Amine Switchable Hydrophilic Solvent Vortex-Assisted Homogeneous Liquid–Liquid Microextraction and GC-MS for the Enrichment and Determination of 2, 6-DIPA Additive in Biodegradable Film
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Internal Standards
2.2. Optimization of Heating Hydrolysis–Extraction Procedures
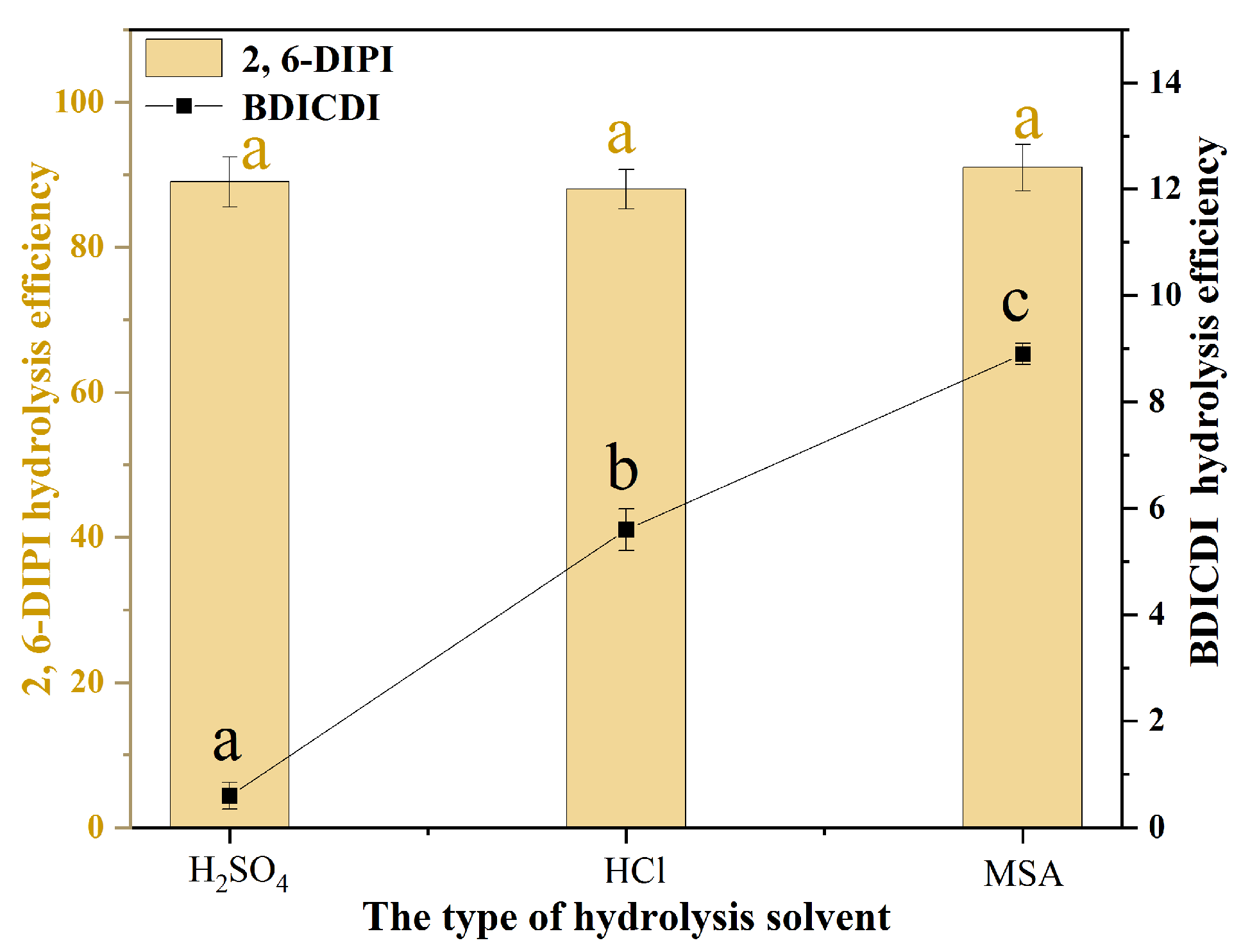
2.2.1. Effect of Type of Hydrolysis Solvent
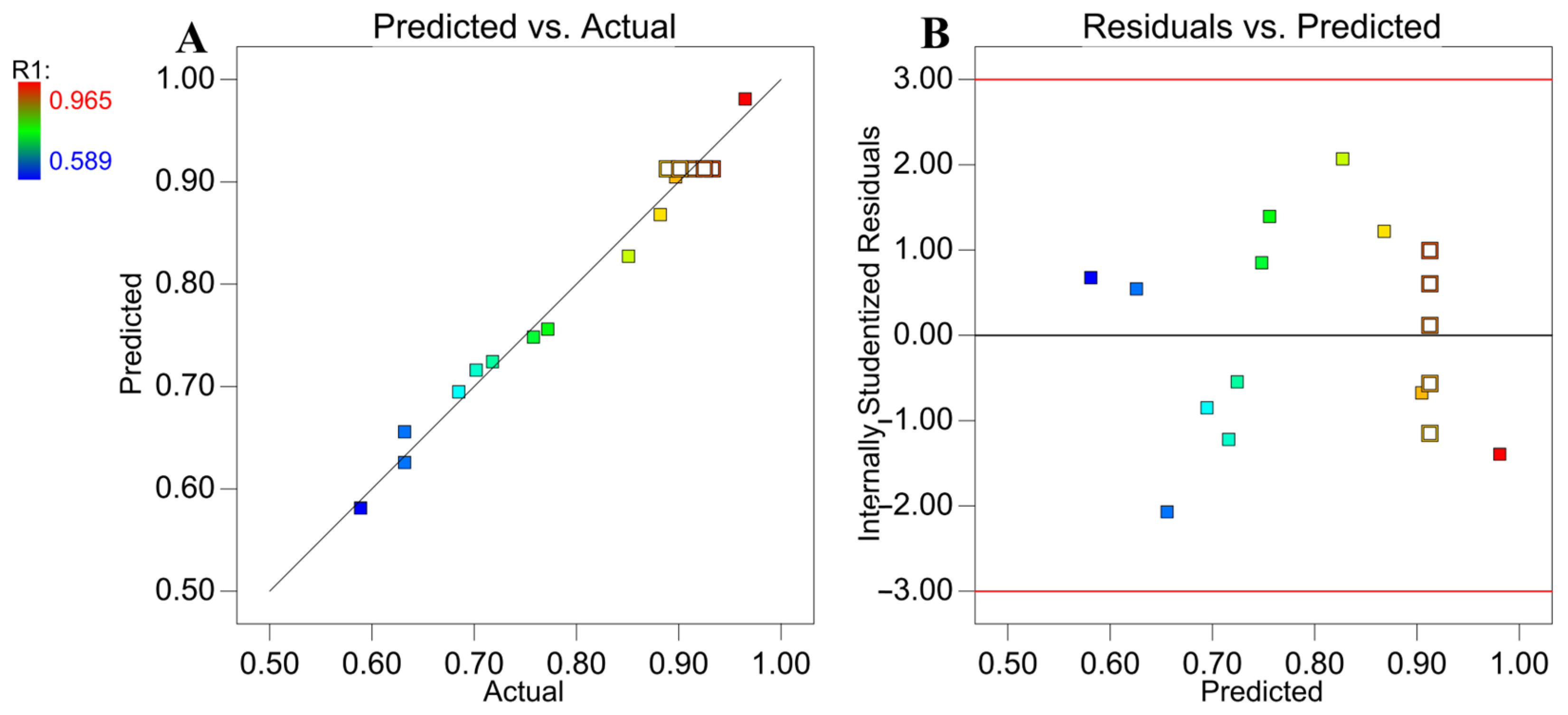
2.2.2. Optimization of 2, 6-DIPI Hydrolysis Efficiency with BBD
2.3. Optimization of Amine SHS-VAHLLME Conditions
2.3.1. Effect of Type of Amines
2.3.2. Effect of DPA Volume
2.3.3. Effect of Transition pH
2.3.4. Effect of Salt Addition and Extraction Time
2.4. Method Validation and Evaluation
2.5. Sample Analysis in PBAT Biodegradable Agricultural Mulching Films
3. Materials and Method
3.1. Reagents, Standard Solutions, and Samples
3.2. Apparatus
3.3. Heating Hydrolysis–Extraction of 2, 6-DIPI and 2, 6-DIPA
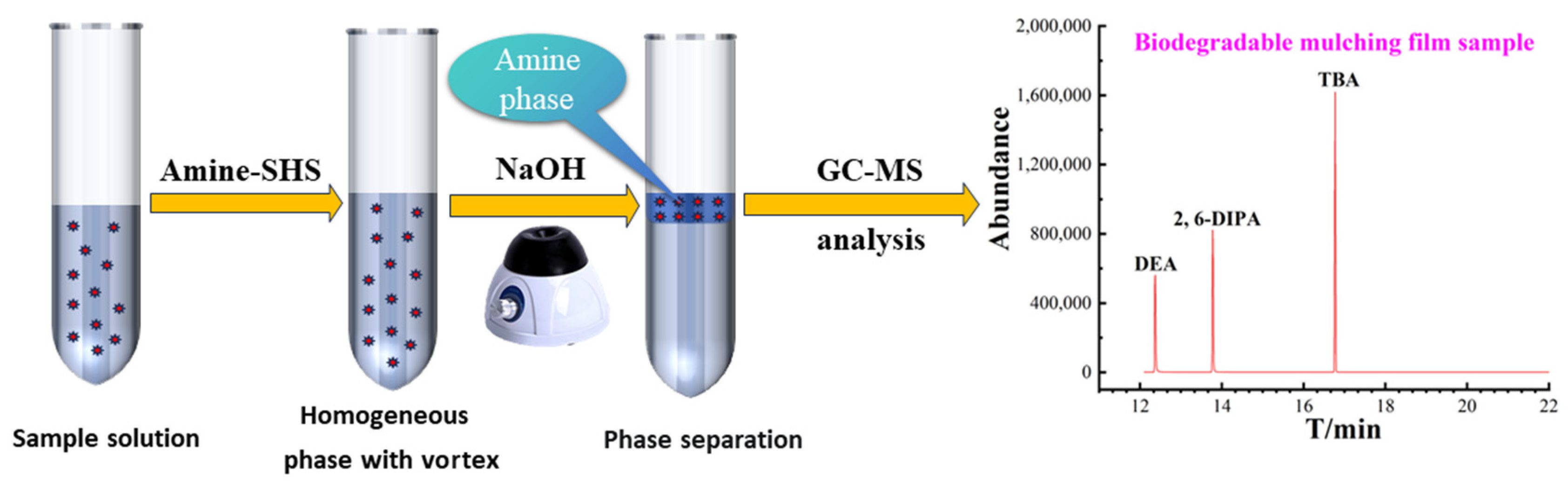
3.4. Amine SHS-VAHLLME Procedures
3.5. Response Surface Methodology Analysis with BBD
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davachi, S.M.; Mokhtare, A.; Torabi, H.; Enayati, M.; Deisenroth, T.; Van Pho, T.; Qu, L.L.; Tücking, K.S.; Abbaspourrad, A. Screening the degradation of polymer microparticles on a chip. ACS Omega 2022, 8, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.S.; Sommaggio, L.R.D.; Marin-Morales, M.A.; Morales, A.R. PBAT biodegradable mulch films: Study of ecotoxicological impacts using Allium cepa, Lactuca sativa and HepG2/C3A cell culture. Chemosphere 2020, 256, 126985. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, M.; Weng, Y.X.; Zhao, Y.; Li, C.T.; Kanwal, A. Degradation of poly (butylene adipate-co-terephthalate) by Stenotrophomonas sp. YCJ1 isolated from farmland soil. J. Environ. Sci. 2021, 103, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.M.; Liang, Y.Q.; Jiang, J.J.; Mo, A.Y.; He, D.F. Organic additives in agricultural plastics and their impacts on soil ecosystems: Compared with conventional and biodegradable plastics. TrAC Trends Anal. Chem. 2023, 166, 117212. [Google Scholar] [CrossRef]
