Structural Characterization of Polygonatum Cyrtonema Polysaccharide and Its Immunomodulatory Effects on Macrophages
Abstract
1. Introduction
2. Results
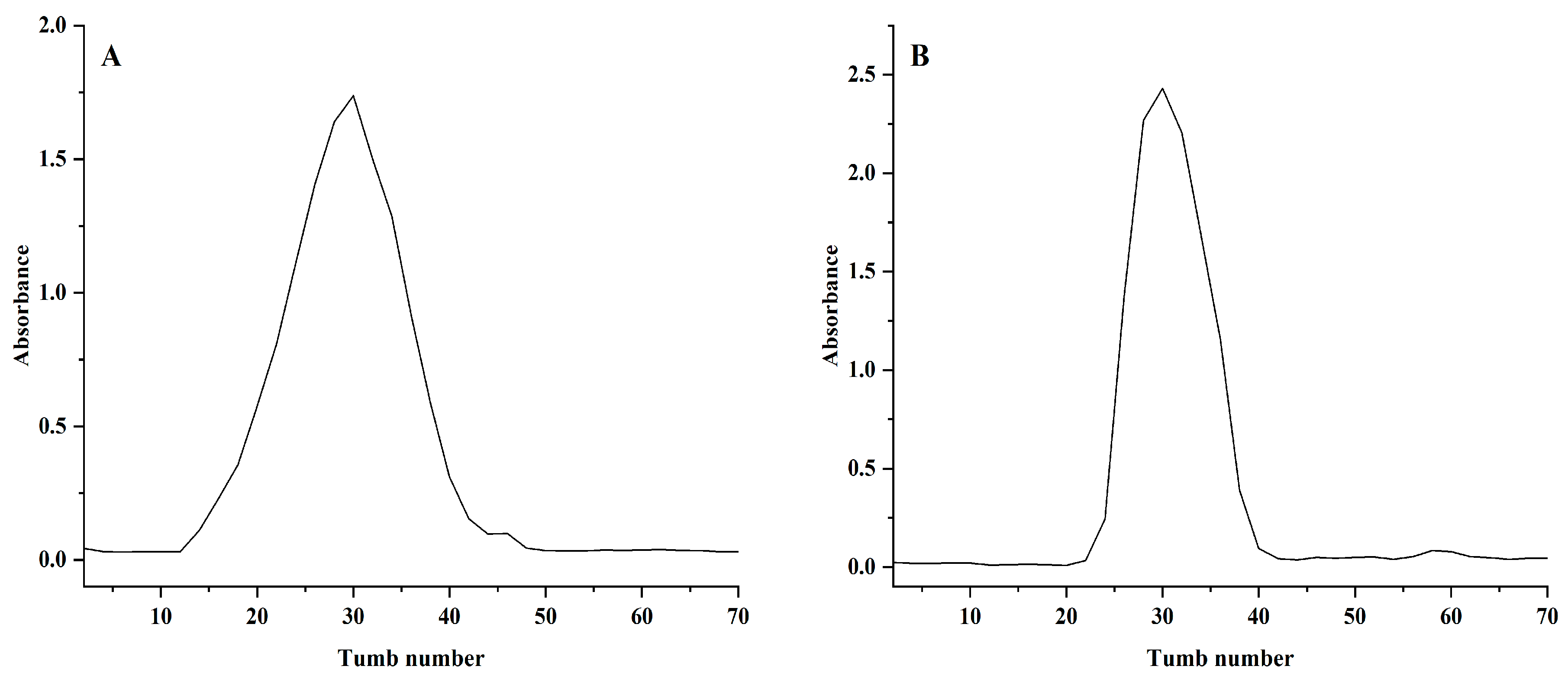
2.1. Purification and Identification of NPCP
2.2. Methylation Analysis
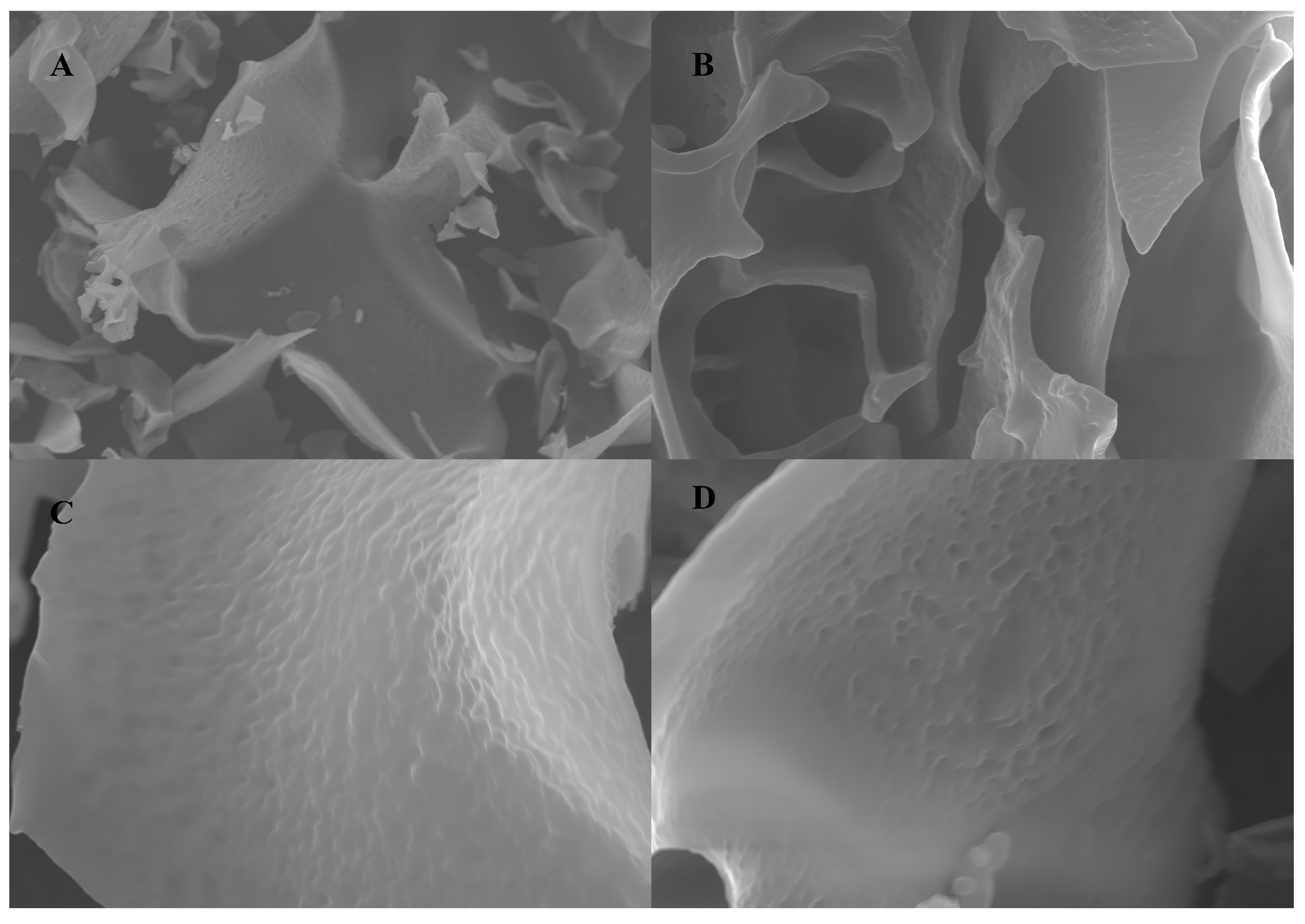
2.3. Scanning Electron Microscopy Analysis of NPCP
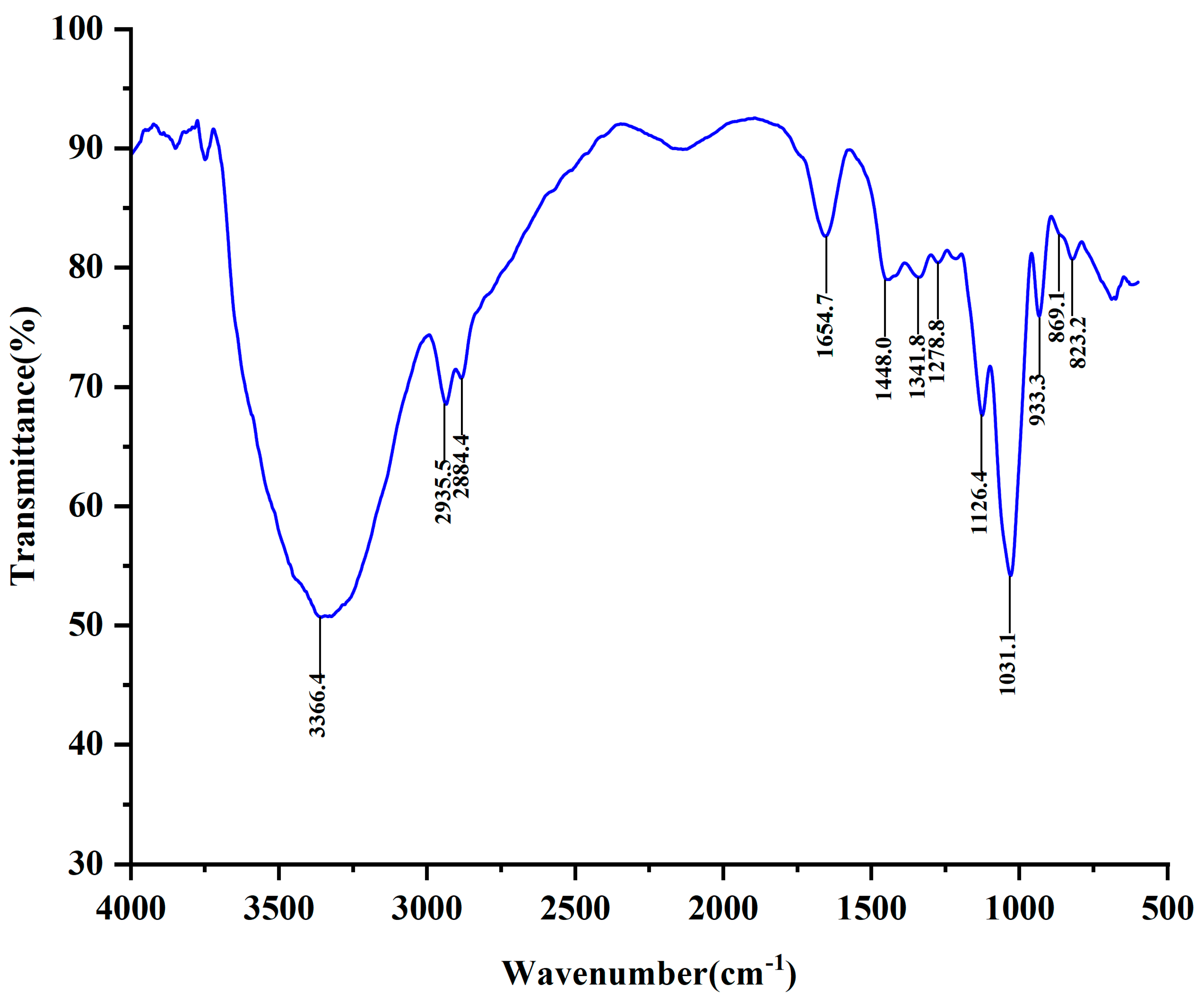
2.4. FT-IR Analysis
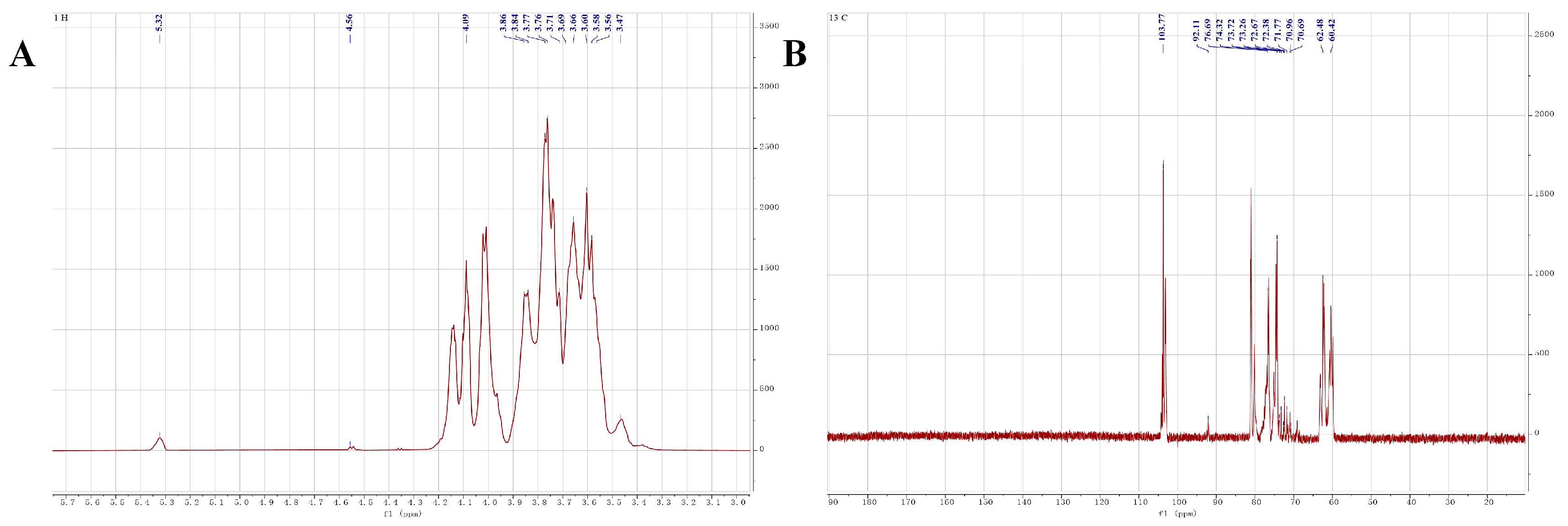
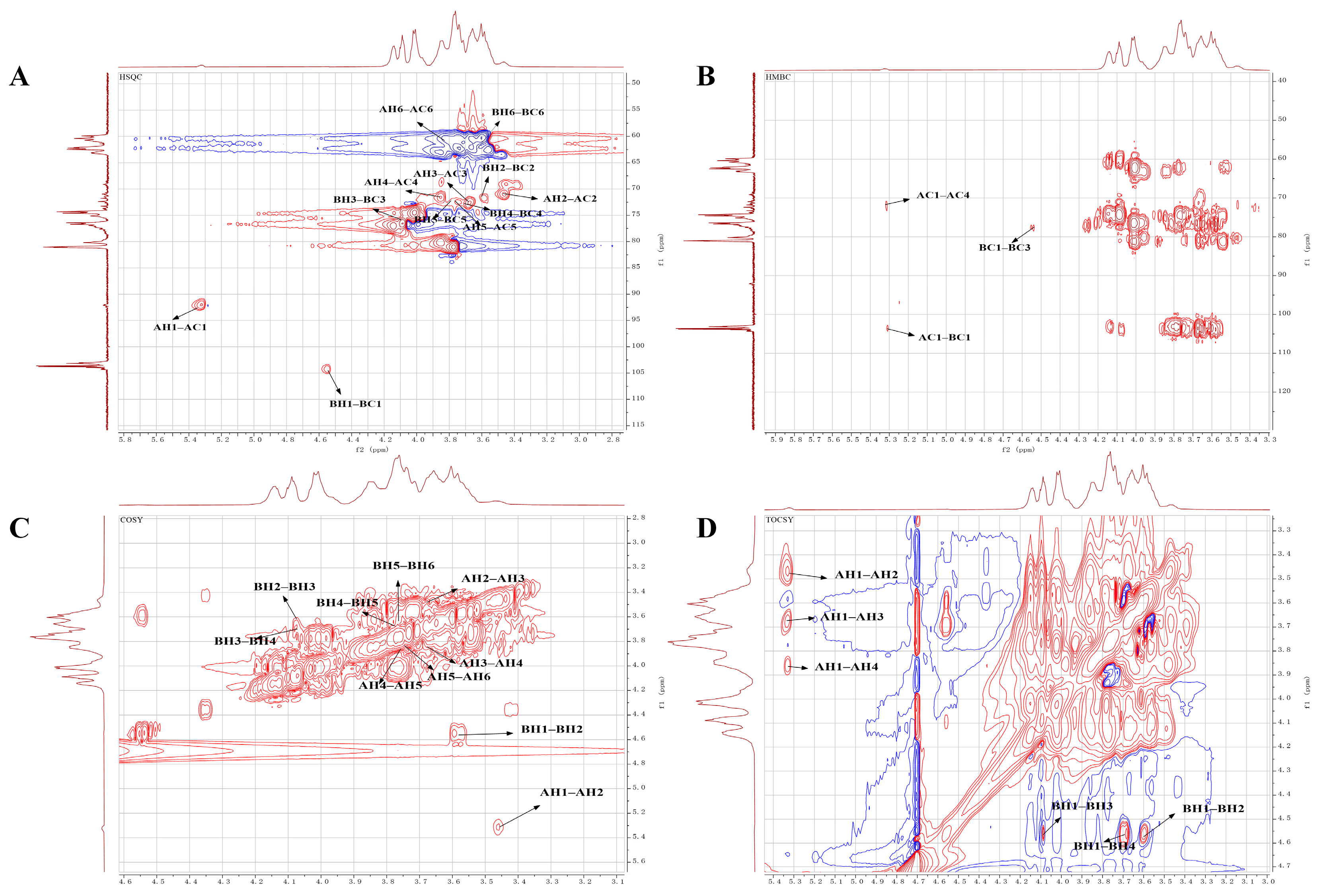
2.5. NMR Analysis
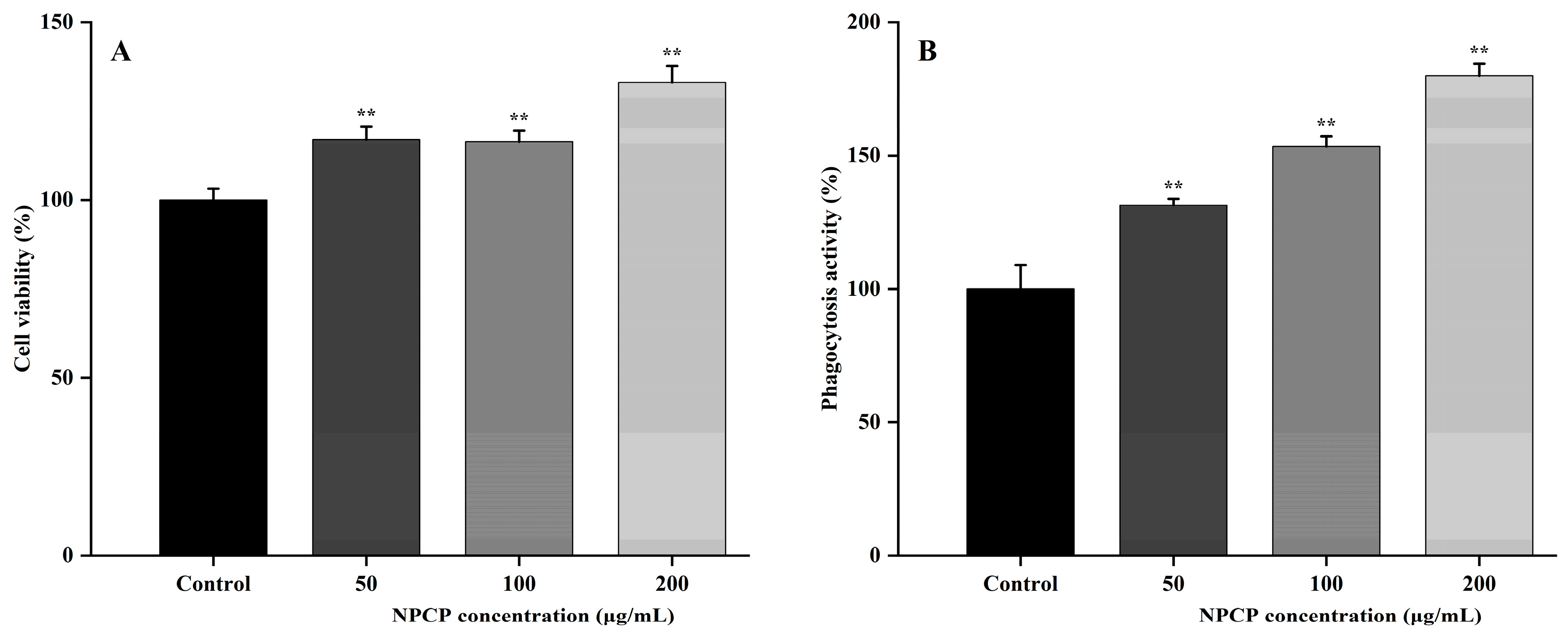
2.6. NPCP Enhanced the Proliferation and Phagocytosis of RAW264.7 Cells
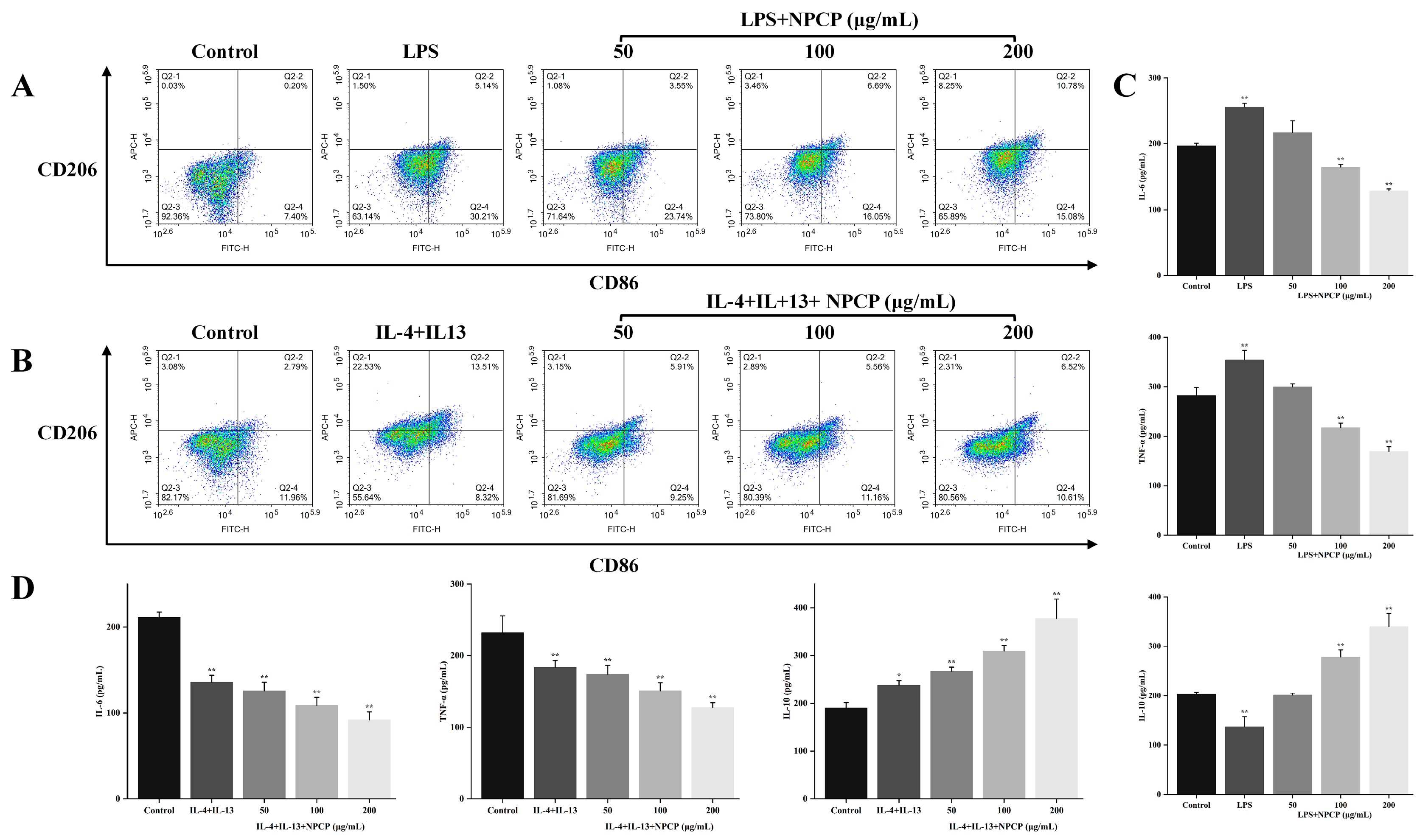
2.7. Effects of NPCP on M1 Macrophages
2.8. Effects of NPCP on M2 Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction, Isolation, and Purification of Polysaccharide from Polygonatum cyrtonema
4.3. Molecular Weight Analysis
4.4. Monosaccharide Composition Analysis
4.5. Methylation Analysis
4.6. Scanning Electron Microscopy (SEM) Analysis
4.7. UV and ATR-FTIR Spectra Analysis
4.8. NMR Analysis
4.9. Immunomodulatory Activity of NPCP on Macrophages Polarization
4.9.1. Cell Culture
4.9.2. Cell Viability Assay
4.9.3. Phagocytic Activity Assay
4.9.4. Cytokine Assay
4.9.5. Flow Cytometry
4.9.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Ye, Q.; Luo, F.; Yan, T. Transcription Factor KLF4 Regulated STAT1 to Promote M1 Polarization of Macrophages in Rheumatoid Arthritis. Aging 2022, 14, 5669–5680. [Google Scholar] [CrossRef]
