Y(OTf)3-Salazin-Catalyzed Asymmetric Aldol Condensation
Abstract
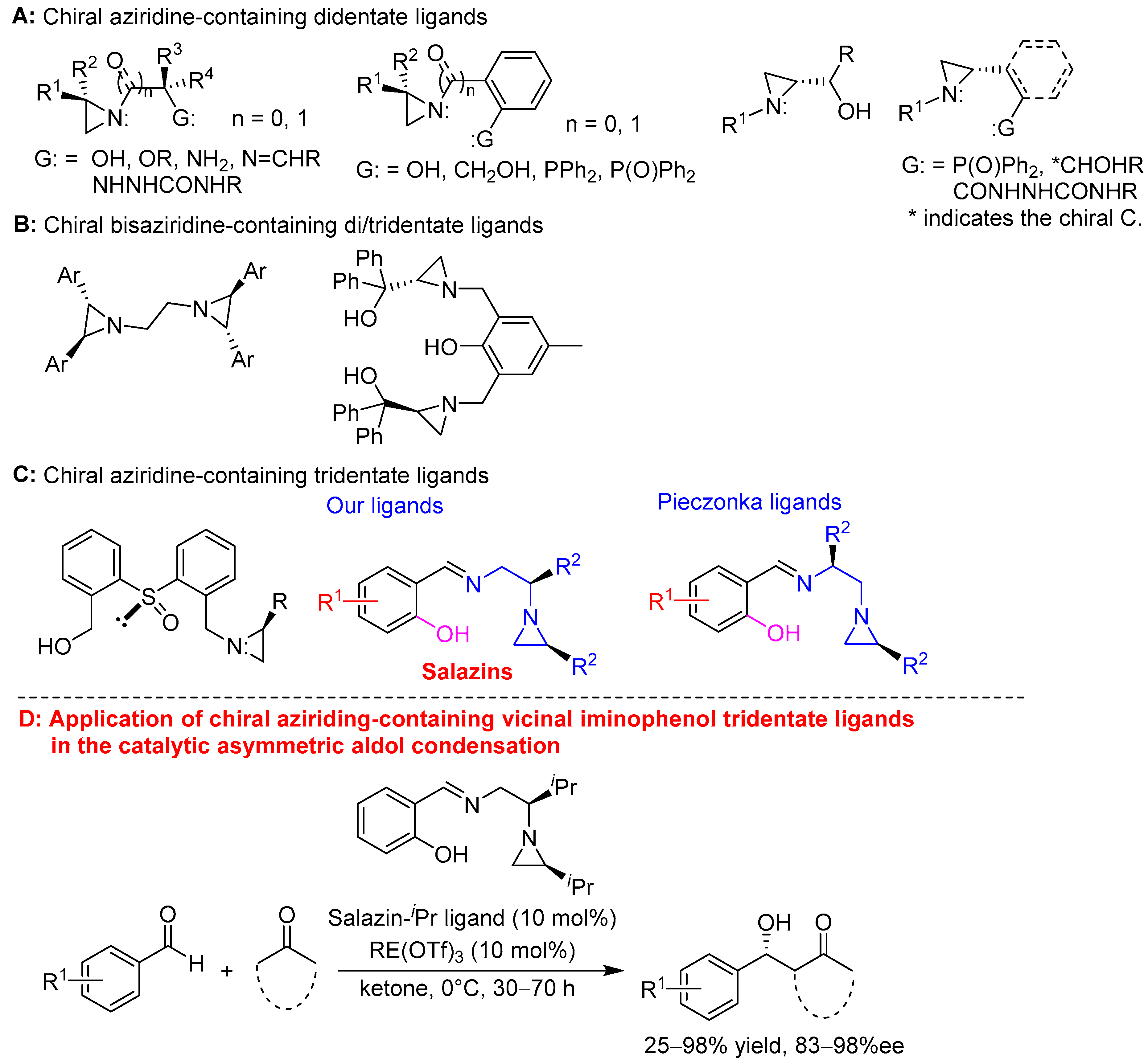
1. Introduction
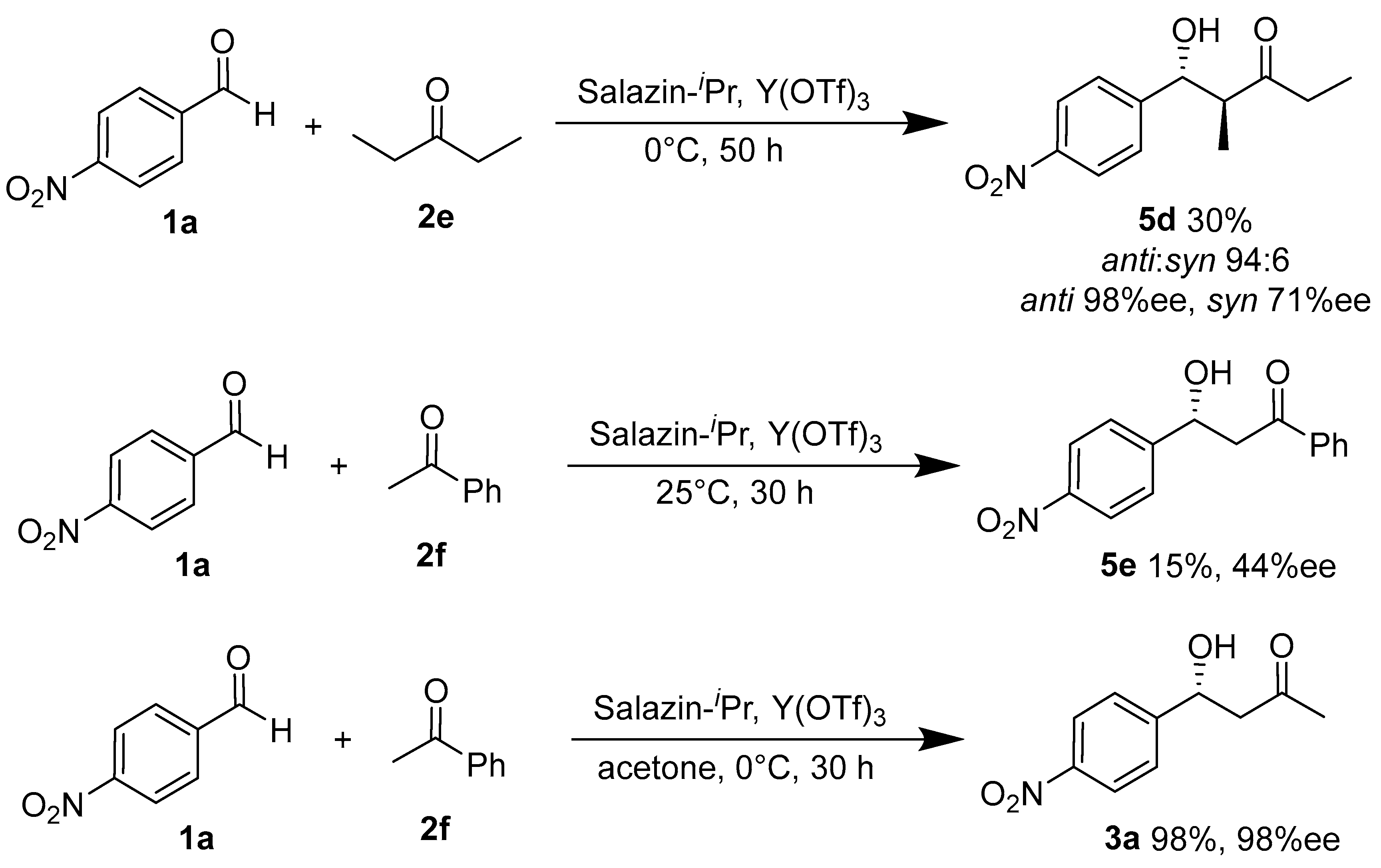
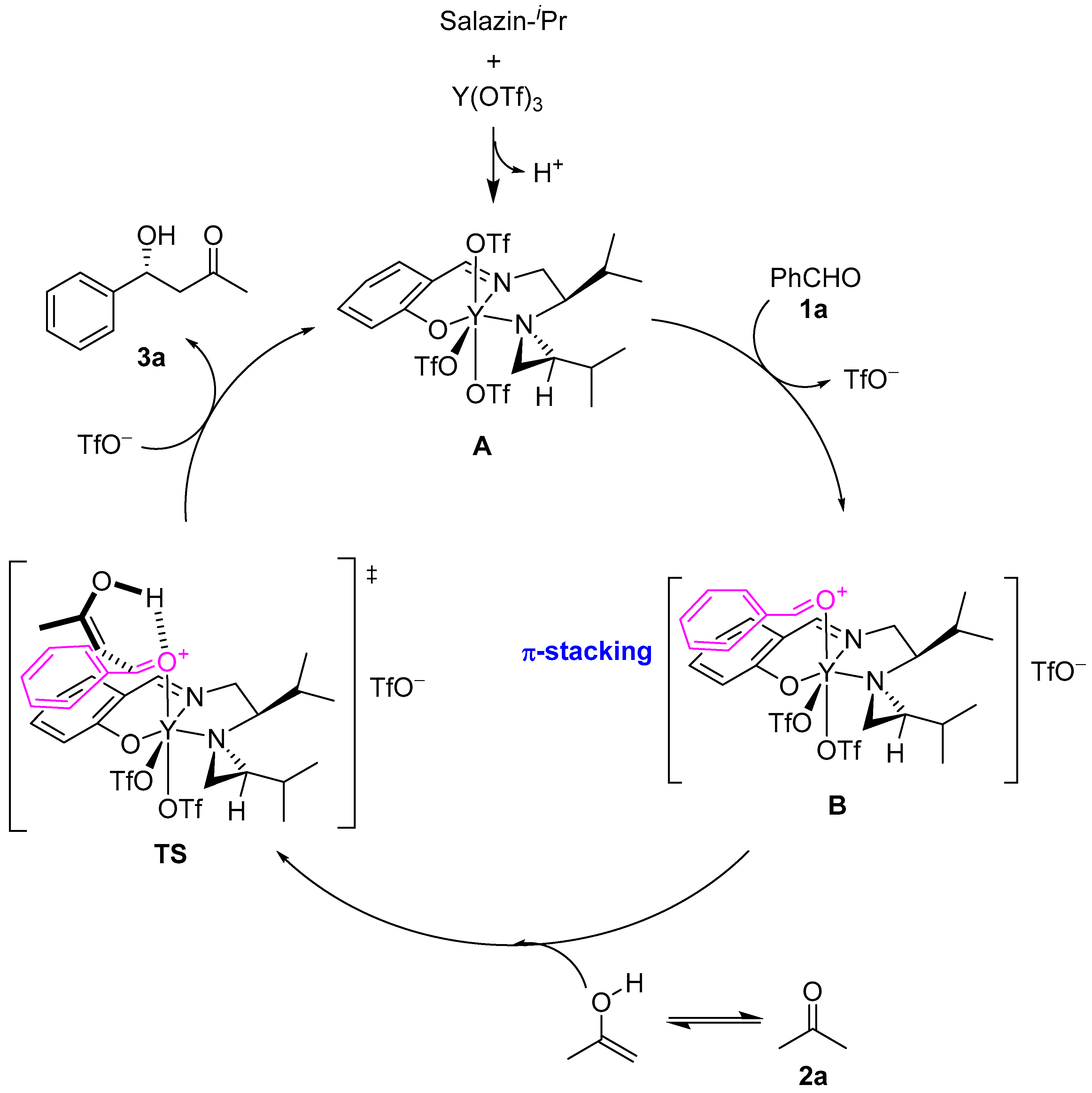
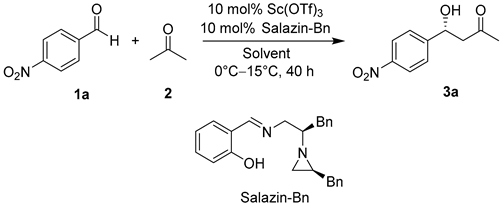
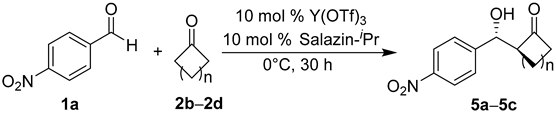
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. General Procedure for the Asymmetric Catalytic Aldol Condensation of Aromatic Aldehydes 1 and Ketones 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, F.; Keller, F.; Vedrenne, E.; Aggarwal, V.K. Stereocontrolled Synthesis of β-Amino Alcohols from Lithiated Aziridines and Boronic Esters. Angew. Chem. Int. Ed. Engl. 2009, 48, 1149–1152. [Google Scholar] [CrossRef]
- Tanner, D. Stereocontrolled synthesis via chiral aziridines. Pure Appl. Chem. 1993, 65, 1319–1328. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, L.B.; Chen, N.; Du, D.-M.; Xu, J.X. Synthesis of NH-aziridines from vicinal amino alcohols via the Wenker reaction: Scope and limitation. Lett. Org. Chem. 2008, 5, 212–217. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, N.; Xu, J.X. An improved and mild Wenker synthesis of aziridines. Synthesis 2010, 2010, 3423–3428. [Google Scholar]
- Jarzyński, S.; Utecht, G.; Leśniak, S.; Rachwalski, M. Highly enantioselective asymmetric reactions involving zinc ions promoted by chiral aziridine alcohols. Tetrahedron Asymmetry 2017, 28, 1774–1779. [Google Scholar] [CrossRef]
- Jarzyński, S.; Leśniak, S.; Pieczonka, A.M.; Rachwalski, M. N-Trityl-aziridinyl alcohols as highly efficient chiral catalysts in asymmetric additions of organozinc species to aldehydes. Tetrahedron Asymmetry 2015, 26, 35–40. [Google Scholar] [CrossRef]
