Comparison of Cytotoxicity and Antioxidant, Antibacterial, and Anti-Inflammatory Activity of Aqueous and Ethanolic Extracts from Malus domestica, Prunus armeniaca, and Prunus cerasus Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Analysis
2.2. Assessment of Antioxidant Activity
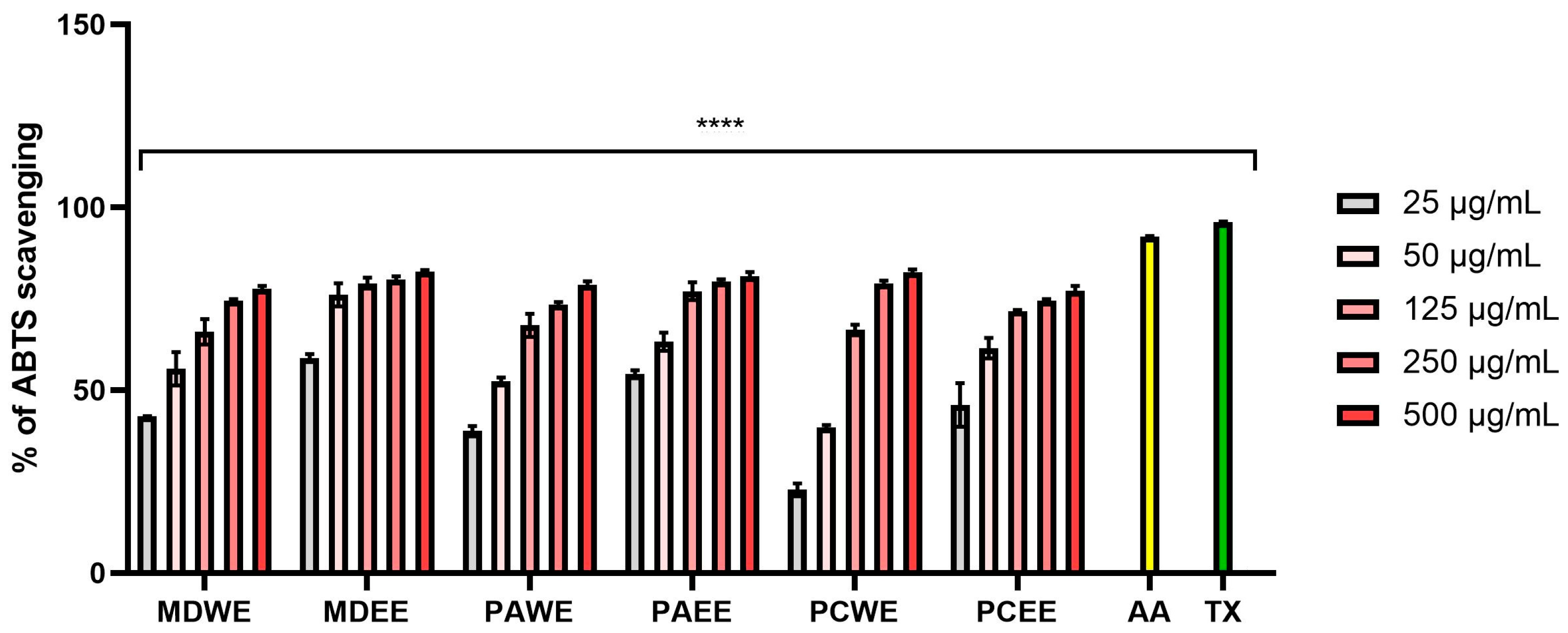
2.2.1. ABTS, DPPH, and FRAP Radical Scavenging
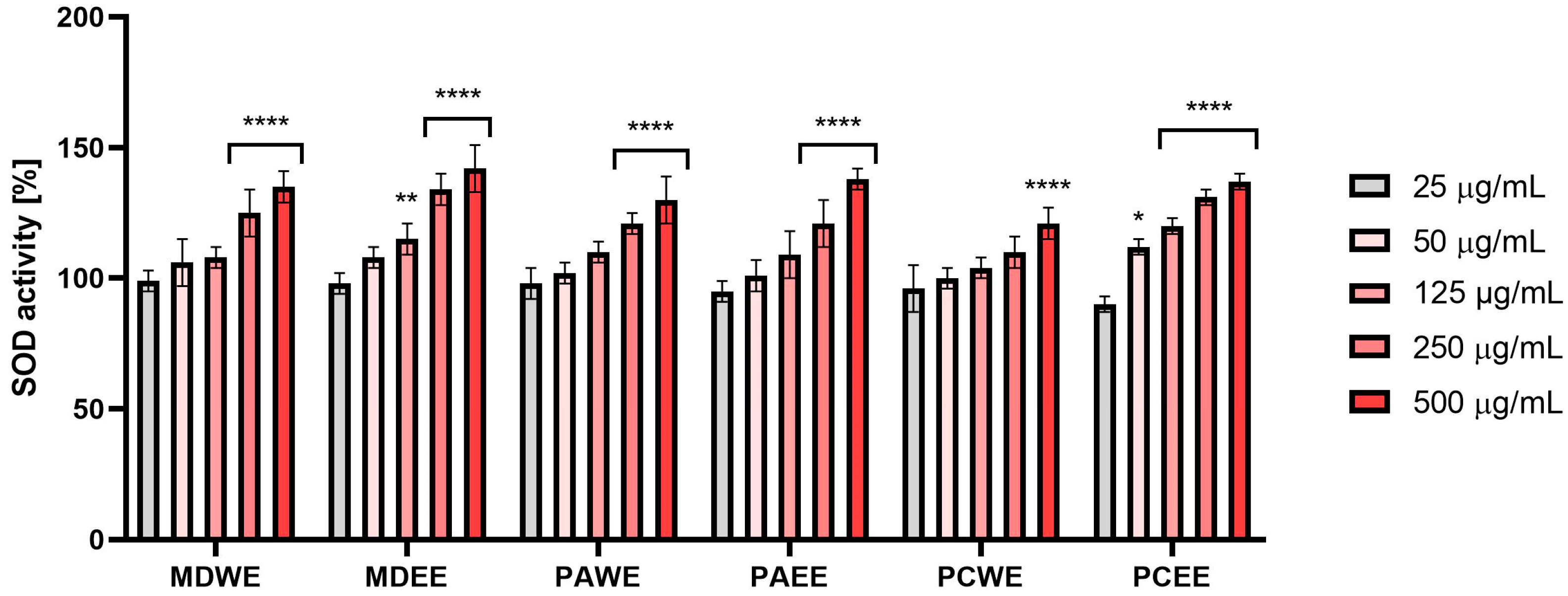
2.2.2. Evaluation of the Effect on Superoxide Dismutase (SOD) Activity
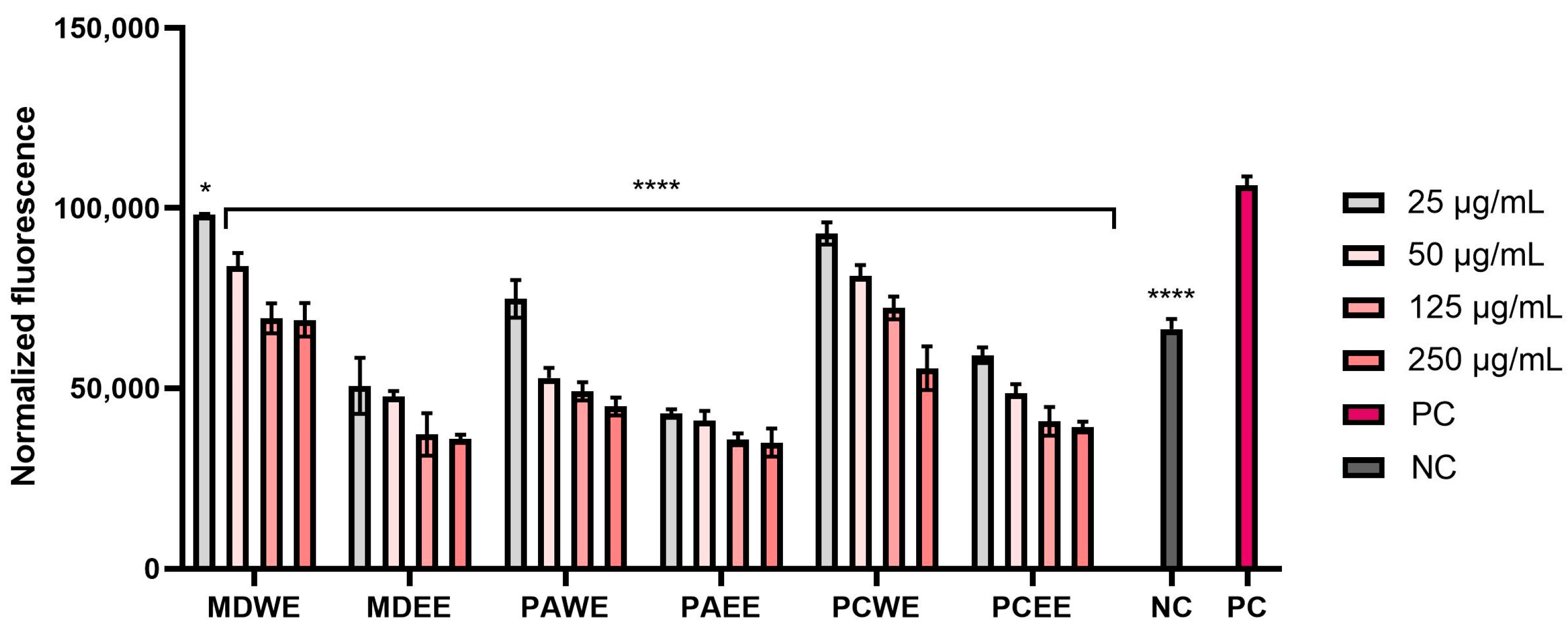
2.2.3. Intracellular ROS Levels in Skin Cells
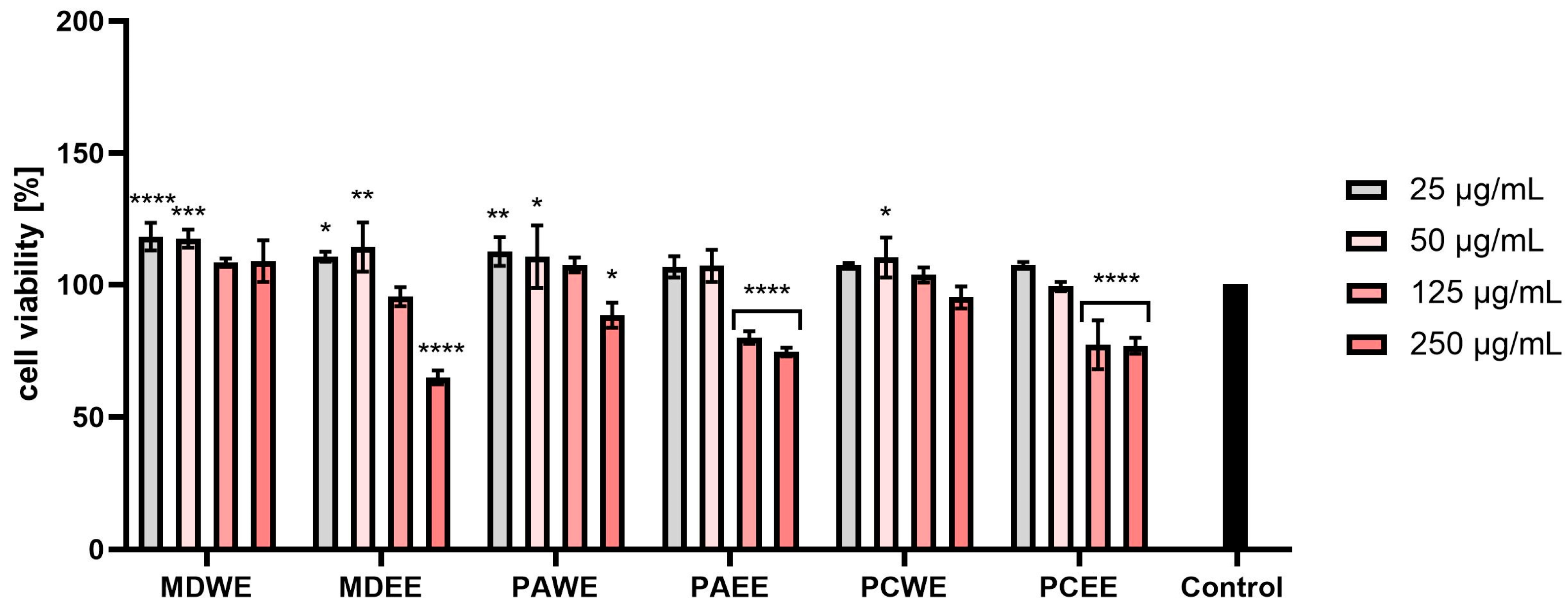