- Quero, L.; Tiaden, A.N.; Hanser, E.; Roux, J.; Laski, A.; Hall, J.; Kyburz, D. miR-221-3p Drives the Shift of M2-Macrophages to a Pro-Inflammatory Function by Suppressing JAK3/STAT3 Activation. Front. Immunol. 2020, 10, 3087. [Google Scholar] [CrossRef]
- Xu, Y.-W.; Xing, R.-X.; Zhang, W.-H.; Li, L.; Wu, Y.; Hu, J.; Wang, C.; Luo, Q.-L.; Shen, J.-L.; Chen, X. Toxoplasma ROP16 I/III Ameliorated Inflammatory Bowel Diseases via Inducing M2 Phenotype of Macrophages. World J. Gastroenterol. 2019, 25, 6634–6652. [Google Scholar] [CrossRef]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 Macrophages Derived from THP-1 Cells Differentially Modulate the Response of Cancer Cells to Etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef]
- Yang, K.; Xie, Y.; Xue, L.; Li, F.; Luo, C.; Liang, W.; Zhang, H.; Li, Y.; Ren, Y.; Zhao, M.; et al. M2 Tumor-Associated Macrophage Mediates the Maintenance of Stemness to Promote Cisplatin Resistance by Secreting TGF-Β1 in Esophageal Squamous Cell Carcinoma. J. Transl. Med. 2023, 21, 26. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, J.; Gao, J.; Zhang, D.; Lin, D.; Lin, J. Polysaccharides of Plantago Asiatica Enhance Antitumor Activity via Regulating Macrophages to M1-like Phenotype. Biomed. Pharmacother. 2023, 159, 114246. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, J.; Xu, J.; Zhang, S.; Bao, Y. Antitumor Effect of a Polysaccharide from Pseudostellaria Heterophylla through Reversing Tumor-Associated Macrophages Phenotype. Int. J. Biol. Macromol. 2022, 220, 816–826. [Google Scholar] [CrossRef]
