Microencapsulation of Essential Oils Using Faba Bean Protein and Chia Seed Polysaccharides via Complex Coacervation Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Microcapsule Yield and Encapsulation Efficiency
2.2. Density, Carr Index, and Hausner Ratio
2.3. Solubility, Moisture Content, and Hygroscopicity
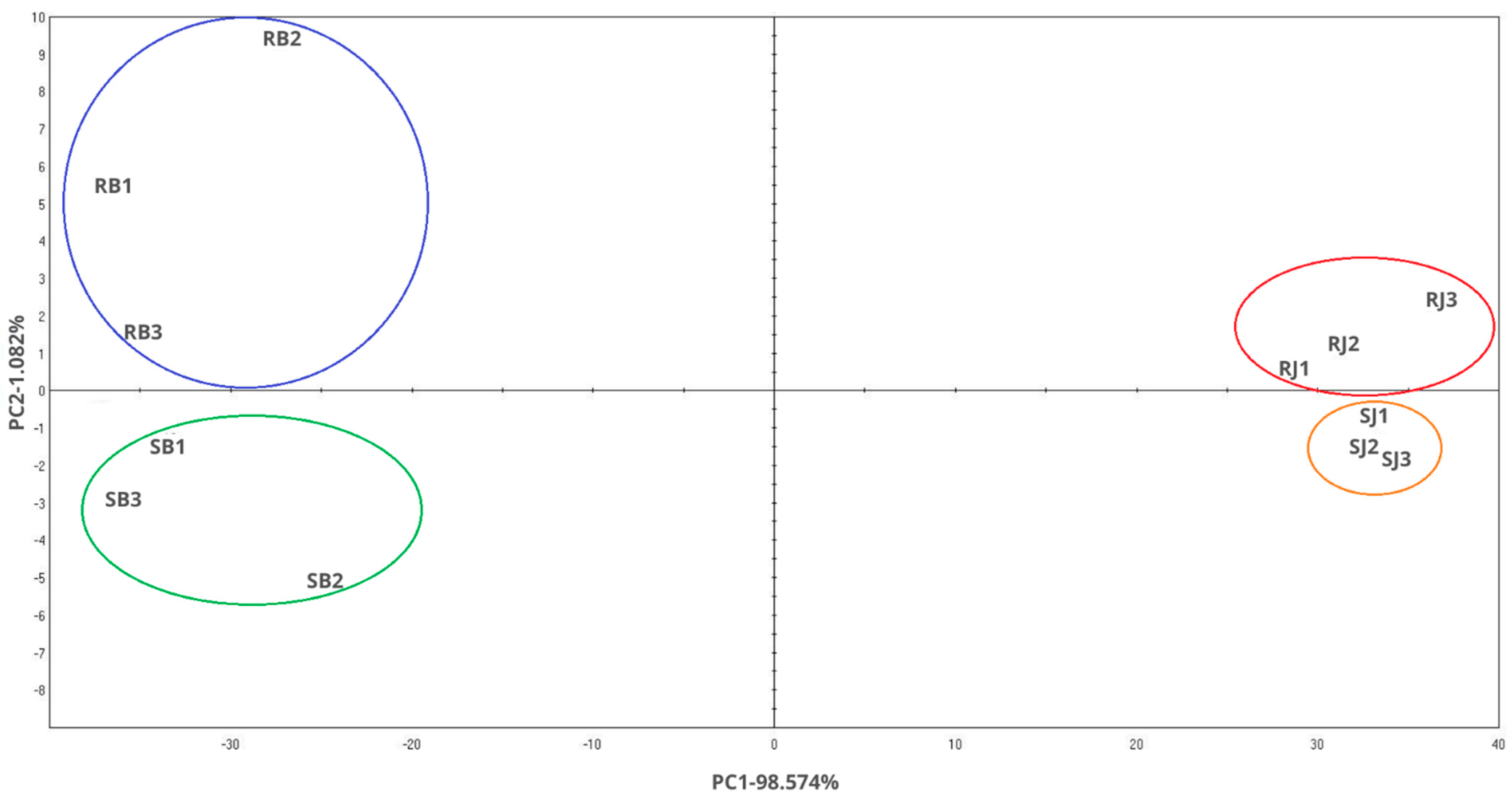
2.4. Color Measurement
| Sample | MY [%] | EE [%] | S [%] | H [%] | MC [%] | ρbulk [g/cm3] | ρtap [g/cm3] | CI [%] | HR | L* | a* | b* | SI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RJ1 | 49.27 ± 0.00 a | 83.23 ± 6.99 ab | 12.58 ± 0.10 a | 62.74 ± 0.00 abcd | 4.78 ± 0.20 a | 0.13 ± 0.00 a | 0.24 ± 0.01 a | 45.83 ± 0.34 a | 1.85 ± 0.01 a | 90.91 ± 1.93 a | 1.53 ± 0.08 ef | 8.91 ± 0.54 a | 0.47 ± 0.00 d |
| RJ2 | 48.89 ± 0.02 e | 84.61 ± 6.68 abc | 28.88 ± 0.10 a | 61.94 ± 0.01 bcd | 4.20 ± 0.23 a | 0.14 ± 0.00 b | 0.24 ± 0.00 e | 45.83 ± 0.67 h | 1.85 ± 0.03 h | 89.35 ± 0.20 e | 0.99 ± 0.09 ac | 7.69 ± 0.32 bd | 0.49 ± 0.00 a |
| RJ3 | 49.01 ± 0.00 b | 74.39 ± 6.60 ab | 27.21 ± 0.06 a | 50.05 ± 0.00 abc | 4.04 ±0.06 a | 0.15 ± 0.00 bc | 0.28 ± 0.00 bc | 46.43 ± 1.63 e | 1.87 ± 0.04 e | 95.93 ± 0.55 b | 1.20 ± 0.26 abd | 8.53 ± 0.29 abc | 0.40 ± 0.01 i |
| RB1 | 48.64 ± 0.02 d | 76.04 ± 6.35 b | 12.64 ± 0.15 a | 66.78 ± 0.00 d | 4.68 ± 0.17 a | 0.13 ± 0.00 e | 0.24 ± 0.01 bc | 45.81 ± 1.17 cd | 1.86 ± 0.06 cd | 87.85 ± 0.94 be | 1.36 ± 0.34 def | 8.35 ± 0.51 abcd | 0.37 ± 0.00 c |
| RB2 | 49.31 ± 0.00 f | 87.85 ± 4.71 ac | 31.70 ± 0.08 a | 76.98 ± 0.00 ae | 7.65 ± 0.10 a | 0.11 ± 0.00 d | 0.21 ± 0.00 e | 47.62 ± 1.05 bc | 1.91 ± 0.03 bc | 88.65 ± 0.18 d | 1.07 ± 0.02 abc | 7.50 ± 0.36 d | 0.45 ± 0.01 f |
| RB3 | 49.04 ± 0.01 g | 77.08 ± 3.21 abc | 22.60 ± 0.06 a | 40.40 ± 0.00 e | 3.72 ± 0.05 a | 0.16 ± 0.00 g | 0.30 ± 0.00 b | 46.67 ± 2.54 g | 1.88 ± 0.03 g | 91.32 ± 0.87 a | 1.09 ± 0.08 abc | 7.63 ± 0.31 d | 0.35 ± 0.00 e |
| SJ1 | 49.17 ± 0.02 c | 79.49 ± 7.25 ab | 8.53 ± 0.10 a | 50.91 ± 0.01 ae | 3.95 ± 0.06 a | 0.16 ± 0.01 c | 0.30 ± 0.01 cd | 46.72 ± 2.95 e | 1.89 ± 0.12 e | 91.70 ± 0.45 a | 1.20 ± 0.08 abd | 7.93 ± 0.72 ac | 0.41 ± 0.00 g |
| SJ2 | 49.27 ± 0.00 c | 85.11 ± 6.92 c | 33.68 ± 0.13 a | 74.97 ± 0.01 ab | 7.46 ± 0.12 a | 0.11 ± 0.00 a | 0.20 ± 0.00 a | 44.28 ± 1.05 a | 1.82 ± 0.03 a | 89.57 ± 0.38 a | 1.56 ± 0.13 f | 9.91 ± 0.58 e | 0.44 ± 0.00 c |
| SJ3 | 49.29 ± 0.01 a | 74.54 ± 4.28 ab | 14.26 ± 0.15 a | 61.00 ± 0.01 cd | 4.20 ± 0.06 a | 0.14 ± 0.00 a | 0.26 ± 0.00 a | 46.17 ± 1.99 a | 1.88 ± 0.02 a | 94.91 ± 0.84 bc | 0.99 ± 0.09 ac | 8.57 ± 1.03 abc | 0.38 ± 0.01 h |