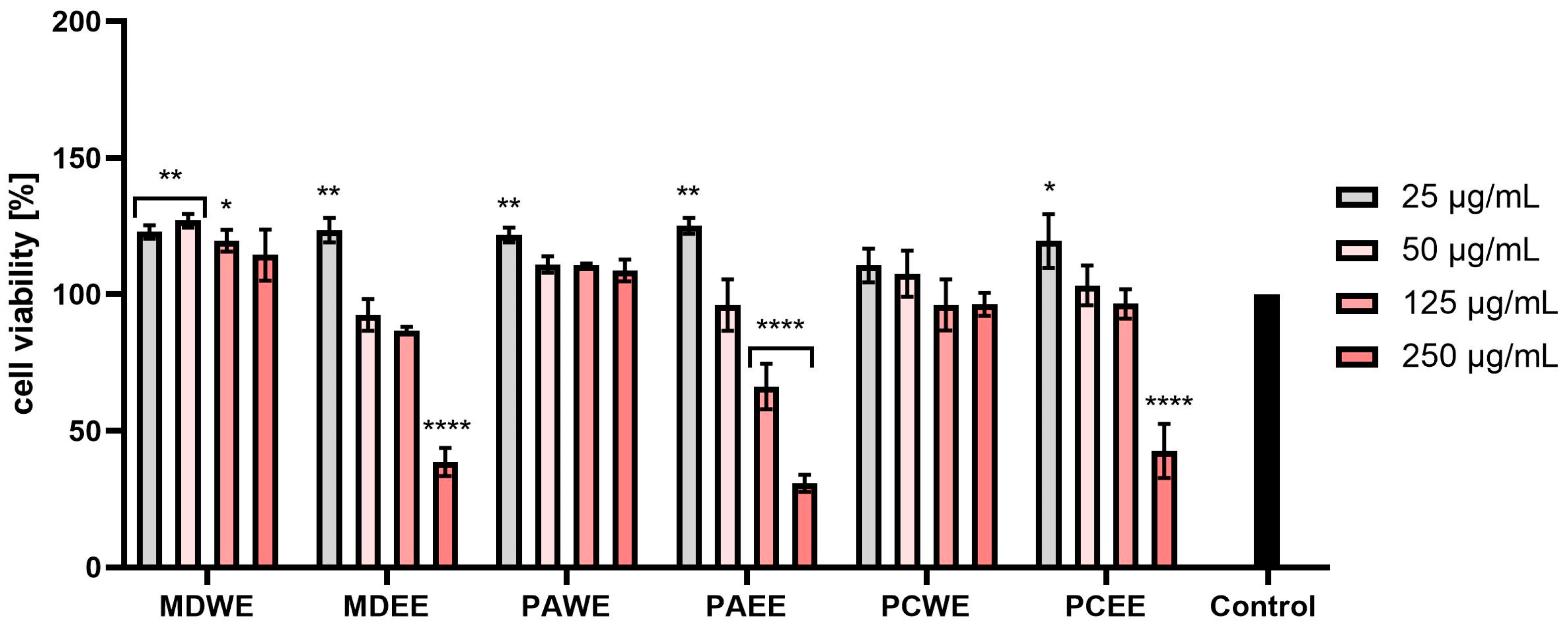
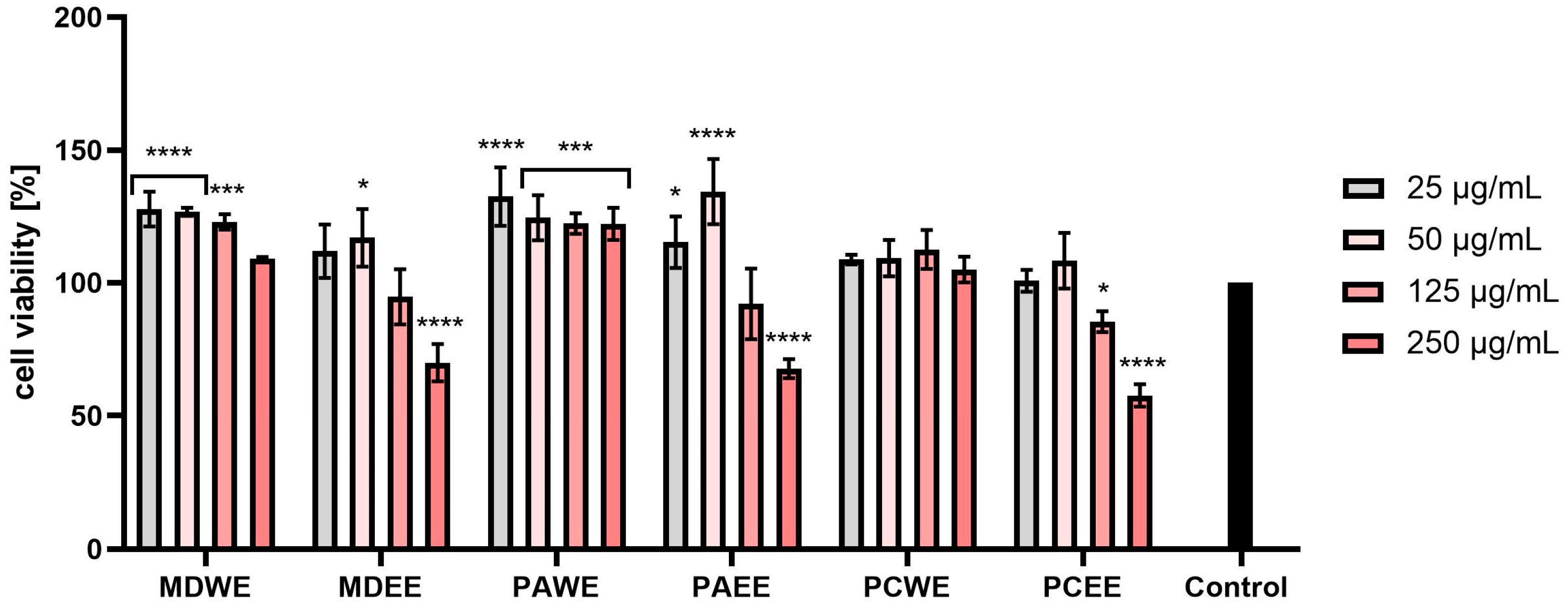
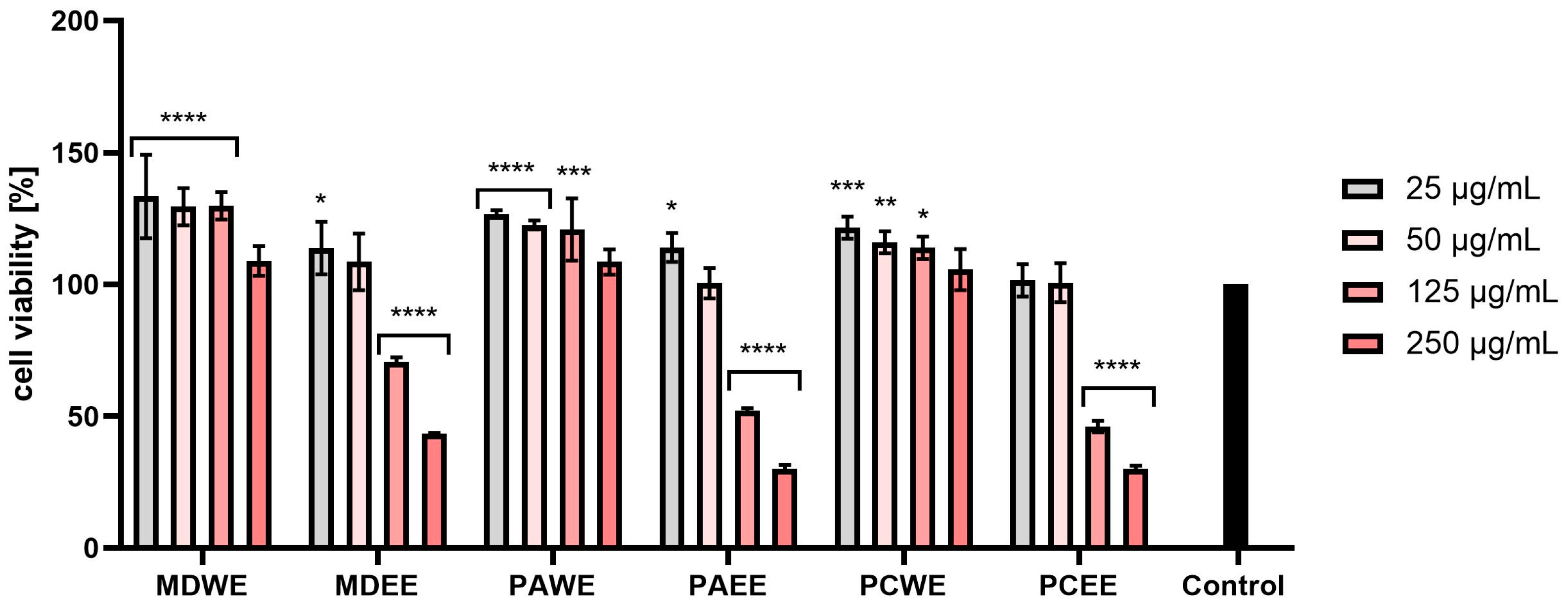
2.3. Cytotoxicity Assessment
2.4. Assessment of Antibacterial Activity
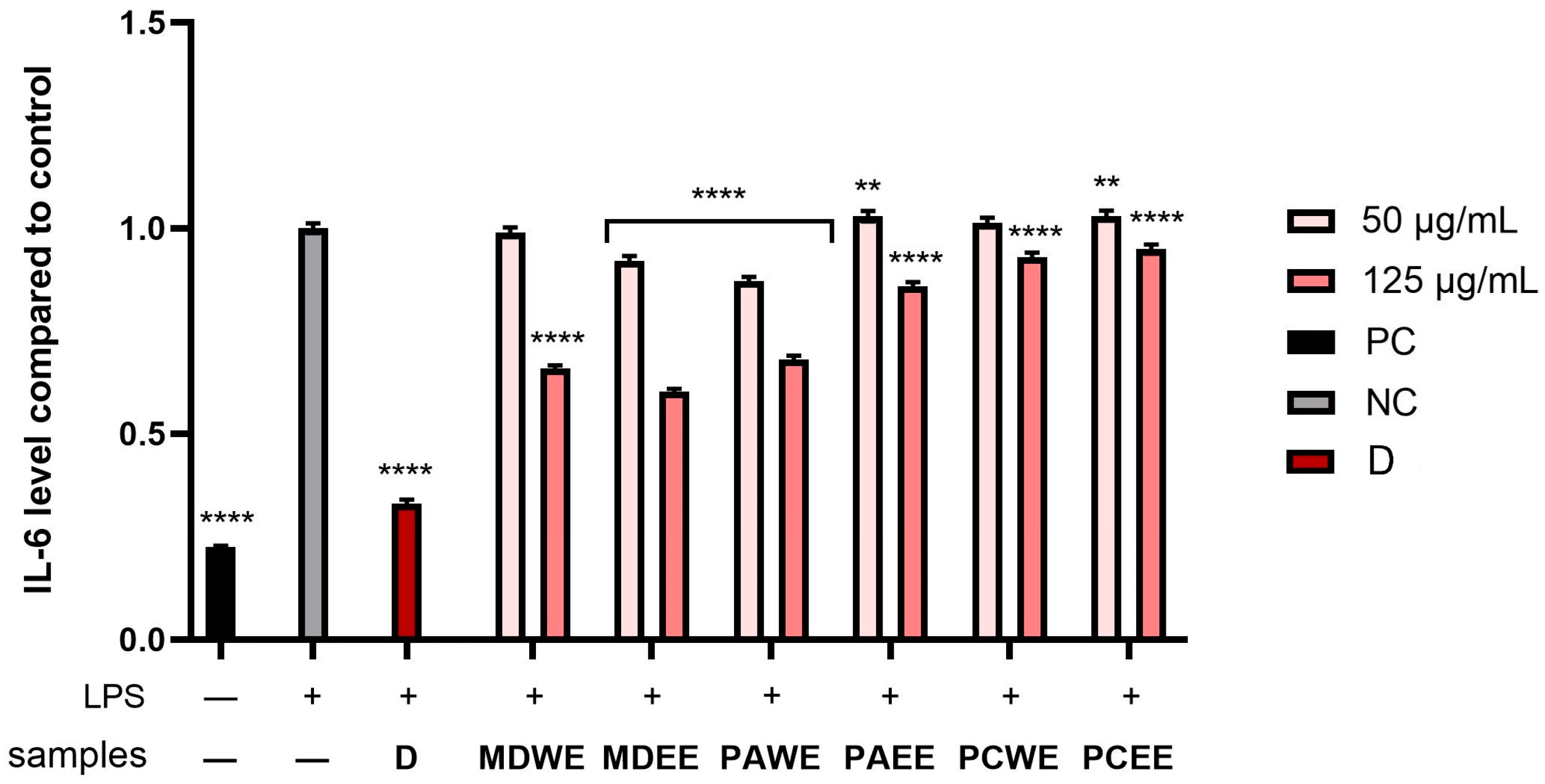
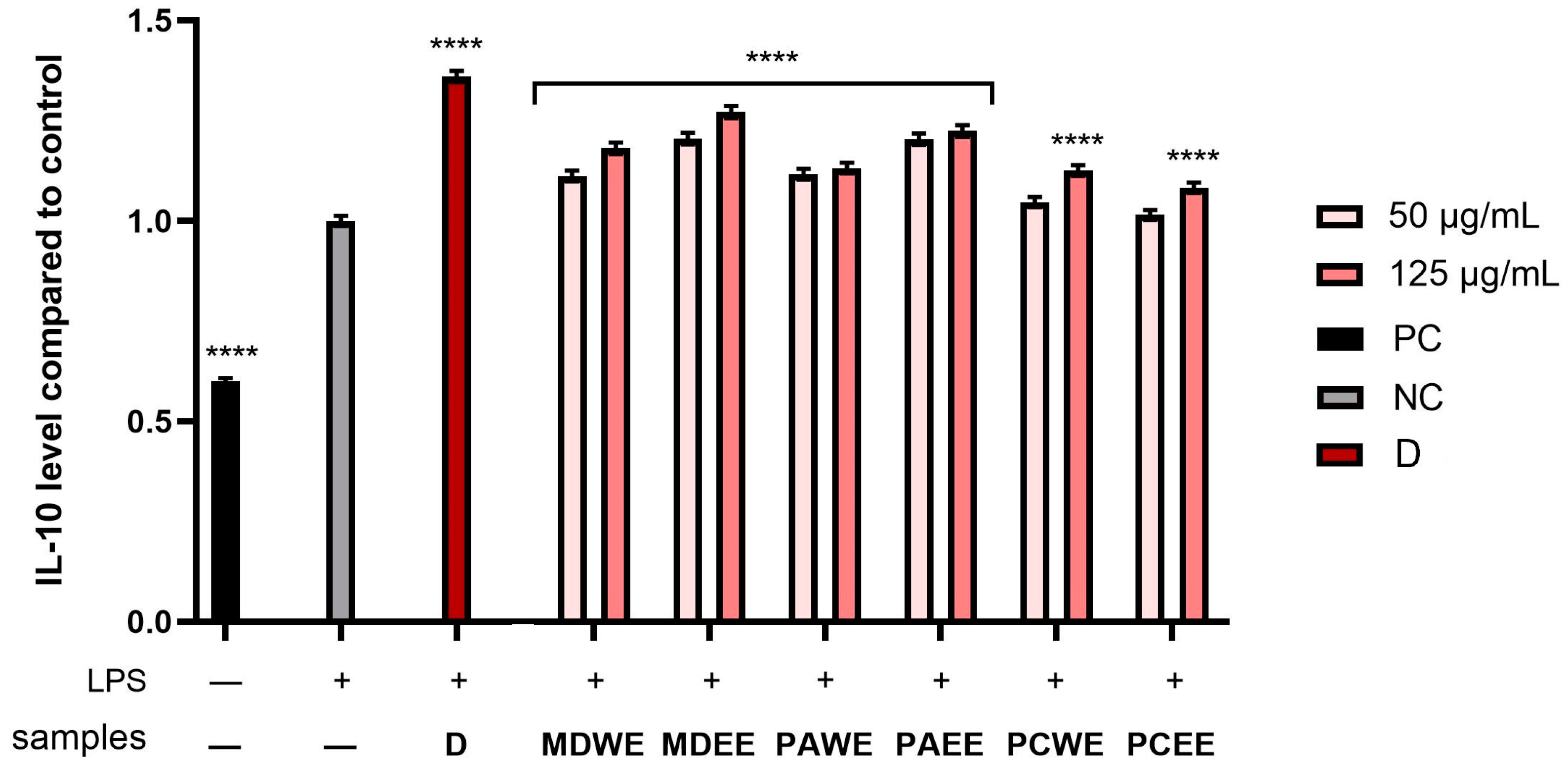
2.5. Assessment of Anti-Inflammatory Activity
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials and Extraction Procedure
3.3. Determination of Biologically Active Compounds
3.4. Determination of Antioxidant Properties
3.4.1. ABTS Scavenging Assay
3.4.2. DPPH (1,1-Diphenyl-2-Picrylhydrazyl) Radical Scavenging Assay