- Yuan, R.; Li, S.; Geng, H.; Wang, X.; Guan, Q.; Li, X.; Ren, C.; Yuan, X. Reversing the Polarization of Tumor-Associated Macrophages Inhibits Tumor Metastasis. Int. Immunopharmacol. 2017, 49, 30–37. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural Characterization and Antioxidant Activities of Bletilla Striata Polysaccharide Extracted by Different Methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef]
- Shao, X.; Li, J.; Zhang, H.; Zhang, X.; Sun, C.; Ouyang, X.; Wang, Y.; Wu, X.; Chen, C. Anti-Inflammatory Effects and Molecular Mechanisms of Bioactive Small Molecule Garlic Polysaccharide. Front. Nutr. 2023, 9, 1092873. [Google Scholar] [CrossRef]
- Meng, M.; Sun, Y.; Qi, Y.; Xu, J.; Sun, J.; Bai, Y.; Han, L.; Han, R.; Hou, L.; Sun, H. Structural Characterization and Induction of Tumor Cell Apoptosis of Polysaccharide from Purple Sweet Potato (Ipomoea batatas (L.) Lam). Int. J. Biol. Macromol. 2023, 235, 123799. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure Characterisation of Polysaccharides in Vegetable “Okra” and Evaluation of Hypoglycemic Activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef]
- Fan, H.; Sun, M.; Li, J.; Zhang, S.; Tu, G.; Liu, K.; Xia, Q.; Jiang, Y.; Liu, B. Structure Characterization and Immunomodulatory Activity of a Polysaccharide from Saposhnikoviae Radix. Int. J. Biol. Macromol. 2023, 233, 123502. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory Activity of a Novel Polysaccharide from Lonicera Japonica in Immunosuppressed Mice Induced by Cyclophosphamide. PLoS ONE 2018, 13, e0204152. [Google Scholar] [CrossRef]
