Oxaloacetate and Ketone Bodies Synergistically Promote Myoblast Differentiation in L6 Cells
Abstract
1. Introduction
2. Results
2.1. Changes in Glucose Concentration in Culture Medium over Time
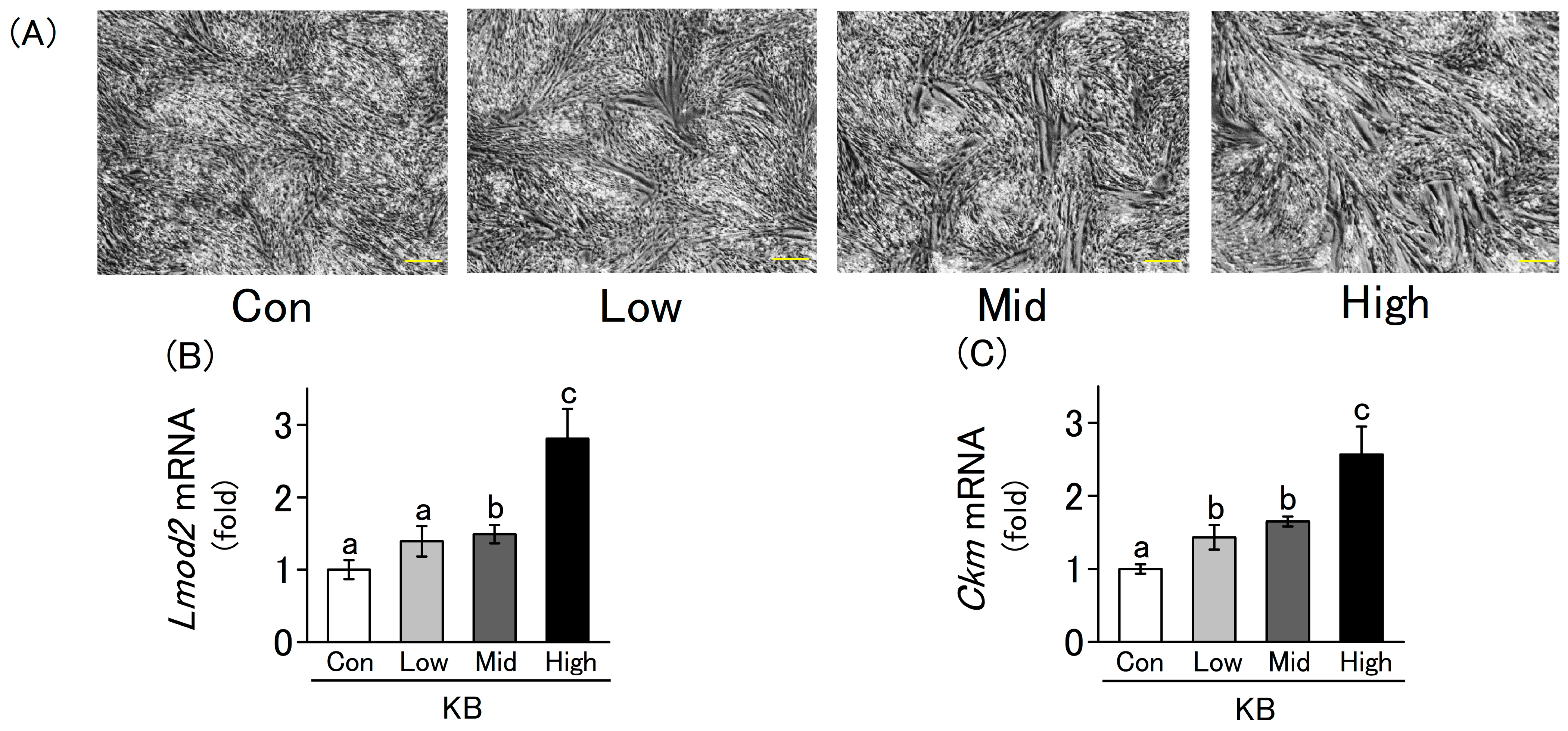
2.2. L6 Cells Differentiation Is Enhanced by Ketone Bodies in a Concentration-Dependent Manner
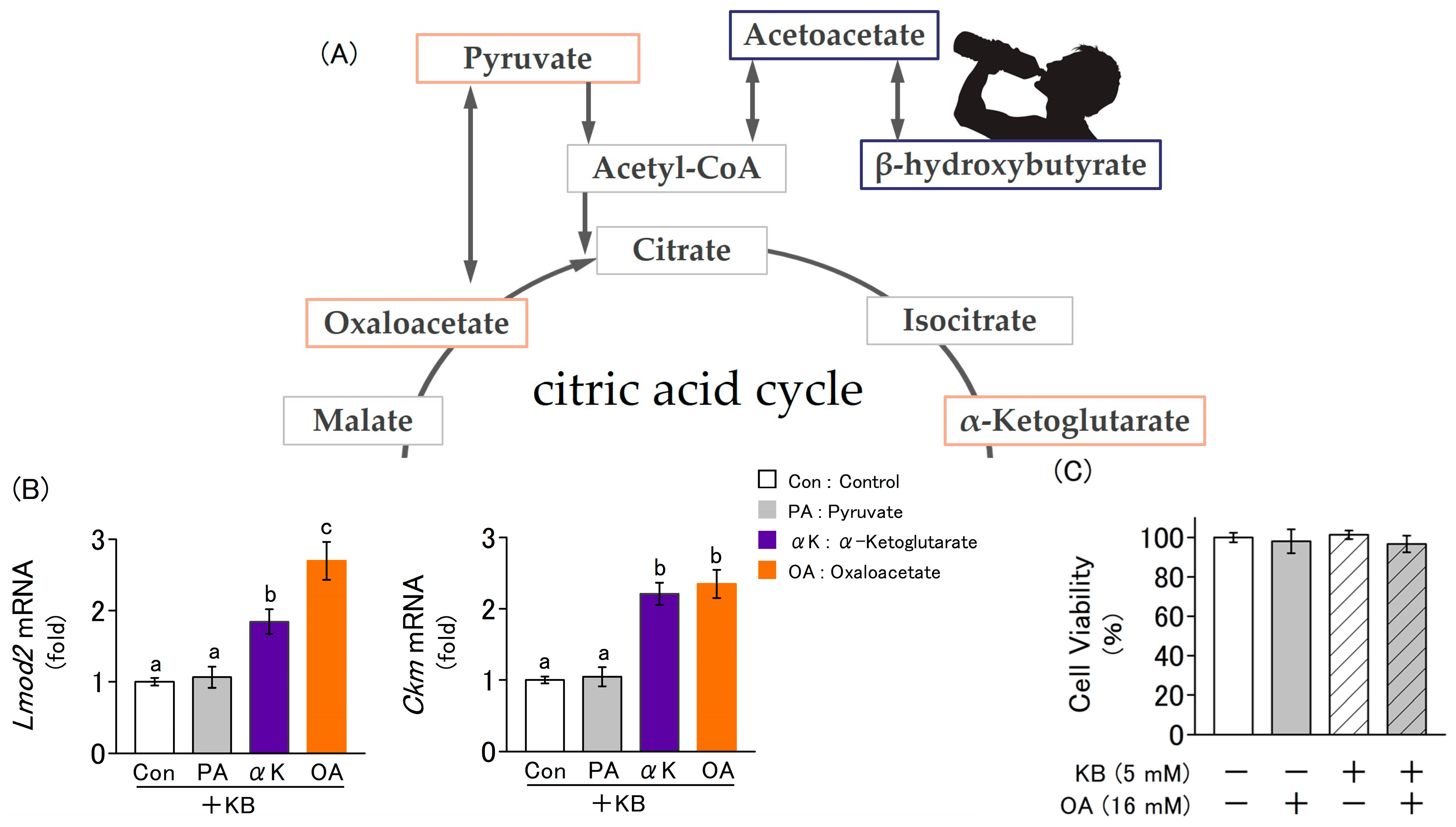
2.3. Fluctuations in the Expression of Myogenic Marker Genes Due to the Addition of Ketone Bodies and Metabolites Related to the Citric Acid Cycle
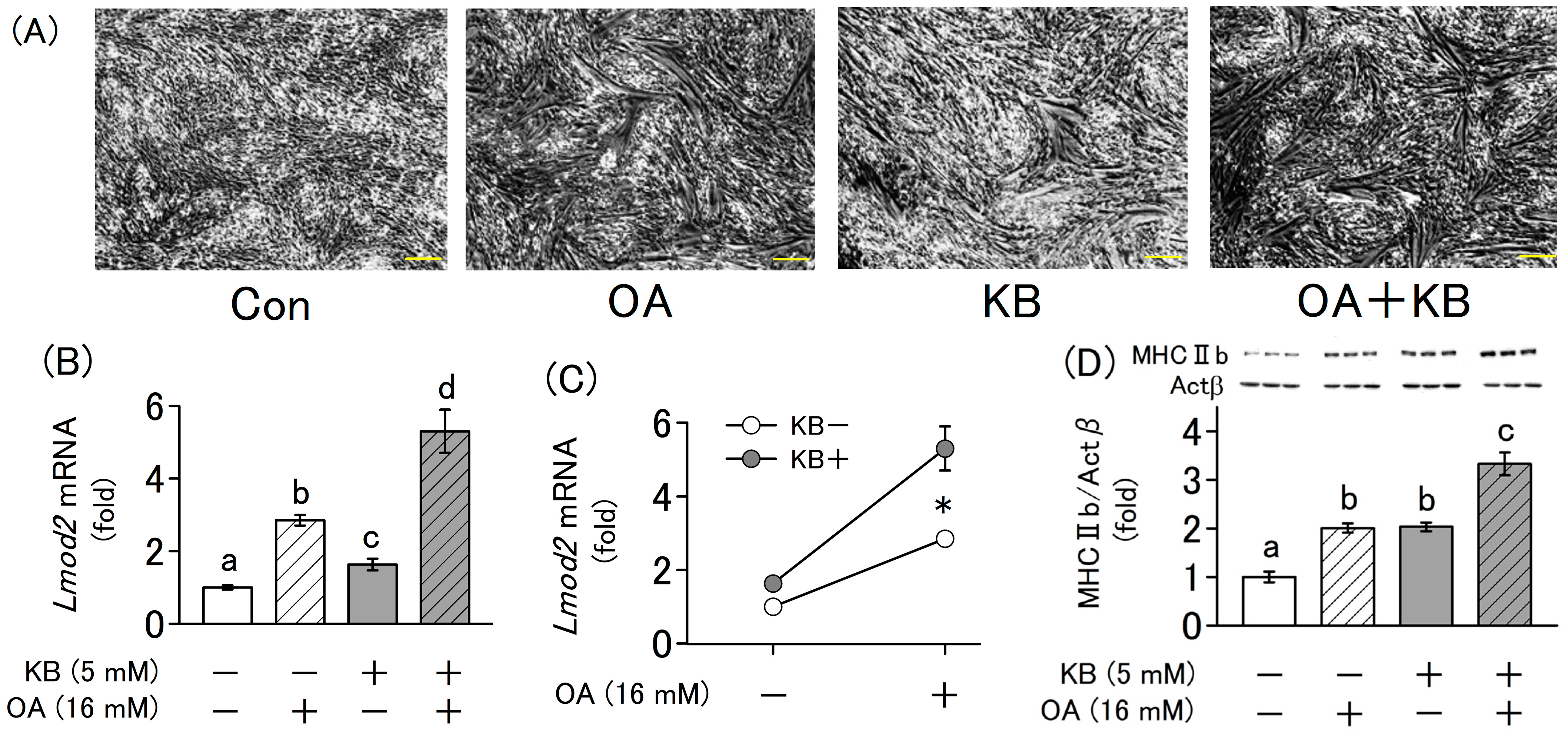
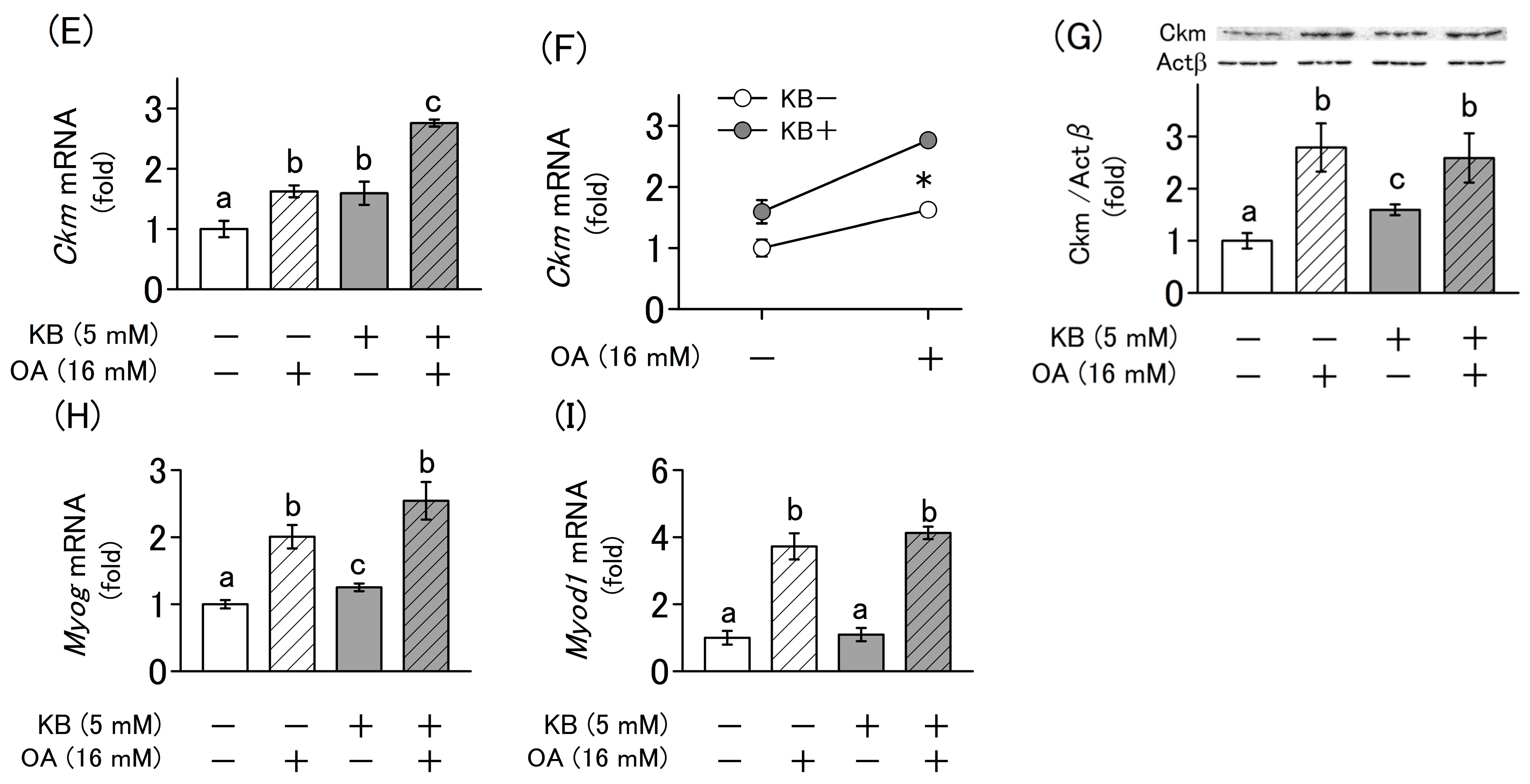
2.4. Synergistic Effect of Ketone Bodies and Oxaloacetate on the Differentiation of L6 Cells
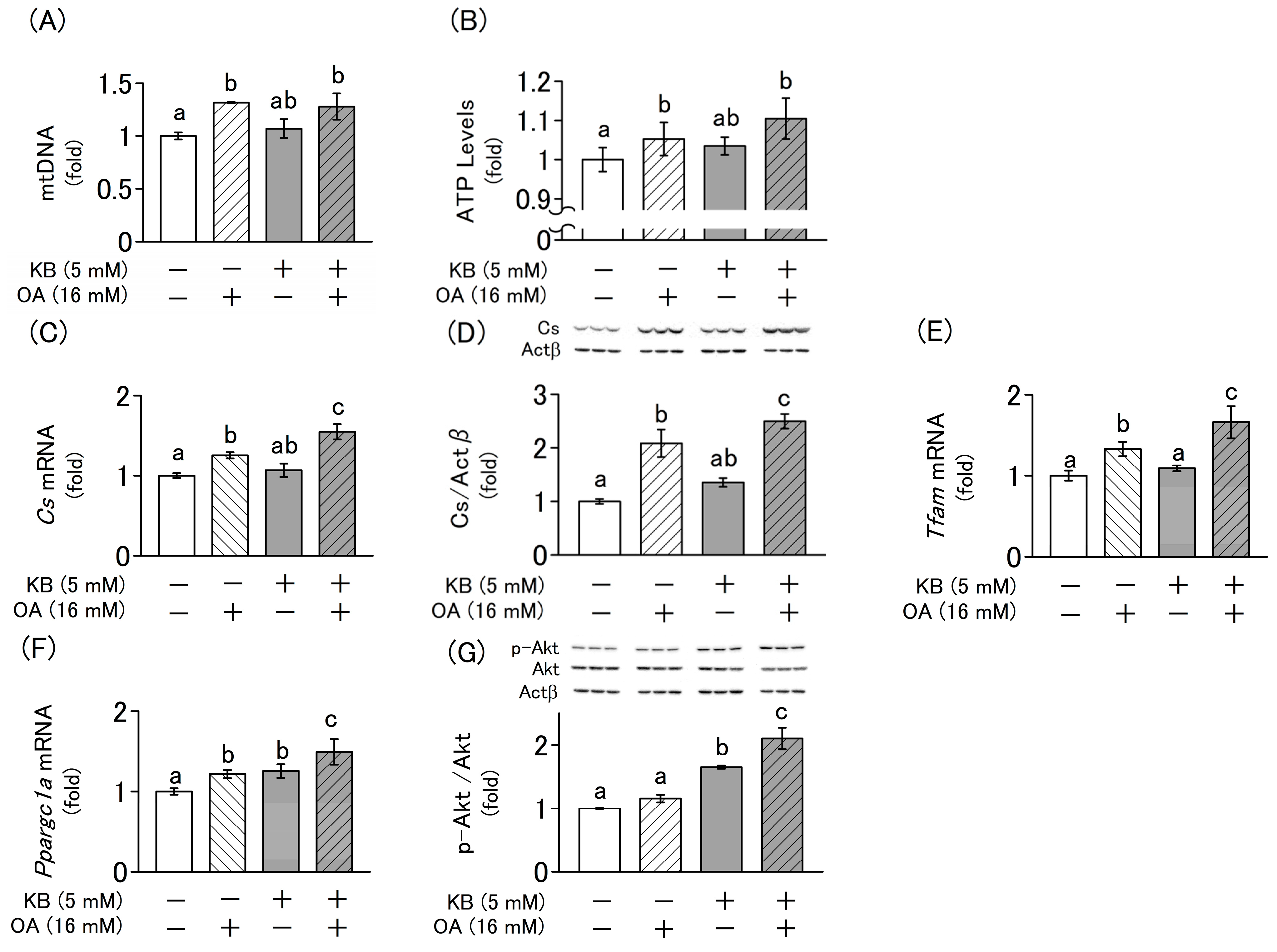
2.5. Effects of Ketone Bodie and Oxaloacetate on the Regulation of Mitochondrial Biogenesis and Cellular Homeostasis in L6 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability
4.3. Real-Time Quantitative PCR
4.4. Mitochondrial DNA Copy Number
4.5. Western Blot Analysis