3.4.3. Determination of Ferric Reducing Antioxidant Power (FRAP Assay)
3.4.4. Determination of Superoxide Dismutase (SOD) Activity
3.4.5. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
3.5. Cytotoxicity Analysis
3.5.1. Cell Culture
3.5.2. Alamar Blue (AB) and Neutral Red (NR) Assays
3.6. Assessment of Antibacterial Activity
3.6.1. Disk Diffusion Assay
3.6.2. Determination of Minimum Inhibitory Concentrations (MIC)
3.7. Assessment of Anti-Inflammatory Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit Wastes as a Valuable Source of Value-Added Compounds: A Collaborative Perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Silva, S.; Costa, E.M. Byproducts as a Sustainable Source of Cosmetic Ingredients. Appl. Sci. 2024, 14, 10241. [Google Scholar] [CrossRef]
- Cendrowski, A.; Jakubowska, Z.; Przybył, J.L. Apple Tree Leaves (Malus domestica Borkh) as a Valuable Source of Polyphenolic Compounds with a High Antioxidant Capacity. Appl. Sci. 2024, 14, 3252. [Google Scholar] [CrossRef]
- Zymonė, K.; Liaudanskas, M.; Lanauskas, J.; Nagelytė, M.; Janulis, V. Variability in the Qualitative and Quantitative Composition of Phenolic Compounds and the In Vitro Antioxidant Activity of Sour Cherry (Prunus cerasus L.) Leaves. Antioxidants 2024, 13, 553. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Profile of Phenolic Compounds of Prunus armeniaca L. Leaf Extract Determined by LC-ESI-QTOF-MS/MS and Their Antioxidant, Anti-Diabetic, Anti-Cholinesterase, and Anti-Inflammatory Potency. Antioxidants 2021, 10, 1869. [Google Scholar] [CrossRef]
- The Fruit and Vegetable Sector in the EU—A Statistical Overview—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=The_fruit_and_vegetable_sector_in_the_EU_-_a_statistical_overview (accessed on 17 April 2025).