| SB1 | 49.18 ± 0.02 h | 65.64 ± 6.45 ab | 18.11 ± 0.06 a | 54.55 ± 0.01 abe | 4.13 ± 0.06 a | 0.14 ± 0.01 a | 0.26 ± 0.00 a | 46.15 ± 0.93 a | 1.86 ± 0.03 a | 92.24 ± 0.96 ac | 1.19 ± 0.08 abd | 8.91 ± 0.62 a | 0.45 ± 0.00 d |
| SB2 | 49.13 ± 0.02 a | 82.42 ± 7.30 ac | 38.38 ± 0.06 a | 63.93 ± 0.00 abc | 4.27 ± 0.11 a | 0.14 ± 0.00 f | 0.25 ± 0.01 a | 48.01 ± 1.05 f | 1.92 ± 0.03 f | 91.46 ± 0.19 d | 1.29 ± 0.06 bde | 8.57 ± 0.05 abc | 0.47 ± 0.00 b |
| SB3 | 48.91 ± 0.01 b | 80.00 ± 4.52 ab | 13.51 ± 0.10 a | 73.00 ± 0.03 cd | 5.02 ± 0.06 a | 0.12 ± 0.00 d | 0.23 ± 0.00 d | 47.82 ± 3.66 b | 1.92 ± 0.09 b | 95.03 ± 0.49 d | 0.90 ± 0.07 c | 7.95 ± 0.35 bcd | 0.37 ± 0.01 ab |
| S.E.M | 0.00 | 54.0 | 0.000014 | 0.000037 | 3.44 | 0.0030 | 79.2 | 780.02 | 135.926 | 0.7 | 0.02080 | 0.281 | 0.000033 |
| Oil | ** | NS | ** | ** | NS | ** | ** | ** | ** | ** | NS | ** | ** |
| MR | ** | * | NS | NS | NS | ** | ** | ** | ** | ** | ** | * | NS |
| EO | ** | NS | * | NS | NS | ** | ** | ** | ** | ** | NS | ** | ** |
| Oil*MR | ** | NS | NS | NS | NS | ** | ** | NS | NS | NS | ** | ** | ** |
| Oil*EO | ** | NS | ** | NS | NS | ** | ** | * | NS | ** | NS | NS | ** |
| MR*EO | ** | * | NS | NS | NS | ** | * | ** | ** | ** | NS | NS | ** |
| Oil*MR*EO | ** | NS | NS | NS | NS | ** | * | ** | ** | NS | NS | NS | ** |
2.5. Particle Size Distribution
2.6. Thermal Stability
| Sample | Ton [°C] | Tmax [°C] | Tend [°C] | ∆H [mJ] |
|---|---|---|---|---|
| RJ1 | 122.81 ± 0.01 | 133.79 ± 0.01 | 156.11 | −523.77 ± 0.01 |
| RJ2 | 154.70 ± 0.01 | 173.79 ± 0.01 | 195.12 ± 0.01 | −438.73 ± 0.01 |
| RJ3 | 87.01 ± 0.01 | 132.08 ± 0.01 | 157.47 ± 0.02 | −361.65 ± 0.01 |
| RB1 | 156.01 ± 0.01 | 158.99 ± 0.02 | 170.42 ± 0.02 | −120.11 ± 0.00 |
| RB2 | 177.52 ± 0.01 | 177.55 ± 0.02 | 181.40 ± 0.00 | −428.38 ± 0.00 |
| RB3 | 120.01 ± 0.02 | 135.35 ± 0.00 | 151.83 ± 0.01 | −376.06 ± 0.01 |
| SJ1 | 105.23 ± 0.00 | 133.89 ± 0.00 | 172.51 ± 0.01 | −398.25 ± 0.00 |
| SJ2 | 145.19 ± 0.00 | 151.69 ± 0.01 | 163.82 ± 0.01 | −413.72 ± 0.00 |
| SJ3 | 98.06 ± 0.01 | 134.72 ± 0.01 | 159.73 ± 0.01 | −369.17 ± 0.01 |
| SB1 | 128.33 ± 0.01 | 128.58 ± 0.01 | 174.08 ± 0.01 | −466.23 ± 0.01 |
| SB2 | 155.01 ± 0.01 | 165.76 ± 0.00 | 177.50 ± 0.01 | −490.12 ± 0.01 |
| SB3 | 56.80 ± 0.01 | 116.90 ± 0.02 | 155.52 ± 0.01 | −392.99 ± 0.00 |
| FB | 48.97 ± 0.01 | 63.60 ± 0.01 | 76.94 ± 0.02 | −37.27 ± 0.01 |
