Low-Hydrophilic HKUST−1/Polymer Extrudates for the PSA Separation of CO2/CH4
Abstract
1. Introduction
2. Results and Discussion
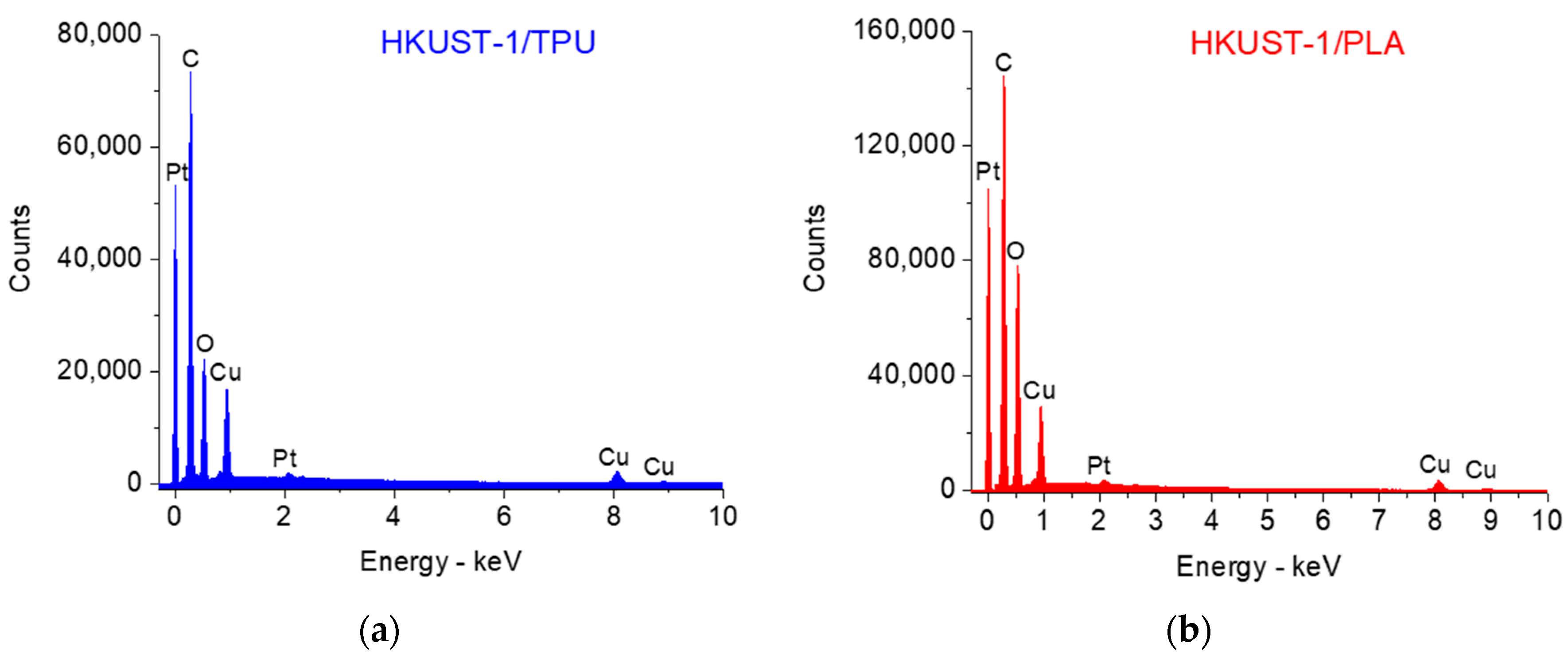
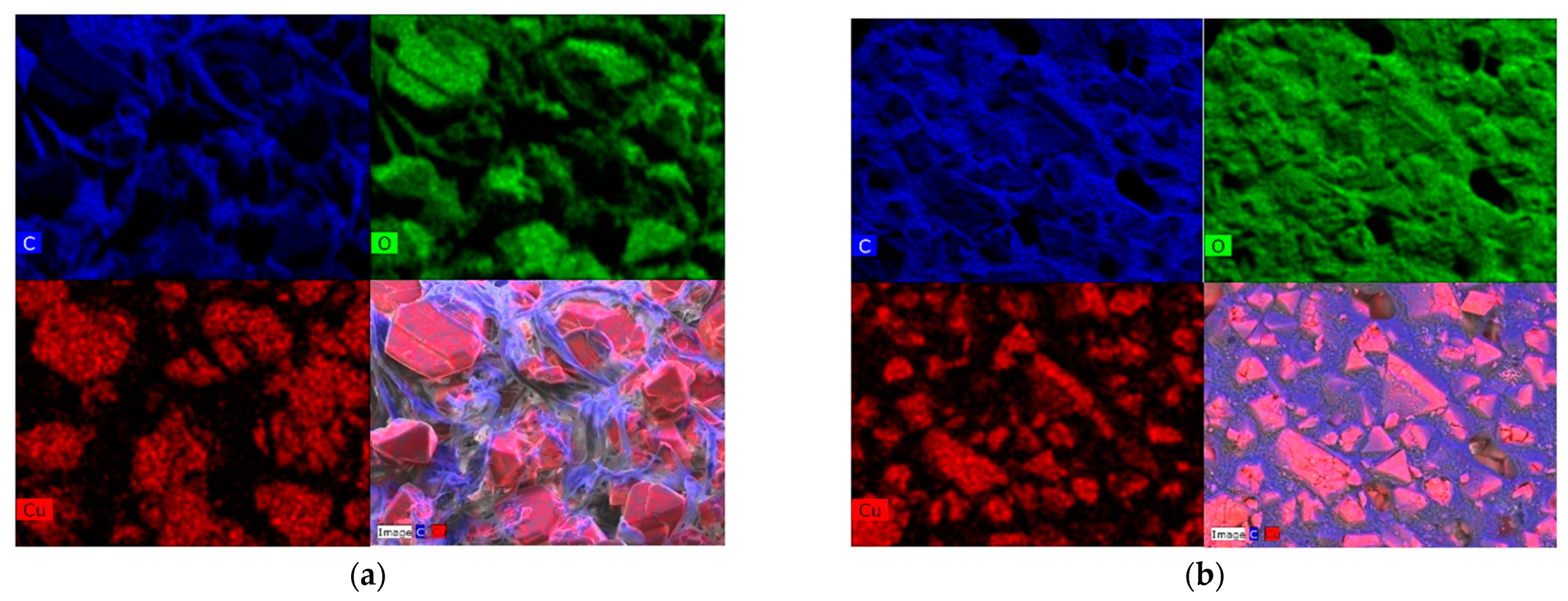
2.1. Sample Characterization
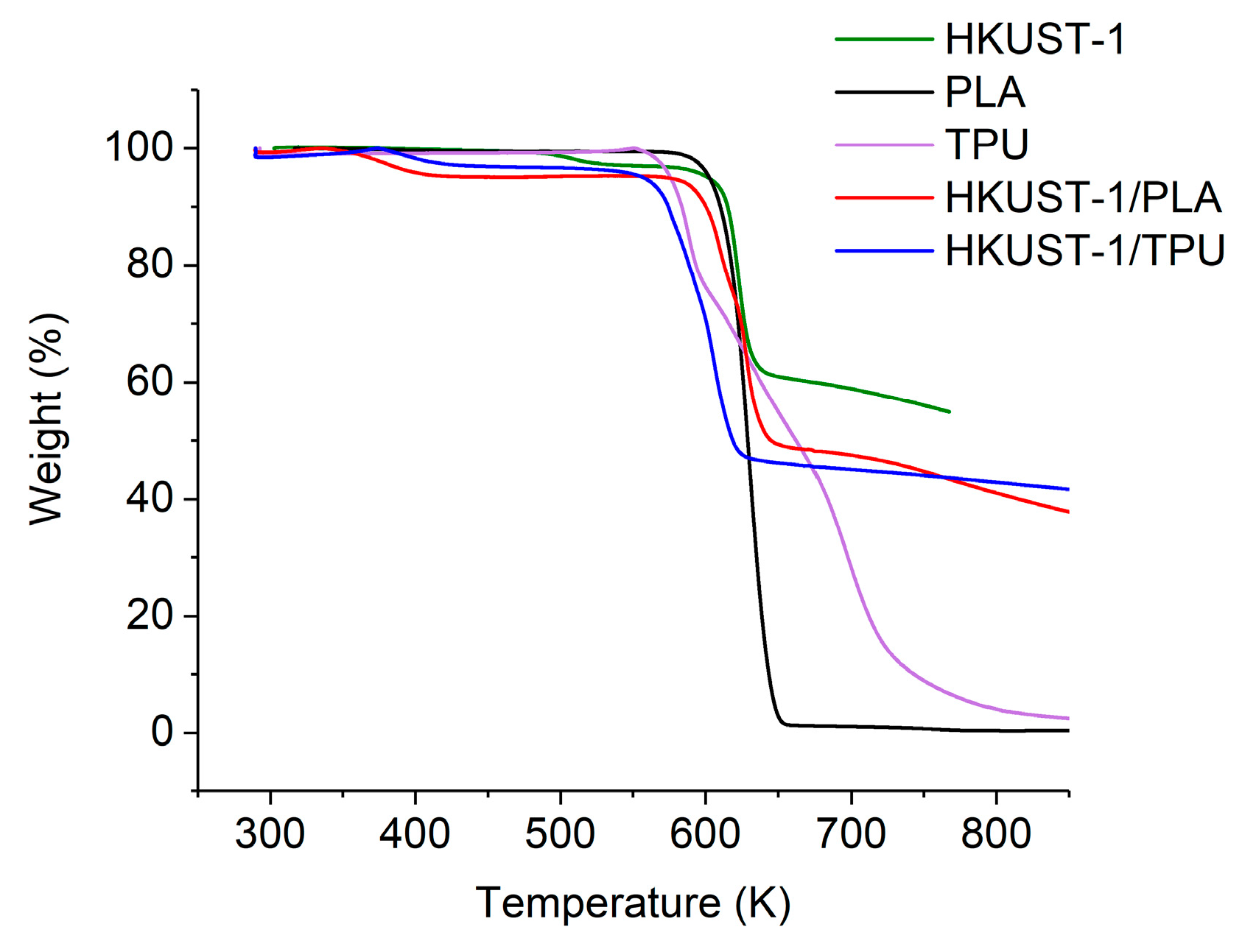
2.2. Thermal and Mechanical Stability
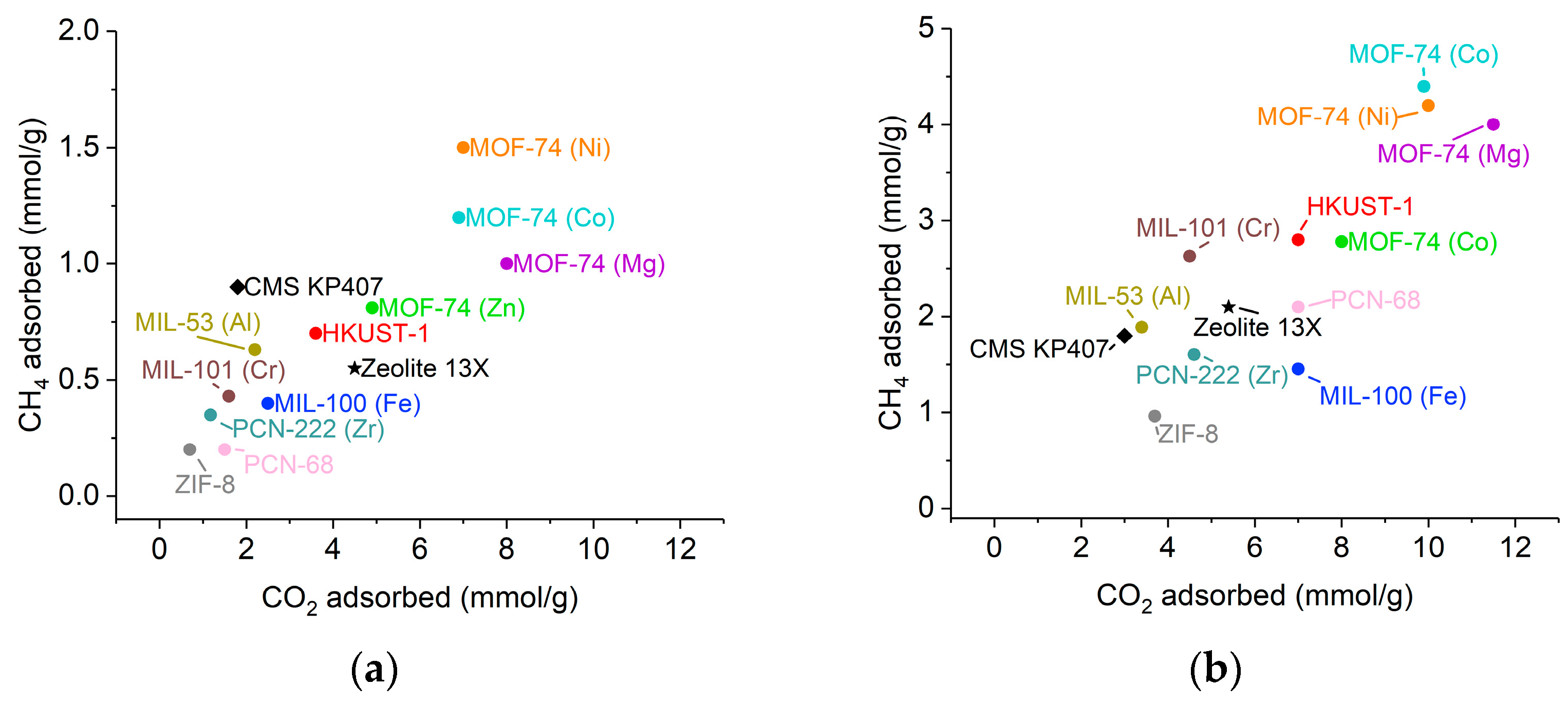
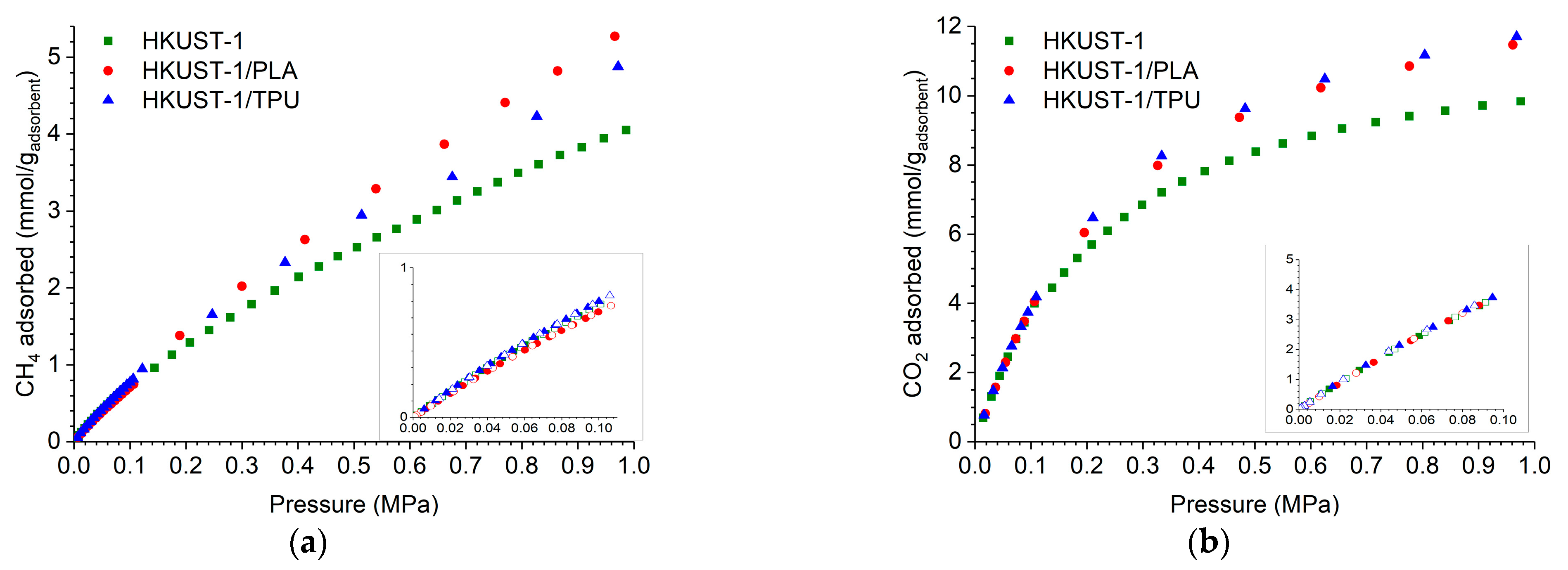
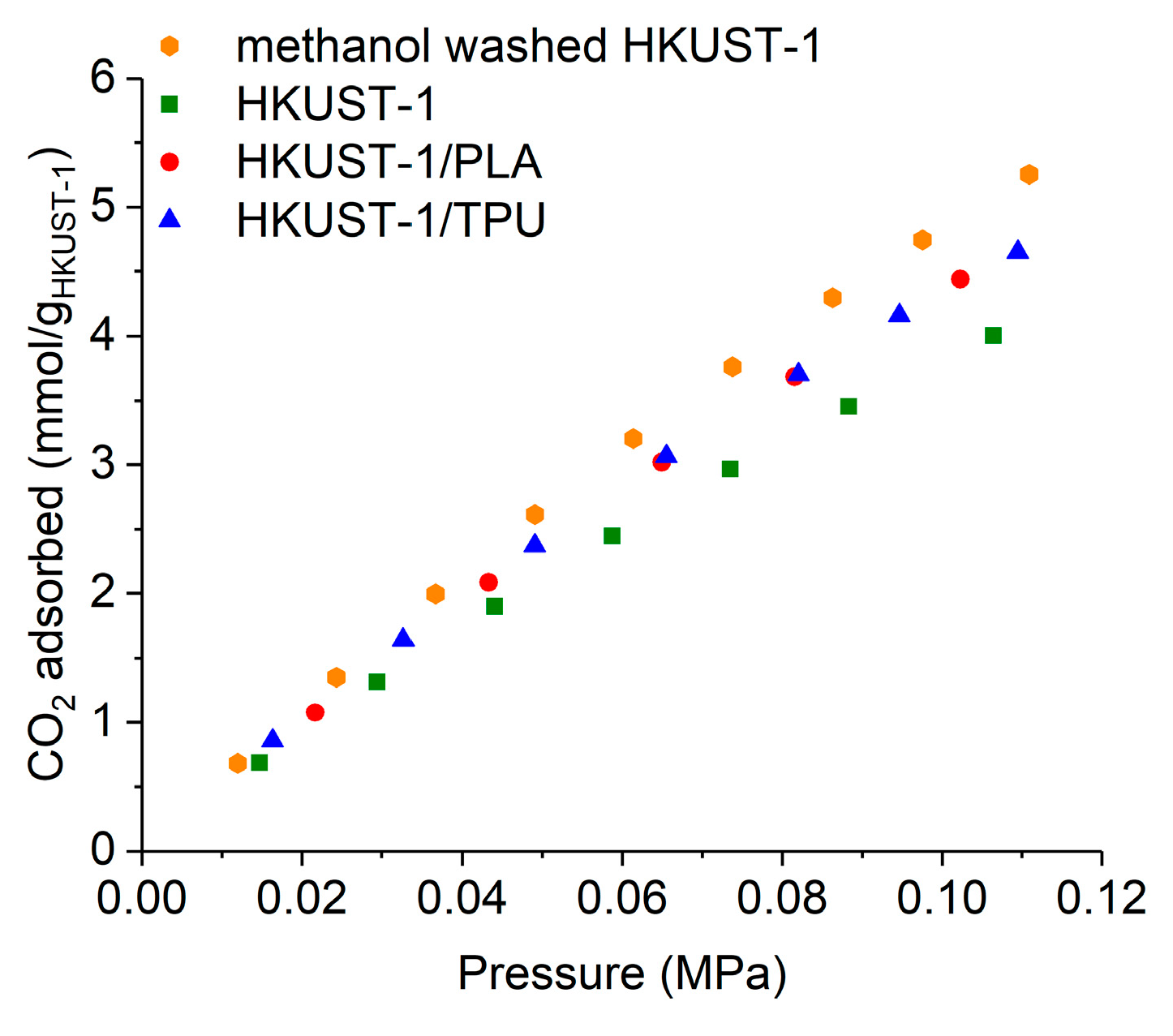
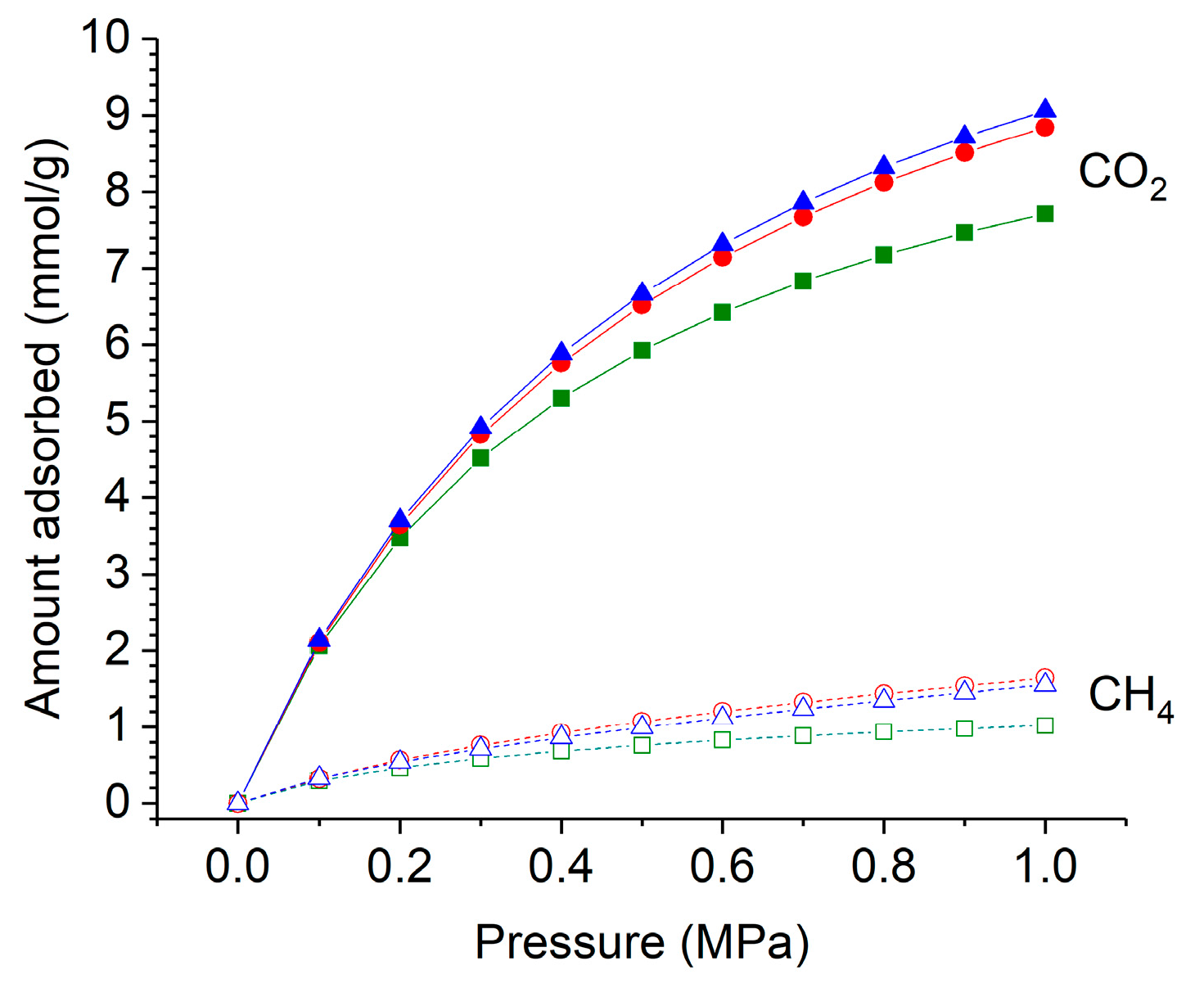
2.3. CO2 and CH4 Adsorption Measurements
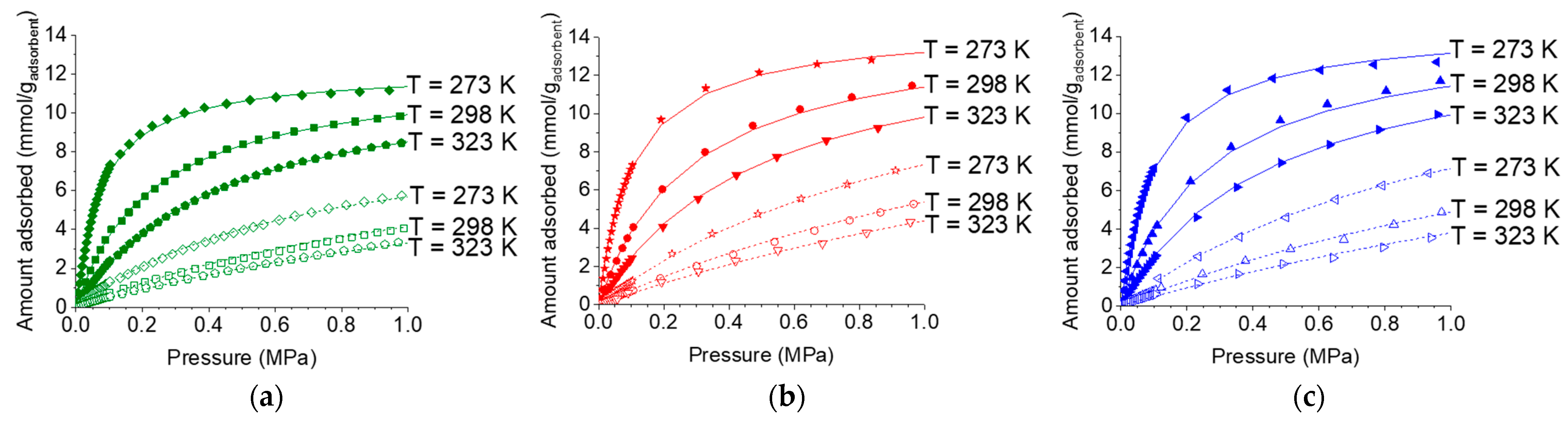
2.4. Effect of Temperature on Adsorption Isotherms
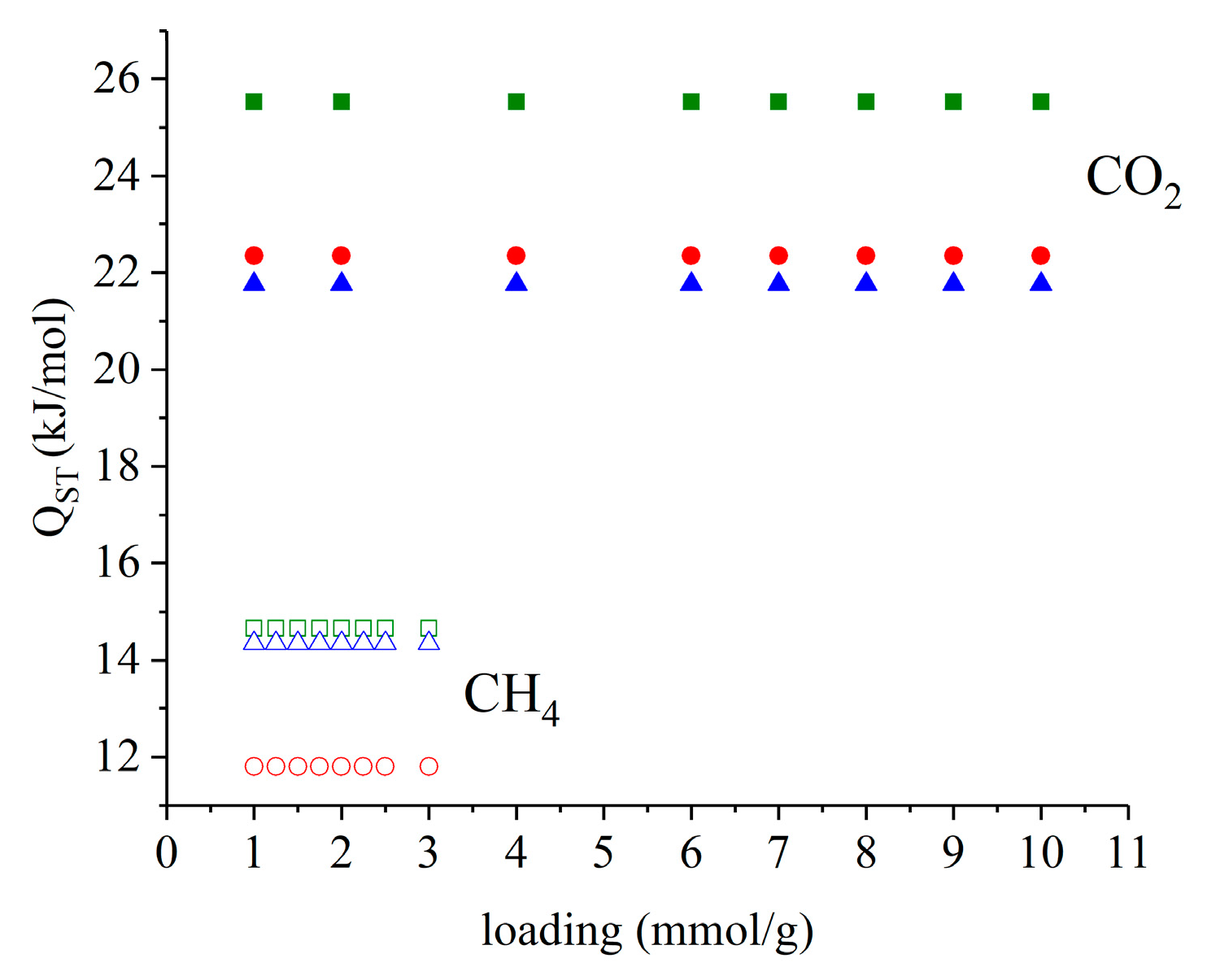
2.5. Temperature Dependent Isotherm Modeling and Isosteric Heat of Adsorption
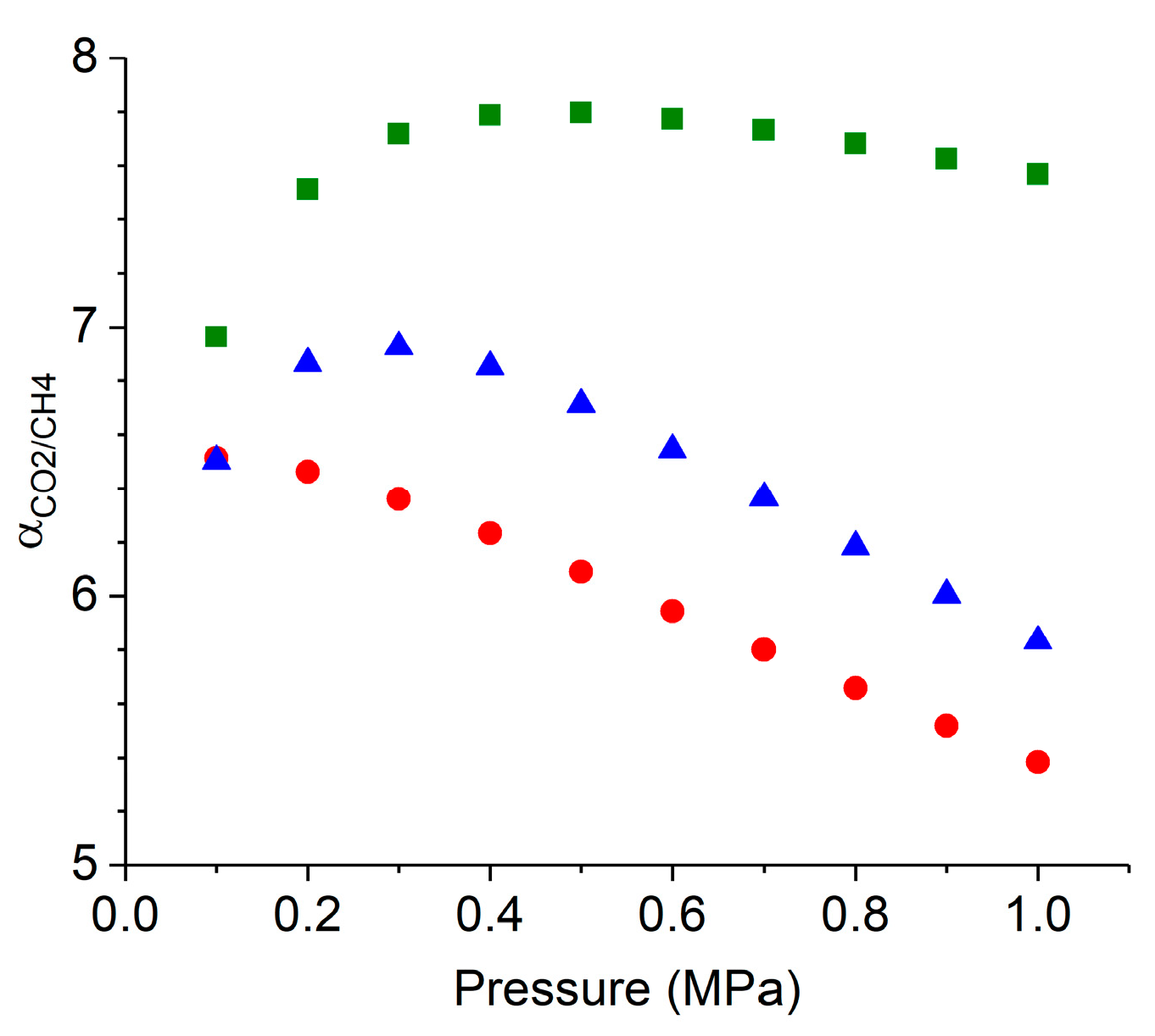
2.6. Prediction of CO2/CH4 Co-Adsorption Isotherm and IAST Selectivites
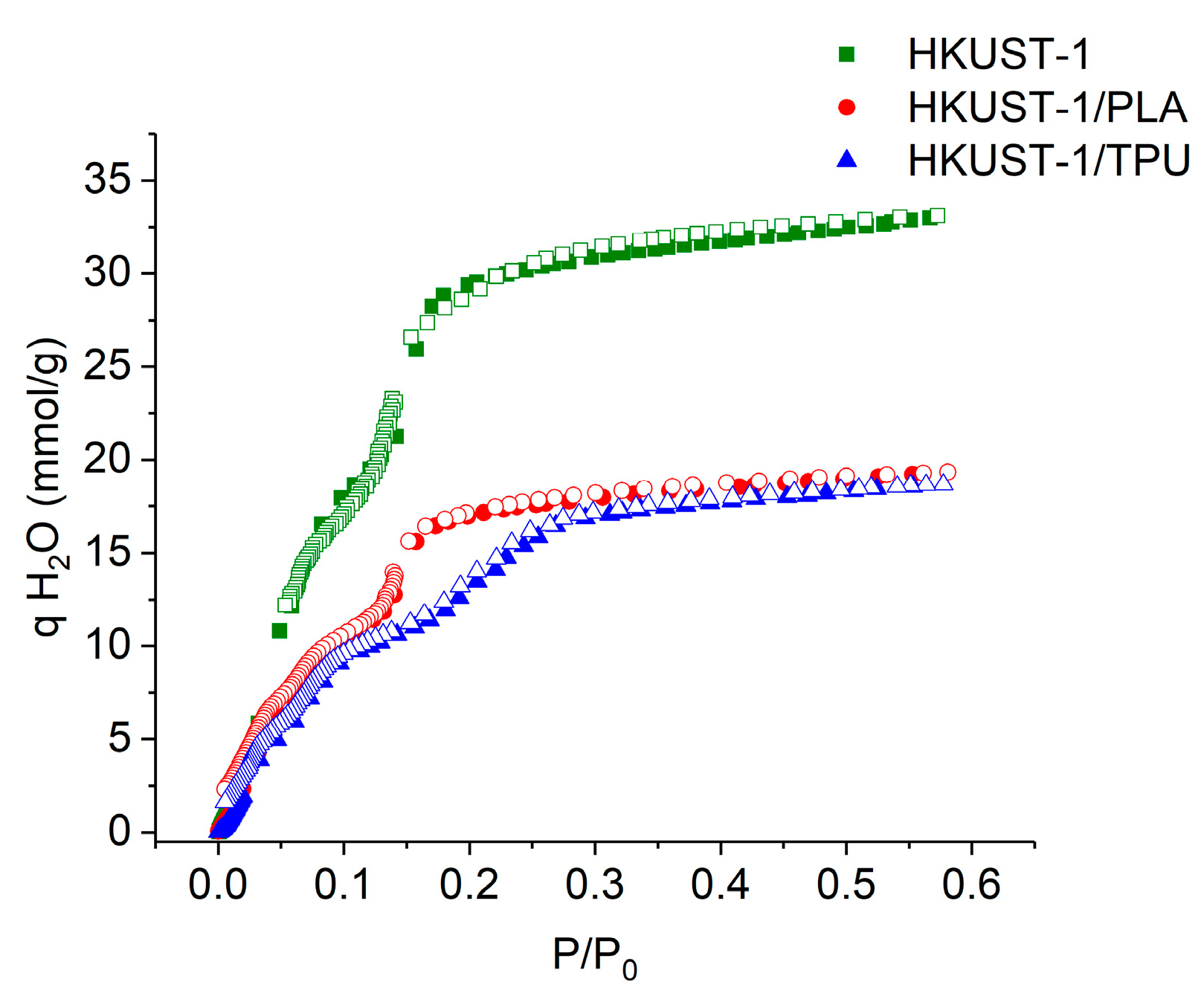
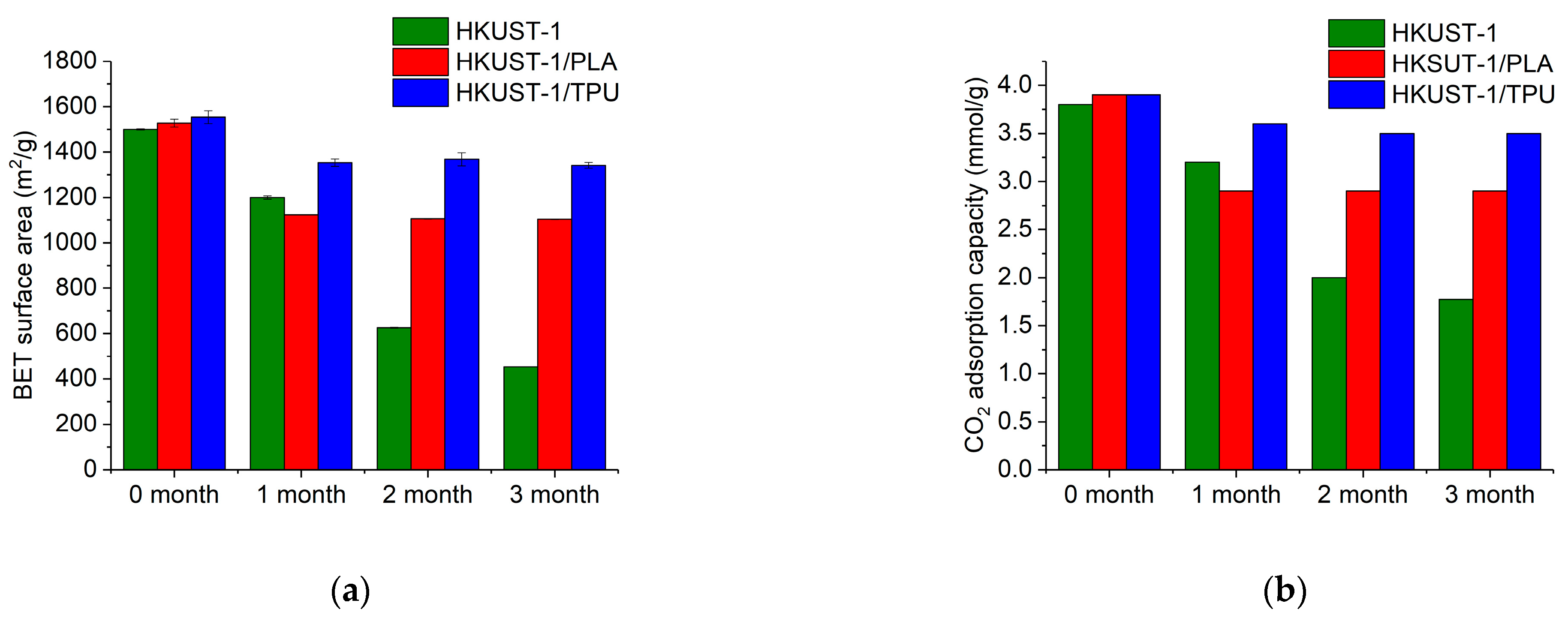
2.7. Aging along Exposure in Humid Conditions
3. Materials and Methods
3.1. Materials
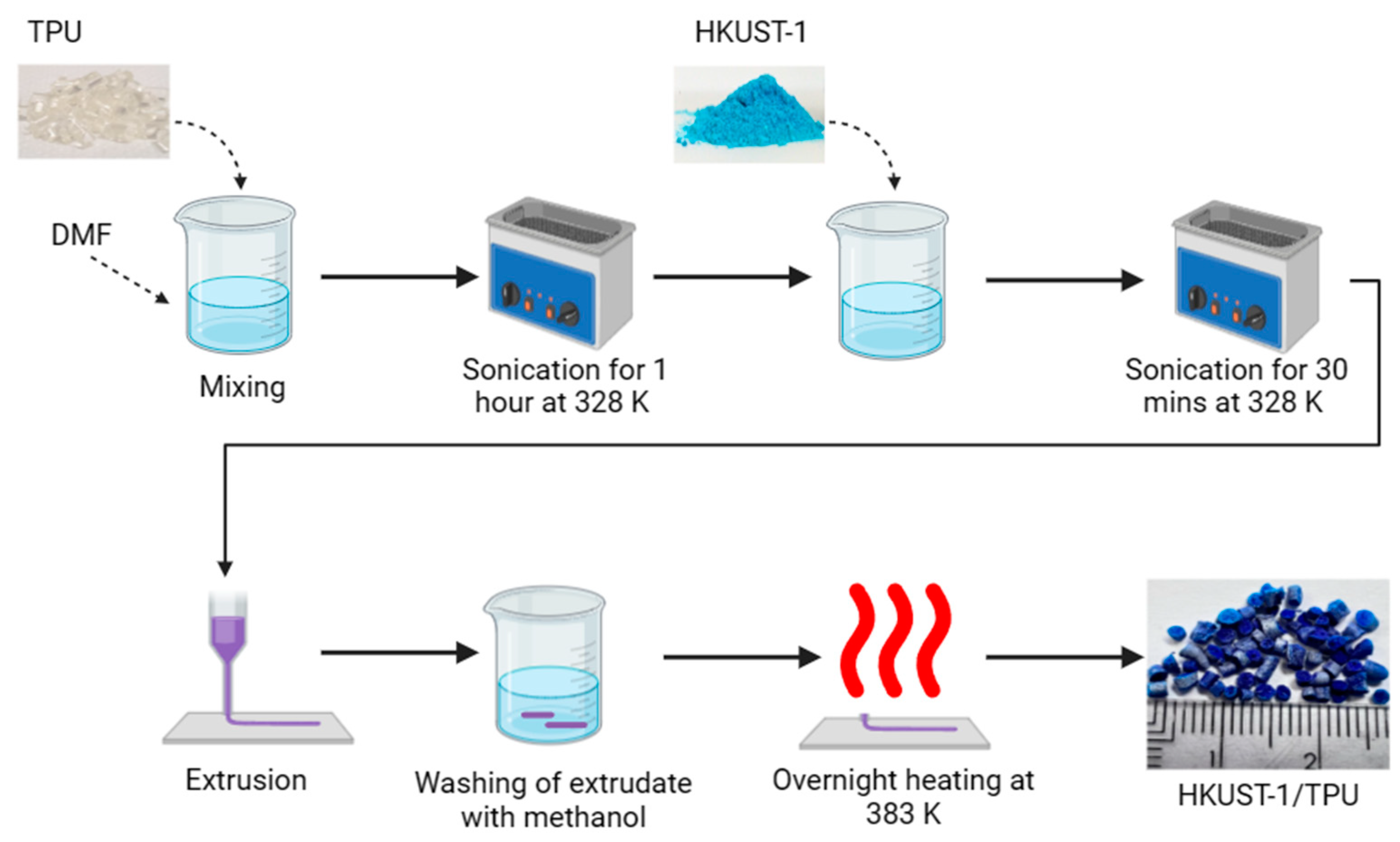
3.2. Synthesis of HKUST−1/TPU Composite
3.3. Scanning Electron Microscopy
3.4. Powder X-ray Diffraction (XRD)
3.5. Thermogravimetric Analysis
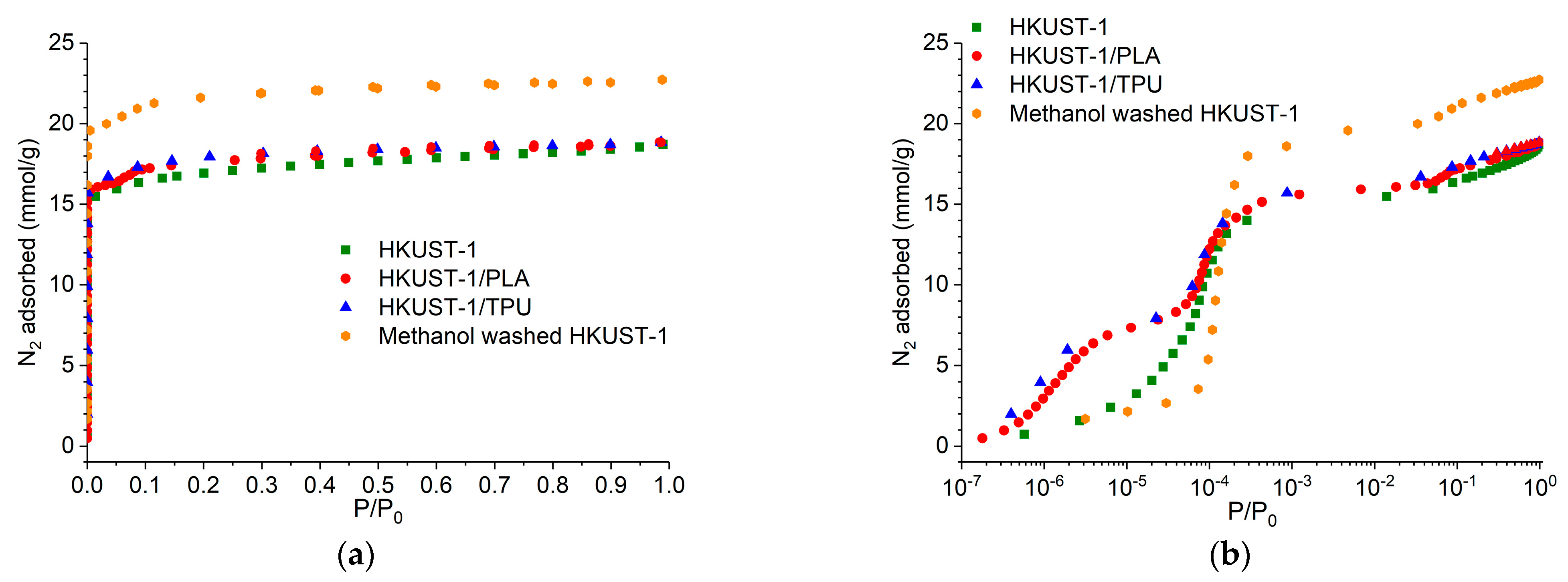
3.6. Characterization of Textural Properties
3.7. Attrition Test
3.8. CO2 and CH4 Adsorption Isotherms
3.9. Isosteric Heat of Adsorption
3.10. Ideal Adsorption Solution Theory (IAST)
3.11. Water Contact Angle
3.12. Water Adsorption Isotherms and Material Aging under Humid Atmosphere
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavičić, J.; Mavar, K.N.; Brkić, V.; Simon, K. Biogas and Biomethane Production and Usage: Technology Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940. [Google Scholar] [CrossRef]
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth. 2020. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth (accessed on 3 January 2024).
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Azelee, I.W. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsana, T.; Jayamuthunagai, J.; Praveenkumar, R.; Chozhavendhan, S.; Iyyappan, J. Biogas production—A review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew. Sustain. Energy Rev. 2018, 90, 570–582. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Tarannum, K.; Chowdhury, A.T.; Rafa, N.; Nuzhat, S.; Kumar, P.S.; Vo, D.-V.N.; Lichtfouse, E.; Mahlia, T.M.I. Biogas upgrading, economy and utilization: A review. Environ. Chem. Lett. 2021, 19, 4137–4164. [Google Scholar] [CrossRef]