- Jelodarian, S.; Ebrahimabadi, A.H.; Kashi, F.J. Evaluation of Antimicrobial Activity of Malus domestica Fruit Extract from Kashan Area. Avicenna J. Phytomed. 2013, 3, 1. [Google Scholar] [PubMed]
- Adamcová, A.; Horna, A.; Šatínský, D. Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity. Pharmaceuticals 2022, 15, 244. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Uğur, Y.; Erdoğan, S.; Yılmaz, I.; Başgel, S. Variation of Composition of Phenolic Compounds in the Apricot (Prunus armeniaca L.) Leaves by Seasons. J. Nat. Prod. Plant Resour. 2018, 8, 32–38. [Google Scholar]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic Extracts of Cherry (Prunus cerasus L.) and Blackcurrant (Ribes nigrum L.) Leaves as Natural Preservatives in Meat Products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Chrzanowski, G.; Sempruch, C.P.; Sprawka, I.G. Investigation of Phenolic Acids in Leaves of Blackcurrant (Ribes nigrum L.) and Sour Cherry (Prunus cerasus L.). Electron. J. Pol. Agric. Univ. 2007, 10, 42. [Google Scholar]
- Hrichi, S.; Rigano, F.; Chaabane-Banaoues, R.; El Majdoub, Y.O.; Mangraviti, D.; Di Marco, D.; Babba, H.; Dugo, P.; Mondello, L.; Mighri, Z.; et al. Identification of Fatty Acid, Lipid and Polyphenol Compounds from Prunus armeniaca L. Kernel Extracts. Foods 2020, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Hammam, W.E.; El-Mahdy El-Tantawi, M.; Yassin, N.A.Z.; Kirollos, F.N.; Abdelhameed, M.F.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. Apple Leaves and Their Major Secondary Metabolite Phlorizin Exhibit Distinct Neuroprotective Activities: Evidence from in Vivo and in Silico Studies. Arab. J. Chem. 2021, 14, 103188. [Google Scholar] [CrossRef]
- Picariello, G.; De Vito, V.; Ferranti, P.; Paolucci, M.; Volpe, M.G. Species- and Cultivar-Dependent Traits of Prunus avium and Prunus cerasus Polyphenols. J. Food Compos. Anal. 2016, 45, 50–57. [Google Scholar] [CrossRef]
- e Silva, S.A.M.; Leonardi, G.R.; Michniak-Kohn, B. An Overview about Oxidation in Clinical Practice of Skin Aging. Bras. Dermatol. 2017, 92, 367. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An Insight on Superoxide Dismutase (SOD) from Plants for Mammalian Health Enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Mihailović, M.; Dinić, S.; Jovanović, J.A.; Uskoković, A.; Grdović, N.; Vidaković, M. The Influence of Plant Extracts and Phytoconstituents on Antioxidant Enzymes Activity and Gene Expression in the Prevention and Treatment of Impaired Glucose Homeostasis and Diabetes Complications. Antioxidants 2021, 10, 480. [Google Scholar] [CrossRef]
- Ermis, A.; Aritici Colak, G.; Acikel-Elmas, M.; Arbak, S.; Kolgazi, M. Ferulic Acid Treats Gastric Ulcer via Suppressing Oxidative Stress and Inflammation. Life 2023, 13, 388. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Punia, A.; Singh, V.; Thakur, A.; Chauhan, N.S. Impact of Caffeic Acid on Growth, Development and Biochemical Physiology of Insect Pest, Spodoptera Litura (Fabricius). Heliyon 2023, 9, e14593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Muema, F.W.; Zhang, Y.L.; Guo, M.Q. Acyl Quinic Acid Derivatives Screened Out from Carissa Spinarum by SOD-Affinity Ultrafiltration LC–MS and Their Antioxidative and Hepatoprotective Activities. Antioxidants 2021, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the Skin: Understanding Formulation and Efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A Review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B.; Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the Antioxidant Potential of Flavonoids as a Subgroup of Polyphenols and Partial Substitute for Synthetic Antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Hussen, N.H.a.; Abdulla, S.K.; Ali, N.M.; Ahmed, V.A.; Hasan, A.H.; Qadir, E.E. Role of Antioxidants in Skin Aging and the Molecular Mechanism of ROS: A Comprehensive Review. Asp. Mol. Med. 2025, 5, 100063. [Google Scholar] [CrossRef]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Krausgruber, T.; Fortelny, N.; Fife-Gernedl, V.; Senekowitsch, M.; Schuster, L.C.; Lercher, A.; Nemc, A.; Schmidl, C.; Rendeiro, A.F.; Bergthaler, A.; et al. Structural Cells Are Key Regulators of Organ-Specific Immune Responses. Nature 2020, 583, 296–302. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The Role of Oxygen in Wound Healing: A Review of the Literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Sowa, A.; Zgórka, G.; Szykuba, A.; Franiczek, R.; Gbikowska, B.; Gamian, A.; Sroka, Z. Analysis of Polyphenolic Compounds in Extracts from Leaves of Some Malus domestica Cultivars: Antiradical and Antimicrobial Analysis of These Extracts. Biomed. Res. Int. 2016, 2016, 6705431. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Sharma, V.; Katoch, P.; Padwad, Y.; Bhushan, S. Phenolic Constituents from Apple Tree Leaves and Their in Vitro Biological Activity. Ind. Crops Prod. 2016, 90, 118–125. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and Oxidative Stress Markers: An Overview of Human Intervention Studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A. Influence of Cherry Leaf-Spot on Changes in the Content of Phenolic Compounds in Sour Cherry (Prunus cerasus L.) Leaves. Physiol. Mol. Plant Pathol. 2014, 86, 28–34. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic Compounds Extracted by Acidic Aqueous Ethanol from Berries and Leaves of Different Berry Plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, D.H.; Sung, J.; Min, I.S.; Lee, S.J. Tart Cherry Extract Containing Chlorogenic Acid, Quercetin, and Kaempferol Inhibits the Mitochondrial Apoptotic Cell Death Elicited by Airborne PM10 in Human Epidermal Keratinocytes. Antioxidants 2021, 10, 443. [Google Scholar] [CrossRef]
- Al-Soufi, M.H.; Alshwyeh, H.A.; Alqahtani, H.; Al-Zuwaid, S.K.; Al-Ahmed, F.O.; Al-Abdulaziz, F.T.; Raed, D.; Hellal, K.; Mohd Nani, N.H.; Zubaidi, S.N.; et al. A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts. Molecules 2022, 27, 5016. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2009; 600p. [Google Scholar] [CrossRef]
