Abstract
The structural diversity of marine natural products is considered a potential resource for the pharmaceutical industry. In our study of marine-derived compounds, one bacterium Bacillus licheniformis S-1 was discovered to have the ability to produce bioactive natural products. After a further chemistry investigation, one novel 4-aminopyridinium derivative, 4-(dimethylamino)-1-(2S-((4hydroxybenzoyl)oxy)propyl)pyridin-1-ium (1), along with 15 known cyclic dipeptides (2–16) were isolated from the bacterium B. licheniformis S-1 derived from a shallow sea sediment. The structures of compounds 1–16 were elucidated through comprehensive NMR spectroscopic and specific optical rotation (OR) data analyses. Compound 6 showed antibacterial activity against Pseudomonas fulva with an MIC value of 50 µg/mL. This is the first study to discover a pyridinium derivative and cyclic dipeptides from B. licheniformis.
1. Introduction
The ocean covers more than three-quarters of the Earth’s surface and is rich in resources, particularly microbial resources [1]. Research has shown that secondary metabolites produced by marine-derived microorganisms possess biological activities such as antibacterial, anti-inflammatory and cytotoxic activities [2,3]. The discovery of these active compounds provides important resources for the study of marine drug lead compounds, effectively addressing the issue of drug sources in the drug development process and possessing significant medicinal value.
Bacillus licheniformis is widely used in industry, environmental protection, graziery and medicine. Pathak et al. isolated alkaline protease from B. licheniformis KBDL4, which remained highly stable under conditions of high pH and high temperature and can be applied as detergents in industry [4]. B. licheniformis SP34 can inhibit the growth of Microcystis aeruginosa DCM4 to improve water quality [5]. B. licheniformis is one of the probiotic strains commonly used in feed additives in graziery [6]. The addition of live B. licheniformis particles on the basis of conventional treatment can reduce treatment time, reduce the level of inflammatory factors and improve treatment effectiveness for children with rotavirus enteritis [7]. While there are a few studies on the secondary metabolites of B. licheniformis, as far as we know, only five compounds have been isolated from the bacteria until now [8,9,10].
Marine-derived B. licheniformis also showed attractive activity and application potential. B. licheniformis AS3 showed a crude oil biodegradation efficiency of 92%, indicating its potential as a biosurfactant-producing bacteria [11]. B. licheniformis VIT02 showed antibacterial activities against Aeromonas hydrophila and Vibrio parahaemolyticus [12]. Phelan et al. isolated three sponge-associated B. licheniformis, which showed good antimicrobial activities against Bacillus cereus, Bacillus megaterium, Listeria innocua and Clostridium sporogenes [13].