- Shah, G.; Ahmad, E.; Pant, K.; Vijay, V. Comprehending the contemporary state of art in biogas enrichment and CO2 capture technologies via swing adsorption. Int. J. Hydrogen Energy 2021, 46, 6588–6612. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Ghazvini, M.F.; Vahedi, M.; Nobar, S.N.; Sabouri, F. Investigation of the MOF adsorbents and the gas adsorptive separation mechanisms. J. Environ. Chem. Eng. 2021, 9, 104790. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Assiri, M.A.; Al-Sehemi, A.G.; Gonfa, G.; Mukhtar, A.; Kareem, F.A.A.; Ayoub, M.; Saqib, S.; Mellon, N.B. Synthesis and characterization of mesoporous MOF UMCM-1 for CO2/CH4 adsorption; an experimental, isotherm modeling and thermodynamic study. Microporous Mesoporous Mater. 2020, 294, 109844. [Google Scholar] [CrossRef]
- Salehi, S.; Anbia, M.; Razavi, F. Improving CO2/CH4 and CO2/N2 adsorptive selectivity of cu-BTC and MOF-derived nanoporous carbon by modification with nitrogen-containing groups. Environ. Prog. Sustain. 2020, 39, 13302. [Google Scholar] [CrossRef]
- Li, C.-N.; Wang, S.-M.; Tao, Z.-P.; Liu, L.; Xu, W.-G.; Gu, X.-J.; Han, Z.-B. Green Synthesis of MOF-801 (Zr/Ce/Hf) for CO2/N2 and CO2/CH4 Separation. Inorg. Chem. 2023, 62, 7853–7860. [Google Scholar] [CrossRef]
- Teo, H.W.B.; Chakraborty, A.; Kayal, S. Evaluation of CH4 and CO2 adsorption on HKUST−1 and MIL-101 (Cr) MOFs employing Monte Carlo simulation and comparison with experimental data. Appl. Therm. Eng. 2017, 110, 891–900. [Google Scholar] [CrossRef]
- Asadi, T.; Ehsani, M.R.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. CO2/CH4 Separation by Adsorption using Nanoporous Metal organic Framework Copper-Benzene-1, 3, 5-tricarboxylate Tablet. Chem. Eng. Technol. 2017, 36, 1231–1239. [Google Scholar] [CrossRef]
- Chong, K.C.; Lai, S.O.; Mah, S.K.; Thiam, H.S.; Chong, W.C.; Shuit, S.H.; Lee, S.S.; Chong, W.E. A Review of HKUST−1 Metal-Organic Frameworks in Gas Adsorption. Conf. Ser. Earth Environ. Sci. 2023, 1135, 012030. [Google Scholar] [CrossRef]
- Yu, D.; Yazaydin, A.O.; Lane, J.R.; Dietzel, P.D.C.; Snurr, R.Q. A combined experimental and quantum chemical study of CO2 adsorption in the metal–organic framework CPO-27 with different metals. Chem. Sci. 2013, 4, 3544–3556. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yildirim, T. High-capacity methane storage in metal−organic frameworks M2 (dhtp): The important role of open metal sites. J. Am. Chem. Soc. 2009, 131, 4995–5000. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, X.; Ruan, L.-W.; Liu, B.; Wang, Y.-M.; Zhu, J.-F.; Qiu, L.-G. Post-combustion CO2 capture with the HKUST−1 and MIL-101 (Cr) metal–organic frameworks: Adsorption, separation and regeneration investigations. Microporous Mesoporous Mater. 2013, 179, 191–197. [Google Scholar] [CrossRef]