4.6. ATP Content
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grefte, S.; Kuijpers-Jagtman, A.M.; Torensma, R.; Von den Hoff, J.W. Skeletal muscle development and regeneration. Stem Cells Dev. 2007, 16, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Shavlakadze, T.; McGeachie, J.; Grounds, M.D. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 2010, 11, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Dulbecco, R.; Freeman, G. Plaque production by the polyoma virus. Virology 1959, 8, 396–397. [Google Scholar] [CrossRef]
- Ackermann, T.; Tardito, S. Cell Culture Medium Formulation and Its Implications in Cancer Metabolism. Trends Cancer 2019, 5, 329–332. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Poff, A.M.; Koutnik, A.P.; Egan, B. Nutritional Ketosis with Ketogenic Diets or Exogenous Ketones: Features, Convergence, and Divergence. Curr. Sports Med. Rep. 2020, 19, 251–259. [Google Scholar] [CrossRef]
- Owen, O.E.; Felig, P.; Morgan, A.P.; Wahren, J.; Cahill, G.F., Jr. Liver and kidney metabolism during prolonged starvation. J. Clin. Investig. 1969, 48, 574–583. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Höhn, S.; Dozières-Puyravel, B.; Auvin, S. History of dietary treatment from Wilder’s hypothesis to the first open studies in the 1920s. Epilepsy Behav. 2019, 101, 106588. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898–1920. [Google Scholar] [CrossRef]
- Leckey, J.J.; Ross, M.L.; Quod, M.; Hawley, J.A.; Burke, L.M. Ketone Diester Ingestion Impairs Time-Trial Performance in Professional Cyclists. Front. Physiol. 2017, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Horii, N.; Miyamoto-Mikami, E.; Fujie, S.; Uchida, M.; Inoue, K.; Iemitsu, K.; Tabata, I.; Nakamura, S.; Tsubota, J.; Tsubota, K.; et al. Effect of Exogenous Acute β-Hydroxybutyrate Administration on Different Modalities of Exercise Performance in Healthy Rats. Med. Sci. Sports Exerc. 