- Sun, S.; Li, K.; Lei, Z.; Xiao, L.; Gao, R.; Zhang, Z. Immunomodulatory Activity of Polysaccharide from Helicteres Angustifolia L. on 4T1 Tumor-Bearing Mice. Biomed. Pharmacother. 2018, 101, 881–888. [Google Scholar] [CrossRef]
- Luo, L.; Qiu, Y.; Gong, L.; Wang, W.; Wen, R. A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes. Molecules 2022, 27, 4821. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.; Tian, Q.; Xiao, L.; Zeng, Z.; Cai, X.; Yan, J.; Li, Q. Microwave-Assisted Degradation of Polysaccharide from Polygonatum sibiricum and Antioxidant Activity. J. Food Sci. 2019, 84, 754–761. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Fu, R.; Ou, J.; Wang, B. Structural Characterization and Anti-Inflammatory Activity of a Novel Polysaccharide PKP2-1 from Polygonatum Kingianum. Front. Nutr. 2023, 10, 1156798. [Google Scholar] [CrossRef]
- Shen, W.-D.; Li, X.-Y.; Deng, Y.-Y.; Zha, X.-Q.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P. Polygonatum Cyrtonema Hua Polysaccharide Exhibits Anti-Fatigue Activity via Regulating Osteocalcin Signaling. Int. J. Biol. Macromol. 2021, 175, 235–241. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhang, H.; Liu, Y.; Wang, W.; You, S.; Hu, X.; Song, M.; Wu, R.; Wu, J. Anti-Cancer Potential of Polysaccharide Extracted From Polygonatum Sibiricum on HepG2 Cells via Cell Cycle Arrest and Apoptosis. Front. Nutr. 2022, 9, 938290. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, H.; Zhao, C.; Li, X.; Wang, Y.; Huang, L.; Gao, W. Purification, Characterization and Immunomodulatory Activity of Fructans from Polygonatum Odoratum and P. Cyrtonema. Carbohydr. Polym. 2019, 214, 44–52. [Google Scholar] [CrossRef]