| CHP | 129.52 ± 0.02 | 139.62 ± 0.02 | 154.18 ± 0.01 | −9663.97 ± 0.02 |
| JEO | 24.71 ± 0.00 | 89.17 ± 0.00 | 140.95 ± 0.01 | −80.00 ± 0.01 |
| BPO | 26.81 ± 0.01 | 97.10 ± 0.02 | 148.11 ± 0.00 | −92.36 ± 0.01 |
| RSO | 184.61 ± 0.01 | 199.29 ± 0.02 | 214.88 ± 0.02 | 102.75 ± 0.01 |
| SBO | 155.34 ± 0.01 | 174.31 ± 0.00 | 198.20 ± 0.02 | 108.87 ± 0.00 |
| T80 | 116.40 ± 0.00 | 138.82 ± 0.02 | 154.42 ± 0.00 | 58.61 ± 0.00 |
2.7. Fourier Transform Infrared Spectroscopy
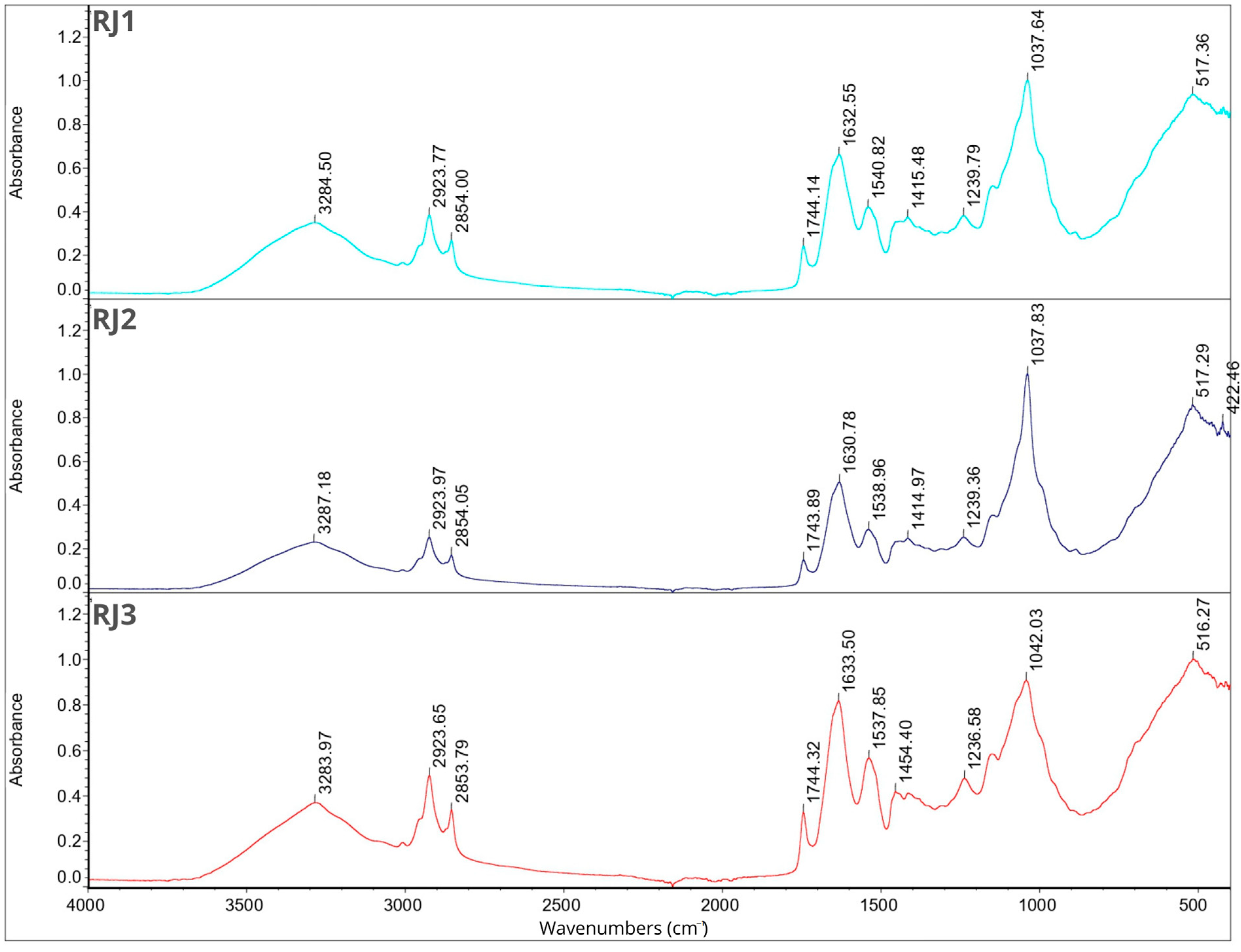
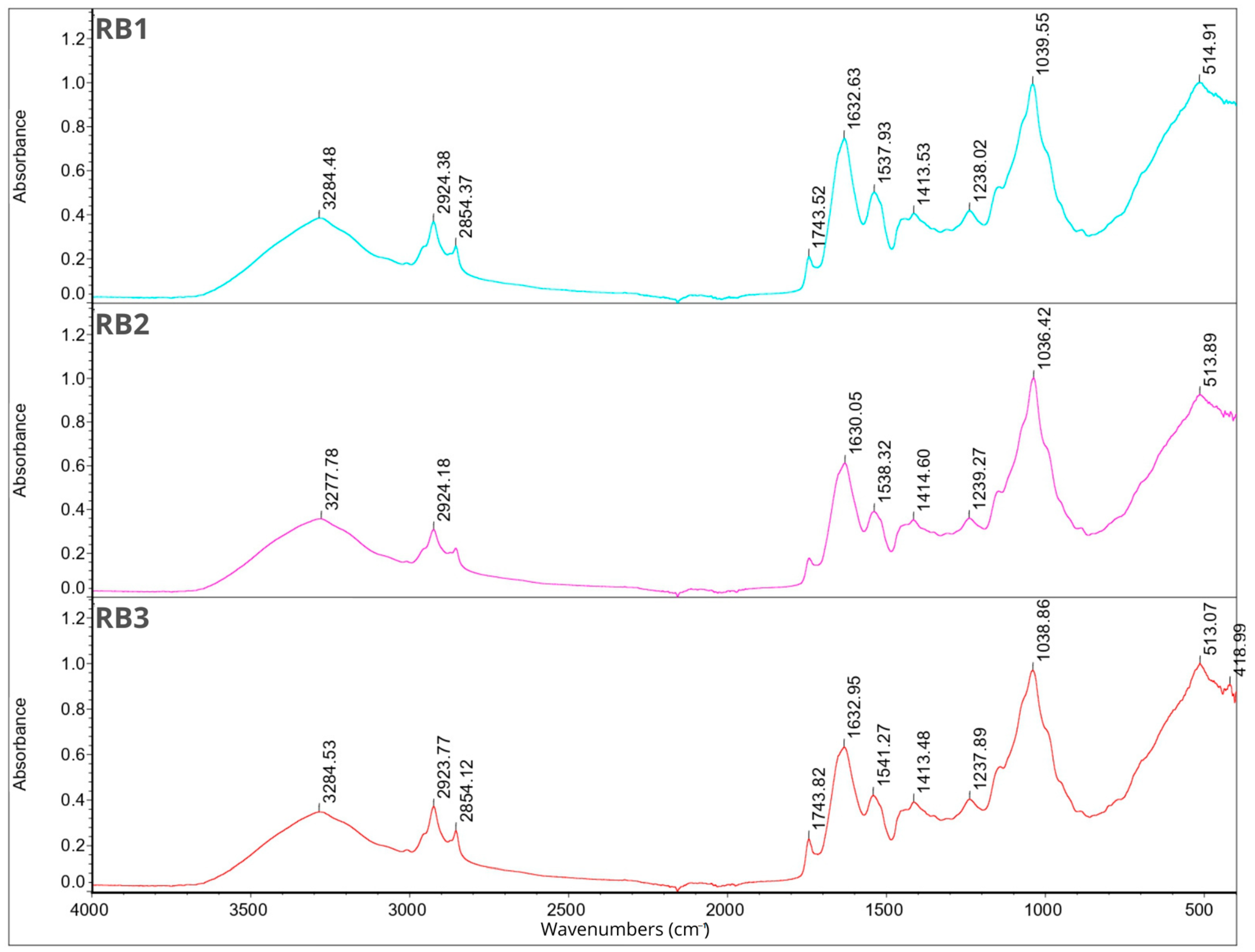
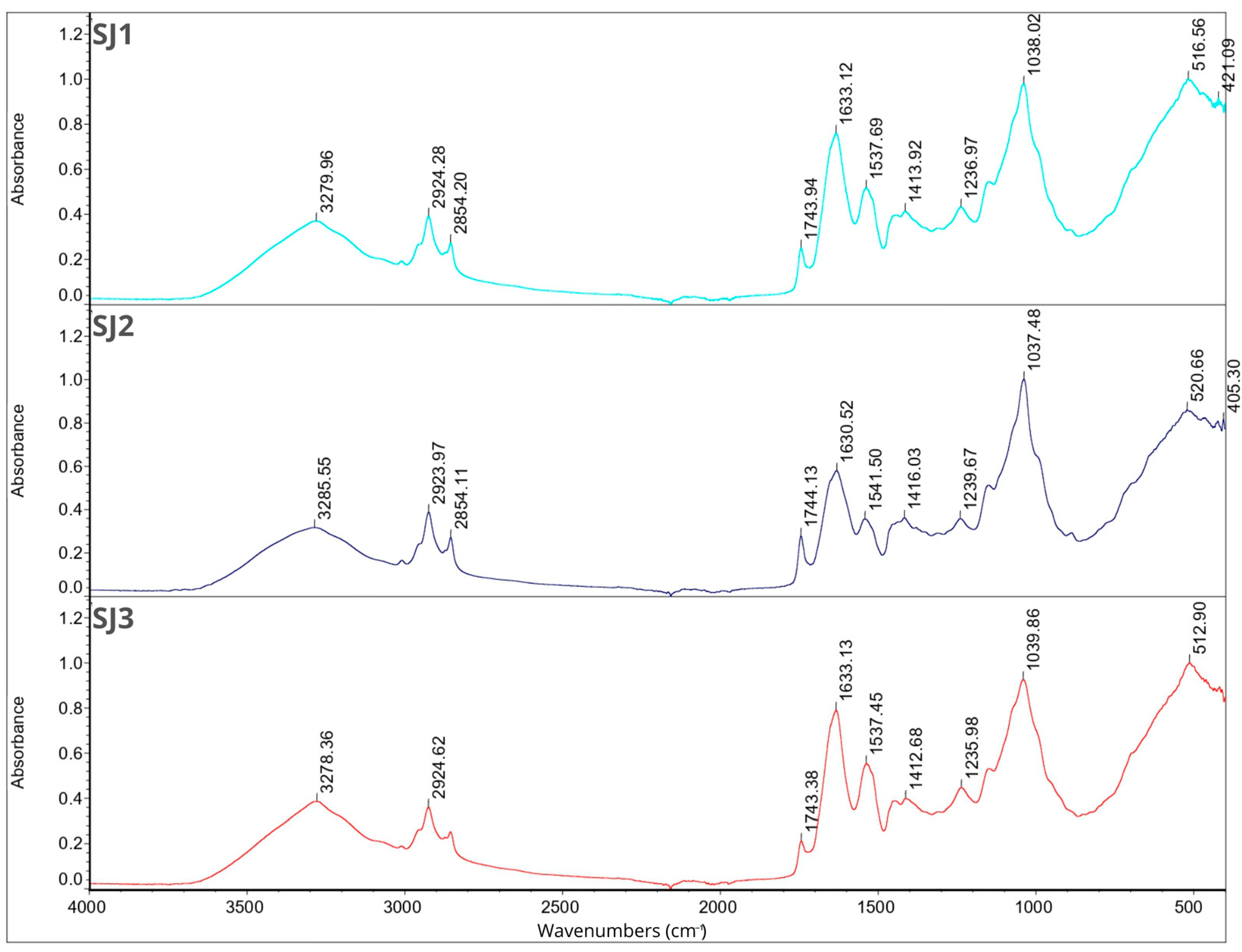
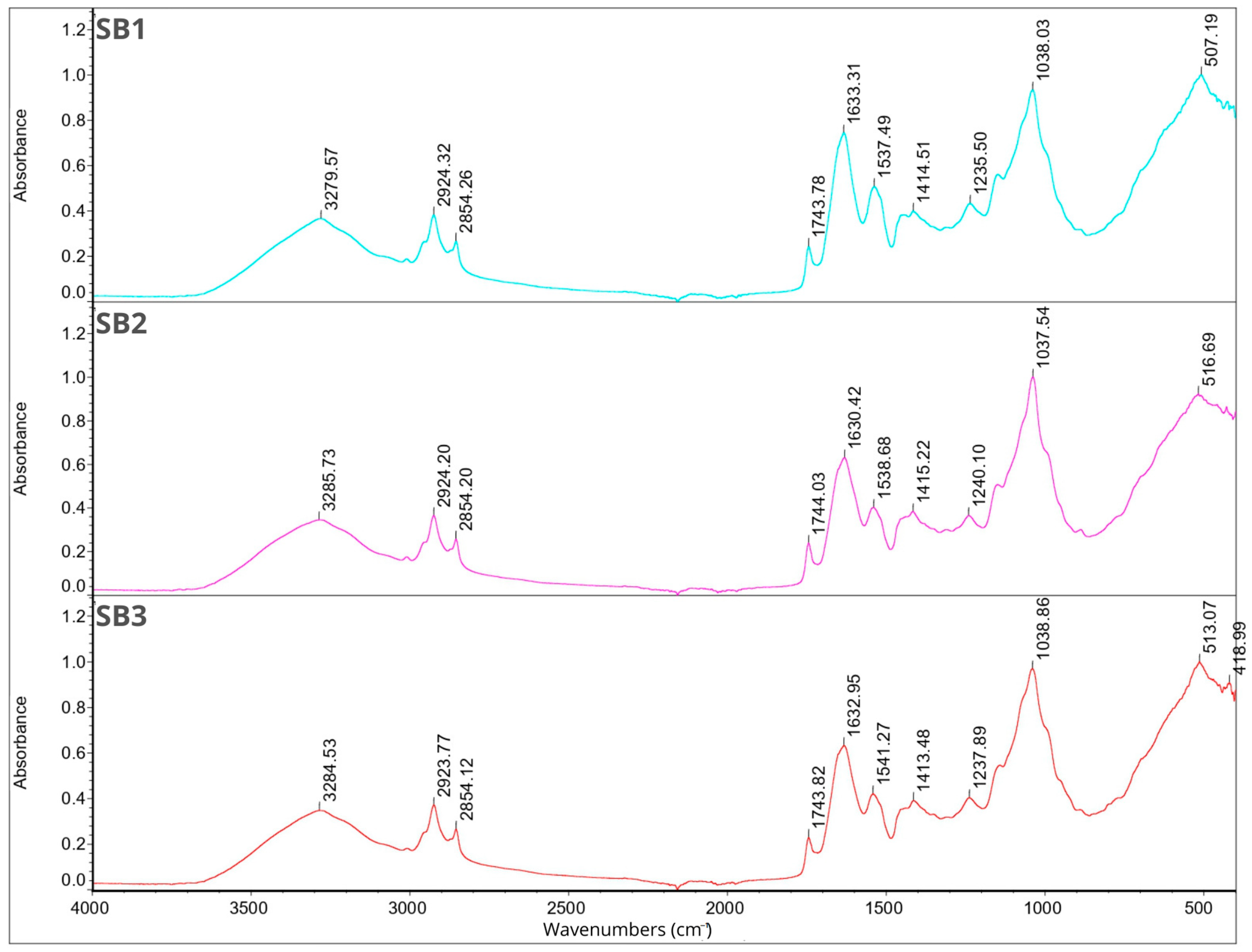
2.8. Smell Pattern
3. Materials and Methods
3.1. Materials
3.2. Chia Polysaccharide Extraction
3.3. Preparation of Coacervates
3.4. Microcapsule Yield and Encapsulation Efficiency
3.5. Density, Carr Index, and Hausner Ratio
3.6. Solubility, Moisture Content, and Hygroscopicity
3.7. Color Measurement
3.8. Particle Size Distribution
3.9. Thermal Stability
3.10. Fourier Transform Infrared Spectroscopy (FT-IR)
3.11. Smell Pattern
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sing, C.E.; Perry, S.L. Recent Progress in the Science of Complex Coacervation. Soft Matter 2020, 16, 2885–2914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shi, H.; Li, Z.; He, C. Recent Advances in Complex Coacervation Design from Macromolecular Assemblies and Emerging Applications. Macromol. Rapid Commun. 2020, 41, e2000149. [Google Scholar] [CrossRef] [PubMed]
- Bastos, L.P.H.; Vicente, J.; dos Santos, C.H.C.; de Carvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of black pepper (Piper nigrum L.) essential oil with gelatin and sodium alginate by complex coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani Gorji, E.; Waheed, A.; Ludwig, R.; Toca-Herrera, J.L.; Schleining, G.; Ghorbani Gorji, S. Complex coacervation of milk proteins with sodium alginate. J. Agric. Food Chem. 2018, 66, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, E.; Razavi, S.M.A. Fabrication and characterisation of milk proteins-chitosan complex coacervates. Int. Dairy J. 2023, 145, 105716. [Google Scholar] [CrossRef]
- Jun-xia, X.; Hai-yan, Y.; Jian, Y. Microencapsulation of sweet orange oil by complex coacervation with soybean protein isolate/gum Arabic. Food Chem. 2011, 125, 1267–1272. [Google Scholar] [CrossRef]