In our efforts to identify new bioactive secondary metabolites from marine microorganisms, we found that Bacillus licheniformis S-1 has the potential to produce antimicrobial secondary metabolites, and further isolation led to one new pyridinium derivative and fifteen cyclic dipeptides.
2. Results
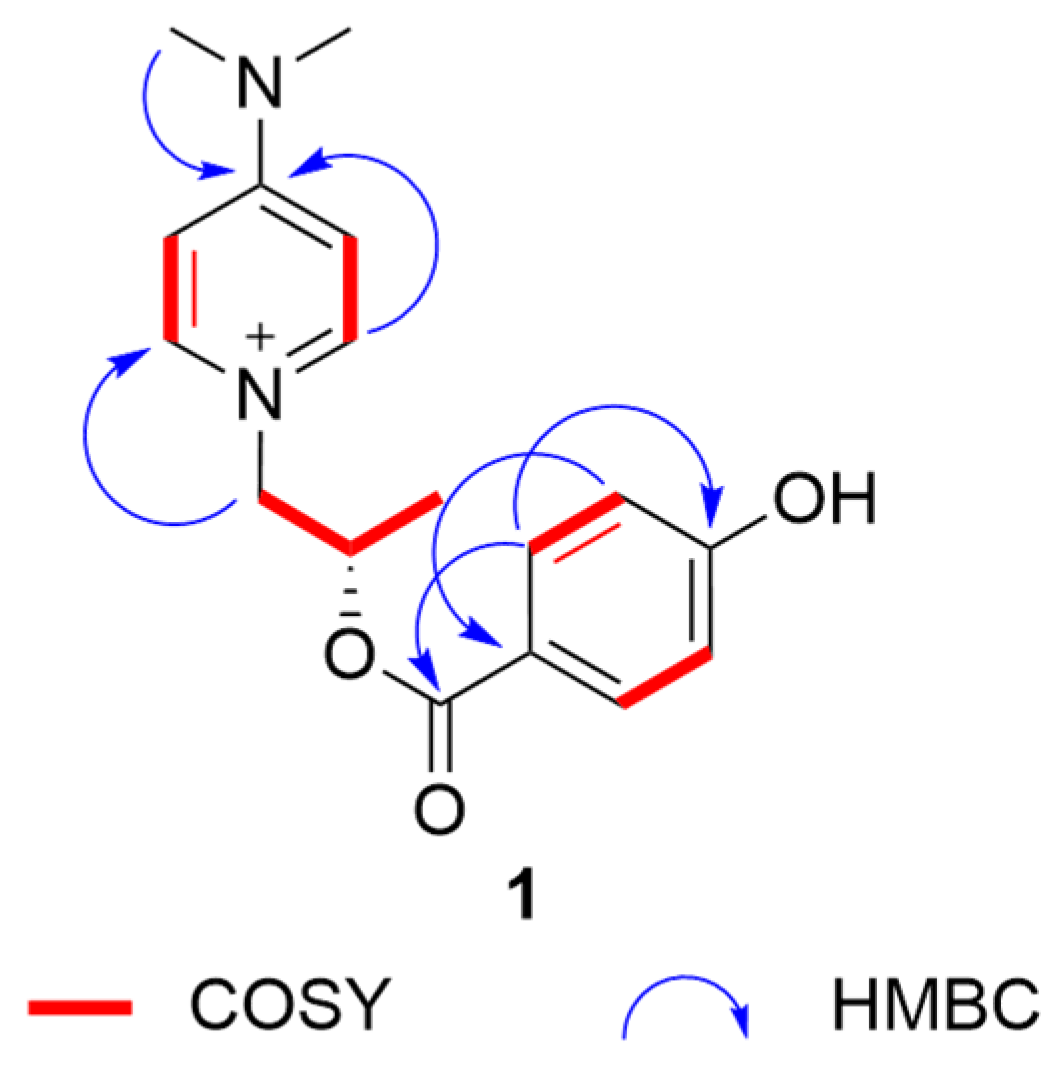
Compound 1 was obtained as colorless needle crystals. Its molecular formula was determined by an HRESIMS (m/z [M]+301.14185, calculated for C17O21N3O2+, 301.15487) spectrum as C17O21N3O2+ (Figure S1), indicating 9 degrees of unsaturation. The UV spectrum exhibited absorption bands at 286 nm, indicating the emergence of conjugated structures. The NMR data (Table 1) of three methyl groups (δH 1.22 (3H, d, 6.3), δC 20.6; δH 3.24 (6H, s), δC 40.2), one methylene (δH 4.23 (1H, dd, 13.7, 2.9), 3.91–3.87 (1H, m), δC 65.0), one methine (δH 4.06–4.00 (1H, m), δC 67.5), four aromatic methines (δH 6.96 (2H, d, 7.8), δC 108.4; δH 8.09 (2H, d, 7.8), δC 143.8) and a quaternary carbon signal (δC 158.1) were almost similar to those of 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium [14], indicating that it has a similar substructural unit to 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium in 1. The only difference between the substructure of 1 and 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium was the high-field shift of C-9 (20.6 in 1 vs. 24.2 in 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium), suggesting the different absolute structure in C-8. The other NMR data of four aromatic methines (δH 6.72 (2H, d, 8.6), δC 115.3; δH 7.82 (2H, d, 8.7), δC 132.3), two quaternary carbon signals (δC 129.8, δC 161.0) and an ester group signal (δC 175.6), combined with the 1H-1H COSY correlations (Figure 1 and Figure S5) of C-15/C-16 and C-18/C-19, the HMBC correlations from H-16/H-18 to C-14, H-15/H-19 to C-17, and H-15/H-19 to C-13, and the downfield shift of C-17 (δC 161.0), suggested the presence of a 4-hydroxybenzoyl ester substructure of 1. The two substructures of 1 were linked through the ester group in C-13. Thus, the planar structure of 1 was determined. The substructure of compound 1 was the same as that of known compound 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium), so the absolute configuration of 1 was further speculated to be 8S by comparing the specific optical rotation data with that of 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium (-79.8 (c 1.0, MeOH) of 1 vs. +1100 of 4-(dimethyllamino)-1-(2R-hydroxypropyl)-pyridinium) [14], and it was named 4-(dimethylamino)-1-(2S-((4hydroxybenzoyl)oxy)propyl)pyridin-1-ium.