- Zhou, W.; Hong, J.; Liu, T.; Li, M.; Jin, H.; Wang, X. Polygonatum Polysaccharide Regulates Macrophage Polarization and Improves LPS-Induced Acute Lung Injury through TLR4-MAPK/NF-κB Pathway. Can. Respir. J. 2022, 2022, 2686992. [Google Scholar] [CrossRef]
- Chen, X.; Fang, D.; Zhao, R.; Gao, J.; Kimatu, B.M.; Hu, Q.; Chen, G.; Zhao, L. Effects of ultrasound-assisted extraction on antioxidant activity and bidirectional immunomodulatory activity of Flammulina velutipes polysaccharide. Int. J. Biol. Macromol. 2019, 140, 505–514. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Omondi Onyango, S. Ultrasound Irradiation Alters the Spatial Structure and Improves the Antioxidant Activity of the Yellow Tea Polysaccharide. Ultrason. Sonochem. 2021, 70, 105355. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, J.; Bi, S.; Chen, D.; Song, L.; Wang, L.; Zi, J.; Yu, R. Isolation, Characterization and Bioactivities of a New Polysaccharide from Annona Squamosa and Its Sulfated Derivative. Carbohydr. Polym. 2016, 152, 287–296. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural Characterization and Antiviral Activity of Lentinan from Lentinus Edodes Mycelia against Infectious Hematopoietic Necrosis Virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef]
- Cai, Z.-N.; Li, W.; Mehmood, S.; Pan, W.-J.; Wang, Y.; Meng, F.-J.; Wang, X.-F.; Lu, Y.-M.; Chen, Y. Structural Characterization, in Vitro and in Vivo Antioxidant Activities of a Heteropolysaccharide from the Fruiting Bodies of Morchella Esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Liu, G.; Xu, H.; Zhang, X. Chemical Elucidation of an Arabinogalactan from Rhizome of Polygonatum Sibiricum with Antioxidant Activities. Int. J. Biol. Macromol. 