ± 0.05 | 1.94 ± 0.22 | 1.34 ± 0.27 | 1.53 ± 0.30 | 2.71 ± 0.16 |
| G″ (MPa) | G′ (MPa) | Tan δ | Tg (°C) 1 | |
|---|---|---|---|---|
| 15-0 | 0.035 | 0.970 | 0.036 | −66.79 |
| 15-5a | 0.013 | 1.508 | 0.008 | −70.14 |
| 15-10a | 0.058 | 1.080 | 0.053 | −60.72 |
| 15-15a | 0.086 | 1.521 | 0.057 | −59.74 |
| 15-5b | 0.062 | 1.014 | 0.061 | −59.81 |
| 15-10b | 0.063 | 1.160 | 0.054 | −59.88 |
| 15-15b | 0.014 | 1.267 | 0.011 | −72.72 |
| 10-0 | 0.064 | 1.097 | 0.059 | −69.31 |
| 10-5a | 0.064 | 1.191 | 0.053 | −79.25 |
| 10-10a | 0.063 | 1.103 | 0.057 | −66.60 |
| 10-15a | 0.062 | 1.161 | 0.054 | −67.00 |
| 10-5b | 0.064 | 1.059 | 0.060 | −61.36 |
| 10-10b | 0.071 | 1.101 | 0.057 | −61.15 |
| 10-15b | 0.071 | 1.301 | 0.053 | −61.05 |
| 5-0 | 0.058 | 0.972 | 0.060 | −61.77 |
| 5-5a | 0.060 | 1.035 | 0.058 | −65.21 |
| 5-10a | 0.068 | 1.081 | 0.063 | −64.31 |
| 5-15a | 0.076 | 1.174 | 0.064 | −60.27 |
| 5-5b | 0.060 | 1.246 | 0.048 | −63.34 |
| 5-10b | 0.097 | 1.267 | 0.077 | −62.34 |
| 5-15b | 0.077 | 1.398 | 0.055 | −52.54 |
| Composite | Intersection (100 °C) | Max. Mass Loss | Reisdual Mass 700 °C (%) | |
|---|---|---|---|---|
| T (°C) | Rate (%/°C) | |||
| 15-0 | 327 | 392 | 0.48 | 0 |
| 10-0 | 351 | 419 | 0.79 | 0 |
| 10-5a | 391 | 447 | 0.72 | 1.17 |
| 10-10a | 395 | 437 | 0.83 | 2.26 |
| 10-15a | 393 | 425 | 0.68 | 2.94 |
| 5-0 | 358 | 435 | 0.65 | 2.27 |
| 5-5b | 377 | 445 | 0.86 | 2.85 |
| 5-10b | 405 | 451 | 0.58 | 8.35 |
| 5-15b | 447 | 550 | 0.5 | 16.16 |
| Group | (%) | (%) | (%) |
|---|---|---|---|
| 15-0 | 3.65 ± 0.55 | 3.59 ± 1.04 | 96.41 ± 1.04 |
| 10-0 | 2.15 ± 0.81 | 1.71 ± 0.86 | 98.29 ± 0.86 |
| 5-0 | 2.23 ± 0.90 | 1.78 ± 0.97 | 98.22 ± 0.97 |
| 10-15a | 1.12 ± 0.80 | 3.49 ± 0.34 | 96.51 ± 0.34 |
| 5-15b | 0.98 ± 0.75 | 3.28 ± 0.64 | 96.72 ±0.64 |
| Group | Contact Angle (°) | Resistivity (1011 Ω/cm) |
|---|---|---|
| 15-0 | 100.4 ± 2.1 | 3.6 ± 0.6 |
| 10-0 | 99.1 ± 1.6 | 3.1 ± 0.8 |
| 5-0 | 99.7 ± 5.0 | 2.5 ± 0.9 |
| 10-15a | 99.3 ± 2.3 | 2.6 ± 0.6 |
| 5-15b | 98.1 ± 3.8 | 2.4 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordoba, A.; Vargas-Coronado, R.F.; Velázquez-Castillo, R.; Cauich-Rodríguez, J.V.; Esquivel, K. Mechanical and Thermal Performance of In-Situ Synthesized PDMS-SiO2 Composite as Electrical Insulating Coatings. Molecules 2025, 30, 2107. https://doi.org/10.3390/molecules30102107
Cordoba A, Vargas-Coronado RF, Velázquez-Castillo R, Cauich-Rodríguez JV, Esquivel K. Mechanical and Thermal Performance of In-Situ Synthesized PDMS-SiO2 Composite as Electrical Insulating Coatings. Molecules. 2025; 30(10):2107. https://doi.org/10.3390/molecules30102107
Chicago/Turabian StyleCordoba, Aldo, Rossana Faride Vargas-Coronado, Rodrigo Velázquez-Castillo, Juan Valerio Cauich-Rodríguez, and Karen Esquivel. 2025. "Mechanical and Thermal Performance of In-Situ Synthesized PDMS-SiO2 Composite as Electrical Insulating Coatings" Molecules 30, no. 10: 2107. https://doi.org/10.3390/molecules30102107
APA StyleCordoba, A., Vargas-Coronado, R. F., Velázquez-Castillo, R., Cauich-Rodríguez, J. V., & Esquivel, K. (2025). Mechanical and Thermal Performance of In-Situ Synthesized PDMS-SiO2 Composite as Electrical Insulating Coatings. Molecules, 30(10), 2107. https://doi.org/10.3390/molecules30102107











