Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis of the Aqueous Extract of Amphiroa anceps
2.2. Characterization of the Aqueous (HA) and Liposome-Formulated Aqueous Extract (NHA) of Amphiroa anceps
2.2.1. FTIR Analysis
2.2.2. Absorption, Size, and Zeta Potential
2.2.3. Surface Morphology and Encapsulation Efficiency
2.2.4. GC-MS Analysis of HA
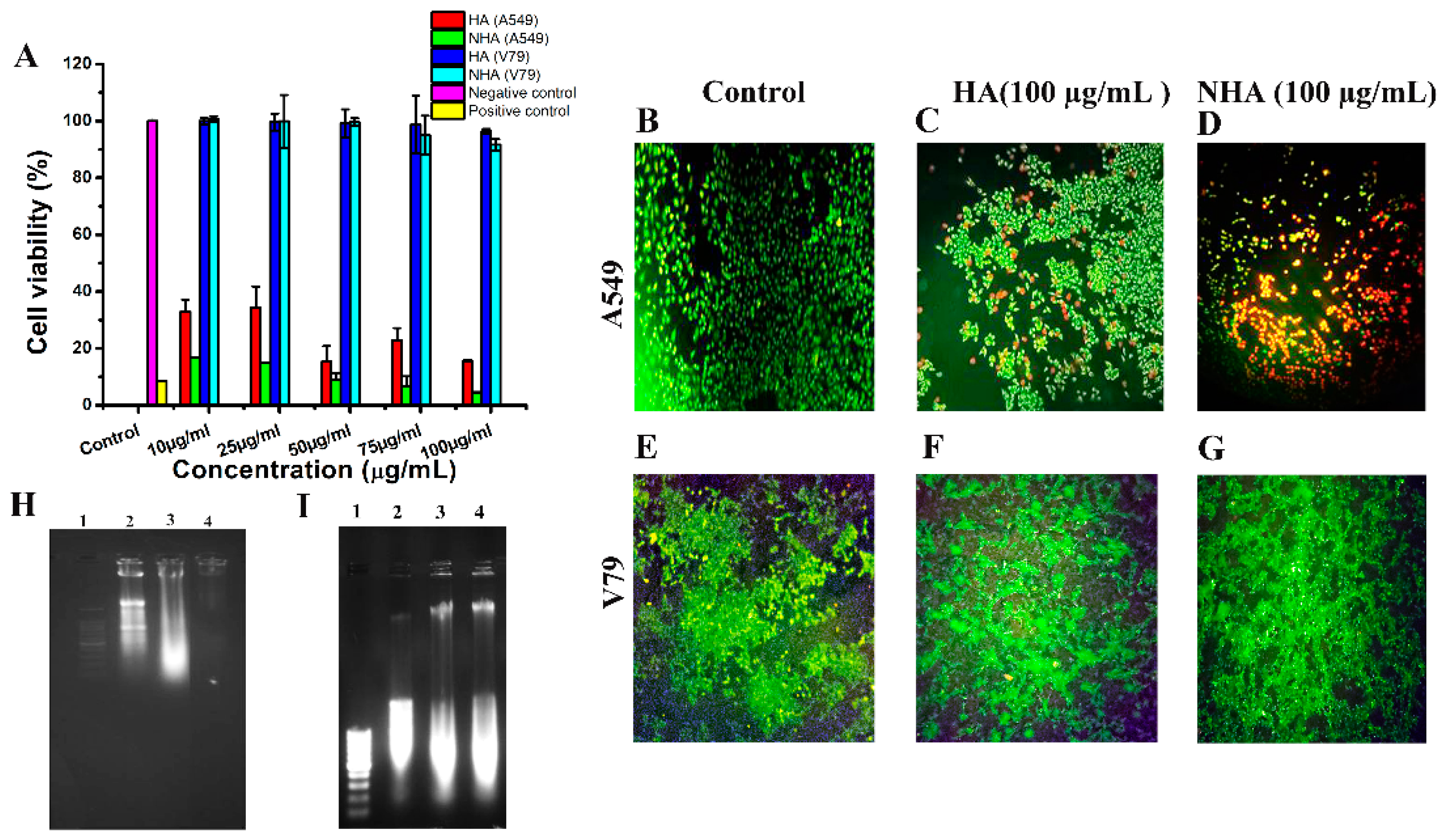
2.3. Cytotoxicity Assay
2.3.1. MTT Assay
2.3.2. Live–Dead Assay
2.3.3. Morphological Change Observation
2.4. Isolation of Apoptotic/Necrotic DNA
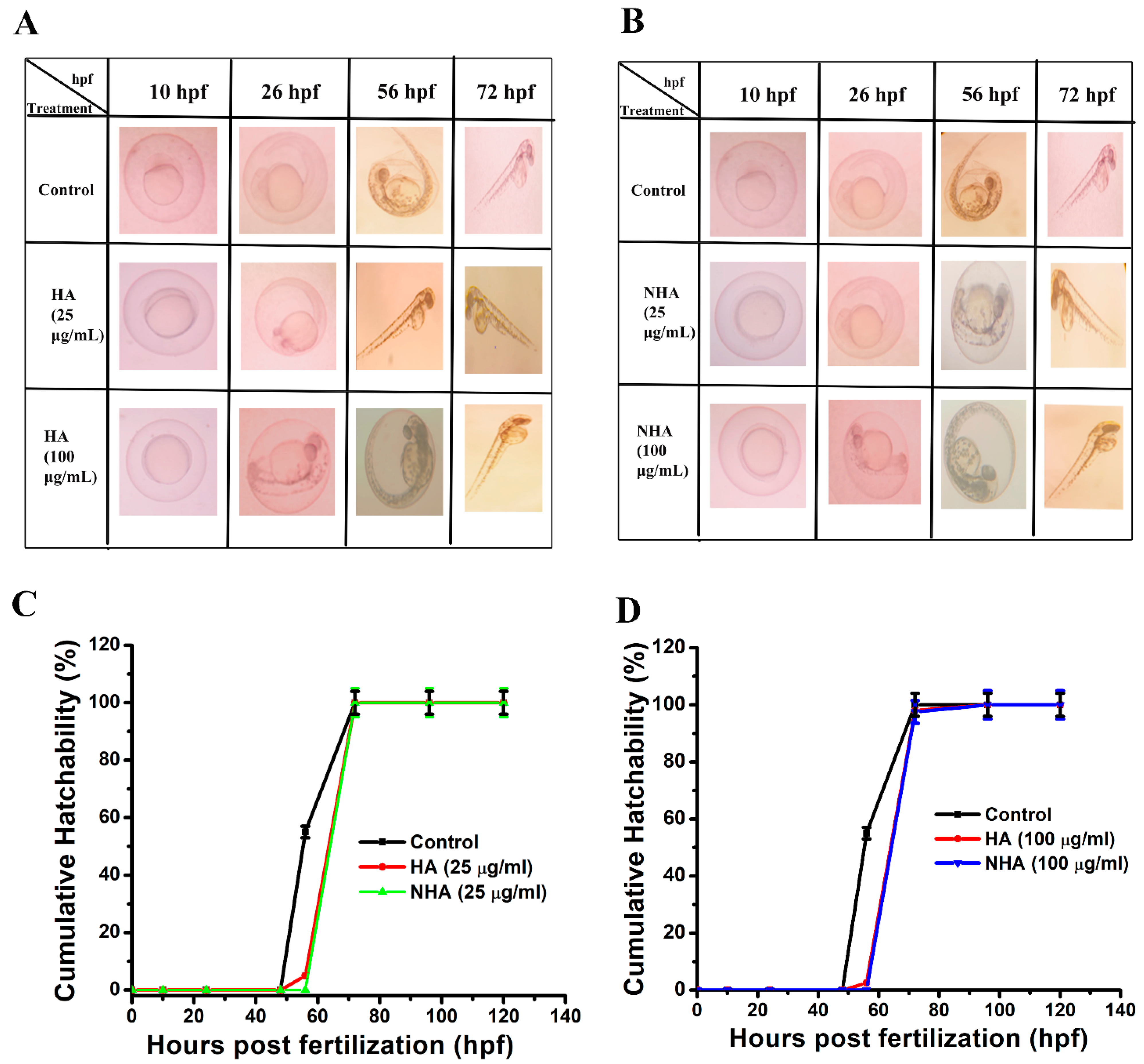
2.5. In Vivo Toxicity Study
3. Material and Methods
3.1. Materials
3.2. Amphiroa Anceps Extract Preparation
3.3. Liposomal Formulation of Aqueous Extract of Amphiroa anceps
3.4. Characterization
3.5. GC-MS Analysis of Aqueous Extract of Amphiroa anceps
3.6. Encapsulation Efficiency
3.7. Cytotoxicity Assays
3.7.1. MTT
3.7.2. Live Dead Assay
3.7.3. Morphological Change Observation
3.8. Isolation of Apoptotic/Necrotic DNA
3.9. Cumulative Hatchability Assay
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Chaturvedi, M.; Das, P.; Stephen, S.; Mathur, P. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J. Med. Res. 2022, 156, 598–607. [Google Scholar]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.; Mofeed, J.; El-Bilawy, E.; Ward, F. Antiviral activity of five filamentous cyanobacteria against coxsackievirus B3 and rotavirus. Arch. Microbiol. 2020, 202, 213–223. [Google Scholar] [CrossRef] [PubMed]
- El-Bilawy, E.H.; Al-Mansori, A.-N.A.; Soliman, S.A.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Sabry, A.E.-N.; Elsharkawy, M.M.; Heflish, A.A.; Behiry, S.I. Antifungal, antiviral, and HPLC analysis of phenolic and flavonoid compounds of Amphiroa Anceps extract. Sustainability 2022, 14, 12253. [Google Scholar] [CrossRef]
- Karthick, M.; Balachandar, M.; Raja, M.; Raj, R.A. Antibacterial activity of red alga Amphiroa anceps (Rhodophyceae: Lithophyllaceae) against selected human pathogens. Sci. Acta Xaver 2019, 10, 15–19. [Google Scholar]
- Kawsar, S.M.; Fujii, Y.; Matsumoto, R.; Yasumitsu, H.; Ozeki, Y. Protein R-phycoerythrin from marine red alga Amphiroa anceps: Extraction, purification and characterization. Phytol. Balc. 2011, 17, 347–354. [Google Scholar]
- Raj, E.D.S. UV–VIS and HPLC studies on Amphiroa anceps (Lamarck) Decaisne. Arab. J. Chem. 2016, 9, S907–S913. [Google Scholar]
