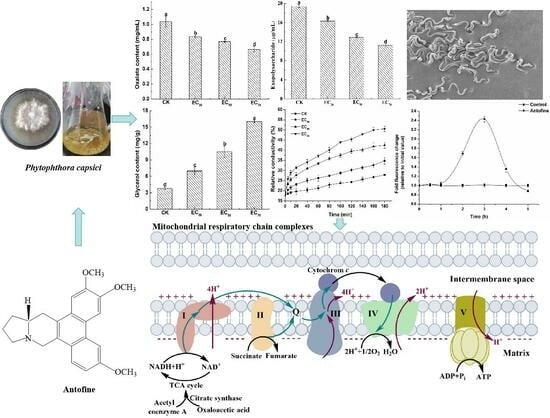
The Effects of Antofine on the Morphological and Physiological Characteristics of Phytophthora capsici
Abstract
1. Introduction
2. Results
2.1. Effect of Antofine on the Mycelial Growth of P. capsici
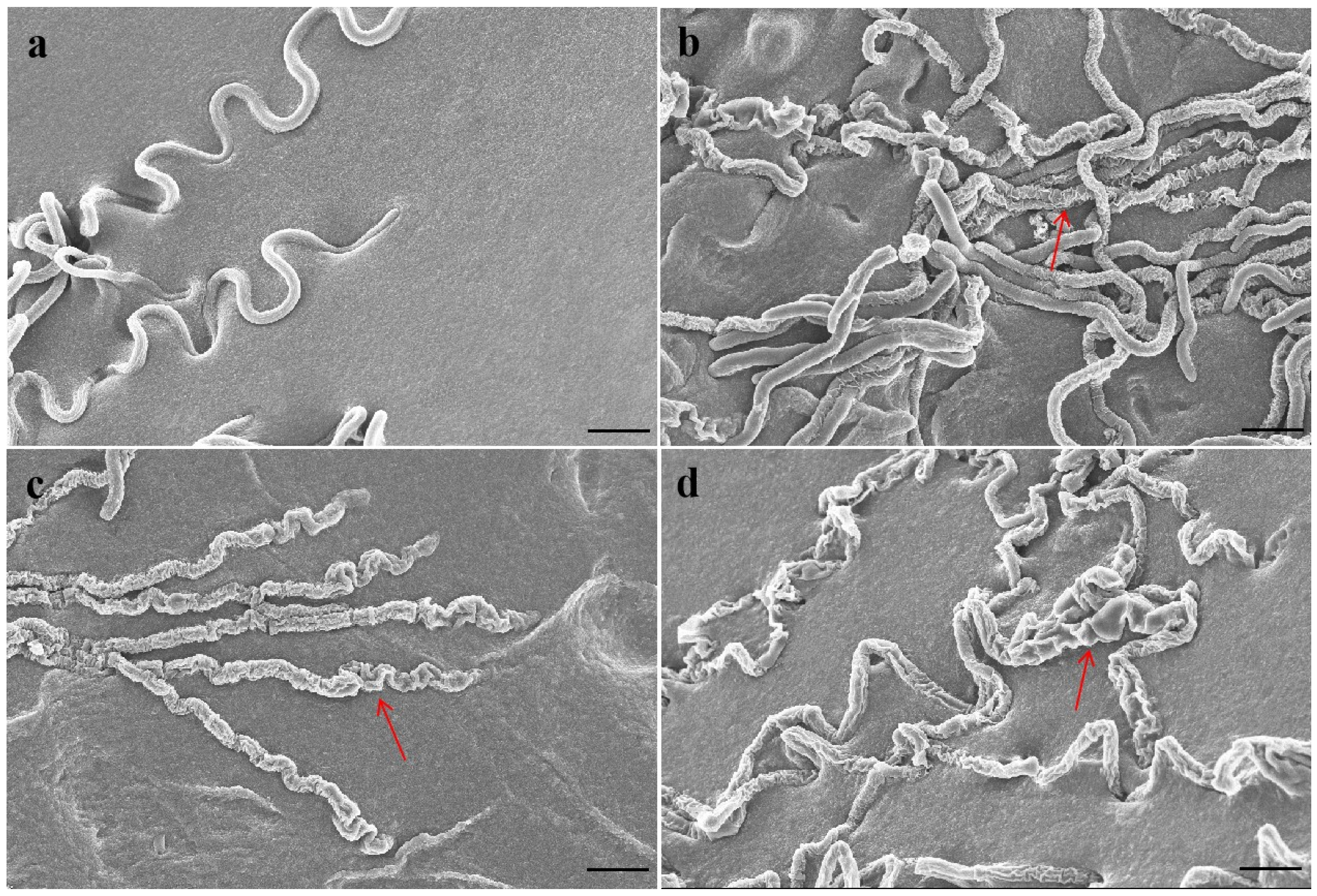
2.2. Effect of Antofine on the Mycelial Morphology of P. capsici
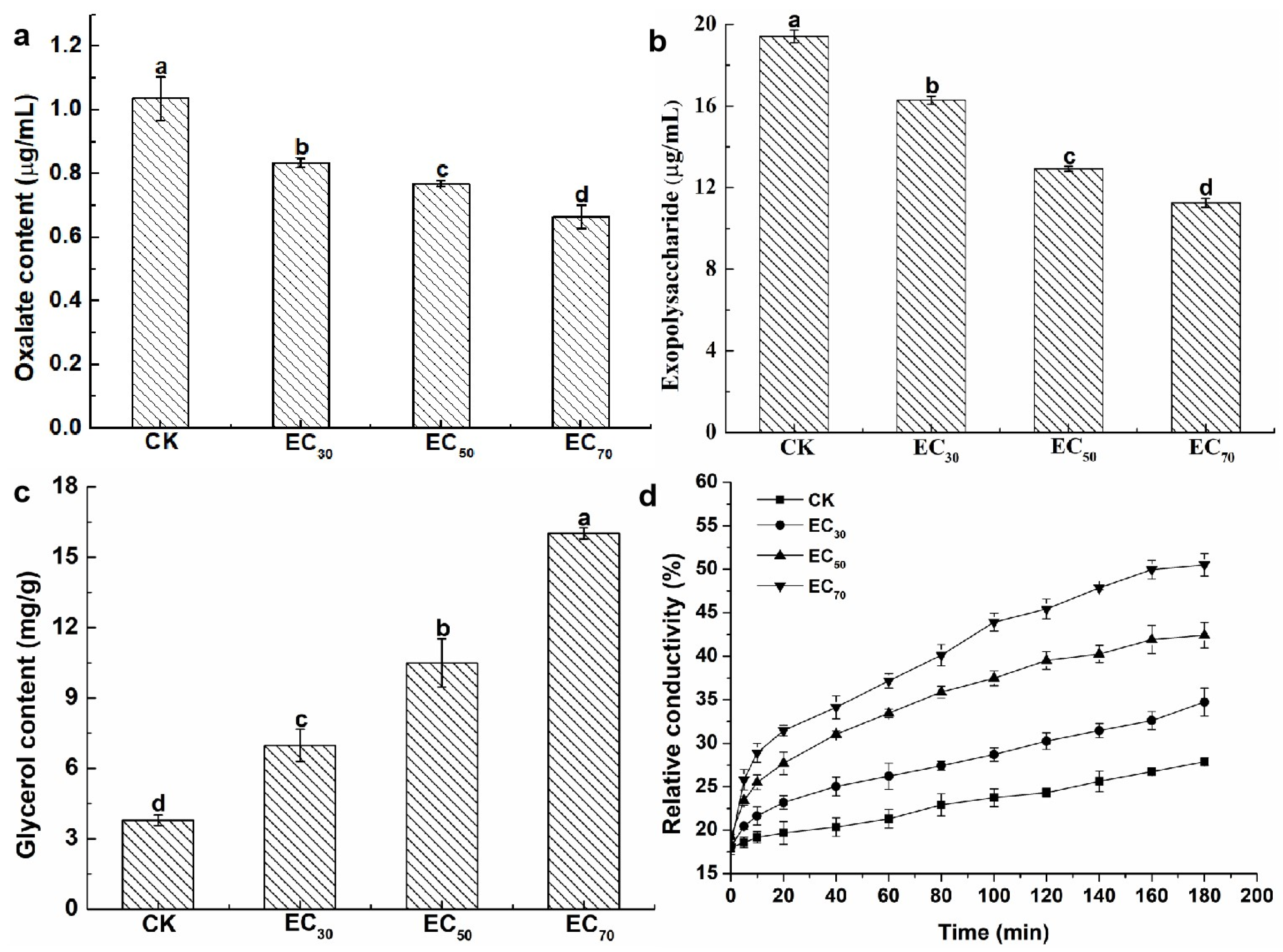
2.3. Effect of Antofine on the Physiological and Biochemical Characteristics of P. capsici
2.4. Effect of Antofine on the Intracellular ROS Contents of P. capsici
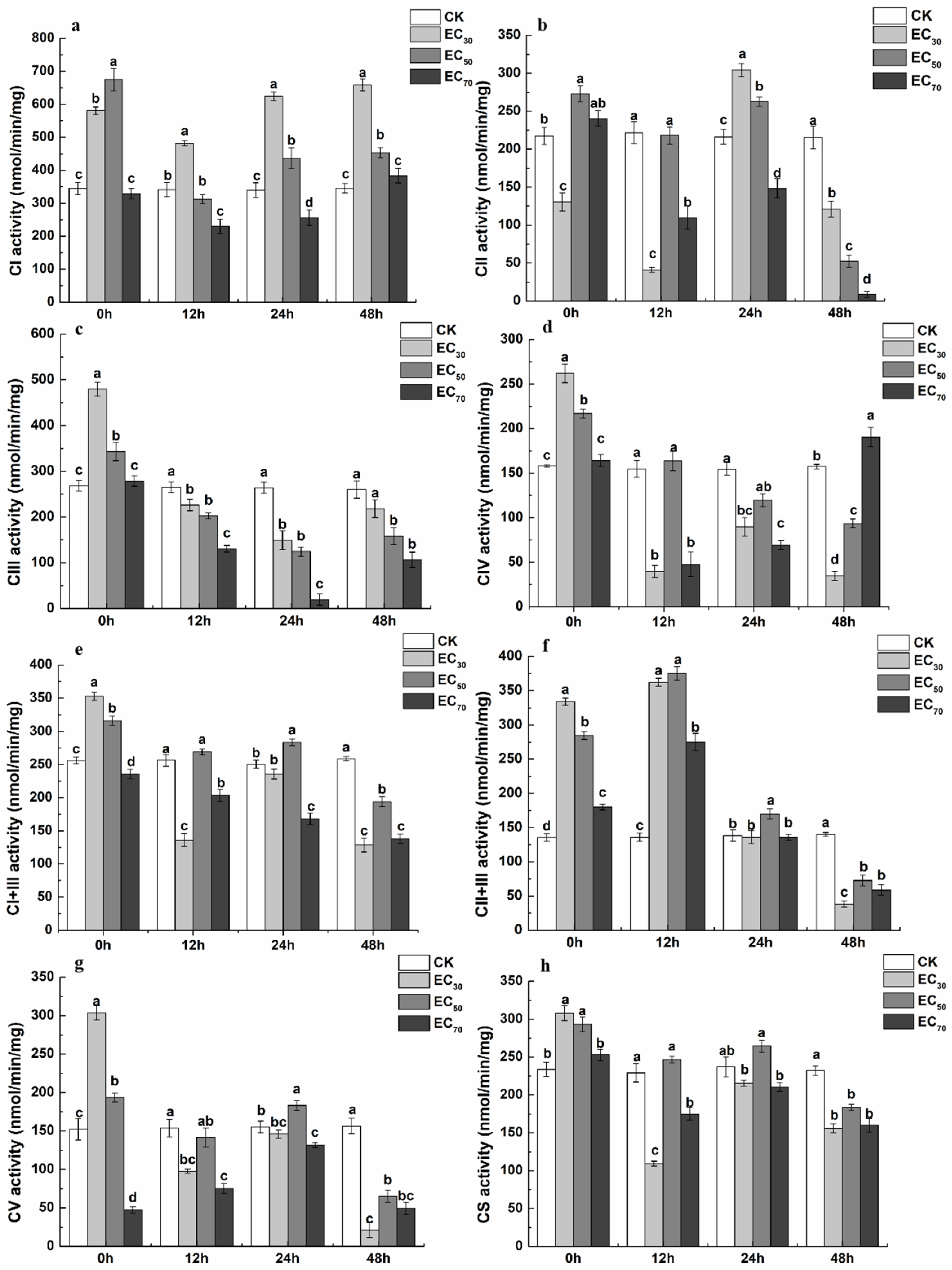
2.5. Effect of Antofine on the Mitochondrial Respiratory Chain Complexes of P. capsici
3. Discussion
4. Materials and Methods
4.1. Chemicals and Pathogen
4.2. Determination of the Effect of Antofine on the Mycelial Growth of P. capsici
4.3. Observation of the Effect of Antofine on the Mycelial Morphology of P. capsici
4.4. Determination of the Effect of Antofine on the Physiological and Biochemical Characteristics of P. capsici
4.4.1. Determination of the Effect of Antofine on the Oxalic Acid Content of P. capsici
4.4.2. Determination of the Effect of Antofine on the Exopolysaccharide Content of P. capsici
4.4.3. Determination of the Effect of Antofine on the Glycerol Content of P. capsici