- Lu, M.; Jones, S.; McKinney, M.; Kandow, A.; Donahoe, R.; Faulk, B.C.; Chen, S.; Lu, Y. Assessment of phthalic acid esters plasticizers in sediments of coastal Alabama, USA: Occurrence, source, and ecological risk. Sci. Total Environ. 2023, 897, 165345. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Binderup, M.L.; Bursch, W.; Castle, L.; Crebelli, R.; Engel, K.H.; Franz, R.; Gontard, N.; Husoy, T.; Jany, K.D.; et al. Scientific opinion on the safety evaluation of the substance bis (2, 6-diisopropylphenyl) carbodiimide for use in food contact materials. EFSA J. 2010, 8, 1928. [Google Scholar]
- Souza, M.C.O.; González, N.; Herrero, M.; Marquès, M.; Rovira, J.; Nadal, N.; Barbosa, F.; Domingo, J.L. Screening of regulated aromatic amines in clothing marketed in Brazil and Spain: Assessment of human health risks. Environ. Res. 2023, 221, 115264. [Google Scholar] [CrossRef]
- Edebali, Ö.; Krupčíková, S.; Goellner, A.; Vrana, B.; Muz, M.; Melymuk, L. Aromatic amines from sources to surface waters. Environ. Sci. Technol. Lett. 2024. [Google Scholar] [CrossRef]
- Cui, H.; Gao, W.C.; Lin, Y.C.; Zhang, J.; Yin, R.S.; Xiang, Z.G.; Zhang, S.; Zhou, S.P.; Chen, W.S.; Cai, K. Development of microwave-assisted extraction and dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry for the determination of organic additives in biodegradable mulch films. Microchem. J. 2021, 160, 105722. [Google Scholar] [CrossRef]
- Lin, Q.B.; Li, B.; Song, H.; Li, X.M. Determination of 7 antioxidants, 8 ultraviolet absorbents, and 2 fire retardants in plastic food package by ultrasonic extraction and ultra performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 730–743. [Google Scholar] [CrossRef]
- Joaquin, H.F.; Jaime, P.M.; Rodrigo, O.T. Applying a green solvent with microwave, ultrasound, and soxhlet extraction techniques to quantify the slip additive cis-1, 3-docosenamide and nine oxidative degradation byproducts in polypropylene samples. Polymers 2023, 15, 3457. [Google Scholar] [CrossRef]
