Lipid Biomarkers in Urban Soils of the Alluvial Area near Sava River, Belgrade, Serbia
Abstract
1. Introduction
2. Materials and Methods
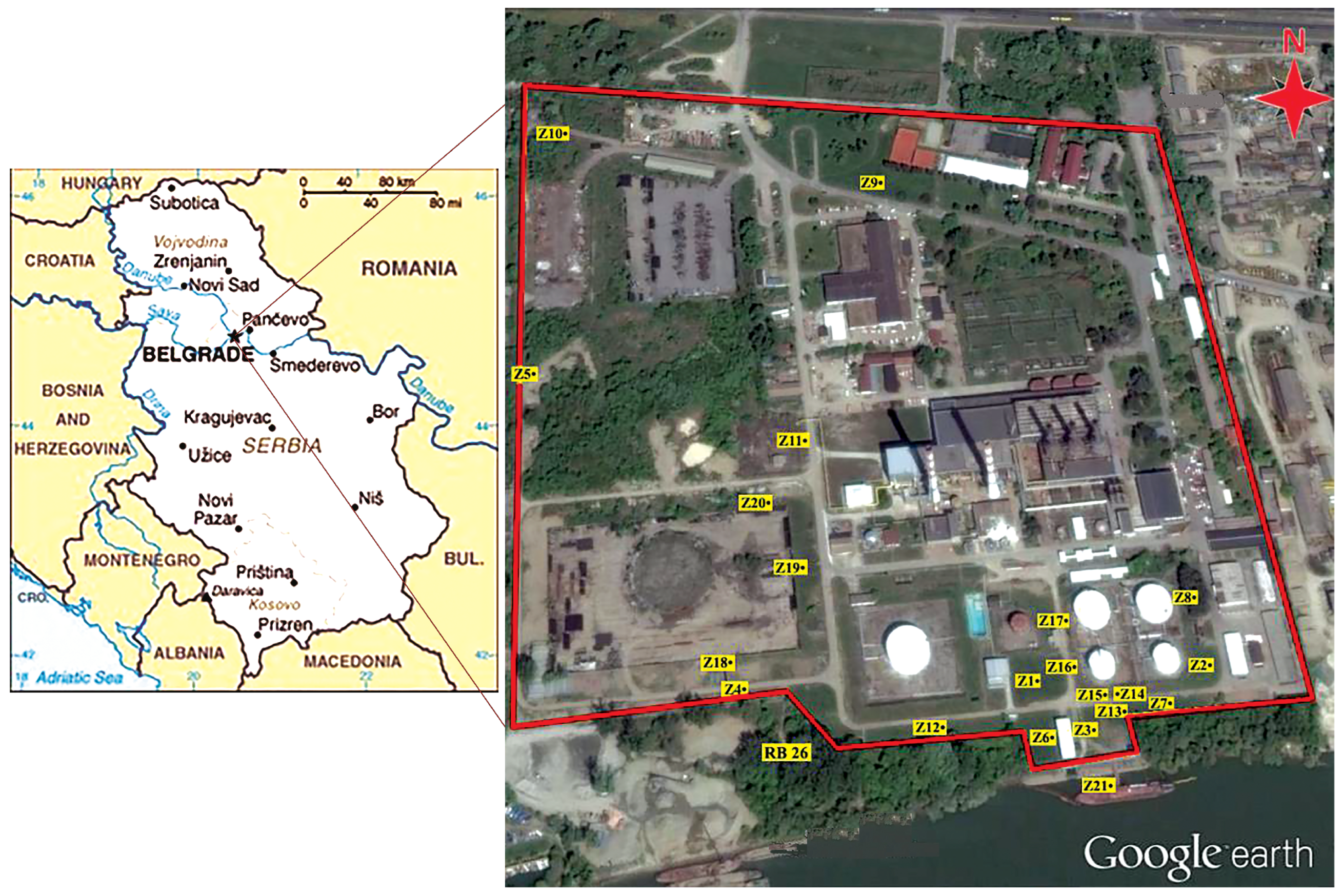
2.1. Study Area
2.2. Sampling and Analytical Strategy
2.3. Multivariate Statistical Analysis
3. Results and Discussion
3.1. Concentrations and Geochemical Characteristics of AHs in Soil
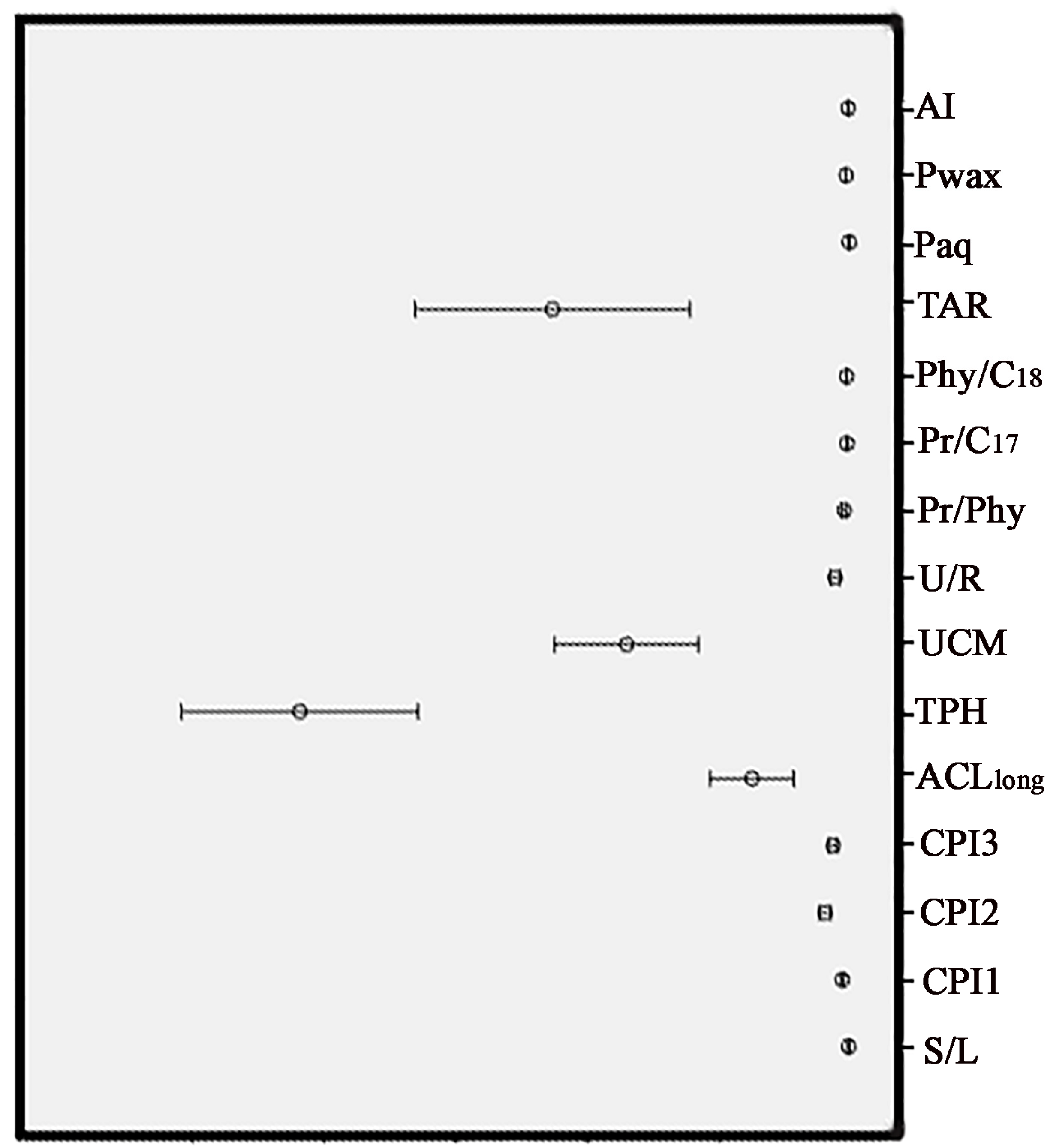
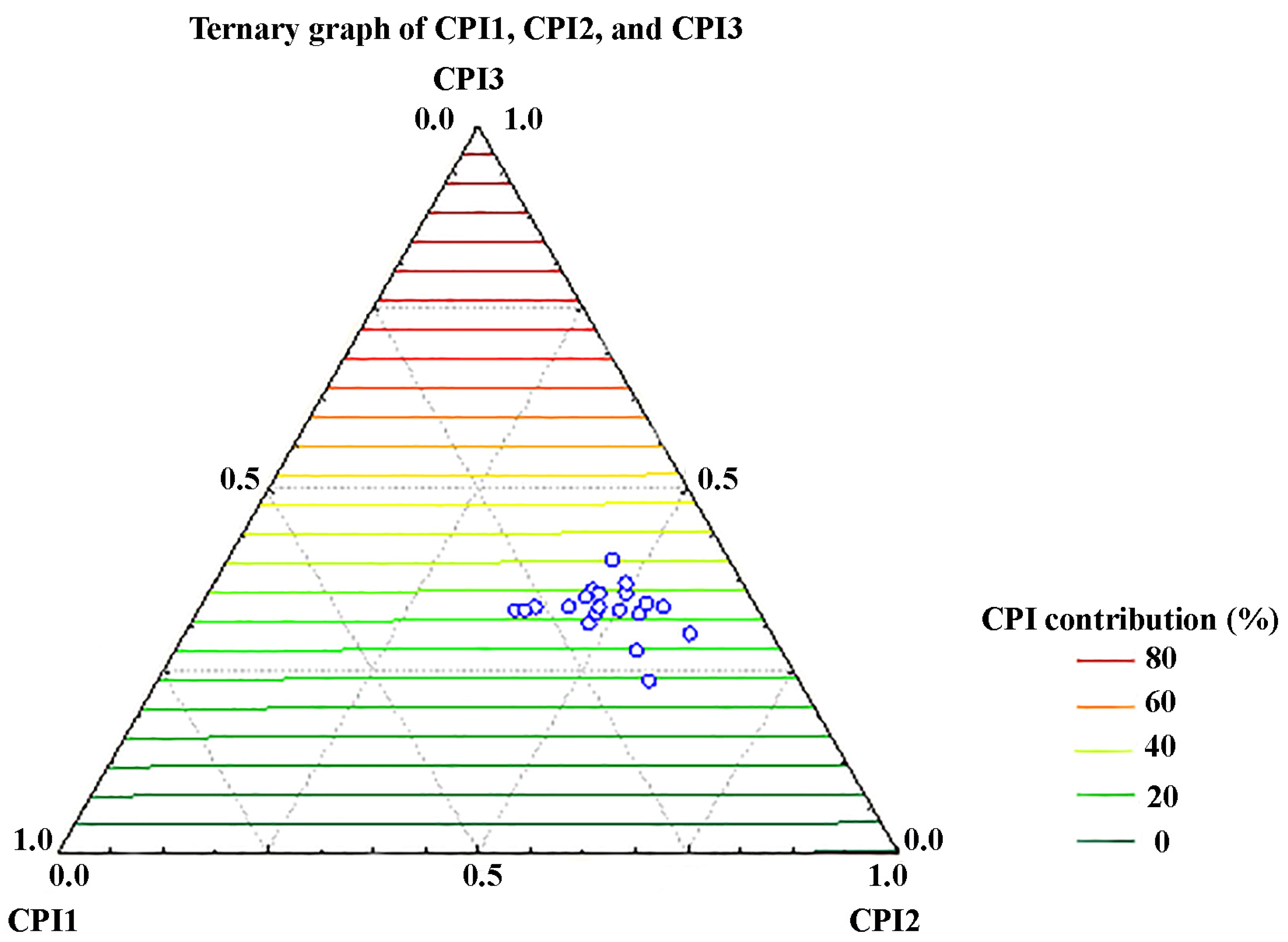
3.2. Characterization of the Soil Samples According to the Organic Geochemical Indices
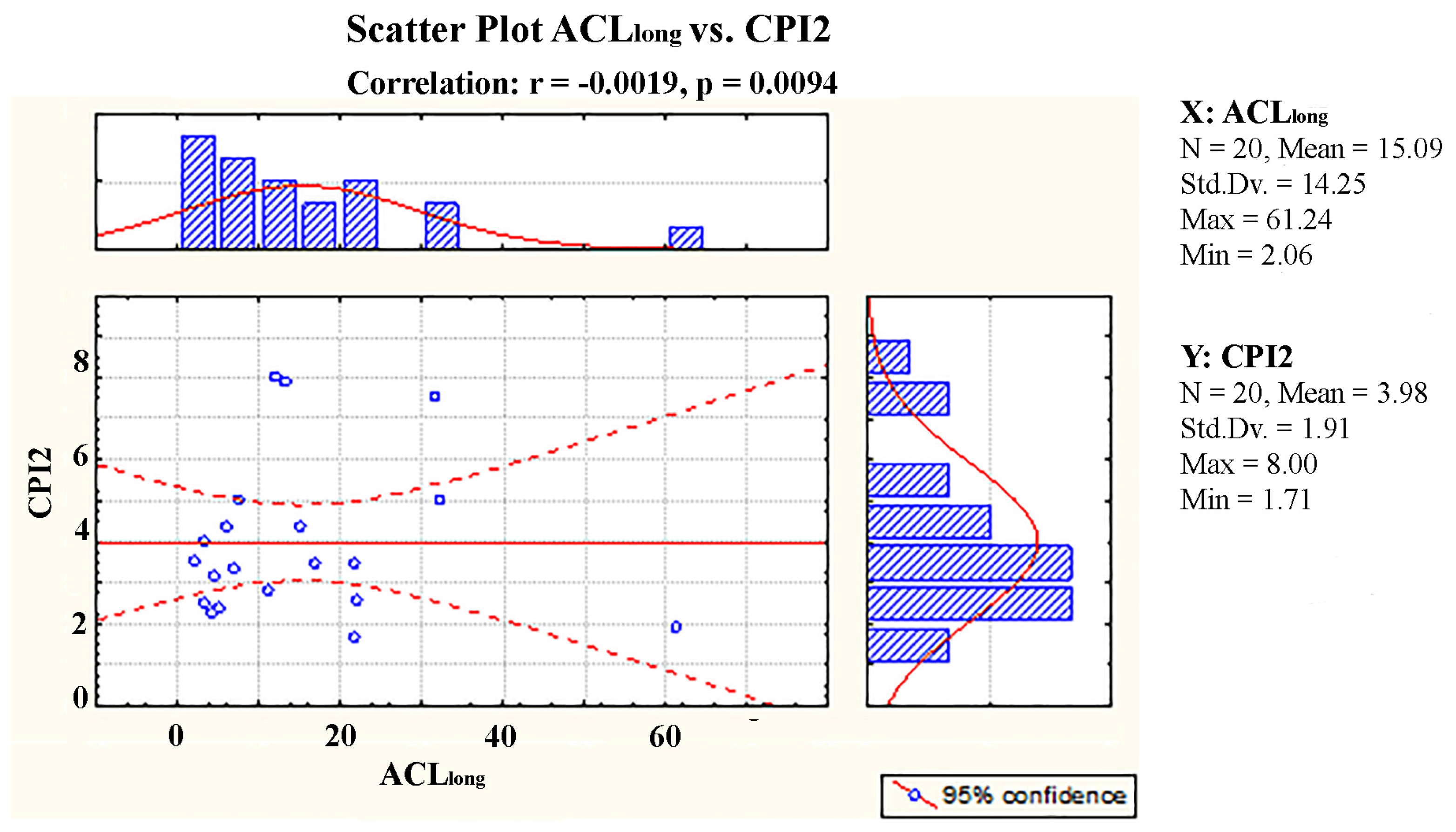
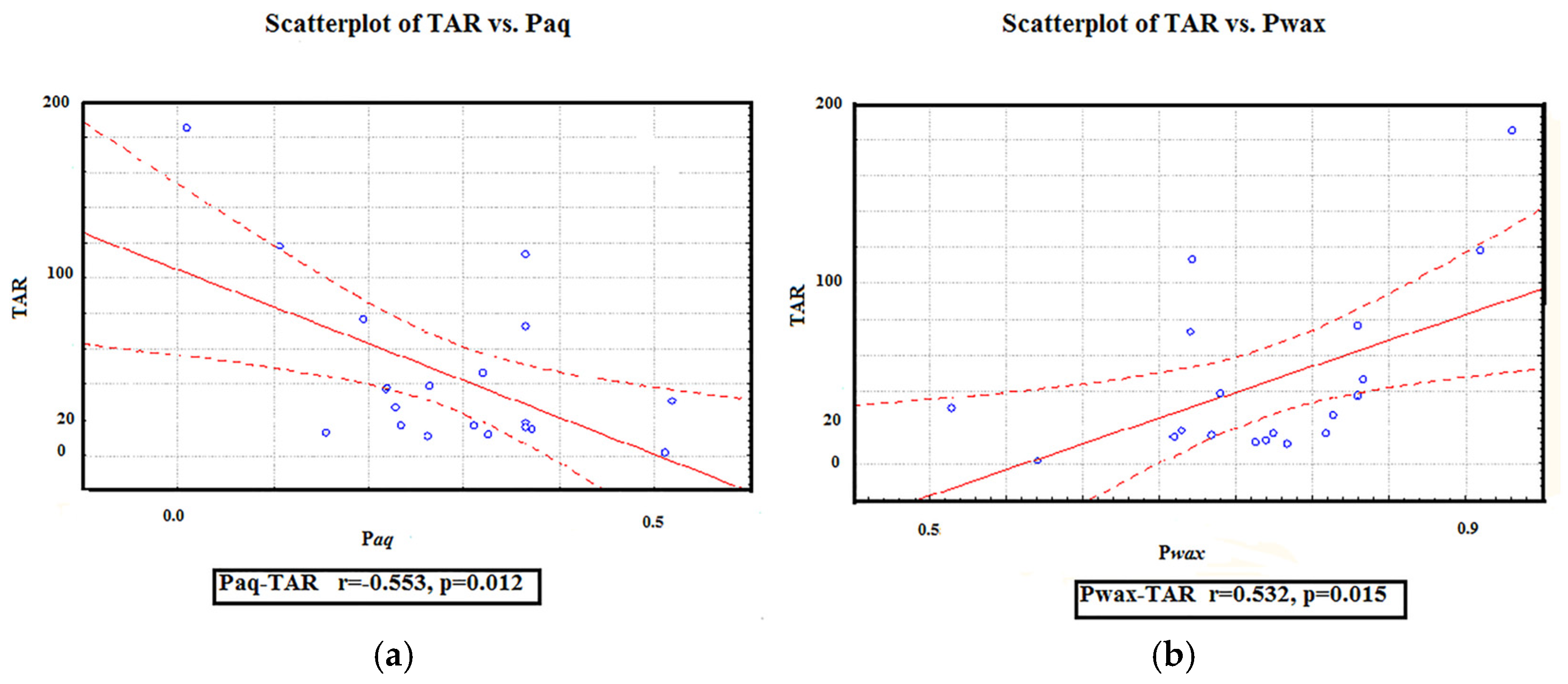
3.3. Correlation Matrix
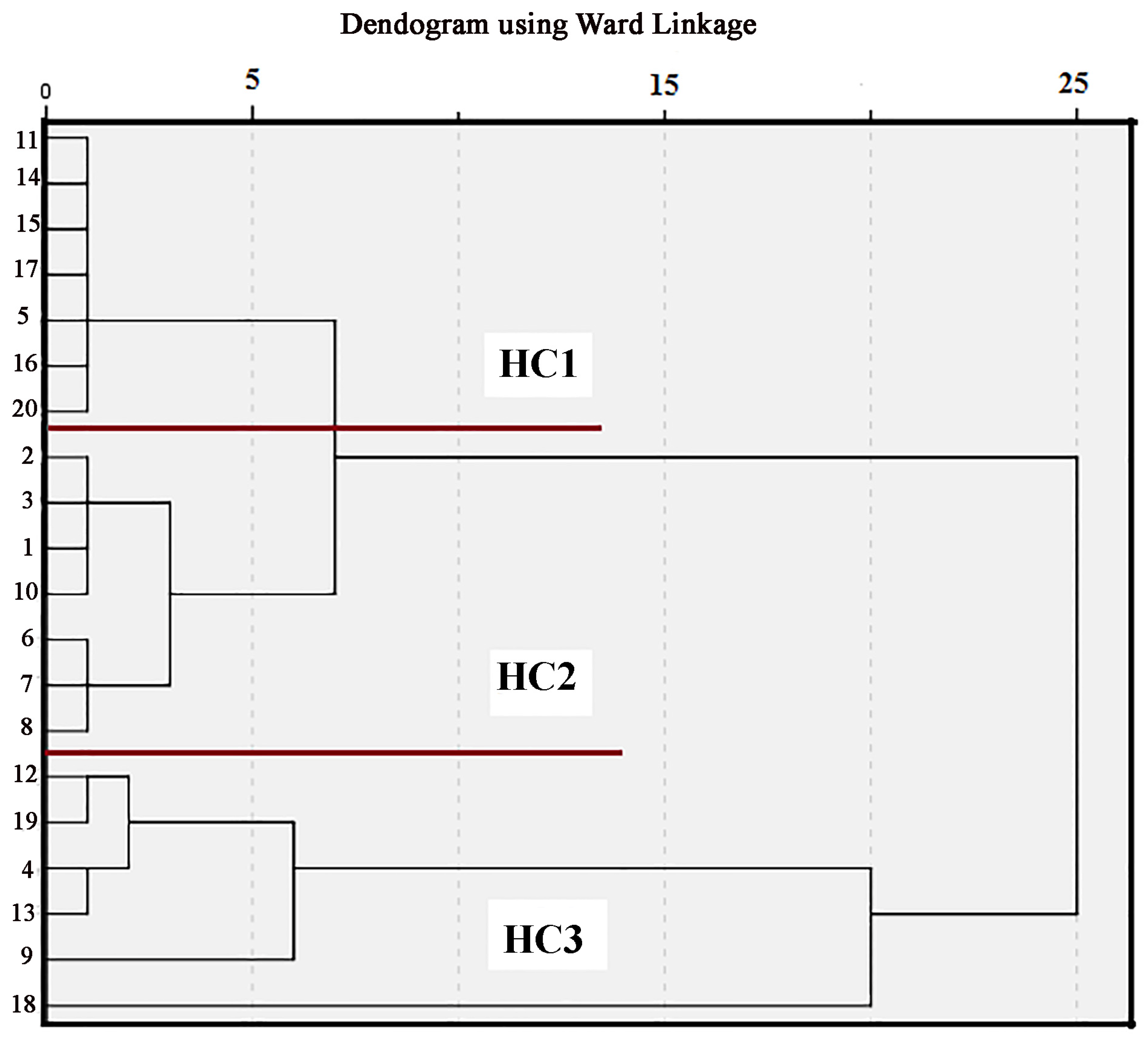
3.4. Q-Mode Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Riordan, R.; Davies, J.; Stevens, C.; Quinton, J.N.; Boyko, C. The ecosystem services of urban soils: A review. Geoderma 2021, 395, 115076. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Ren, Y.; Luo, X.S.; Zhu, Y.G. Urban soil and human health: A review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef]
- UNEP. Global Assessment of Soil Pollution—Summary for Policy Makers; FAO: Rome, Italy, 2021. [Google Scholar]
- Pérez, A.P.; Natalia Rodríguez Eugenio. Status of Local Soil Contamination in Europe: Revision of the Indicator “Progress in the Management Contaminated Sites in Europe”; EUR 29124 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-80072-6. [Google Scholar] [CrossRef]
- Latimer, J.S.; Hoffman, E.J.; Hoffman, G.; Fasching, J.L.; Quinn, J.G. Sources of petroleum hydrocarbons in urban runoff. Water Air Soil Pollut. 1990, 52, 1–21. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, C.; Wang, L.; Tang, J. Fingerprint analysis reveals sources of petroleum hydrocarbons in soils of different geographical oilfields of China and its ecological assessment. Sci. Rep. 2022, 12, 4808. [Google Scholar] [CrossRef]
- Stout, S.; Wang, Z. Standard Handbook Oil Spill Environmental Forensics: Fingerprinting and Source Identification, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128096598. [Google Scholar]
- Mohamed, L.A.; El Zokm, G.M.; El Deeb, K.Z.; Okbah, M.A. A new record for aliphatic and polycyclic aromatic hydrocarbon accumulation in the surficial sediments of El Mex Bay, Alexandria, Egypt after 19 years from the first one. Egypt. J. Aquat. Res. 2016, 42, 375–383. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Al-Mutlaq, K.F.; El-Mubarak, A.H.; Al-Saleh, M.A.; El-Otaibi, M.T.; Ibrahim, S.M.M.; Simoneit, B.R.T. Occurrence and sources of natural and anthropogenic lipid tracers in surface soils from arid urban areas of Saudi Arabia. Environ. Pollut. 2016, 208, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Petroleum Hydrocarbon Fingerprints of Water and Sediment Samples of Buffalo River Estuary in the Eastern Cape Province, South Africa. J. Anal. Methods Chem. 2017, 2017, 2629365. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.M.; Cattani, P.A.; Da Cunha, L.P.; César, R.; Figueira, R.C.L.; Martins, C.C. Petroleum biomarkers as tracers of low-level chronic oil contamination of coastal environments: A systematic approach in a subtropical mangrove. Environ. Pollut. 2019, 249, 1060–1070. [Google Scholar] [CrossRef]