- Tanna, B.; Choudhary, B.; Mishra, A.; Yadav, S.; Chauhan, O.; Elansary, H.O.; Shokralla, S.; El-Abedin, T.K.Z.; Mahmoud, E.A. Biochemical and Anti-proliferative activities of seven abundant tropical red seaweeds confirm nutraceutical potential of Grateloupia indica. Arab. J. Chem. 2022, 15, 103868. [Google Scholar] [CrossRef]
- Girigoswami, A.; Girigoswami, K. Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes 2023, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Ps, S.S.; Guha, A.; Deepika, B.; Udayakumar, S.; Nag, M.; Lahiri, D.; Girigoswami, A.; Girigoswami, K. Nanocargos designed with synthetic and natural polymers for ovarian cancer management. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, T.; CH, M.N.; Anburaj, G.; Ahmed, S.S.; Gopinath, V.; Munuswamy-Ramanujam, G.; Rao, S.K.; Kamath, M.S. Controlled release of kaempferol from porous scaffolds augments in-vitro osteogenesis in human osteoblasts. J. Drug Deliv. Sci. Technol. 2023, 83, 104396. [Google Scholar] [CrossRef]
- Balaji, S.; Karthikeyan, R.; Kiran, V.; Yuvaraj, B.; Nagaraj, S.; Manivannan, S.; Narayan, S. Platelet Lysate as a Promising Medium for Nanocarriers in the Management and Treatment of Ocular Diseases. Curr. Ophthalmol. Rep. 2022, 10, 19–41. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A. Smart nanocarriers as an emerging platform for cancer therapy: A review. Molecules 2021, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Sanei-Dehkordi, A.; Ghasemian, A.; Zarenezhad, E.; Qasemi, H.; Nasiri, M.; Osanloo, M. Nanoliposomes containing three essential oils from the Artemisia genus as effective larvicides against Aedes aegypti and Anopheles stephensi. Sci. Rep. 2023, 13, 11002. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Co-encapsulation of curcumin and vitamin D3 in mixed phospholipid nanoliposomes using a continuous supercritical CO2 assisted process. J. Taiwan Inst. Chem. Eng. 2022, 132, 104120. [Google Scholar] [CrossRef]
- Eid, M.M. Characterization of Nanoparticles by FTIR and FTIR-Microscopy. In Handbook of Consumer Nanoproducts; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–30. [Google Scholar]