- Li, G.Y.; Chen, Q.H.; Su, C.R.; Wang, H.; He, S.; Liu, J.; Nag, A.; Yuan, Y. Soy protein-polysaccharide complex coacervate under physical treatment: Effects of pH, ionic strength and polysaccharide type. Innov. Food Sci. Emerg. Technol. 2021, 68, 102612. [Google Scholar] [CrossRef]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.E.; Maugard, T. Complex coacervation of pea protein isolate and tragacanth gum: Comparative study with commercial polysaccharides. Innov. Food Sci. Emerg. Technol. 2021, 69, 102641. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Phase behavior and complex coacervation of concentrated pea protein isolate-beet pectin solution. Food Chem. 2020, 307, 125536. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Kidwai, M.K.; Siddiq, M.; Sidhu, J.S. Faba (broad) bean production, processing, and nutritional profile. In Dry Beans and Pulses: Production, Processing, and Nutrition; Wiley: Hoboken, NJ, USA, 2022; pp. 359–381. [Google Scholar]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.). Agronomy 2019, 9, 209. [Google Scholar] [CrossRef]
- Arogundade, L.A.; Tshay, M.; Shumey, D.; Manazie, S. Effect of ionic strength and/or pH on Extractability and physico-functional characterization of broad bean (Vicia faba L.) Protein concentrate. Food Hydrocoll. 2006, 20, 1124–1134. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Makri, E.; Papalamprou, E.; Doxastakis, G. Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocoll. 2005, 19, 583–594. [Google Scholar] [CrossRef]
- Capitani, M.I.; Corzo-Rios, L.J.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A.; Nolasco, S.M.; Tomás, M.C. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J. Food Eng. 2015, 149, 70–77. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Complex Coacervation Between Gelatin and Chia Mucilage as an Alternative of Encapsulating Agents. J. Food Sci. 2019, 84, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.H.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Kurek, M. Coacervation as a Novel Method of Microencapsulation of Essential Oils—A Review. Molecules 2022, 27, 5142. [Google Scholar] [CrossRef] [PubMed]
- Naderi, B.; Keramat, J.; Nasirpour, A.; Aminifar, M. Complex coacervation between oak protein isolate and gum Arabic: Optimization & functional characterization. Int. J. Food Prop. 2020, 23, 1854–1873. [Google Scholar] [CrossRef]
- Mitra, H.; Pushpadass, H.A.; Franklin, M.E.E.; Ambrose, R.P.K.; Ghoroi, C.; Battula, S.N. Influence of moisture content on the flow properties of basundi mix. Powder Technol. 2017, 312, 133–143. [Google Scholar] [CrossRef]