- Haynes, H.M.; Taylor, K.G.; Rothwell, J.; Byrne, P. Characterisation of road-dust sediment in urban systems: A review of a global challenge. J. Soils Sediments 2020, 20, 4194–4217. [Google Scholar] [CrossRef]
- Grigoriadou, A.; Schwarzbauer, J.; Georgakopoulos, A. Organic geochemical parameters for estimation of petrogenicinputs in the coastal area of Kavala City, Greece. J. Soils Sediments 2008, 8, 253–262. [Google Scholar] [CrossRef]
- Jovančićević, B.; Antić, M.; Pavlović, I.; Vrvić, M.; Beškoski, V.; Kronimus, A.; Schwarzbauer, J. Transformation of Petroleum Saturated Hydrocarbons 434 during Soil Bioremediation Experiments. Water Air Soil Pollut. 2008, 190, 299–307. [Google Scholar] [CrossRef]
- Maioli, O.L.G.; Rodrigues, K.C.; Knoppers, B.A.; Azevedo, D.A. Pollution source evaluation using petroleum and aliphatic hydrocarbons in surface sediments from two Brazilian estuarine systems. Org. Geochem. 2010, 41, 966–970. [Google Scholar] [CrossRef]
- Samelak, I.; Balaban, M.; Vidović, N.; Koljančić, N.; Antić, M.; Solević-Knudsen, T.; Jovančićević, B. The application of alkane biological markers in the assessment of the origin of oil pollutants in the soil and recent river sediments (river Vrbas, Bosnia and Herzegovina). J. Serb. Chem. Soc. 2018, 83, 1167–1175. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; Liu, J.; He, X. Multiple lipid biomarkers record organic matter sources and paleoenvironmental changes in the East China Sea coast over the past 160 years. Holocene 2021, 31, 16–27. [Google Scholar] [CrossRef]
- Ielpo, P.; Leardi, R.; Pappagallo, G.; Uricchio, V.F. Tools based on multivariate statistical analysis for classification of soil and groundwater in Apulian agricultural sites. Environ. Sci. Pollut. Res. Int. 2017, 24, 13967–13978. [Google Scholar] [CrossRef] [PubMed]
- Dević, J.G.; Ilić, V.M.; Zildzović, N.S.; Avdalović, S.J.; Miletić, B.S.; Bulatović, S.S.; Vrvić, M.M. Investigation of potentially toxic elements in urban sediments in Belgrade, Serbia. J. Environ. Sci. Health A 2020, 55, 765–775. [Google Scholar] [CrossRef]
- Vrvić, M.M. (Ed.) Project for Remediation of the Contaminated Site at the Location of New Belgrade Thermal Plant; Brem Group: Belgrade, Serbia, 2015. (In Serbian) [Google Scholar]
- Bulatović, S.; Maric, N.; Solevic Knudsen, T.; Avdalović, J.; Ilic, M.; Jovančićević, B.; Vrvic, M. Bioremediation of groundwater contaminated with petroleum hydrocarbons applied at a site in Belgrade (Serbia). J. Serb. Chem. Soc. 2020, 85, 1067–1081. [Google Scholar] [CrossRef]