2021, 190, 730–738. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Yan, A.; Feng, L.; Wan, Y. Fractionation, Structure and Conformation Characterization of Polysaccharides from Anoectochilus Roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Luo, L.; Zhou, Z.; Wang, Y.; Gao, T.; Yang, L.; Peng, T.; Wu, M. Structures of Fructan and Galactan from Polygonatum Cyrtonema and Their Utilization by Probiotic Bacteria. Carbohydr. Polym. 2021, 267, 118219. [Google Scholar] [CrossRef]
- Abdelhady, E.I.; Abd El-Hady, H.I.; Badran, S.G.; Rabie, M. TNF-α versus IL-6 Genes Expression Levels in Active Rheumatoid Arthritis: Clinical and Laboratory Determinants. Egypt. J. Immunol. 2023, 30, 01–10. [Google Scholar] [CrossRef]
- Matsuno, H.; Yudoh, K.; Katayama, R.; Nakazawa, F.; Uzuki, M.; Sawai, T.; Yonezawa, T.; Saeki, Y.; Panayi, G.S.; Pitzalis, C.; et al. The Role of TNF-a in the Pathogenesis of Inflammation and Joint Destruction in Rheumatoid Arthritis (RA): A Study Using a Human RA/SCID Mouse Chimera. Rheumatology. 2002, 41, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Wakisaka, S.; Suzuki, N.; Takeba, Y.; Shimoyama, Y.; Nagafuchi, H.; Takeno, M.; Saito, N.; Yokoe, T.; Kaneko, A.; Asai, T.; et al. Modulation by Proinflammatory Cytokines of Fas/Fas Ligand-Mediated Apoptotic Cell Death of Synovial Cells in Patients with Rheumatoid Arthritis (RA). Clin. Exp. Immunol. 2001, 114, 119–128. [Google Scholar] [CrossRef]