- Rachwalski, M.; Jarzyński, S.; Jasiński, M.; Leśniak, S. Mandelic acid derived α-aziridinyl alcohols as highly efficient ligands for asymmetric additions of zinc organyls to aldehydes. Tetrahedron Asymmetry 2013, 24, 689–693. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Jarzyński, S.; Rachwalski, M. Aziridinylethers as highly enantioselective ligands for the asymmetric addition of organozinc species to carbonyl compounds. Tetrahedron Asymmetry 2015, 26, 148–151. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Jarzyński, S.; Wujkowska, Z.; Leśniak, S.; Rachwalski, M. Zinc(II) mediated asymmetric aldol condensation catalyzed by chiral aziridine ligands. Tetrahedron Lett. 2015, 56, 6506–6507. [Google Scholar] [CrossRef]
- Jarzyński, S.; Rachwalski, M.; Pieczonka, A.M.; Wujkowska, Z.; Leśniak, S. Highly efficient conjugate additions of diethylzinc to enones promoted by chiral aziridine alcohols and aziridine ethers. Tetrahedron Asymmetry 2015, 26, 924–927. [Google Scholar] [CrossRef]
- Rachwalski, M.; Jarzyński, S.; Leśniak, S. Aziridine ring-containing chiral ligands as highly efficient catalysts in asymmetric synthesis. Tetrahedron Asymmetry 2013, 24, 421–425. [Google Scholar] [CrossRef]
- Adam, E.M.; Pieczonka, M.; Rachwalski, M.; Leśniak, S. Synthesis of chiral 1-(2-aminoalkyl)aziridines via the self-opening reaction of aziridine. ARKIVOC 2017, 2017, 223–234. [Google Scholar]
- Pieczonka, A.M.; Marciniak, L.; Rachwalski, M.; Leśniak, S. Enantiodivergent aldol condensation in the presence of aziridine/acid/water systems. Symmetry 2020, 12, 930. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Rachwalski, M. Direct asymmetric aldol condensation catalyzed by aziridine semicarbazide zinc(II) complexes. Tetrahedron Lett. 2014, 55, 2373–2375. [Google Scholar] [CrossRef]