Table 1.
The 1H and 13C NMR data of compound 1.
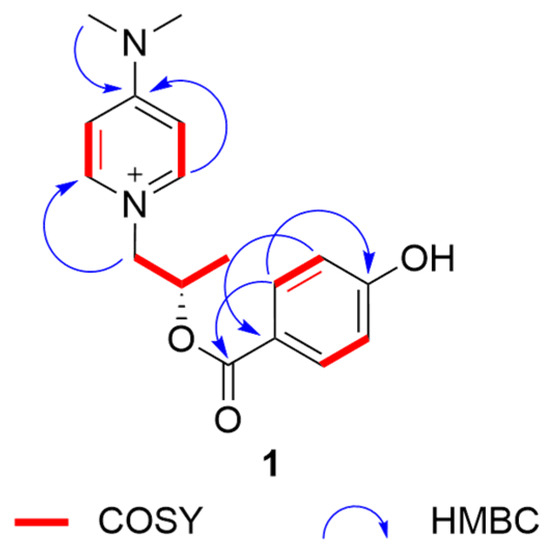
Figure 1.
Key HMBC and COSY correlations of 1.
The structures of 2–16 were determined to be cyclo(D-Leu-L-Leu)(2) [15,16], cyclo(L-leucyl-L-leucyl)(3) [17,18], cyclo-(L-Pro-L-Ile)(4) [19,20], cyclo-(L-Pro-D-Val)(5) [21,22], cyclo (D-Val-D-Pro)(6) [23], cyclo(L-Pro-L-Val)(7) [24,25], cyclo (D-pro-L-val)(8) [26], cyclo (L-Ala-L-Pro)(9) [27], cyclo-(L-Pro-D-Leu)(10) [20,28], cyclo-(D-Pro-D-Leu)(11) [29], cyclo(L-Phe-L-Pro(12) [30], cyclo-(L-Phe-D-Pro)(13) [31], cyclo (L-Pro-L-Tyr)(14) [32], cyclo-(4methyl-D-Pro-L-Nva)(15) [20] and cyclo-(L-4hydroxyl-Pro-L-Leu)(16) [33] (Figure 2), respectively, by comparing their NMR and specific OR data (Table S1) with those in the literature.
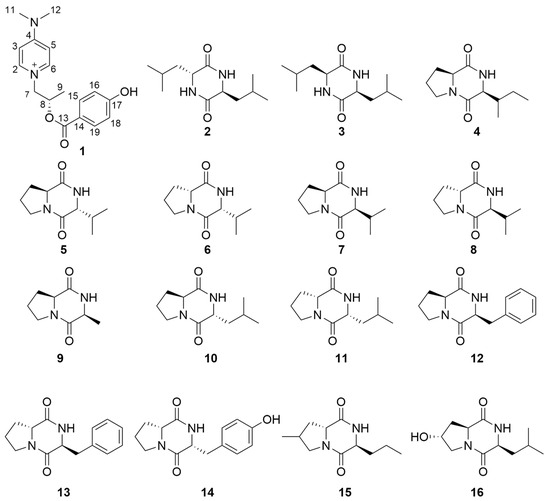
Figure 2.
Structures of compounds 1–16.
All compounds were tested for their inhibitory activities against fifteen pathogenic microbes. Only compound 6 showed antibacterial activity against P. fulva with an MIC value of 50 µg/mL.