- Das, P.; Zeng, Q.; Leybros, A.; Gabriel, J.C.P.; Tay, C.Y.; Lee, J.M. Enhanced extraction of brominated flame retardants from e-waste plastics. Chem. Eng. J. 2023, 469, 144126. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.W.; Lin, Q.B.; Hu, C.Y.; Su, Q.Z.; Wu, Y.M. Determination of polymer additives-antioxidants, ultraviolet stabilizers, plasticizers and photoinitiators in plastic food package by accelerated solvent extraction coupled with high-performance liquid chromatography. J. Chromatogr. Sci. 2015, 53, 1026–1035. [Google Scholar] [CrossRef]
- Cai, K.; Lin, Y.C.; Ma, Y.F.; Yang, Z.X.; Yu, L.; Zhang, J.; Xu, D.Q.; Zeng, R.Z.; Gao, W.C. Determination of residual diisocyanates and related diamines in biodegradable mulch films using N-ethoxycarbonylation derivatization and GC-MS. Molecules 2022, 27, 6754. [Google Scholar] [CrossRef]
- Pouech, C.; Lafay, F.; Wiest, L.; Baudot, R.; Léonard, D.; Cren-Olivé, C. Monitoring the extraction of additives and additive degradation products from polymer packaging into solutions by multi-residue method including solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry analysis. Anal. Bioanal. Chem. 2014, 406, 1493–1507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Lin, Q.B.; Song, X.C.; Chen, S.; Zhong, H.N.; Nerin, C. Discrimination of virgin and recycled polyethylene based on volatile organic compounds using a headspace GC-MS coupled with chemometrics approach. Food Packag. Shelf Life 2020, 26, 100553. [Google Scholar] [CrossRef]
- Zhang, S.J.; Chen, Y.H.; Liu, S.H.; Li, Y.Y.; Zhao, H.H.; Chen, Q.Q.; Hou, X.H. Dissolution-precipitation method concatenated sodium alginate/MOF-derived magnetic multistage pore carbon magnetic solid phase extraction for determination of antioxidants and ultraviolet stabilizers in polylactic acid food contact plastics. Talanta 2024, 270, 125487. [Google Scholar] [CrossRef]
- Alshana, U.; Hassan, M.; Al-Nidawi, M.; Yilmaz, E.; Soylak, M. Switchable-hydrophilicity solvent liquid-liquid microextraction. TrAC Trends Anal. Chem. 2020, 131, 116025. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef]
- Elik, A.; Altunay, A.Ö.; Lanjwani, M.F.; Tuzen, M. A new ultrasound-assisted liquid-liquid microextraction method utilizing a switchable hydrophilicity solvent for spectrophotometric determination of nitrite in food samples. J. Food Compos. Anal. 2023, 119, 105267. [Google Scholar] [CrossRef]