- Madathil, D.; Chidambaram, R. In-silico analysis of bioactive compounds extracted from seaweed Amphiroa anceps on the pathogenicity of bacteria. Indian J. Geo-Mar. Sci. (IJMS) 2022, 51, 379–387. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2011; pp. 63–70. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Nanoencapsulated myricetin to improve antioxidant activity and bioavailability: A study on zebrafish embryos. Chemistry 2021, 4, 1–17. [Google Scholar] [CrossRef]
- Mofeed, J.; Deyab, M.; Sabry, A.E.-N.; Ward, F. In vitro anticancer activity of five marine seaweeds extract from Egypt against human breast and colon cancer cell lines. Preprint 2021. [Google Scholar] [CrossRef]
- Gowtham, P.; Girigoswami, K.; Pallavi, P.; Harini, K.; Gurubharath, I.; Girigoswami, A. Alginate-Derivative Encapsulated Carbon Coated Manganese-Ferrite Nanodots for Multimodal Medical Imaging. Pharmaceutics 2022, 14, 2550. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Janani, G.; Girigoswami, A.; Girigoswami, K. Advantages of nanomedicine over the conventional treatment in Acute myeloid leukemia. J. Biomater. Sci. Polym. Ed. 2023, 35, 415–441. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Christopher, J.; Chamcheu; Mukhtar, H. Impact of nanotechnology in cancer: Emphasis on nanochemoprevention. Int. J. Nanomed. 2012, 7, 591–605. [Google Scholar]
- Lubobi, S.; Matunda, C.; Kumar, V.; Omboki, B. Isolation of bioactive secondary metabolites from seaweeds Amphiroa anceps against chicken meat associated pathogens. J. Antimicrob. Agents 2016, 2, 2. [Google Scholar]
- Thirumalai, A.; Girigoswami, K.; Harini, K.; Pallavi, P.; Gowtham, P.; Girigoswami, A. A review of the current state of probiotic nanoencapsulation and its future prospects in biomedical applications. Biocatal. Agric. Biotechnol. 2024, 57, 103101. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, anticancer activity and phytochemical analysis of green algae, Chaetomorpha collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef]
- Kumar, L.; Brice, J.; Toberer, L.; Klein-Seetharaman, J.; Knauss, D.; Sarkar, S.K. Antimicrobial biopolymer formation from sodium alginate and algae extract using aminoglycosides. PLoS ONE 2019, 14, e0214411. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Fatima, S.; Velurajan, S. A guide to phytochemical analysis. Int. J. Adv. Res. Innov. Ideas Educ. 2019, 5, 236–245. [Google Scholar]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Preparation and characterization of ATP-loaded chitosan/alginate nanoparticles for therapeutic applications. Appl. Chem. Eng. 2023, 6. [Google Scholar] [CrossRef]