- BS EN ISO 16703; Soil Quality—Determination of Content of Hydrocarbon in the Range C10 to C40 by Gas Chromatography. 2011. Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030236051 (accessed on 25 December 2024).
- DIN EN 14345; Characterization of Waste—Determination of Hydrocarbon Content by Gravimetry. 2008. Available online: https://www.en-standard.eu/din-en-14345-characterization-of-waste-determination-of-hydrocarbon-content-by-gravimetry/?srsltid=AfmBOopMZa2RAxc-zW-rVHFyE22jJv1Z8YM9l-Cp4Zk5XyXScgiremkd (accessed on 25 December 2024).
- Lukić, M.; Avdalović, J.; Gojgić-Cvijović, G. Industrial-scale bioremediation of a hydrocarbon-contaminated aquifer’s sediment at the location of a heating plant, Belgrade, Serbia. Clean Technol. Environ. Policy 2024, 26, 1785–1798. [Google Scholar] [CrossRef]
- Dević, G.; Đorđević, D.; Sakan, S. Natural and anthropogenic factors affecting the groundwater quality in Serbia. Sci. Total Environ. 2014, 468–469, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, W.; Chen, G.; Lu, Y.; Feng, C.; Tu, X.M. Correlation and agreement: Overview and clarification of competing concepts and measures. Shanghai Arch. Psychiatry 2016, 28, 115–120. [Google Scholar] [CrossRef]
- Thomas, C.L.; Jansen, B.; van Loon, E.E.; Wiesenberg, G.L. Transformation of n-alkanes from plant to soil: A review. Soil 2021, 7, 785–809. [Google Scholar] [CrossRef]
- Cranwell, P.A.; Eglinton, G.; Robinson, N. Lipids of aquatic organisms as potential contributors to lacustrine sediments-II. Org. Geochem. 1987, 11, 513–527. [Google Scholar] [CrossRef]
- Nott, C.J.; Xie, S.; Avsejs, L.A.; Maddy, D.; Chambers, F.M.; Evershed, R.P. n-alkane distribution in ombrotrophic mires as indicators of vegetation change related to climatic variation. Org. Geochem. 2000, 31, 231–235. [Google Scholar] [CrossRef]
- Pancost, R.D.; Baas, M.; Van Geel, B.; Sinninghe Damsté, J.S. Biomarkers as proxies for plant inputs to peats: An example from a sub-boreal ombrotrophic bog. Org. Geochem. 2002, 33, 675–690. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Díaz-Bautista, A.; Aldasoro, J.J.; Torres, T.; Gallego, J.L.R.; Moreno, L.; Estébanez, C. n-Alkan-2-ones in peat-forming plants from the Roñanzas ombrotrophic bog (Asturias, northern Spain). Org. Geochem. 2011, 42, 586–592. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Torres, T.; Delgado, A.; Julia, R.; Lucini, M.; Llamas, F.J.; Reyes, E.; Soler, V.; Valle, M. The palaeoenvironmental and palaeohydrological evolution of Padul Peat Bog (Granada, Spain) over one million years, from elemental, isotopic, and molecular organic geochemical proxies. Org. Geochem. 2004, 35, 1243–1260. [Google Scholar] [CrossRef]