- Sofi, B.A.; Wani, I.A.; Masoodi, F.A.; Saba, I.; Muzaffar, S. Effect of gamma irradiation on physicochemical properties of broad bean (Vicia faba L.) starch. LWT—Food Sci. Technol. 2013, 54, 63–72. [Google Scholar] [CrossRef]
- dos Santos, F.S.; de Figueirêdo, R.M.F.; Queiroz, A.J.d.M.; Paiva, Y.F.; Moura, H.V.; Silva, E.T.d.V.; Ferreira, J.P.d.L.; de Melo, B.A.; Carvalho, A.J.d.B.A.; Lima, M.d.S.; et al. Influence of Dehydration Temperature on Obtaining Chia and Okra Powder Mucilage. Foods 2023, 12, 569. [Google Scholar] [CrossRef]
- Akseli, I.; Hilden, J.; Katz, J.M.; Kelly, R.C.; Kramer, T.T.; Mao, C.; Osei-Yeboah, F.; Strong, J.C. Reproducibility of the measurement of bulk/tapped density of pharmaceutical powders between pharmaceutical laboratories. J. Pharm. Sci. 2018, 108, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Airouyuwa, J.O.; Kaewmanee, T. Microencapsulation of Moringa oleifera leaf extracts with vegetable protein as wall materials. Food Sci. Technol. Int. 2019, 25, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.S.; Ramachandra, C.T.; Hiregoudar, S.; Nidoni, U.; Ram, J.; Kammar, M. Influence of processing conditions on functional and reconstitution properties of milk powder made from Osmanabadi goat milk by spray drying. Small Rumin. Res. 2014, 119, 130–137. [Google Scholar] [CrossRef]
- Bordón, M.G.; Paredes, A.J.; Camacho, N.M.; Penci, M.C.; González, A.; Palma, S.D.; Ribotta, P.D.; Martinez, M.L. Formulation, spray-drying and physicochemical characterization of functional powders loaded with chia seed oil and prepared by complex coacervation. Powder Technol. 2021, 391, 479–493. [Google Scholar] [CrossRef]
- Xin, X.; Essien, S.; Dell, K.; Woo, M.W.; Baroutian, S. Effects of Spray-Drying and Freeze-Drying on Bioactive and Volatile Compounds of Smoke Powder Food Flavouring. Food Bioprocess Technol. 2022, 15, 785–794. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.I.; Zamudio-Flores, P.B.; Ortega-Rivas, E.; Perez-Vega, S.; Sepulveda, D.R. Effect of water content on the flowability of hygroscopic powders. J. Food Eng. 2017, 205, 12–17. [Google Scholar] [CrossRef]
- Newman, A.W.; Reutzel-Edens, S.M.; Zografi, G. Characterization of the "hygroscopic" properties of active pharmaceutical ingredients. J. Pharm. Sci. 2008, 97, 1047–1059. [Google Scholar] [CrossRef]