- Lorenzo-Parodi, N.; Leitner, E.; Schmidt, T.C. Comparison of gas chromatographic techniques for the analysis of iodinated derivatives of aromatic amines. Anal. Bioanal. Chem. 2023, 415, 3313–3325. [Google Scholar] [CrossRef]
- Katthanet, K.; Supo, S.; Jaroensan, J.; Khiaophong, W.; Kachangoon, R.; Ponhong, K.; Pramual, P.; Vichapong, J. Preconcentration of heterocyclic aromatic amines in edible fried insects using surfactant-assisted hydrophobic deep eutectic solvent for homogeneous liquid–liquid microextraction prior to HPLC. ACS Omega 2024, 9, 3962–3970. [Google Scholar] [CrossRef]
- Perez, M.Â.F.; Daniel, D.; Padula, M.; do Lago, C.L.; Bottoli, C.B.G. Determination of primary aromatic amines from cooking utensils by capillary electrophoresis-tandem mass spectrometry. Food Chem. 2021, 362, 129902. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.H.; Du, H.Y.; Wang, F.; Mou, S.F.; Haddad, P.R. Organic analysis by ion chromatography: 1. Determination of aromatic amines and aromatic diisocyanates by cation-exchange chromatography with amperometric detection. J. Chromatogr. A 2002, 956, 215–220. [Google Scholar] [CrossRef]
- Lorenzo-Parodi, N.; Kaziur-Cegla, W.; Schmidt, T.C. Automation and optimization of the sample preparation of aromatic amines for their analysis with GC–MS. Green Anal. Chem. 2023, 6, 100071. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Yüksel, D.E.; Ballice, L.; Cengiz, N.; Sağlam, M.; Yüksel, M. Aromatic sulfonic acid-catalyzed conversion of safflower stalk into levulinic acid. Biomass Conv. Bioref. 2024, 14, 1105–1116. [Google Scholar] [CrossRef]
- Antonoplis, R.A.; Blanch, H.W.; Freitas, R.P.; Sciamanna, A.F.; Wilke, C.R. Production of sugars from wood using high-pressure hydrogen chloride. Biotechnol. Bioeng. 1983, 25, 2757–2773. [Google Scholar] [CrossRef]
- Stloukal, P.; Jandikova, G.; Koutny, M.; Sedlařík, V. Carbodiimide additive to control hydrolytic stability and biodegradability of PLA. Polym. Test. 2016, 54, 19–28. [Google Scholar] [CrossRef]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef]