- Bush, R.T.; Mclnerney, F.A. Influence of temperature and C4 abundance on n-alkane chain length distributions across the central USA. Org. Geochem. 2015, 79, 65–73. [Google Scholar] [CrossRef]
- Gennadiev, A.N.; Pikovskii, Y.I.; Tsibart, A.S.; Smirnova, M.A. Hydrocarbons in Soils: Origin, Composition and Behavior (Review). Soil. Chem. 2015, 48, 1195–1209. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, D.; Liu, X.; Xie, H.; Lou, X.; Zeng, C. Combined microbial degradation of crude oil under alkaline conditions by Acinetobacter baumannii and Talaromyces sp. Chemosphere 2021, 273, 129666. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Hollebone, B.; Brown, C.; Landriault, M. Characteristics of Bicyclic Sesquiterpanes in Crude Oils and Petroleum Products. J. Chromatogr. A 2009, 1216, 4475–4484. [Google Scholar] [CrossRef]
- Elfadly, A.A.; Ahmed, O.E.; El Nady, M.M. Assessing of organic content in surface sediments of Suez Gulf, Egypt depending on normal alkanes, terpanes and steranes biological markers indicators. Egypt. J. Pet. 2016, 26, 969–979. [Google Scholar] [CrossRef]
- Silva, T.R.; Lopes, S.R.P.; Spörl, G.; Knoppers, B.A.; Azevedo, D.A. Evaluation of anthropogenic inputs of hydrocarbons in sediment cores from a tropical Brazilian estuarine system. Microchem. J. 2013, 109, 178–188. [Google Scholar] [CrossRef]
- Frysinger, G.S.; Gaines, R.B.; Li, X.; Reddy, C.M. Resolving the Unresolved Complex Mixture in Petroleum-Contaminated Sediments. Environ. Sci. Technol. 2003, 37, 1653–1662. [Google Scholar] [CrossRef]
- Zegouagh, Y.; Derenne, S.; Largeau, C.; Bardoux, G.; Mariotti, A. Organic matter sources and early diagenetic in Arctic surface sediments (Lena River delta and Laptev Sea, Eastern Siberia) II. Molecular and isotopic studies of hydrocarbons. Org. Geochem. 1998, 28, 571–583. [Google Scholar] [CrossRef]
- Readman, J.W.; Fillmann, G.; Tolosa, I.; Bartocci, J.; Villeneuve, J.P.; Catinni, C.; Mee, L.D. Petroleum and PAH contamination of the Black Sea. Mar. Pollut. Bull. 2002, 44, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Vane, C.H.; Kim, A.W.; Beriro, D.; Cave, M.R.; Lowe, S.R.; Lopes dos Santos, R.A.; Ferreira, A.M.P.J.; Collins, C.; Nathanail, C.P.; Moss-Hayes, V. Persistent Organic Pollutants in Urban Soils of Central London, England, UK: Measurement and Spatial Modelling of Black Carbon (BC), Petroleum Hydrocarbons (TPH), Polycyclic Aromatic Hydrocarbons (PAH) and Polychlorinated Biphenyls (PCB). Adv. Environ. Eng. Res. 2021, 2, 12. [Google Scholar] [CrossRef]
- RS Official Gazette. The Regulation on the Program for the Systematic Monitoring of Soil Quality, Soil Degradation Risk Assessment Indicators and Methodology for the Development of Remediation Programs, (No. 88/2010) (in Serbian). 2010. Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2010_11/t11_0317.htm (accessed on 25 December 2024).