- Leśniak, S.; Pieczonka, A.M.; Jarzyński, S.; Justyna, K.; Rachwalski, M. Synthesis and evaluation of the catalytic properties of semicarbazides derived from N-triphenylmethyl-aziridine-2-carbohydrazides. Tetrahedron Asymmetry 2013, 24, 1341–1344. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Zawisza, A.; Le’sniak, S.; Rachwalski, M. Highly efficient asymmetric Morita–Baylis–Hillman reaction promoted by chiral aziridine-phosphines. Catalysts 2022, 12, 394. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Lésniak, S.; Rachwalski, M. Chiral aziridine phosphines as highly effective promoters of asymmetric Rauhut–Currier reaction. Symmetry 2022, 14, 1631. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Lésniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective Mannich reaction promoted by chiral phosphinoyl-aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef]
- Tanner, D.; Johansson, F.; Harden, A.; Andersson, P.G. A comparative study of C2-symmetric bis(aziridine) ligands in some transition metal-mediated asymmetric transformations. Tetrahedron 1998, 54, 15731–15738. [Google Scholar] [CrossRef]
- Tanner, D.; Harden, A.; Johansson, F.; Wyatt, P.; Andersson, P.G.; Zhang, S.Y.; Zhao, S.H.; Ciglic, M.I.; Haugg, M.; Trabesinger-Rüf, N.; et al. Asymmetric catalysis via chiral aziridines. Acta Chem. Scand. 1996, 50, 361–368. [Google Scholar] [CrossRef][Green Version]
- Andersson, P.G.; Harden, A.; Tanner, D.; Norrby, P.-O. Studies of Allylic Substitution Catalysed by a Palladium Complex of a C2-Symmetric Bis(aziridine): Preparation and NMR Spectroscopic Investigation of a Chiral π-Allyl Species. Chem.-Eur. J. 1995, 1, 12–16. [Google Scholar] [CrossRef]
- Tanner, D.; Andersson, P.G.; Harden, A.; Somfai, P. C2-symmetric bis(aziridines): A new class of chiral ligands for transition metal-mediated asymmetric synthesis. Tetrahedron Lett. 1994, 35, 4631–4634. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leśniak, S.; Kiełbasiński, P. Highly enantioselective addition of phenylethynylzinc to aldehydes using aziridine-functionalized tridentate sulfinyl ligands. Tetrahedron Asymmetry 2010, 21, 2687–2689. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leśniak, S.; Kiełbasiński, P. Highly enantioselective conjugate addition of diethylzinc to enones using aziridine-functionalized tridentate sulfinyl ligands. Tetrahedron Asymmetry 2010, 21, 1890–1892. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Sznajder, E.; Kiełbasiński, P. New highly efficient aziridine-functionalized tridentate sulfinyl catalysts for enantioselective diethylzinc addition to carbonyl compounds. Tetrahedron Asymmetry 2009, 20, 2311–2314. [Google Scholar] [CrossRef]
- Chen, X.P.; Lin, C.; Du, H.G.; Xu, J.X. Efficient direct synthesis of aziridine-containing chiral tridentate ligands by the iminium-mediated self-ring opening reaction of enantiopure aziridines and salicylaldehydes. Adv. Synth. Catal. 2019, 361, 1647–1661. [Google Scholar] [CrossRef]
- Ma, L.G.; Xu, J.X. Nucleophilic ring opening reaction of unsymmetric aziridines and its regioselectivity. Prog. Chem. (Huaxue Jinzhan) 2004, 16, 220–235. [Google Scholar]
- Wu, Y.-H.; Zhang, L.-Y.; Wang, N.-X.; Xing, Y.L. Recent advances in the rare-earth metal triflates catalyzed organic reactions. Catal. Rev. 2020, 64, 679–715. [Google Scholar] [CrossRef]
- Feng, X.M.; Wang, Z.; Liu, X.L. Chiral Lewis acid rare-earth metal complexes in enantioselective catalysis. In Topics in Organometallic Chemistry; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Ma, L.G.; Jiao, P.; Zhang, Q.H.; Du, D.-M.; Xu, J.X. Ligand and substrate π-stacking interaction contro-lled enantioselectivity in the asymmetric aziridination. Tetrahedron Asymmetry 2007, 18, 878–884. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Chen, Y.; Zheng, C.-Q.; Yin, Y.; Wang, L.; Sun, X.-Q. Highly enantioselective aldol reactions catalyzed by reusable upper rim-functionalized calix[4]arene-based l-proline organocatalyst in aqueous conditions. Tetrahedron 2017, 73, 78–85. [Google Scholar] [CrossRef]