- Hamon, L.; Jolimaître, E.; Pirngruber, G.D. CO2 and CH4 separation by adsorption using Cu-BTC metal−organic framework. Ind. Eng. Chem. Res 2010, 49, 7497–7503. [Google Scholar] [CrossRef]
- Xian, S.; Peng, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. Highly enhanced and weakened adsorption properties of two MOFs by water vapor for separation of CO2/CH4 and CO2/N2 binary mixtures. Chem. Eng. J. 2015, 270, 385–392. [Google Scholar] [CrossRef]
- Chanut, N.; Wiersum, A.D.; Lee, U.-H.; Hwang, Y.K.; Ragon, F.; Chevreau, H.; Bourrelly, S.; Kuchta, B.; Chang, J.-S.; Serre, C.; et al. Observing the effects of shaping on gas adsorption in metal-organic frameworks. Eur. J. Inorg. Chem. 2016, 27, 4416–4423. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Kayal, S.; Chakraborty, A. Activated carbon (type Maxsorb-III) and MIL-101 (Cr) metal organic framework based composite adsorbent for higher CH4 storage and CO2 capture. Chem. Eng. J. 2018, 334, 780–788. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, D.; Sun, D.; Zhou, H.-C. An isoreticular series of metal–organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. 2010, 122, 5485–5489. [Google Scholar] [CrossRef]
- Lv, D.; Shi, R.; Chen, Y.; Chen, Y.; Wu, H.; Zhou, X.; Xi, H.; Li, Z.; Xia, Q. Selective adsorptive separation of CO2/CH4 and CO2/N2 by a water resistant zirconium–porphyrin metal–organic framework. Ind. Eng. Chem. Res. 2018, 57, 12215–12224. [Google Scholar] [CrossRef]
- Awadallah-F, A.; Hillman, F.; Al-Muhtaseb, S.A.; Jeong, H.K. Adsorption equilibrium and kinetics of nitrogen, methane and carbon dioxide gases onto ZIF-8, Cu10%/ZIF-8, and Cu30%/ZIF-8. Ind. Eng. Chem. Res. 2019, 58, 6653–6661. [Google Scholar] [CrossRef]
- Rocha, L.A.; Andreassen, K.A.; Grande, C.A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chem. Eng. Sci. 2017, 164, 148–157. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Ren, J.; Dyosiba, X.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Review on the current practices and efforts towards pilot-scale production of metal-organic frameworks (MOFs). Coord. Chem. Rev. 2017, 352, 187–219. [Google Scholar] [CrossRef]