4.4.4. Determination of the Effect of Antofine on the Cell Membrane Permeability of P. capsici
4.5. Determination of the Effect of Antofine on the Intracellular ROS Content of P. capsici
4.6. Determination of the Effect of Antofine on the Mitochondrial Respiratory Chain Complexes of P. capsici
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, N.; Kang, W.H.; Lee, J.; Yeom, S.I. Development of clustered resistance gene analogs-based markers of resistance to Phytophthora capsici in chili pepper. BioMed Res. Int. 2019, 2019, 1093186. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, G.; Yang, J.; Li, L.; Li, P.; Xu, S.; Feng, X.; Chen, Y. Evaluation of inhibitory effect and mechanism of Euphorbia factor L3 against Phytophthora capsici. Molecules 2023, 28, 2958. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.F.; Shen, D.Y.; Wu, Y.R.; Xu, H.; Dou, D.L. RNA-seq for comparative transcript profiling of Phytophthora capsici during its interaction with Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 2018, 102, 193–199. [Google Scholar] [CrossRef]
- Saltos, L.A.; Monteros-Altamirano, Á.; Reis, A.; Garcés-Fiallos, F.R. Phytophthora capsici: The diseases it causes and management strategies to produce healthier vegetable crops. Hortic. Bras. 2022, 40, 5–17. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.M.; Parada-Rojas, C.H.; Hansen, Z.; Vogel, G.; Smart, C.; Hausbeck, M.K.; Carmo, R.M.; Huitema, E.; Naegele, R.P.; Kousik, C.S.; et al. Phytophthora capsici: Recent progress on fundamental biology and disease management 100 years after its description. Annu. Rev. Phytopathol. 2023, 61, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Wang, T.; Zhao, W.; Li, P.; Ding, J.; Gao, Z. Activity of ten fungicides against Phytophthora capsici isolates resistant to metalaxyl. J. Phytopathol. 2012, 160, 717–722. [Google Scholar] [CrossRef]
- Volynchikova, E.; Kim, K.D. Biological control of oomycete soilborne diseases caused by Phytophthora capsici, Phytophthora infestans, and Phytophthora nicotianae in solanaceous crops. Mycobiology 2022, 50, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Yang, C.W.; Chen, W.L.; Wu, P.L.; Tseng, H.Y.; Lee, S.J. Anti-inflammatory mechanisms of phenanthroindolizidine alkaloids. Mol. Pharmacol. 2006, 69, 749–758. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Lu, L.; Gong, Y.; Han, W.; Piao, G. Natural indole-containing alkaloids and their antibacterial activities. Arch. Pharm. 2020, 353, e2000120. [Google Scholar] [CrossRef]