- Vlasserou, I.; Sfetsa, M.; Gerokonstantis, D.-T.; Kokotos, C.G.; Moutevelis-Minakakis, P. Combining prolinamides with 2-pyrrolidinone: Novel organocatalysts for the asymmetric aldol reaction. Tetrahedron 2018, 74, 2338–2349. [Google Scholar] [CrossRef]
- Chen, G.; Fu, X.; Li, C.; Wu, C.; Miao, Q. Highly efficient direct a larger-scale aldol reactions catalyzed by a flexible prolinamide based-metal Lewis acid bifunctional catalyst in the presence of water. J. Organomet. Chem. 2012, 702, 19–26. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Chen, Y.X.; Xu, J.X. π-Stacking-controlled dearomatic sulfur-shifted ene reaction of ketenes and polycyclic arylthiiranes: Access to areno[d]-ε-thiolactones. J. Org. Chem. 2024, 89, 4749–4759. [Google Scholar] [CrossRef] [PubMed]
- Li, B.N.; Wang, Y.K.; Du, D.-M.; Xu, J.X. Notable and obvious ketene substituent-dependent effect of temperature on the stereoselectivity in the Staudinger reaction. J. Org. Chem. 2007, 72, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X. Recent advances in π-stacking interaction-controlled asymmetric synthesis. Molecules 2024, 29, 1454. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Yu, M.; Wu, X.; Zhang, Y.; Zhao, G. 4,4′-Disubstituted L-prolines as highly enantioselective catalysts for direct aldol reactions. Adv. Synth. Catal. 2006, 348, 2223–2228. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, F.; Yu, L.T.; Cui, X.; Gong, L.Z.; Mi, A.Q.; Jiang, Y.Z.; Wu, Y.D. Novel small organic molecules for a highly enantioselective direct aldol reaction. J. Am. Chem. Soc. 2003, 125, 5262–5263. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, A.S.; Kostenko, A.A.; Gerasimchuk, V.V.; Zlotin, S.G. Stereospecific diaza-Cope rearrangement as an efficient tool for the synthesis of DPEDA pyridine analogs and related C2-symmetric organocatalysts. Org. Biomol. Chem. 2017, 15, 7028–7033. [Google Scholar] [CrossRef]