- Wolf, M.E.; Vandezande, J.E.; Schaefer, H.F. Catalyzed reaction of isocyanates (RNCO) with water. Phys. Chem. Chem. Phys. 2021, 23, 18535–18546. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Liao, H.B.; Dai, Y.T.; Qi, Y.L.; Zou, Z. Characterization and anti-ultraviolet radiation activity of proanthocyanidin-rich extracts from Cinnamomum camphora by ultrasonic-assisted method. Molecules 2024, 29, 796. [Google Scholar] [CrossRef]
- Pashaei, H.; Ghaemi, A.; Nasiri, M.; Karami, B. Experimental modeling and optimization of CO2 absorption into piperazine solutions using RSM-CCD methodology. ACS Omega 2020, 5, 8432–8448. [Google Scholar] [CrossRef]
- Vanderveen, J.R.; Durelle, J.; Jessop, P.G. Design and evaluation of switchable-hydrophilicity solvents. Green Chem. 2014, 16, 1187–1197. [Google Scholar] [CrossRef]
- Shahvandi, S.K.; Banitaba, M.H.; Ahmar, H. Development of a new pH assisted homogeneous liquid-liquid microextraction by a solvent with switchable hydrophilicity: Application for GC-MS determination of methamphetamine. Talanta 2018, 184, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Oenning, A.L.; Birk, L.; Eller, S.; de Oliveira, T.F.; Merib, J.; Carasek, E. A green and low-cost method employing switchable hydrophilicity solvent for the simultaneous determination of antidepressants in human urine by gas chromatography-mass spectrometry detection. J. Chromatogr. B 2020, 1143, 122069. [Google Scholar] [CrossRef]
- Lv, H.H.; Zi, A.; Zhang, Z.H.; Chen, Y.; Zhu, G.H.; Li, Z.G.; Lee, M.W. Ultrasound-assisted switchable hydrophilic solvent-based homogeneous liquid–liquid microextraction for the determination of triazole fungicides in environmental water by GC-MS. Anal. Methods 2022, 14, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, H.; Nejati-Yazdinejad, M.; Najafi, M.; Hasheminasab, K.S. Switchable hydrophilicity solvent-based homogenous liquid–liquid microextraction (SHS-HLLME) combined with GC-FID for the quantification of methadone and tramadol. Chromatographia 2018, 81, 1063–1070. [Google Scholar] [CrossRef]
- Terzi, M.; Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Utilization of a pH-switchable hydrophilicity solvent for the microextraction of clomipramine from human urine samples. J. Chromatogr. B 2024, 1235, 124060. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Fu, R.Y.; Lu, Q.X.; Ren, T.Z.; Guo, X.L.; Di, X. Switchable hydrophilicity solvent for extraction of pollutants in food and environmental samples: A review. Microchem. J. 2023, 189, 108566. [Google Scholar] [CrossRef]