- Silva, L.A.; Sinnecker, P.; Cavalari, A.A.; Sato, A.C.K.; Perrechil, F.A. Extraction of chia seed mucilage: Effect of ultrasound application. Food Chem. Adv. 2022, 1, 100024. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, B.; Jaafari, M.R.; Golabpour, A.; Momtazi-Borojeni, A.A.; Karimi, M.; Eslami, S. Application of ensemble machine learning approach to assess the factors affecting size and polydispersity index of liposomal nanoparticles. Sci. Rep. 2023, 13, 18012. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; van der Goot, A.J. Modifying Faba Bean Protein Concentrate Using Dry Heat to Increase Water Holding Capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Dhull, S.B. Chia seed (Salvia hispanica L.) mucilage (a heteropolysaccharide): Thermal, pasting, rheological behaviour and its utilization. Int. J. Biol. Macromol. 2019, 140, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.A.; Vera, C.N.; Zúñiga-López, M.C.; Moncada, M.; Haros, C.M. Physicochemical and functional properties of soluble fiber extracted from two phenotypes of chia (Salvia hispanica L.) seeds. J. Food Compos. Anal. 2021, 104, 104138. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Microencapsulation of Mentha spicata essential oil by spray drying: Optimization, characterization, release kinetics of essential oil from microcapsules in food models. Ind. Crop. Prod. 2020, 154, 112694. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar]
- Shiko, G.; Gladden, L.; Sederman, A.; Connolly, P.; Butler, J. Degradation of polysorbates 20 and 80: Studies on thermal autoxidation and hydrolysis. J. Pharm. Sci. 2011, 100, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar]
- Barchi, J.J., Jr.; Strain, C.N. The effect of a methyl group on structure and function: Serine vs. threonine glycosylation and phosphorylation. Front. Mol. Biosci. 2023, 10, 1117850. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Grdadolnik, J. Saturation effects in FTIR spectroscopy: Intensity of amide I and amide II bands in protein spectra. Acta Chim. Slov. 2003, 50, 777–788. [Google Scholar]
- Babault, N.; Païzis, C.; Deley, G.; Guérin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: A double-blind, randomized, Placebo-controlled clinical trial vs. Whey protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, I.; Wetie, A.G.N.; Woods, A.G.; Darie, C.C. Automatic Determination of Disulfide Bridges in Proteins. J. Lab. Autom. 2012, 17, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.; Khalifa, R.E.; El Sohaimy, S.A. Functional properties of chia seed mucilage supplemented in low fat yoghurt. Alex. Sci. Exch. J. 2018, 39, 450–459. [Google Scholar] [CrossRef]