- Sanches, F.P.J.; Böhm, E.M.; Böhm, G.M.; Montenegro, G.O.; Silveira, L.A.; Betemps, G.R. Determination of hydrocarbons transported by urban runoff in sediments of São Gonçalo Channel (Pelotas–RS, Brazil). Mar. Pollut. Bull. 2017, 114, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Ines, Z.; Amina, B.; Mahmoud, R.; Dalila, S.-M. Aliphatic and aromatic biomarkers for petroleum hydrocarbon monitoring in Khniss Tunisian-coast, (Mediterranean Sea). Procedia Environ. Sci. 2013, 18, 211–220. [Google Scholar] [CrossRef]
- Bomboi, M.T.; Hernández, A. Hydrocarbons in urban runoff: Their contribution to the wastewaters. Water Res. 1991, 25, 557–565. [Google Scholar] [CrossRef]
- Moghaddam, A.; Hashemi, S.H.; Ghadiri, A. Aliphatic hydrocarbons in urban runoff sediments: A case study from the megacity of Tehran, Iran. J. Environ. Health Sci. Eng. 2021, 19, 205–216. [Google Scholar] [CrossRef]
- Poynter, J.; Eglinton, G. Molecular composition of three sediments from Hole 717C: The Bengal Fan. Proc. Ocean. Drill. Program Sci. Results 1990, 116, 155–161. [Google Scholar] [CrossRef]
- Jeng, W.L. Higher plant n-alkane average chain length as an indicator of petrogenic hydrocarbon contamination in marine sediments. Mar. Chem. 2006, 102, 242–251. [Google Scholar] [CrossRef]
- Schefuß, E.; Ratmeyer, V.; Stuut, J.B.W.; Jansen, J.H.F.; Sinninghe-Damste, S.J. Carbon isotope analysis of n-alkanes in dust from the lower atmosphere over the central eastern Atlantic. Geochim. Cosmochim. Acta 2003, 67, 1757–1767. [Google Scholar] [CrossRef]
- Ficken, K.J.; Li, B.; Swain, D.; Eglinton, G. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org. Geochem. 2000, 31, 745–749. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, W.; Meyers, P.A.; Xie, S. Lipid biomarkers in the Zoigê-Hongyuan peat deposit: Indicators of Holocene climate changes in West China. Org. Geochem. 2007, 38, 1927–1940. [Google Scholar] [CrossRef]
- Salisu, N.; Augie, N.M.; Sani, A.A.; Abbott, G. Geochemical applications and limitations of pristane to phytane ratio in determining the redox condition of North Sea oil and Kimmeridge source rocks’ paleoenvironment of deposition Dutse. J. Pure Appl. Sci. 2015, 1, 96–103. [Google Scholar]
- Oraegbunam, C.; Osuji, L.; Onojake, M.; Abrakasa, S. Geochemical Appraisal of the Depositional Environment and Source Organic Matter of Crude Oils from Some Oil Fields in Bayelsa State, Nigeria. Iran. J. Oil Gas Sci. Technol. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Cheng, X.; Hou, D.; Zhou, X.; Liu, J.; Diao, H.; Wei, L. Geochemical Characterization of the Eocene Coal-Bearing Source Rocks, Xihu Sag, East China Sea Shelf Basin, China: Implications for Origin and Depositional Environment of Organic Matter and Hydrocarbon Potential. Minerals 2021, 11, 909. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide Vol. 1: Biomarkers and Isotopes in the Environment and Human History, 2nd ed.; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Hakimi, M.; Al-Matary, A.; Ahmed, A. Bulk geochemical characteristics and carbon isotope composition of oils from the Sayhut sub-basin in the Gulf of Aden with emphasis on organic matter input, age and maturity. Egypt. J. Pet. 2018, 27, 361–370. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Gonza’lez-Vila, F.J.; Polvillo, O.; Boski, T.; Moura, D.; de Andre’s, J.R. Biomarker patterns in a time-resolved holocene/terminal Pleistocene sedimentary sequence from the Guadiana river estuarine area (SW Portugal/Spain border). Org. Geochem. 2003, 34, 1601–1613. [Google Scholar] [CrossRef]
- Avdalović, J.; Đurić, A.; Miletić, S.; Ilić, M.; Milić, J.; Vrvić, M.M. Treatment of a mud pit by bioremediation. Waste Manag. Res. 2016, 34, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Beškoski, P.V.; Gojgić-Cvijović, G.; Milić, J.; Ilić, M.; Miletić, S.; Šolević, T.; Vrvić, M.M. Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil)—A field experiment. Chemosphere 2011, 8, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Boski, T.; González-Vila, F.J.; de la Rosa, J.M.; González-Pérez, José A. Discerning natural and anthropogenic organic matter inputs to salt marsh sediments of Ria Formosa lagoon (South Portugal). Environ. Sci. Pollut. Res. 2020, 27, 28962–28985. [Google Scholar] [CrossRef]
- Vogts, A.; Moossen, H.; Rommerskirchen, F.; Rullkötter, J. Distribution patterns and stable carbon isotopic composition of alkanes and alkan-1-ols from plant waxes of African rain forest and savanna C3 species. Org. Geochem. 2009, 40, 1037–1054. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, M.; Eglinton, G.; Lu, H.; Huang, C.Y. Leaf wax lipids as paleovegetational and paleoenvironmental proxies for the Chinese Loess Plateau over the last 170kyr. Quat. Sci. Rev. 2006, 25, 575–594. [Google Scholar] [CrossRef]
- Rommerskirchen, F.; Plader, A.; Eglinton, G.; Chikaraishi, Y.; Rullkotter, J. Chemotaxonomic significance of distribution and stable carbon isotopic composition of long-chain alkanes and alkan-1-ols in C4 grass waxes. Org. Geochem. 2006, 37, 1303–1332. [Google Scholar] [CrossRef]
- Bourbonniere, R.A.; Meyers, P.A. Sedimentary geolipid records of historical changes in the watersheds and productivities of Lakes Ontario and Erie. Limnol. Oceanogr. 1996, 41, 352–359. [Google Scholar] [CrossRef]

| Sample | Extracted Organic Matter (mg/kg) | I Fraction (mg/kg) | II Fraction (mg/kg) | III Fraction (mg/kg) | Range n-Alkanes | Max n-Alkanes |
|---|---|---|---|---|---|---|
| Z-1 | 317.23 | / | 34.95 | 110.22 | C10-33 | C20, C29 |
| Z-2 | 395.23 | 29.06 | 46.50 | 129.81 | C10-33 | C29 |
| Z-3 | 361.80 | 38.19 | 48.24 | 132.66 | C10-33 | C29 |
| Z-4 | 102.59 | 14.66 | 3.66 | 18.32 | C10-33 | C29 |