- Shao, L.l.; Xi, Y.W.; Weng, Y.X. Research progress in degradation characteristics of poly (lactic acid) composites. China Plast. 2022, 36, 155–164. [Google Scholar]
- Ullah, N.; Tuzen, M. A comprehensive review on recent developments and future perspectives of switchable solvents and their applications in sample preparation techniques. Green Chem. 2023, 25, 1729. [Google Scholar] [CrossRef]
- Cai, K.; Gao, W.C.; Li, X.; Lin, Y.C.; Li, D.C.; Quan, W.X.; Zhao, R.J.; Ren, X.L. Development and application of portable reflectometric spectroscopy combined with solid-phase extraction for determination of potassium in flue-cured tobacco leaves. ACS Omega 2023, 8, 20730–20738. [Google Scholar] [CrossRef]



| Uncoded Variable Factors | Response Factor | |||
|---|---|---|---|---|
| Nos. | A | B | C | Extraction Recovery |
| 1 | 0.5 | 50 | 1.75 | 63.2% |
| 2 | 4 | 110 | 1.75 | 85.1% |
| 3 | 0.5 | 80 | 3 | 88.2% |
| 4 | 0.5 | 80 | 0.5 | 77.2% |
| 5 | 2.25 | 110 | 0.5 | 68.5% |
| 6 | 2.25 | 80 | 1.75 | 90.1% |
| 7 | 0.5 | 110 | 1.75 | 71.8% |
| 8 | 2.25 | 50 | 0.5 | 58.9% |
| 9 | 2.25 | 110 | 3 | 89.7% |
| 10 | 4 | 50 | 1.75 | 63.2% |
| 11 | 2.25 | 80 | 1.75 | 92.5% |
| 12 | 2.25 | 80 | 1.75 | 93.3% |
| 13 | 4 | 80 | 3 | 96.5% |
| 14 | 2.25 | 50 | 3 | 75.8% |
| 15 | 4 | 80 | 0.5 | 70.2% |
| 16 | 2.25 | 80 | 1.75 | 88.9% |
| 17 | 2.25 | 80 | 1.75 | 91.5% |
| ANOVA | Model F and p-value | 50.59 and <0.0001 | ||
| Variable importance A | 0.059 | |||
| Variable importance B | <0.0001 | |||
| Variable importance C | <0.0001 | |||
| Lack-of-fit p-value | 0.1941 | |||
| Coefficient of variation | 22.71 | |||
| R2 | 0.985 | |||
| Spiked/ µg g−1 | Recovery of Repeated Samples/% | Mean Recovery/% | Inter-Day Precision /RSD% | Intra-Day Precision /RSD% | Stability /% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 6 | ||||||
| Low-concentration samples | 64.8 | 94.5 | 105.4 | 103.6 | 97.7 | 104.2 | 101.1 | 4.7 | 4.1 | 2.4 |