- Golmakani, A.; Nabavi, S.A.; Wadi, B.; Manovic, V. Advances, challenges, and perspectives of biogas cleaning, upgrading, and utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Álvarez, J.R.; Sánchez-González, E.; Pérez, E.; Schneider-Revueltas, E.; Martínez, A.; Tejeda-Cruz, A.; Islas-Jácome, A.; González-Zamora, E.; Ibarra, I.A. Structure stability of HKUST−1 towards water and ethanol and their effect on its CO2 capture properties. Dalton Trans. 2017, 46, 9192–9200. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal–organic framework (HKUST−1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef]
- Ediati, R.; Dewi, S.K.; Hasan, M.R.; Kahardina, M.; Murwani, I.K.; Nadjib, M. Mesoporous HKUST−1 synthesized using solvothermal method. Rasayan J. Chem. 2019, 12, 1653–1659. [Google Scholar] [CrossRef]
- Morales, E.M.C.; Méndez-Rojas, M.A.; Torres-Martínez, L.M.; Garay-Rodríguez, L.F.; López, I.; Uflyand, I.E.; Kharisov, B.I. Ultrafast synthesis of HKUST−1 nanoparticles by solvothermal method: Properties and possible applications. Polyhedron 2021, 210, 115517. [Google Scholar] [CrossRef]
- Nobar, S.N. Cu-BTC synthesis, characterization and preparation for adsorption studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- Guo, L.; Du, J.; Li, C.; He, G.; Xiao, Y. Facile synthesis of hierarchical micro-mesoporous HKUST−1 by a mixed-linker defect strategy for enhanced adsorptive removal of benzothiophene from fuel. Fuel 2021, 300, 120955. [Google Scholar] [CrossRef]
- Armstrong, M.; Sirous, P.; Shan, B.; Wang, R.; Zhong, C.; Liu, J.; Mu, B. Prolonged HKUST−1 functionality under extreme hydrothermal conditions by electrospinning polystyrene fibers as a new coating method. Microporous Mesoporous Mater. 2018, 270, 34–39. [Google Scholar] [CrossRef]
- Vehrenberg, J.; Vepsäläinen, M.; Macedo, D.S.; Rubio-Martinez, M.; Webster, N.A.S.; Wessling, M. Steady-state electrochemical synthesis of HKUST−1 with polarity reversal. Microporous Mesoporous Mater. 2020, 303, 110218. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Macedo, D.S.; Gong, H.; Rubio-Martinez, M.; Bayatsarmadi, B.; He, B. Electrosynthesis of HKUST−1 with flow-reactor post-processing. Appl. Sci. 2021, 11, 3340. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, T.; Cai, K.; Chen, P.; Liu, F.; Tao, D.-J. Rapid mechanochemical construction of HKUST−1 with enhancing water stability by hybrid ligands assembly strategy for efficient adsorption of SF6. Chem. Eng. J. 2022, 437, 135364. [Google Scholar] [CrossRef]
- Stolar, T.; Batzdorf, L.; Lukin, S.; Žilić, D.; Motillo, C.; Friščić, T.; Emmerling, F.; Halasz, I.; Užarević, K. In situ monitoring of the mechanosynthesis of the archetypal metal–organic framework HKUST−1: Effect of liquid additives on the milling reactivity. Inorg. Chem. 2017, 56, 6599–6608. [Google Scholar] [CrossRef]