- Monteiro, N.O.; Monteiro, T.d.M.; Nogueira, T.S.R.; Cesar, J.R.; Nascimento, L.P.S.; Campelo, K.A.; Silveira, G.R.; Antunes, F.; de Oliveira, D.B.; de Carvalho Junior, A.R.; et al. Antihypertensive activity of the alkaloid aspidocarpine in normotensive Wistar rats. Molecules 2022, 27, 6895. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, Y.; Liu, J.; Kang, Y.; Li, R.; Wang, J. The anti-tumor activity and mechanism of alkaloids from Aconitum szechenyianum Gay. Bioorg. Med. Chem. Lett. 2016, 26, 380–387. [Google Scholar] [CrossRef]
- Hao, F.S.; Tao, L.; Liu, J.M.; Ma, Y.; Zhang, J.; Wang, W.; Yan, W.; Wang, B.; Wang, X.F.; Chen, X.Y.; et al. Cynanchum komarovii extract for the treatment of rheumatoid arthritis by acting on synovial cells in vitro and in vivo. J. Ethnopharmacol. 2023, 317, 116825. [Google Scholar] [CrossRef]
- Wiegrebe, W.; Budzikiewicz, H.; Faber, L. Alkaloide aus Cynanchum vincetoxicum (L.) Pers. 3. Mitt.: 14-Hydroxy-2,3,6-trimethoxy-9,11,12,13, 13a,14-hexahydro-dibenzo[f,h]-pyrrolo[1,2-b]-isochinolin. Arch. Pharm. 1970, 303, 1009–1012. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, S.K.; Min, H.Y.; Lee, T.; Lee, J.; Cheng, M.S.; Kim, S. Synthesis and structure–activity studies of antofine analogues as potential anticancer agents. Bioorg. Med. Chem. Lett. 2007, 17, 97–100. [Google Scholar] [CrossRef]