- Hashizume, M.; Mihara, M. Atherogenic Effects of TNF-α and IL-6 via up-Regulation of Scavenger Receptors. Cytokine 2012, 58, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Peranteau, W.H.; Zhang, L.; Muvarak, N.; Badillo, A.T.; Radu, A.; Zoltick, P.W.; Liechty, K.W. IL-10 Overexpression Decreases Inflammatory Mediators and Promotes Regenerative Healing in an Adult Model of Scar Formation. J. Invest. Dermatol. 2008, 128, 1852–1860. [Google Scholar] [CrossRef]
- Bromberg, J.S. IL-10 Immunosuppression in Transplantation. Curr. Opin. Immunol. 1995, 7, 639–643. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, H.; Wang, C.; Li, H.; Zhang, Q.; Bai, J. M2A and M2C Macrophage Subsets Ameliorate Inflammation and Fibroproliferation in Acute Lung Injury Through Interleukin 10 Pathway. Shock 2017, 48, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cao, Q.; Zheng, D.; Sun, Y.; Wang, C.; Yu, X.; Wang, Y.; Lee, V.W.S.; Zheng, G.; Tan, T.K.; et al. Discrete Functions of M 2a and M 2c Macrophage Subsets Determine Their Relative Efficacy in Treating Chronic Kidney Disease. Kidney Int. 2013, 84, 745–755. [Google Scholar] [CrossRef] [PubMed]



| Retention Time | Methylated Sugar | Type of Linkage | Mass Fragments (m/z) | Molar Ratios% |
|---|---|---|---|---|
| 44.23 | 2,3,4,6-Me4-Galp | T-linked Galp | 43, 87, 101, 129, 145, 161, 205 | 3.38 |
| 45.59 | 2,4,6-Me3-Galp | 1,3-linked Galp | 43, 87, 101, 117, 129, 145, 161, 205, 233 | 4.43 |
| 48.29 | 2,3,6-Me3-Glcp | 1,4-linked Glcp | 43, 87, 101, 117, 129, 161, 173, 233 | 47.35 |
| 49.02 | 2,3,4,6-Me4-Glcp | T-linked Glcp | 43, 87, 101, 117, 129, 161, 173, 189, 203 | 8.01 |