| 129.6 | 95.0 | 92.5 | 99.2 | 95.7 | 98.3 | 96.1 | 2.8 | 2.6 | −1.3 | |
| High-concentration samples | 1411.2 | 98.4 | 97.4 | 103.8 | 105.0 | 98.3 | 100.6 | 3.5 | 3.2 | 2.2 |
| 2822.4 | 96.7 | 97.0 | 96.8 | 102.3 | 97.0 | 98.0 | 2.5 | 2.3 | 1.8 | |
| Samples | 2, 6-DIPA Content/µg g−1 | Samples | 2, 6-DIPA Content/µg g−1 |
|---|---|---|---|
| PBAT-1 | 2875.5 ± 276.5 1 | PBAT-6 | 144.9 ± 16.4 |
| PBAT-2 | 131.9 ± 16.8 | PBAT-7 | 2729.5 ± 255.9 |
| PBAT-3 | 85.1 ± 10.6 | PBAT-8 | 153.2 ± 12.3 |
| PBAT-4 | 103.3 ± 8.8 | PBAT-9 | 108.9 ± 11.7 |
| PBAT-5 | 99.2 ± 15.5 | PBAT-10 | 105.3 ± 22.3 |
| Levels | Variable Factors | ||
|---|---|---|---|
| H2SO4 Concentration (A)/M | Heating Temperature (B)/°C | Hydrolysis–Extraction Time (C)/h | |
| Low (−1) | 1.0 | 50 | 0.5 |
| Middle (0) | 2.5 | 80 | 1.75 |
| High (1) | 4.0 | 110 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Liu, Q.; Lin, Y.; Yang, X.; Liu, Q.; Pan, W.; Gao, W. Amine Switchable Hydrophilic Solvent Vortex-Assisted Homogeneous Liquid–Liquid Microextraction and GC-MS for the Enrichment and Determination of 2, 6-DIPA Additive in Biodegradable Film. Molecules 2024, 29, 2068. https://doi.org/10.3390/molecules29092068
Cai K, Liu Q, Lin Y, Yang X, Liu Q, Pan W, Gao W. Amine Switchable Hydrophilic Solvent Vortex-Assisted Homogeneous Liquid–Liquid Microextraction and GC-MS for the Enrichment and Determination of 2, 6-DIPA Additive in Biodegradable Film. Molecules. 2024; 29(9):2068. https://doi.org/10.3390/molecules29092068
Chicago/Turabian StyleCai, Kai, Qiang Liu, Yechun Lin, Xingyou Yang, Qi Liu, Wenjie Pan, and Weichang Gao. 2024. "Amine Switchable Hydrophilic Solvent Vortex-Assisted Homogeneous Liquid–Liquid Microextraction and GC-MS for the Enrichment and Determination of 2, 6-DIPA Additive in Biodegradable Film" Molecules 29, no. 9: 2068. https://doi.org/10.3390/molecules29092068
APA StyleCai, K., Liu, Q., Lin, Y., Yang, X., Liu, Q., Pan, W., & Gao, W. (2024). Amine Switchable Hydrophilic Solvent Vortex-Assisted Homogeneous Liquid–Liquid Microextraction and GC-MS for the Enrichment and Determination of 2, 6-DIPA Additive in Biodegradable Film. Molecules, 29(9), 2068. https://doi.org/10.3390/molecules29092068