3. Materials and Methods
3.1. General Experimental Procedure
Optical rotations were recorded using a JASCO P-1020 digital polarimeter (JASCO, Tokyo, Japan). UV spectra were measured on an Implen Gmbh NanoPhotometer N50 Touch (Implen, Munich, Germany). NMR spectra were measured using a Bruker AVANCE NEO (Bruker, Fällanden, Switzerland) at 600 MHz for 1H and 151 MHz for 13C in CDCl3 or DMSO. HRESIMS spectra were recorded using a Thermo Scientific LTQ Orbitrap XL spectrometer (Thermo Fisher Scientific, Bremen, Germany). HPLC separation was performed using a Waters 2989 UV/Visible detector (Waters, Milford, MA, USA) with a Kromasil 100-5-C18 HPLC column (Kromasil, Göteborg, Sweden) used at 30 °C.
3.2. Bacterial Material
The strain S-1 was isolated from the marine sediment collected from the Huanghai Sea in Qingdao, China, in 2021. It was identified as Bacillus licheniformis through the sequence analysis of the 16S rDNA internal spacer (ITS) fr (GenBank accession number PP506587). This bacterial strain is preserved at Shandong University of Science and Technology.
3.3. Fermentation, Extraction and Isolation
The strain B. licheniformis S-1 was cultured on Nutrient Broth (NB) agar plates at 37 °C for 3 d. Subsequently, it was cultivated in a NB liquid medium in 30 Erlenmeyer flasks (300 mL in each 500 mL flask) at room temperature in static. According to the actual growth state of B. licheniformis S-1, a 40 d period of fermentation was selected.
Each fermented culture medium was inactivated by adding 50 mL ethyl acetate (EtOAc) in static for 3 d. The mixture was filtered through two layers of gauze, and then the mycelia and the medium were separated. The mycelia and the medium were extracted three times with EtOAc, respectively. All the extracts were combined and then evaporated to dryness using a rotary evaporator to afford residue (2.46 g).
According to the polarity shown by a thin-layer chromatography (TLC) experiment, the developing agent with a retention factor value of 0.2 was selected as the mobile phase. The residue was separated into three fractions (Fr.1–Fr.3) on silica gel, with a gradient of EtOAc–PE (0–100%). Fr.1 was isolated by gel chromatography and eluted with CH2Cl2–MeOH (0–10%) to afford two fractions (Fr.1.1, Fr.1.2). Fr.1.1 was purified with HPLC eluted with 30% MeOH–H2O (2 mL/min) to give compounds 2 (2.3 mg, tR 5.418 min), 5 (1.8 mg, tR 30.230 min) and 4 (5.9 mg, tR 33.725 min). Fr.1.2 was purified with HPLC eluted with 40% MeOH–H2O (2 mL/min) to give compounds 1 (3.3 mg, tR 5.359 min) and 10 (4.6 mg, tR 17.910 min). Fr.2 was isolated by gel chromatography and eluted with CH2Cl2–MeOH (0–10%) to afford three fractions (Fr.2.1, Fr.2.2 and Fr.2.3). Fr.2.2 was isolated by HPLC eluted with 60% MeOH–H2O (2 mL/min) to afford Fr.2.2.1 and compound 3 (2.7 mg, tR 16.369 min). Fr.2.2.1 was isolated by HPLC eluted with 40% MeOH–H2O (2 mL/min) to afford four fractions: Fr.2.2.1.1, Fr.2.2.1.2, Fr.2.2.1.3, and Fr.2.2.1.4. Fr.2.2.1.1 was purified with HPLC eluted with 30% MeOH–H2O (2 mL/min) to give compounds 7 (2.7 mg, tR 14.240 min) and 6 (5 mg, tR 15.605 min). Fr.2.2.1.2 was purified with HPLC eluted with 30% MeOH–H2O to give compounds 15 (2.9 mg, tR 27.515 min) and 11 (7.1 mg, tR 29.976 min). Fr.2.2.1.3 was purified with HPLC eluted with 40% MeOH–H2O (2 mL/min) to give compound 12 (7.2 mg, tR 22.618 min). Fr.3 was isolated by gel chromatography and eluted with CH2Cl2–MeOH (0–10%) to afford two fractions (Fr.3.1, Fr.3.2). Fr.3.1 was purified with HPLC eluted with 40% MeOH–H2O to give compounds 9 (2.1 mg, tR 6.650 min), 14 (5.2 mg, tR 8.406 min), 8 (3.2 mg, tR 9.618 min), 16 (2.9 mg, tR 11.900 min), 13 (9.5 mg, tR 22.558 min) and 12 (3.2 mg, tR 27.547 min).