| Z-5 | 242.01 | 21.64 | 37.38 | 27.55 | C10-33 | C29 |
| Z-6 | 312.30 | 18.26 | 38.35 | 73.05 | C10-33 | C29 |
| Z-7 | 476.08 | 11.90 | 71.41 | 130.92 | C10-33 | C29 |
| Z-8 | 267.14 | / | 27.07 | 68.59 | C10-33 | C27, C29 |
| Z-9 | 318.17 | 19.75 | 24.14 | 61.44 | C10-33 | C31 |
| Z-10 | 1388.03 | 8.29 | 24.86 | 82.87 | C10-33 | C20 |
| Z-11 | 241.46 | 31.22 | 33.31 | 70.77 | C10-33 | C31 |
| Z-12 | 278.34 | / | 17.04 | 58.70 | C10-33 | C29 |
| Z-13 | 431.20 | 7.70 | 30.80 | 128.33 | C10-33 | C31 |
| Z-14 | 344.50 | 14.76 | 27.07 | 54.14 | C10-33 | C31 |
| Z-15 | 678.21 | 18.63 | 26.08 | 89.43 | C10-33 | C31 |
| Z-16 | 1429 | 12.99 | 110.39 | 82.25 | C10-33 | C22/C27 |
| Z-17 | 556.42 | 7.25 | 18.12 | 27.19 | C10-33 | C31 |
| Z-18 | 231.80 | 2.37 | 18.92 | 73.32 | C10-33 | C29 |
| Z-19 | 217.13 | / | / | 60.10 | C10-33 | C31 |
| Z-20 | 167.57 | 1.90 | / | 36.18 | C10-33 | C29 |
| Sample | S/L | ACL | CPI1 | CPI2 | CPI3 | TAR | Paq | Pwax | AI | TPH | UCM | U/R | Pr/Phy | Pr/nC17 | Phy/nC18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z-1 | 0.31 | 21.90 | 2.57 | 1.30 | 1.98 | 12.32 | 0.33 | 0.76 | 0.47 | 70.5 | 54.80 | 3.10 | 0.65 | 0.89 | 0.63 |
| Z-2 | 0.37 | 6.90 | 3.38 | 0.92 | 2.13 | 16.75 | 0.31 | 0.77 | 0.31 | 66.0 | 70.40 | 6.50 | 0.56 | 0.57 | 0.54 |
| Z-3 | 0.32 | 12.13 | 8.00 | 1.33 | 3.99 | 16.66 | 0.23 | 0.81 | 0.13 | 49.6 | 76.90 | 5.60 | 0.32 | 0.91 | 0.95 |
| Z-4 | 0.20 | 5.00 | 2.37 | 1.58 | 2.00 | 72.96 | 0.36 | 0.70 | 0.34 | 67.1 | 53.8 | 1.50 | 2.57 | 0.78 | 0.80 |
| Z-5 | 0.19 | 11.07 | 2.84 | 1.15 | 2.26 | 27.33 | 0.23 | 0.81 | 0.79 | 70.4 | 3.01 | 0.18 | 0.74 | 0.64 | 0.63 |
| Z-6 | 0.18 | 15.00 | 4.36 | 1.28 | 3.11 | 37.45 | 0.22 | 0.83 | 0.27 | 57.1 | 30.1 | 0.94 | 0.73 | 0.63 | 0.67 |
| Z-7 | 0.23 | 32.29 | 5.04 | 1.02 | 3.12 | 47.16 | 0.32 | 0.83 | 0.35 | 59.5 | 56.7 | 2.48 | 0.37 | 0.49 | 0.62 |
| Z-8 | 0.18 | 61.24 | 1.94 | 1.47 | 1.70 | 39.72 | 0.26 | 0.74 | 0.46 | 77.7 | 42.3 | 2.08 | 0.95 | 0.58 | 0.70 |
| Z-9 | 0.05 | 31.65 | 7.57 | 2.33 | 6.64 | 185.11 | 0.08 | 0.93 | 0.59 | 88.0 | 48.8 | 3.98 | 1.31 | 0.64 | 0.73 |
| Z-10 | 2.26 | 21.71 | 3.45 | 1.06 | 1.39 | 1.315 | 0.51 | 0.62 | 0.37 | 77.5 | 78.4 | 3.70 | 0.10 | 0.52 | 0.65 |
| Z-11 | 0.29 | 7.51 | 5.03 | 1.22 | 3.24 | 12.70 | 0.15 | 0.77 | 0.52 | 79.6 | 20.2 | 0.83 | 0.26 | 0.77 | 0.98 |
| Z-12 | 0.25 | 2.06 | 3.51 | 1.16 | 2.33 | 113.82 | 0.36 | 0.72 | 0.44 | 77.0 | 2.9 | 0.73 | 1.41 | 0.64 | 0.78 |
| Z-13 | 0.22 | 6.00 | 4.37 | 1.89 | 3.38 | 76.82 | 0.19 | 0.83 | 0.56 | 78.4 | 12.2 | 3.75 | 1.90 | 0.60 | 0.56 |
| Z-14 | 0.51 | 3.18 | 4.01 | 1.75 | 2.67 | 11.56 | 0.26 | 0.79 | 0.51 | 79.5 | 21.4 | 3.28 | 1.18 | 0.68 | 0.59 |
| Z-15 | 0.43 | 4.30 | 2.30 | 0.93 | 1.58 | 18.54 | 0.36 | 0.71 | 0.50 | 70.9 | 16.6 | 2.25 | 1.60 | 0.72 | 0.78 |
| Z-16 | 0.68 | 3.28 | 2.51 | 0.79 | 1.28 | 30.92 | 0.51 | 0.56 | 0.28 | 77.9 | 7.7 | 1.00 | 2.15 | 0.77 | 0.70 |