- Biswas, K.; Janani, G.; Udayakumar, S.; Deepika, B.; Girigoswami, K. Rough edges of reduced graphene oxide (rGO) sheets elicit anticancerous activities: An in vitro study. Results Chem. 2023, 6, 101207. [Google Scholar] [CrossRef]
- Ghosh, R.; Girigoswami, K. NADH dehydrogenase subunits are overexpressed in cells exposed repeatedly to H2O2. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2008, 638, 210–215. [Google Scholar] [CrossRef]
- Harini, K.; Alomar, S.Y.; Vajagathali, M.; Manoharadas, S.; Thirumalai, A.; Girigoswami, K.; Girigoswami, A. Niosomal Bupropion: Exploring Therapeutic Frontiers through Behavioral Profiling. Pharmaceuticals 2024, 17, 366. [Google Scholar] [CrossRef]


| S. No. | Name of the Compound | Retention Time | Peak Area% | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|
| 1 | Propanamide, 3,3,3-trifluoro-2-(trifluoromethyl)- | 3.165 | 21.96 | 195 | C4H3F6NO |
| 2 | Decanoic acid, methyl ester | 15.345 | 3.03 | 186 | C11H22O2 |
| 3 | n-Hexadecanoic acid | 15.715 | 50.36 | 256 | C16H32O2 |
| 4 | 4-Tridecene, (Z)- | 17.015 | 2.48 | 182 | C13H26 |
| 5 | Octadecanoic acid | 17.740 | 31.64 | 284 | C18H36O2 |
| 6 | n-Nonadecanol-1 | 19.215 | 17.86 | 284 | C19H40O |
| 7 | 2-Pyrazoline-3-carboxylic acid, 5-hydroxy-1-(4-methylbenzoyl)-5-phenyl-, methyl ester | 19.975 | 16.59 | 338 | C19H18N2O4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janani, G.; Girigoswami, A.; Deepika, B.; Udayakumar, S.; Girigoswami, K. Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management. Molecules 2024, 29, 2077. https://doi.org/10.3390/molecules29092077
Janani G, Girigoswami A, Deepika B, Udayakumar S, Girigoswami K. Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management. Molecules. 2024; 29(9):2077. https://doi.org/10.3390/molecules29092077
Chicago/Turabian StyleJanani, Gopalarethinam, Agnishwar Girigoswami, Balasubramanian Deepika, Saranya Udayakumar, and Koyeli Girigoswami. 2024. "Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management" Molecules 29, no. 9: 2077. https://doi.org/10.3390/molecules29092077
APA StyleJanani, G., Girigoswami, A., Deepika, B., Udayakumar, S., & Girigoswami, K. (2024). Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management. Molecules, 29(9), 2077. https://doi.org/10.3390/molecules29092077