3.4. Antimicrobial Activity Assays
The antimicrobial assay was evaluated by broth microdilution in 96-well plates with a sample concentration of 50 µg/mL [34]. Fifteen pathogenic microbes, namely, Canidia albicans (ATCC10231), Aeromonas salmonicida (ATCC7965D), Escherichia coli (ATCC 25922), Comamonas terrigena, Aeromonas hydrophila (ATCC 7966), Photobacterium angustum (ATCC 33975), Photobacterium halotolerans, Vibrio anguillarum (ATCC 19109), Vibrio harveyi (ATCC BAA-2752), Pseudomonas aeruginosa (ATCC 10145), Pseudomonas aeruginosa (ATCC 10145), Pseudomonas fulva (ATCC 31418), Staphylococcus aureus (ATCC 27154), Enterobacter cloacae (ATCC 39978) and Xanthomonas axonopodis, were used. Ciprofloxacin was used as the positive control and DMSO as the negative control.
4. Conclusions and Discussions
In summary, one new pyridinium derivative, 4-(dimethylamino)-1-(2S-((4hydroxybenzoyl)oxy)propyl)pyridin-1-ium (1), along with fifteen cyclic dipeptides were isolated from Bacillus licheniformis S-1. Compound 6 showed antibacterial activity against P. fulva. This is the first study to discover a pyridinium derivative and cyclic dipeptides from Bacillus licheniformis. The results demonstrate the significance of secondary metabolites isolated from marine-derived microorganisms in providing innovative structural templates for new drug development.
Although B. licheniformis is a common soil-dwelling bacteria, there are few reports of its activity from marine sources, and studies on its secondary metabolites are also limited. In the preparation experiment, the crude extract of B. licheniformis S-1 showed antimicrobial activity, but only compound 1 was active among the isolated compounds, which may be because the antimicrobial activity of the crude extract comprised a synergistic effect of a variety of compounds, or because the active compounds were lost during the separation process.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30010007/s1. The HRESIMS and NMR spectra of compounds 1–16 (Figures S1–S50), a graphical representation of the separation process (Figure S51), specific OR of compounds 1–16 (Table S1), antimicrobial activities of compounds 1–16 (50 µg/mL) (Tables S2–S3), Acronym list (Table S4).
Author Contributions
Data curation, Y.W.; Investigation, Y.L.; Methodology, T.S.; Project administration, T.S.; Resources, B.W.; Software, T.S.; Supervision, T.S.; Validation, H.W., Y.W. and G.W.; Writing—original draft, H.W.; Writing—review and editing, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82104029 and 21868011); and the Talent Support Program of Shandong University of Science and Technology in 2019–2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chukwudulue, U.M.; Barger, N.; Dubovis, M.; Luzzatto Knaan, T. Natural products and pharmacological properties of symbiotic bacillota (firmicutes) of marine macroalgae. Mar. Drugs 2023, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Zeng, Y.B. Novel bioactive natural products from marine-derived Penicillium fungi: A review (2021–2023). Mar. Drugs 2024, 22, 191. [Google Scholar] [CrossRef]
- Yang, G.; Lin, M.; Kaliaperumal, K.; Lu, Y.; Qi, X.; Jiang, X.; Xu, X.; Gao, C.; Liu, Y.; Luo, X. Recent advances in anti-inflammatory compounds from marine microorganisms. Mar. Drugs 2024, 22, 424. [Google Scholar] [CrossRef]