- Ntouros, V.; Kousis, I.; Pisello, A.L.; Assimakopoulos, M.N. Binding Materials for MOF Monolith Shaping Processes: A Review towards Real Life Application. Energies 2022, 15, 1489. [Google Scholar] [CrossRef]
- Cousin-Saint-Remi, J.; Finoulst, A.-L.; Jabbour, C.; Baron, G.V.; Denayer, J.F.M. Selection of binder recipes for the formulation of MOFs into resistant pellets for molecular separations by fixed-bed adsorption. Microporous Mesoporous Mater. 2020, 304, 109322. [Google Scholar] [CrossRef]
- Hastürk, E.; Höfert, S.-P.; Topalli, B.; Schlüsener, C.; Janiak, C. Shaping of MOFs via freeze-casting method with hydrophilic polymers and their effect on textural properties. Microporous Mesoporous Mater. 2020, 295, 109907. [Google Scholar] [CrossRef]
- Rozaini, M.T.; Grekov, D.I.; Bustam, M.A.; Pré, P. Shaping of HKUST−1 via Extrusion for the Separation of CO2/CH4 in Biogas. Separations 2023, 10, 487. [Google Scholar] [CrossRef]
- Yu, W.; Sun, L.; Li, M.; Li, M.; Lei, W.; Wei, C. FDM 3D printing and properties of PBS/PLA blends. Polymers 2023, 15, 4305. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, R.A.; Subeshan, B.; Asmatulu, E. Fabrication of hydrophobic PLA filaments for additive manufacturing. J. Mater. Sci. 2022, 57, 8987–9001. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Mohammadi, M.; Carreau, P.J. Effect of TPU hard segment content on the rheological and mechanical properties of PLA/TPU blends. J. Appl. Polym. Sci 2020, 137, 49387. [Google Scholar] [CrossRef]
- Lis-Bartos, A.; Smieszek, A.; Frańczyk, K.; Marycz, K. Fabrication, characterization, and cytotoxicity of thermoplastic polyurethane/poly (lactic acid) material using human adipose derived mesenchymal stromal stem cells (hASCs). Polymers 2018, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Oliaei, E.; Kaffashi, B.; Davoodi, S. Investigation of structure and mechanical properties of toughened poly (l-lactide)/thermoplastic poly (ester urethane) blends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Muñoz-Chilito, J.; Lara-Ramos, J.A.; Marín, L.; Machuca-Martínez, F.; Correa-Aguirre, J.P.; Hidalgo-Salazar, M.A.; García-Navarro, S.; Roca-Blay, L.; Rodríguez, L.A.; Mosquera-Vargas, E.; et al. Morphological Electrical and Hardness Characterization of Carbon Nanotube-Reinforced Thermoplastic Polyurethane (TPU) Nanocomposite Plates. Molecules 2023, 28, 3598. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, T.K.; Varadarajan, K.M. Strong, stretchable and ultrasensitive MWCNT/TPU nanocomposites for piezoresistive strain sensing. Compos. Part B 2019, 117, 107285. [Google Scholar] [CrossRef]