| Glycosyl Residues | Chemical Shift δ (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | H6b | |
| →4)-α-D-Glcp-(1→ | 5.32 92.11 | 3.47 70.69 | 3.66 72.67 | 3.86 73.72 | 3.76 73.26 | 3.84 60.42 | 3.69 |
| →3)-β-D-Gal-(1→ | 4.56 103.77 | 3.61 71.96 | 4.07 76.69 | 3.69 72.81 | 3.77 72.51 | 3.66 62.48 | 3.58 |
| α-D-Glcp-(1→ | 5.32 92.11 | 3.46 70.99 | 3.67 72.42 | 3.72 71.72 | 3.76 72.46 | 3.84 60.42 | 3.69 |
| β-D-Gal-(1→ | 4.56 103.77 | 3.61 71.96 | 3.65 71.88 | 3.69 72.81 | 3.77 72.51 | 3.66 62.48 | 3.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, R.; Luo, L.; Zhang, R.; Zhou, X.; Wang, W.; Gong, L. Structural Characterization of Polygonatum Cyrtonema Polysaccharide and Its Immunomodulatory Effects on Macrophages. Molecules 2024, 29, 2076. https://doi.org/10.3390/molecules29092076
Wen R, Luo L, Zhang R, Zhou X, Wang W, Gong L. Structural Characterization of Polygonatum Cyrtonema Polysaccharide and Its Immunomodulatory Effects on Macrophages. Molecules. 2024; 29(9):2076. https://doi.org/10.3390/molecules29092076
Chicago/Turabian StyleWen, Ruiding, Lu Luo, Runcheng Zhang, Xudong Zhou, Wei Wang, and Limin Gong. 2024. "Structural Characterization of Polygonatum Cyrtonema Polysaccharide and Its Immunomodulatory Effects on Macrophages" Molecules 29, no. 9: 2076. https://doi.org/10.3390/molecules29092076
APA StyleWen, R., Luo, L., Zhang, R., Zhou, X., Wang, W., & Gong, L. (2024). Structural Characterization of Polygonatum Cyrtonema Polysaccharide and Its Immunomodulatory Effects on Macrophages. Molecules, 29(9), 2076. https://doi.org/10.3390/molecules29092076