- Aly, R. Microbial Infections of Skin and Nails. In Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Allemailem, K.S. Antimicrobial Potential of Naturally Occurring Bioactive Secondary Metabolites. J. Pharm. Bioallied Sci. 2021, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents from Apocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist. Updat. 2020, 51, 100695. [Google Scholar] [CrossRef]
- Irshad, A.; Jawad, R.; Mushtaq, Q.; Spalletta, A.; Martin, P.; Ishtiaq, U. Determination of Antibacterial and Antioxidant Potential of Organic Crude Extracts from Malus domestica, Cinnamomum Verum and Trachyspermum Ammi. Sci. Rep. 2025, 15, 976. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and Antimicrobial Properties of Dried Portuguese Apple Variety (Malus domestica Borkh. Cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef]
- Rehab; El-Desoukey, M.; Almuhsin, S.; Almuhsin, A. The Phytochemical and Antimicrobial Effect of Mallus domestica (Apple) Dried Peel Powder Extracts on Some Animal Pathogens as Eco-Friendly. Int. J. Vet. Sci. 2018, 7, 88–92. [Google Scholar]
- Horvacki, N.M.; Milinčić, D.D.; Jović, M.D.; Dramićanin, A.M.; Fotirić-Akšić, M.M.; Pešić, M.B.; Milojković-Opsenica, D.M. Bioassay-Guided Evaluation of Antimicrobial Properties and Profile of Bioactive Compounds from Leaf, Peel and Mesocarp of Four Apple Cultivars (Malus domestica Borkh.) Grown in Serbia: Application of HPTLC-EDA and UHPLC Q-ToF MS Techniques. Food Chem. 2025, 467, 142336. [Google Scholar] [CrossRef]
- Jaya, S.; Hs, L. Antimicrobial activity of fruits of Prunus armeniaca (L.). J. Drug Deliv. Ther. 2012, 2, 163. [Google Scholar] [CrossRef]
- Yiǧit, D.; Yiǧit, N.; Mavi, A. Antioxidant and Antimicrobial Activities of Bitter and Sweet Apricot (Prunus armeniaca L.) Kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, R.; Mahmood, A.; Ahmad, Z.; Bibi, N.; Kazmi, S.U. Flavonoid Glycosides from Prunus Armeniaca and the Antibacterial Activity of a Crude Extract. Arch. Pharm. Res. 2007, 30, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Sabir, S.; Mehr, P.; Shah, A.; Mehmood, A.; Shakil, M.; Sohail, M.; Habib, T.; Awan, M.S.; And Kashmir, J. Antibacterial, Antioxidant Activity and Phyto-Chemical Screening of Prunus armeniaca (L.) Var. (Hari & Khobani) Leaf Extracts. Kepes 2023, 21, 26–32. [Google Scholar] [CrossRef]
- Coccia, A.; Carraturo, A.; Mosca, L.; Masci, A.; Bellini, A.; Campagnaro, M.; Lendaro, E. Effects of Methanolic Extract of Sour Cherry (Prunus cerasus L.) on Microbial Growth. Int. J. Food Sci. Technol. 2012, 47, 1620–1629. [Google Scholar] [CrossRef]
- Berroukche, A.; Benreguieg, M.; Terras, M.; Fares, S.; Dellaoui, H.; Lansari, W.; Zerarki, I.; Tahir, A.; Dehkal, B. Antibacterial Effects of Prunus cerasus and Chamaemelum Nobile against Drug Resistant Strains Induced Urinary Disorders. East. Afr. Sch. J. Med. Sci. 2018, 1, 26–31. [Google Scholar]
- Piccirillo, C.; Demiray, S.; Silva Ferreira, A.C.; Pintado, M.E.; Castro, P.M.L. Chemical Composition and Antibacterial Properties of Stem and Leaf Extracts from Ginja Cherry Plant. Ind. Crops Prod. 2013, 43, 562–569. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Pernin, A.; Guillier, L.; Dubois-Brissonnet, F. Inhibitory Activity of Phenolic Acids against Listeria Monocytogenes: Deciphering the Mechanisms of Action Using Three Different Models. Food Microbiol. 2019, 80, 18–24. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial Activity of Phenolic Acids against Commensal, Probiotic and Pathogenic Bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Bai, J.; Wu, Y.; Bu, Q.; Zhong, K.; Gao, H. Comparative Study on Antibacterial Mechanism of Shikimic Acid and Quinic Acid against Staphylococcus Aureus through Transcriptomic and Metabolomic Approaches. LWT 2022, 153, 112441. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Alkufeidy, R.M.; Ameer Altuwijri, L.; Aldosari, N.S.; Alsakabi, N.; Dawoud, T.M. Antimicrobial and Synergistic Properties of Green Tea Catechins against Microbial Pathogens. J. King Saud. Univ. Sci. 2024, 36, 103277. [Google Scholar] [CrossRef]
- Mita, S.R.; Husni, P.; Putriana, N.A.; Maharani, R.; Hendrawan, R.P.; Dewi, D.A. A Recent Update on the Potential Use of Catechins in Cosmeceuticals. Cosmetics 2024, 11, 23. [Google Scholar] [CrossRef]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-Tuning of the Hydrophobicity of Caffeic Acid: Studies on the Antimicrobial Activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus Aureus Clinical Strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Nilforoushzadeh, M.A.; Ahmadi Ashtiani, H.R.; Hashemian, F. Effect of Apple (Malus domestica) Stem Cells on UVB-Induced Damage Skin with Anti-Inflammatory Properties: An In Vivo Study. Adv. Mater. Sci. Eng. 2022, 2022, 2417766. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- Mansoori, S.; Dini, A.; Chai, S.C. Effects of Tart Cherry and Its Metabolites on Aging and Inflammatory Conditions: Efficacy and Possible Mechanisms. Ageing Res. Rev. 2021, 66, 101254. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Göttingerová, M.; Kumšta, M.; Rampáčková, E.; Kiss, T.; Nečas, T. Analysis of Phenolic Compounds and Some Important Analytical Properties in Selected Apricot Genotypes. HortScience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- Khajuria, V.; Gupta, S.; Sharma, N.; Tiwari, H.; Bhardwaj, S.; Dutt, P.; Satti, N.; Nargotra, A.; Bhagat, A.; Ahmed, Z. Kaempferol-3-o-β-d-Glucuronate Exhibit Potential Anti-Inflammatory Effect in LPS Stimulated RAW 264.7 Cells and Mice Model. Int. Immunopharmacol. 2018, 57, 62–71. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Q.; Hou, R.; Sun, H.; Tang, Q.; Wang, H.; Hao, Z.; Kang, S.; Xu, T.; Wu, S. Kaempferol-3-O-Glucorhamnoside Inhibits Inflammatory Responses via MAPK and NF-ΚB Pathways In Vitro and In Vivo. Toxicol. Appl. Pharmacol. 2019, 364, 22–28. [Google Scholar] [CrossRef]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-β-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-ΚB and AP-1 Pathways. Int. J. Mol. Sci. 2021, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Kum, H.; Roh, K.B.; Shin, S.; Jung, K.; Park, D.; Jung, E. Evaluation of Anti-Acne Properties of Phloretin in Vitro and in Vivo. Int. J. Cosmet. Sci. 2016, 38, 85–92. [Google Scholar] [CrossRef]