- Pathak, A.P.; Deshmukh, K.B. Alkaline protease production, extraction and characterization from alkaliphilic Bacillus licheniformis KBDL4: A Lonar soda lake isolate. Indian J. Exp. Biol. 2012, 50, 569–576. [Google Scholar] [PubMed]
- Liu, J.; Yang, C.; Chi, Y.; Wu, D.; Dai, X.; Zhang, X.; Igarashi, Y.; Luo, F. Algicidal characterization and mechanism of Bacillus licheniformis Sp34 against Microcystis aeruginosa in Dianchi Lake. J. Basic Microbiol. 2019, 59, 1112–1124. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. Mysore 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yu, Q.Q. Effects of Bacillus licheniformis granules, live combined with conventional treatment on children with rotavirus enteritis. Med. J. Chin. People’s Health 2024, 36, 51–53. [Google Scholar] [CrossRef]
- Devi, P.; Wahidullah, S.; Rodrigues, C.; Souza, L.D. The sponge-associated bacterium Bacillus licheniformis SAB1: A Source of antimicrobial compounds. Mar. Drugs 2010, 8, 1203–1212. [Google Scholar] [CrossRef]
- Batrakov, S.G.; Rodionova, T.A.; Esipov, S.E.; Polyakov, N.B.; Sheichenko, V.I.; Shekhovtsova, N.V.; Lukin, S.M.; Panikov, N.S.; Nikolaev, Y.A. A novel lipopeptide, an inhibitor of bacterial adhesion, from the thermophilic and halotolerant subsurface Bacillus licheniformis strain 603. Biochim. Biophys. Acta 2003, 1634, 107–115. [Google Scholar] [CrossRef]
- Jeong, M.-H.; Lee, Y.-S.; Cho, J.-Y.; Ahn, Y.-S.; Moon, J.-H.; Hyun, H.-N.; Cha, G.-S.; Kim, K.-Y. Isolation and characterization of metabolites from Bacillus licheniformis MH48 with antifungal activity against plant pathogens. Microb. Pathog. 2017, 110, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.A.; Prabhu, N.S.; Rajasekar, A.; Parthipan, P.; AlSalhi, M.S.; Devanesan, S.; Govarthanan, M. Bio-electrokinetic remediation of crude oil contaminated soil enhanced by bacterial biosurfactant. J. Hazard. Mater. 2021, 405, 124061. [Google Scholar] [CrossRef]
- Mondal, H.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Antibacterial activity of Bacillus licheniformis isolated from marine sediments and its effect in treating Aeromonas hydrophila infection in freshwater prawn, Macrobrachium rosenbergii. Aquacult. Int. 2023, 31, 3071–3093. [Google Scholar] [CrossRef]
- Phelan, R.; O’halloran, J.; Kennedy, J.; Morrissey, J.; Dobson, A.; O’gara, F.; Barbosa, T. Diversity and bioactive potential of endospore-forming bacteria cultured from the marine sponge Haliclona simulans. J. Appl. Microbiol. 2012, 112, 65–78. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.-J.; Wu, S.-Y.; Chen, G.-Y.; Song, X.-P.; Han, C.-R. Chemical constituents from Scutellaria hainanensis C. Y. Wu. Biochem. Syst. Ecol. 2019, 82, 1–12. [Google Scholar] [CrossRef]
- Lu, X.; Shen, Y.; Zhu, Y.; Xu, Q.; Liu, X.; Ni, K.; Cao, X.; Zhang, W.; Jiao, B. Diketopiperazine constituents of marine Bacillus subtilis. Chem. Nat. Compd. 2009, 45, 290–292. [Google Scholar] [CrossRef]
- Chen, R.Q.; Luo, X.C.; Lin, H.W.; Jiao, W.H. Secondary metabolites of a marine sponge associated Streptomyces parvulus 162432. Chin. Herb. Med. 2023, 54, 4104–4110. [Google Scholar]