2023, 55, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Prins, P.J.; Koutnik, A.P.; D’Agostino, D.P.; Rogers, C.Q.; Seibert, J.F.; Breckenridge, J.A.; Jackson, D.S.; Ryan, E.J.; Buxton, J.D.; Ault, D.L. Effects of an Exogenous Ketone Supplement on Five-Kilometer Running Performance. J. Hum. Kinet. 2020, 72, 115–127. [Google Scholar] [CrossRef]
- O’Malley, T.; Myette-Cote, E.; Durrer, C.; Little, J.P. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl. Physiol. Nutr. Metab. 2017, 42, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzym. Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Metabolic and Signaling Roles of Ketone Bodies in Health and Disease. Annu. Rev. Nutr. 2021, 41, 49–77. [Google Scholar] [CrossRef]
- Gough, S.M.; Casella, A.; Ortega, K.J.; Hackam, A.S. Neuroprotection by the Ketogenic Diet: Evidence and Controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef]
- Shefer, G.; Yablonka-Reuveni, Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 2005, 290, 281–304. [Google Scholar]
- Pasut, A.; Jones, A.E.; Rudnicki, M.A. Isolation and culture of individual myofibers and their satellite cells from adult skeletal muscle. J. Vis. Exp. 2013, 73, e50074. [Google Scholar]
- Furuichi, Y.; Kawabata, Y.; Aoki, M.; Mita, Y.; Fujii, N.L.; Manabe, Y. Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Front. Cell Dev. Biol. 2021, 9, 640399. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Bruss, M.D.; Arias, E.B.; Lienhard, G.E.; Cartee, G.D. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 2005, 54, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ohanna, M.; Sobering, A.K.; Lapointe, T.; Lorenzo, L.; Praud, C.; Petroulakis, E.; Sonenberg, N.; Kelly, P.A.; Sotiropoulos, A.; Pende, M. Atrophy of S6K1 (-/-) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat. Cell Biol. 2005, 7, 286–294. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Lipsky, M.M.; Trump, B.F.; Hsu, I.C. Neutral red (NR) assay for cell viability and xenobiotic-induced cytotoxicity in primary cultures of human and rat hepatocytes. Cell Biol. Toxicol. 1990, 6, 219–234. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuki, Y.; Nanashima, N.; Sasaki, Y.; Kojima-Yuasa, A.; Norikura, T. Oxaloacetate and Ketone Bodies Synergistically Promote Myoblast Differentiation in L6 Cells. Molecules 2025, 30, 2101. https://doi.org/10.3390/molecules30102101
Onuki Y, Nanashima N, Sasaki Y, Kojima-Yuasa A, Norikura T. Oxaloacetate and Ketone Bodies Synergistically Promote Myoblast Differentiation in L6 Cells. Molecules. 2025; 30(10):2101. https://doi.org/10.3390/molecules30102101
Chicago/Turabian StyleOnuki, Yuji, Naoki Nanashima, Yutaro Sasaki, Akiko Kojima-Yuasa, and Toshio Norikura. 2025. "Oxaloacetate and Ketone Bodies Synergistically Promote Myoblast Differentiation in L6 Cells" Molecules 30, no. 10: 2101. https://doi.org/10.3390/molecules30102101
APA StyleOnuki, Y., Nanashima, N., Sasaki, Y., Kojima-Yuasa, A., & Norikura, T. (2025). Oxaloacetate and Ketone Bodies Synergistically Promote Myoblast Differentiation in L6 Cells. Molecules, 30(10), 2101. https://doi.org/10.3390/molecules30102101