- Edwards, M.L.; Ritter, H.W.; Stemerick, D.M.; Stewart, K.T. Mannich bases of 4-phenyl-3-buten-2-one. A new class of antiherpes agent. J. Med. Chem. 1983, 26, 431–436. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Shaw, D.M.; Longbottom, D.A.; Gold, J.B.; Ley, S.V. Organocatalysis with proline derivatives: Improved catalysts for the asymmetric Mannich, nitro-Michael and aldol reactions. Org. Biomol. Chem. 2005, 3, 84–96. [Google Scholar] [CrossRef]
- Guo, G.; Wu, Y.; Zhao, X.; Wang, J.; Zhang, L.; Cui, Y. Polymerization of L-proline functionalized styrene and its catalytic performance as a supported organocatalyst for direct enantioselective aldol reaction. Tetrahedron Asymmetry 2016, 27, 740–746. [Google Scholar] [CrossRef]
- Da, C.-S.; Che, L.-P.; Guo, Q.-P.; Wu, F.-C.; Ma, X.; Jia, Y.-N. 2,4-Dinitrophenol as an effective cocatalyst: Greatly improving the activities and enantioselectivities of primary amine organocatalysts for asymmetric aldol reactions. J. Org. Chem. 2009, 74, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Downey, C.W.; Johnson, M.W. A tandem enol silane formation-Mukaiyama aldol reaction mediated by TMSOTf. Tetrahedron Lett. 2007, 48, 3559–3562. [Google Scholar] [CrossRef]
- Li, L.; Gou, S.H.; Liu, F. Highly stereoselective direct aldol reactions catalyzed by a bifunctional chiral diamine. Tetrahedron Asymmetry 2014, 25, 193–197. [Google Scholar] [CrossRef]

 | |||
|---|---|---|---|
| Entry | Solvent | Yield/% | ee/% |
| 1 | PhMe | 3 | 38 |
| 2 | EtOH | 98 | 53 |
| 3 | DCM | 10 | 60 |
| 4 | THF | 20 | 49 |
| 5 | acetone | 96 | 87 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Metal Salt | Ligand | Temp./°C | Time/h | Yield/% | ee/% |
| 1 | Sc(OTf)3 | Salazin-Bn | 15 | 40 | 96 | 81 |
| 2 | Sc(OTf)3 | Salazin-tBu-Bn | 15 | 40 | 96 | 70 |
| 3 | Sc(OTf)3 | Salazin-Br-Bn | 15 | 40 | 55 | 78 |