- Dai, X.; Cao, Y.; Shi, X.; Wang, X. Non-isothermal crystallization kinetics, thermal degradation behavior and mechanical properties of poly (lactic acid)/MOF composites prepared by melt-blending methods. RSC Adv. 2016, 6, 71461–71471. [Google Scholar] [CrossRef]
- Gregor-Svetec, D.; Leskovšek, M.; Leskovar, B.; Elesini, U.S.; Vrabič-Brodnjak, U. Analysis of PLA composite filaments reinforced with lignin and polymerised-lignin-treated NFC. Polymers 2021, 13, 2174. [Google Scholar] [CrossRef]
- Yang, T.; Hu, J.; Wang, P.; Edeleva, M.; Cardon, L.; Zhang, J. Two-step approach based on fused filament fabrication for high performance graphene/thermoplastic polyurethane composite with segregated structure. Compos. Part A Appl. Sci. Manuf. 2023, 174, 107719. [Google Scholar] [CrossRef]
- Bhoria, N.; Basina, G.; Pokhrel, J.; Reddy, K.S.K.; Anastasiou, S.; Balasubramanian, V.V.; AlWahedi, Y.F.; Karanikolos, G.N. Functionalization effects on HKUST−1 and HKUST−1/graphene oxide hybrid adsorbents for hydrogen sulfide removal. J. Hazard. Mater. 2020, 394, 122565. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltra, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and characterization of an SWCNT@ HKUST−1 composite: Enhancing the CO2 adsorption properties of HKUST−1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Kanbur, Y.; Tayfun, U. Investigating mechanical, thermal, and flammability properties of thermoplastic polyurethane/carbon nanotube composites. J. Thermoplast. Compos. Mater. 2018, 31, 1661–1675. [Google Scholar] [CrossRef]
- Wu, W.; Huang, W.; Tong, Y.; Huang, J.; Wu, J.; Cao, X.; Zhang, Q.; Yu, B.; Li, R.K.Y. Self-assembled double core-shell structured zeolitic imidazole framework-8 as an effective flame retardant and smoke suppression agent for thermoplastic polyurethane. Appl. Surf. Sci. 2023, 610, 155540. [Google Scholar] [CrossRef]
- Evans, K.A.; Kennedy, Z.C.; Arey, B.W.; Christ, J.F.; Schaef, H.T.; Nune, S.K.; Erikson, R.L. Chemically active, porous 3D-printed thermoplastic composites. ACS Appl. Mater. Interfaces 2018, 10, 15112–15121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Qi, X.; Zhang, W.; Wang, D.-Y. Size tailored bimetallic metal-organic framework (MOF) on graphene oxide with sandwich-like structure as functional nano-hybrids for improving fire safety of epoxy. Compos. Part B 2020, 188, 107881. [Google Scholar] [CrossRef]