- Wu, C.S.; Lin, S.C.; Li, S.; Chiang, Y.C.; Bracci, N.; Lehman, C.W.; Tang, K.T.; Lin, C.C. Phloretin Alleviates Dinitrochlorobenzene-Induced Dermatitis in BALB/c Mice. Int. J. Immunopathol. Pharmacol. 2020, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Zagórska-Dziok, M.; Mokrzyńska, A.; Wójciak, M.; Sowa, I. Apiaceae Bioferments Obtained by Fermentation with Kombucha as an Important Source of Active Substances for Skin Care. Molecules 2025, 30, 983. [Google Scholar] [CrossRef]
- Wójciak, M.; Paduch, R.; Drozdowski, P.; Żuk, M.; Wójciak, W.; Tyszczuk-Rotko, K.; Feldo, M.; Sowa, I. Ultra-Performance Liquid Chromatography and Mass Spectrometry Characterization, and Antioxidant, Protective, and Anti-Inflammatory Activity, of the Polyphenolic Fraction from Ocimum Basilicum. Molecules 2024, 29, 5043. [Google Scholar] [CrossRef]
- Wójciak, M.; Paduch, R.; Drozdowski, P.; Wójciak, W.; Żuk, M.; Płachno, B.J.; Sowa, I. Antioxidant and Anti-Inflammatory Effects of Nettle Polyphenolic Extract: Impact on Human Colon Cells and Cytotoxicity Against Colorectal Adenocarcinoma. Molecules 2024, 29, 5000. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors Influencing the Antioxidant Activity Determined by the ABTS.+ Radical Cation Assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Grauzdytė, D.; Pukalskas, A.; Viranaicken, W.; El Kalamouni, C.; Venskutonis, P.R. Protective Effects of Phyllanthus Phillyreifolius Extracts against Hydrogen Peroxide Induced Oxidative Stress in HEK293 Cells. PLoS ONE 2018, 13, e0207672. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Zagórska-Dziok, M.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Sowa, I.; Wójciak, M. Comparison of Anti-Inflammatory and Antibacterial Properties of Raphanus Sativus L. Leaf and Root Kombucha-Fermented Extracts. Int. J. Mol. Sci. 2024, 25, 5622. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]




| Bacteria | Plant Species | Zone of Inhibition [mm] | |||||
|---|---|---|---|---|---|---|---|
| Water Extract | Water–Ethanol Extract | ||||||
| 50 µg/mL | 250 µg/mL | 500 µg/mL | 50 µg/mL | 250 µg/mL | 500 µg/mL | ||
| Staphylococcus aureus | M. domestica | 5 | 7 | 9 | 7 | 10 | 13 |
| P. armeniaca | 4 | 7 | 10 | 6 | 9 | 15 | |
| P. cerasus | 7 | 12 | 9 | 4 | 7 | 10 | |
| Staphylococcus epidermidis | M. domestica | 4 | 7 | 11 | 6 | 9 | 14 |
| P. armeniaca | 3 | 6 | 8 | 8 | 11 | 18 | |
| P. cerasus | nd | nd | nd | nd | 5 | 8 | |
| Bacillus subtilis | M. domestica | 5 | 7 | 10 | 5 | 8 | 11 |
| P. armeniaca | nd | 5 | 7 | 6 | 9 | 10 | |
| P. cerasus | nd | nd | nd | 4 | 10 | 10 | |
| Staphylococcus capitis | M. domestica | nd | 12 | 9 | 6 | 16 | 12 |
| P. armeniaca | nd | 5 | 7 | 4 | 8 | 10 | |
| P. cerasus | 4 | 9 | 7 | 6 | 13 | 10 | |
| Micrococcus luteus | M. domestica | nd | nd | nd | nd | nd | nd |
| P. armeniaca | nd | nd | 5 | nd | 8 | 10 | |
| P. cerasus | nd | nd | nd | nd | nd | nd | |
| Yersinia enterocolitica | M. domestica | nd | nd | nd | nd | nd | nd |
| P. armeniaca | nd | 4 | 7 | 6 | 8 | 9 | |
| P. cerasus | nd | nd | nd | nd | nd | nd | |
| Pseudomonas aeruginosa | M. domestica | nd | 5 | 7 | 6 | 8 | 11 |
| P. armeniaca | nd | 4 | 7 | 5 | 8 | 10 | |
| P. cerasus | nd | 5 | 7 | 5 | 8 | 11 | |
| Bacteria | Plant Species | Minimum Inhibitory Concentration MIC [µg/mL] | |
|---|---|---|---|
| Water Extract | Water–Ethanol Extract | ||
| Staphylococcus aureus | M. domestica | 350 | 250 |
| P. armeniaca | 350 | 250 | |
| P. cerasus | 300 | 350 | |
| Staphylococcus epidermidis | M. domestica | 250 | 250 |
| P. armeniaca | 350 | 250 | |
| P. cerasus | nd | 400 | |
| Bacillus subtilis | M. domestica | 350 | 250 |
| P. armeniaca | 400 | 250 | |
| P. cerasus | nd | 300 | |
| Staphylococcus capitis | M. domestica | 300 | 150 |
| P. armeniaca | 400 | 300 | |
| P. cerasus | 400 | 350 | |
| Micrococcus luteus | M. domestica | nd | nd |
| P. armeniaca | 600 | 450 | |
| P. cerasus | nd | nd | |
| Yersinia enterocolitica | M. domestica | nd | nd |
| P. armeniaca | 400 | 300 | |
| P. cerasus | nd | nd | |
| Pseudomonas aeruginosa | M. domestica | 350 | 250 |
| P. armeniaca | 400 | 300 | |
| P. cerasus | 450 | 300 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska-Dziok, M.; Ziemlewska, A.; Wójciak, M.; Sowa, I.; Wąsik-Szczepanek, E.; Nizioł-Łukaszewska, Z. Comparison of Cytotoxicity and Antioxidant, Antibacterial, and Anti-Inflammatory Activity of Aqueous and Ethanolic Extracts from Malus domestica, Prunus armeniaca, and Prunus cerasus Leaves. Molecules 2025, 30, 2085. https://doi.org/10.3390/molecules30102085
Zagórska-Dziok M, Ziemlewska A, Wójciak M, Sowa I, Wąsik-Szczepanek E, Nizioł-Łukaszewska Z. Comparison of Cytotoxicity and Antioxidant, Antibacterial, and Anti-Inflammatory Activity of Aqueous and Ethanolic Extracts from Malus domestica, Prunus armeniaca, and Prunus cerasus Leaves. Molecules. 2025; 30(10):2085. https://doi.org/10.3390/molecules30102085
Chicago/Turabian StyleZagórska-Dziok, Martyna, Aleksandra Ziemlewska, Magdalena Wójciak, Ireneusz Sowa, Ewa Wąsik-Szczepanek, and Zofia Nizioł-Łukaszewska. 2025. "Comparison of Cytotoxicity and Antioxidant, Antibacterial, and Anti-Inflammatory Activity of Aqueous and Ethanolic Extracts from Malus domestica, Prunus armeniaca, and Prunus cerasus Leaves" Molecules 30, no. 10: 2085. https://doi.org/10.3390/molecules30102085
APA StyleZagórska-Dziok, M., Ziemlewska, A., Wójciak, M., Sowa, I., Wąsik-Szczepanek, E., & Nizioł-Łukaszewska, Z. (2025). Comparison of Cytotoxicity and Antioxidant, Antibacterial, and Anti-Inflammatory Activity of Aqueous and Ethanolic Extracts from Malus domestica, Prunus armeniaca, and Prunus cerasus Leaves. Molecules, 30(10), 2085. https://doi.org/10.3390/molecules30102085