- Yang, Z.-D.; Li, Z.-J.; Zhao, J.-W.; Sun, J.-H.; Yang, L.-J.; Shu, Z.-M. Secondary metabolites and PI3K inhibitory activity of Colletotrichum gloeosporioides, a fungal endophyte of Uncaria rhynchophylla. Curr. Microbiol. 2019, 76, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Lai, T.K.; Saha, A.; Selvin, J.; Mukherjee, J. Structural elucidation and antimicrobial activity of a diketopiperazine isolated from a Bacillus sp. associated with the marine sponge Spongia officinalis. Nat. Prod. Res. 2021, 35, 2315–2323. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-Diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Adamczeski, M.; Reed, A.R.; Crews, P. New and known diketopiperazines from the caribbean sponge, calyx cf. podatypa. J. Nat. Prod. Lloydia 1995, 58, 201–208. [Google Scholar] [CrossRef]
- Danh, C.Đ.; Nam, V.V.; Hương, Đ.T.M.; Quyên, V.T.; Murphy, B.T.; Thạch, T.Đ.; Minh, C.V.; Cường, P.V. Nghiên cứu một số hợp chất thứ cấp từ chủng xạ khuẩn Streptomyces sp.(G065). Vietnam. J. Chem. 2017, 55, 19. [Google Scholar]
- Fu, H.C.; Zhong, H.M. Studies on the chemical constituents of marine bacterium Pseudomonas sp. J. Qingdao Univ. Sci. Technol. 2009, 30, 31–33. [Google Scholar]
- Lv, H.N.; Chen, H.; Qu, J.; Li, Y.; Ma, S.G.; Liu, Y.B. Study on secondary metabolites of endophytic fungi Trichoderma harzianum. Mod. Chin. Med. 2015, 17, 427–430. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Zhou, Z.-F.; Guan, X.-K.; Deng, J.-S.; Li, G.-H. Metabolites from a global regulator engineered strain of Pseudomonas lurida and their inducement of trap formation in Arthrobotrys oligospora. Chem. Biol. Technol. Agric. 2024, 11, 1–13. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.H.; Yun, B.-S.; Pyun, Y.R.; Kim, C.-J. Cyclo (D-Pro-L-Val), a specific ß-glucosidase inhibitor produced by Aspergillus sp F70609. J. Antibiot. 2001, 54, 179–181. [Google Scholar] [CrossRef]
- Zeng, X.R.; Jiao, W.H.; Tang, J.S.; Gao, H.; Hong, K.; Jia, L.I.; Yao, X.S. Secondary metabolites from marine actinomycete Streptomyces sp.(No. 30701). Chin. J. Med. Chem. 2010, 20, 298–303. [Google Scholar] [CrossRef]
- Lin, Z.H.; Ding, W.P.; Li, Y.Q.; Chen, R.W.; Gao, Y.L.; Yin, H. Chemical constituents of marine derived actinomycete Demequina litorisediminis SCSIO 53428. Cent. South Pharm. 2020, 18, 213–217. [Google Scholar]
- Yang, B.; Dong, J.; Zhou, X.; Yang, X.; Lee, K.J.; Wang, L.; Zhang, S.; Liu, Y. Proline-containing dipeptides from a marine sponge of a Callyspongia species. Helv. Chim. Acta 2009, 92, 1112–1117. [Google Scholar] [CrossRef]
- Hwang, J.; Jang, H.-J.; Kim, J.; Park, C.; Kim, Y.; Lim, C.-H.; Lee, S.; Rho, M.-C. Lactococcus lactis KR-050L inhibit IL-6/STAT3 activation. J. Appl. Microbiol. 2017, 122, 1412–1422. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Gao, H.; Lin, H.; Hong, K.; Yao, X. Study of anti-MSRA bioactive constituents from a marine actinomycetes Micromonospora sp. (No. 69). Chin. J. Mar. Drugs 2010, 29, 16–21. [Google Scholar] [CrossRef]
- Wang, G.; Dai, S.; Chen, M.; Wu, H.; Xie, L.; Luo, X.; Li, X. Two diketopiperazine cyclo (pro-phe) isomers from marine bacteria Bacillus subtilis sp. 13-2. Chem. Nat. Compd. 2010, 46, 583–585. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef]
- QV, C.L.; Yang, X.; Zhang, S.M.; Xie, Z.P.; Jin, H.Z. Study of diketopiperazines from the marine-derived Streptomyces sp. 223. Chin. J. Mar. Drugs 2015, 34, 23–28. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.Q.; Wang, Z.M.; Zheng, L.; Yu, Y.Y.; Dai, J.J.; Shi, D.Y. Bioactivity-guided screening of antimicrobial secondary metabolites from antarctic cultivable fungus Acrostalagmus luteoalbus CH-6 combined with molecular networking. Mar. Drugs 2022, 20, 334. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).