| Z-17 | 0.35 | 21.80 | 1.71 | 1.24 | 1.45 | 14.57 | 0.37 | 0.71 | 0.85 | 75.3 | 17.0 | 1.38 | 1.76 | 0.65 | 0.49 |
| Z-18 | 0.19 | 16.96 | 3.46 | 1.32 | 2.64 | 37.94 | 0.22 | 0.83 | 0.45 | 244 | 33.7 | 2.48 | 1.10 | 0.12 | 0.80 |
| Z-19 | 0.13 | 13.31 | 7.92 | 2.21 | 6.01 | 118.05 | 0.11 | 0.91 | 0.58 | 109 | 17.2 | 1.80 | 2.18 | 0.63 | 0.49 |
| Z-20 | 0.32 | 4.40 | 3.17 | 1.26 | 2.26 | 15.57 | 0.36 | 0.73 | 0.24 | 102 | 18.0 | 1.30 | 0.05 | 0.39 | 0.63 |
| S/L | CPI2 | CPI1 | CPI3 | AI | ACL | TAR | Pwax | Paq | Phy/C18 | Pr/C17 | Pr/Phy | TPH | UCM | U/R | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S/L | |||||||||||||||
| CPI2 | −0.18 | ||||||||||||||
| CPI1 | −0.33 | 0.58 * | |||||||||||||
| CPI3 | −0.38 | 0.91 ** | 0.79* | ||||||||||||
| AI | −0.17 | −0.16 | 0.3 | 0.09 | |||||||||||
| ACL | −0.09 | 0.06 | −0.41 | 0.06 | 0.19 | ||||||||||
| TAR | −0.39 | 0.51 * | 0.70 * | 0.73 ** | 0.2 | −0.58 * | |||||||||
| Pwax | −0.62 ** | 0.68 * | 0.69 * | 0.83 ** | 0.32 | −0.3 | 0.89 | ||||||||
| Paq | 0.68 ** | −0.65 * | −0.71 ** | −0.81 ** | −0.29 | 0.33 | −0.49 | −0.92 ** | |||||||
| Phy/C18 | −0.05 | 0.12 | 0.02 | 0.03 | −0.02 | −0.05 | −0.03 | −0.16 | |||||||
| Pr/C17 | −0.06 | 0.18 | −0.17 | 0.03 | −0.37 | −0.13 | −0.03 | −0.09 | 0.03 | ||||||
| Pr/Phy | −0.02 | −0.08 | 0.35 | 0.09 | 0.3 | −0.03 | 0.45 | 0.09 | 0.39 | 0.48 | |||||
| TPH | −0.09 | −0.14 | −0.13 | 0.09 | 0.1 | 0.04 | 0.09 | 0.19 | −0.55 | 0.78 * | 0.013 | ||||
| UCM | 0.35 | 0.23 | −0.35 | 0.06 | −0.49 | −0.13 | 0.14 | 0.04 | 0.07 | −070 ** | −0.16 | −0.6 | −0.15 | ||
| U/R | 0.18 | 0.18 | 0.13 | 0.21 | −0.33 | −0.32 | −0.03 | 0.2 | −0.09 | −0.23 | −0.08 | 0.2 | −0.09 | 0.72 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dević, G.; Bulatović, S.; Avdalović, J.; Marić, N.; Milić, J.; Ilić, M.; Šolević Knudsen, T. Lipid Biomarkers in Urban Soils of the Alluvial Area near Sava River, Belgrade, Serbia. Molecules 2025, 30, 154. https://doi.org/10.3390/molecules30010154
Dević G, Bulatović S, Avdalović J, Marić N, Milić J, Ilić M, Šolević Knudsen T. Lipid Biomarkers in Urban Soils of the Alluvial Area near Sava River, Belgrade, Serbia. Molecules. 2025; 30(1):154. https://doi.org/10.3390/molecules30010154
Chicago/Turabian StyleDević, Gordana, Sandra Bulatović, Jelena Avdalović, Nenad Marić, Jelena Milić, Mila Ilić, and Tatjana Šolević Knudsen. 2025. "Lipid Biomarkers in Urban Soils of the Alluvial Area near Sava River, Belgrade, Serbia" Molecules 30, no. 1: 154. https://doi.org/10.3390/molecules30010154
APA StyleDević, G., Bulatović, S., Avdalović, J., Marić, N., Milić, J., Ilić, M., & Šolević Knudsen, T. (2025). Lipid Biomarkers in Urban Soils of the Alluvial Area near Sava River, Belgrade, Serbia. Molecules, 30(1), 154. https://doi.org/10.3390/molecules30010154