- ASTM D4058-96; Standard Test Method for Attrition and Abrasion of Catalysts and Catalyst Carriers. ASTM International: West Conshohocken, PA, USA, 2020.
- Shah, B.B.; Kundu, T.; Zhao, D. Mechanical properties of shaped metal-organic frameworks. Top. Curr. Chem. 2019, 377, 25. [Google Scholar] [CrossRef] [PubMed]
- Khabzina, Y.; Dhainaut, J.; Ahlhelm, M.; Richter, H.-J.; Reinsch, H.; Stock, N.; Farrusseng, D. Synthesis and shaping scale-up study of functionalized UiO-66 MOF for ammonia air purification filters. Ind. Eng. Chem. Res. 2018, 57, 8200–8208. [Google Scholar] [CrossRef]
- Supronowicz, B.; Mavrandonakis, A.; Heine, T. Interaction of small gases with the unsaturated metal centers of the HKUST−1 metal organic framework. J. Phys. Chem. C 2013, 117, 14570–14578. [Google Scholar] [CrossRef]
- García-Pérez, E.; Gascón, J.; Morales-Flórez, V.; Castillo, J.M.; Kapteijn, F.; Calero, S. Identification of adsorption sites in Cu-BTC by experimentation and molecular simulation. Langmuir 2009, 25, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.S.; Park, S.; Kang, D.W.; Kim, D.W.; Kang, M.; Choi, D.S.; Choe, J.H.; Hong, C.S. Moisture-tolerant diamine-appended metal–organic framework composites for effective indoor CO2 capture through facile spray coating. Chem. Eng. J. 2022, 433, 133856. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Denny, M.S., Jr.; Peterson, G.W.; Mahle, J.J.; Cohen, S.M. Enhanced aging properties of HKUST−1 in hydrophobic mixed-matrix membranes for ammonia adsorption. Schem. Sci. 2016, 7, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chae, Y.S.; Kang, D.W.; Kang, M.; Choe, J.H.; Kim, S.; Kim, J.Y.; Jeong, Y.W.; Hong, C.S. Shaping of a metal–organic framework–polymer composite and its CO2 adsorption performances from humid indoor air. ACS Appl. Mater. Interfaces 2021, 13, 25421–25427. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zhang, Z.; Huang, H.; Zhong, C.; Mei, D. Theoretical insights into the initial hydrolytic breakdown of HKUST−1. J. Phys. Chem. C 2019, 124, 1991–2001. [Google Scholar] [CrossRef]
- Todaro, M.; Alessi, A.; Sciortino, L.; Agnello, S.; Cannas, M.; Gelardi, F.M.; Buscarino, G. Investigation by Raman Spectroscopy of the Decomposition Process of HKUST−1 upon Exposure to Air. J. Spectrosc. 2016, 2016, 8074297. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sircar, S.; Mohr, R.; Ristic, C.; Rao, M.B. Isosteric heat of adsorption: Theory and experiment. J. Phys. Chem. B 1999, 103, 6539–6546. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, J. Differential heat of adsorption and isosteres. Langmuir 2017, 33, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Walton, K.S.; Sholl, D.S. Predicting multicomponent adsorption: 50 years of the ideal adsorbed solution theory. AIChE J. 2015, 61, 2757–2762. [Google Scholar] [CrossRef]