- Chiang, J.H.; Ong, D.S.M.; Ng, F.S.K.; Hua, X.Y.; Tay, W.L.W.; Henry, C.J. Application of chia (Salvia hispanica) mucilage as an ingredient replacer in foods. Trends Food Sci. Technol. 2021, 115, 105–116. [Google Scholar] [CrossRef]
- Cebi, N.; Arici, M.; Sagdic, O. The famous Turkish rose essential oil: Characterization and authenticity monitoring by FTIR, Raman and GC–MS techniques combined with chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crop. Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Maeh, R.K.; Jaaffar, A.I.; Al-Azawi, K.F. Preparation of Juniperus extract and detection of its antimicrobial and antioxidant activity. Iraqi J. Agric. Sci. 2019, 50, 1153–1161. [Google Scholar]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Novel applications of black pepper essential oil as an antioxidant agent in sodium caseinate and chitosan based active edible films. Int. J. Biol. Macromol. 2024, 254, 128045. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef]
- Cebi, N.; Taylan, O.; Abusurrah, M.; Sagdic, O. Detection of orange essential oil, isopropyl myristate, and benzyl alcohol in lemon essential oil by FTIR spectroscopy combined with chemometrics. Foods 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, C.; Shao, Y.; Zhou, J. Characterization of rapeseed oil using FTIR-ATR spectroscopy. J. Food Sci. Eng. 2014, 4, 244–249. [Google Scholar]
- Rohman, A.; Ariani, R. Authentication of Nigella sativa seed oil in binary and ternary mixtures with corn oil and soybean oil using FTIR spectroscopy coupled with partial least square. Sci. World J. 2013, 2013, 740142. [Google Scholar] [CrossRef]
- Nair, L.M.; Stephens, N.V.; Vincent, S.; Raghavan, N.; Sand, P.J. Determination of polysorbate 80 in parenteral formulations by high-performance liquid chromatography and evaporative light scattering detection. J. Chromatogr. A 2003, 1012, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Perez, M.D.T.; González, H.D.; Kurek, M.A. Microencapsulation of Juniper and Black Pepper Essential Oil Using the Coacervation Method and Its Properties after Freeze-Drying. Foods 2023, 12, 4345. [Google Scholar] [CrossRef] [PubMed]
- De Melo Ramos, F.; Silveira Júnior, V.; Prata, A.S. Assessing the Vacuum Spray Drying Effects on the Properties of Orange Essential Oil Microparticles. Food Bioprocess Technol. 2019, 12, 1917–1927. [Google Scholar] [CrossRef]