| 4 | Sc(OTf)3 | Salazin-NO2-iPr | 15 | 40 | 91 | 86 |
| 5 | Sc(OTf)3 | Salazin-Br-iPr | 15 | 40 | 89 | 87 |
| 6 | Sc(OTf)3 | Salazin-I-iPr | 15 | 40 | 60 | 80 |
| 7 | Sc(OTf)3 | Salazin-iPr | 15 | 26 | 98 | 88 |
| 8 | Y(OTf)3 | Salazin-iPr | 15 | 26 | 98 | 88 |
| 9 | La(OTf)3 | Salazin-iPr | 15 | 26 | 93 | 83 |
| 10 | Ce(OTf)3 | Salazin-iPr | 15 | 26 | 91 | 86 |
| 11 | Eu(OTf)3 | Salazin-iPr | 15 | 26 | 98 | 85 |
| 12 | Gd(OTf)3 | Salazin-iPr | 15 | 26 | 98 | 87 |
| 13 | Lu(OTf)3 | Salazin-iPr | 15 | 26 | 96 | 87 |
| 14 | Zn(OTf)2 | Salazin-iPr | 15 | 40 | 90 | 70 |
| 15 | Sc(OTf)3 | Salazin-iPr | 40 | 20 | 98 | 85 |
| 16 | Sc(OTf)3 | Salazin-iPr | 20 | 26 | 98 | 88 |
| 17 | Sc(OTf)3 | Salazin-iPr | 0 | 30 | 98 | 98 |
| 18 | Y(OTf)3 | Salazin-iPr | 40 | 20 | 96 | 89 |
| 19 | Y(OTf)3 | Salazin-iPr | 20 | 26 | 98 | 88 |
| 20 | Y(OTf)3 | Salazin-iPr | 0 | 30 | 98 | 98 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Product 5 | n | Yield/% | anti:syn | ee of anti/% | ee of syn/% |
| 1 | 5a | 1 | 25 | 53:47 | 83 | 69 |
| 2 | 5b | 2 | 81 | 83:17 | 96 | - b |
| 3 | 5c | 3 | 94 | 94:6 | 91 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Chen, N.; Yang, Z.; Xu, J. Y(OTf)3-Salazin-Catalyzed Asymmetric Aldol Condensation. Molecules 2024, 29, 1963. https://doi.org/10.3390/molecules29091963
Wang C, Chen N, Yang Z, Xu J. Y(OTf)3-Salazin-Catalyzed Asymmetric Aldol Condensation. Molecules. 2024; 29(9):1963. https://doi.org/10.3390/molecules29091963
Chicago/Turabian StyleWang, Chengzhuo, Ning Chen, Zhanhui Yang, and Jiaxi Xu. 2024. "Y(OTf)3-Salazin-Catalyzed Asymmetric Aldol Condensation" Molecules 29, no. 9: 1963. https://doi.org/10.3390/molecules29091963
APA StyleWang, C., Chen, N., Yang, Z., & Xu, J. (2024). Y(OTf)3-Salazin-Catalyzed Asymmetric Aldol Condensation. Molecules, 29(9), 1963. https://doi.org/10.3390/molecules29091963