- Heymans, N.; SVaesen; De Weireld, G. A complete procedure for acidic gas separation by adsorption on MIL-53 (Al). Microporous Mesoporous Mater. 2012, 154, 93–99. [Google Scholar] [CrossRef]
- Simon, C.M.; Smit, B.; Haranczyk, M. pyIAST: Ideal adsorbed solution theory (IAST) Python package. Comput. Phys. Commun. 2016, 200, 364–380. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.H.; Kim, J. User-friendly graphical user interface software for ideal adsorbed solution theory calculations. Korean J. Chem. Eng. 2018, 35, 214–221. [Google Scholar] [CrossRef]
- Rasband, W.S. “ImageJ”. U.S. National Institutes of Health, Bethesda, Maryland. Available online: https://imagej.nih.gov/ij/ (accessed on 8 February 2023).



| Sample | SBET (m2/g) | Micropore Volume (cm3/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| HKUST−1 | 1500 | 0.46 | 0.65 |
| HKUST−1/TPU | 1557 | 0.50 | 0.65 |
| HKUST−1/PLA | 1528 | 0.54 | 0.65 |
| Methanol-washed HKUST−1 | 1956 | 0.60 | 0.79 |
| Sample | Attrition Loss (% wt) | Reference |
|---|---|---|
| HKUST−1/TPU | 0.4 | This study |
| HKUST−1/PLA | 0.5 | [46] |
| Zeolite 3A | ≤0.2 | [65] |
| Zeolite 4A | ≤0.2 | |
| Zeolite 5A | ≤0.2 | |
| Zeolite 13X | ≤0.2 | |
| AC-Norit RZN1 | 0.2 | [66] |
| UiO-66 extrudate | 1.4 |
| Polymer | Average Contact Angle (°) |
|---|---|
| PLA | 66.9 |
| TPU | 90.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozaini, M.T.; Grekov, D.I.; Bustam, M.A.; Pré, P. Low-Hydrophilic HKUST−1/Polymer Extrudates for the PSA Separation of CO2/CH4. Molecules 2024, 29, 2069. https://doi.org/10.3390/molecules29092069
Rozaini MT, Grekov DI, Bustam MA, Pré P. Low-Hydrophilic HKUST−1/Polymer Extrudates for the PSA Separation of CO2/CH4. Molecules. 2024; 29(9):2069. https://doi.org/10.3390/molecules29092069
Chicago/Turabian StyleRozaini, Muhamad Tahriri, Denys I. Grekov, Mohamad Azmi Bustam, and Pascaline Pré. 2024. "Low-Hydrophilic HKUST−1/Polymer Extrudates for the PSA Separation of CO2/CH4" Molecules 29, no. 9: 2069. https://doi.org/10.3390/molecules29092069
APA StyleRozaini, M. T., Grekov, D. I., Bustam, M. A., & Pré, P. (2024). Low-Hydrophilic HKUST−1/Polymer Extrudates for the PSA Separation of CO2/CH4. Molecules, 29(9), 2069. https://doi.org/10.3390/molecules29092069