- Fernandes, R.V.d.B.; Borges, S.V.; Botrel, D.A.; de Oliveira, C.R. Physical and chemical properties of encapsulated rosemary essential oil by spray drying using whey protein-inulin blends as carriers. Int. J. Food Sci. Technol. 2013, 49, 1522–1529. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Andean Blueberry (Vaccinium meridionale Sw.) Anthocyanins Using Prickly Pear (Opuntia ficus indica L.) Peel Mucilage or Gum Arabic: A Comparative Study. Foods 2023, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Guzek, D.; Sun, D.W.; Wierzbicka, A. Differentiation of chill-stored and frozen pork necks using electronic nose with ultra-fast gas chromatography. J. Food Process Eng. 2017, 40, e12540. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Brodowska, M.; Sun, D.W.; Wierzbicka, A. Diet with linseed oil and organic selenium yields low n-6/n-3 ratio pork Semimembranosus meat with unchanged volatile compound profiles. Int. J. Food Sci. 2018, 53, 1838–1846. [Google Scholar] [CrossRef]
| Oil | Essential Oil | FP/CHP | Code |
|---|---|---|---|
| Rapeseed | Juniper | 1:1 | RJ1 |
| 1:2 | RJ2 | ||
| 2:1 | RJ3 | ||
| Black pepper | 1:1 | RB1 | |
| 1:2 | RB2 | ||
| 2:1 | RB3 | ||
| Soybean | Juniper | 1:1 | SJ1 |
| 1:2 | SJ2 | ||
| 2:1 | SJ3 | ||
| Black pepper | 1:1 | SB1 | |
| 1:2 | SB2 | ||
| 2:1 | SB3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska, A.; Szpicer, A.; Górska-Horczyczak, E.; Kurek, M.A. Microencapsulation of Essential Oils Using Faba Bean Protein and Chia Seed Polysaccharides via Complex Coacervation Method. Molecules 2024, 29, 2019. https://doi.org/10.3390/molecules29092019
Napiórkowska A, Szpicer A, Górska-Horczyczak E, Kurek MA. Microencapsulation of Essential Oils Using Faba Bean Protein and Chia Seed Polysaccharides via Complex Coacervation Method. Molecules. 2024; 29(9):2019. https://doi.org/10.3390/molecules29092019
Chicago/Turabian StyleNapiórkowska, Alicja, Arkadiusz Szpicer, Elżbieta Górska-Horczyczak, and Marcin Andrzej Kurek. 2024. "Microencapsulation of Essential Oils Using Faba Bean Protein and Chia Seed Polysaccharides via Complex Coacervation Method" Molecules 29, no. 9: 2019. https://doi.org/10.3390/molecules29092019
APA StyleNapiórkowska, A., Szpicer, A., Górska-Horczyczak, E., & Kurek, M. A. (2024). Microencapsulation of Essential Oils Using Faba Bean Protein and Chia Seed Polysaccharides via Complex Coacervation Method. Molecules, 29(9), 2019. https://doi.org/10.3390/molecules29092019