- Kwon, Y.; Song, J.; Lee, H.; Kim, E.Y.; Lee, K.; Lee, S.K.; Kim, S. Design, synthesis, and biological activity of sulfonamide analogues of antofine and cryptopleurine as potent and orally active antitumor agents. J. Med. Chem. 2015, 58, 7749–7762. [Google Scholar] [CrossRef]
- Xin, Z.T.; OuYang, Q.L.; Wan, C.P.; Che, J.X.; Li, L.; Chen, J.Y.; Tao, N.G. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.R.; Wang, J.; Lu, J.J.; Huo, Y.B. Extraction separation and antifungal activities of Cynanchum komarovii Al. Iljinski. World Sci. Res. J. 2023, 9, 52–59. [Google Scholar] [CrossRef]
- Yang, G.X.; Ma, G.L.; Li, H.; Huang, T.; Xiong, J.; Hu, J.F. Advanced natural products chemistry research in China between 2015 and 2017. Chin. J. Nat. Med. 2018, 16, 881–906. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Oh, J.; Kim, G.D.; Kim, S.; Lee, S.K. Antofine, a natural phenanthroindolizidine alkaloid, suppresses angiogenesis via regulation of AKT/mTOR and AMPK pathway in endothelial cells and endothelial progenitor cells derived from mouse embryonic stem cells. Food Chem. Toxicol. 2017, 107, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.Y.; Liu, Y.X.; Huang, H.C.; Du, F.; Huang, L.L.; Wu, J.Q.; Li, Y.W.; Zhu, S.S.; Yang, M. Benzothiazole inhibits the growth of Phytophthora capsici through inducing apoptosis and suppressing stress responses and metabolic detoxification. Pestic. Biochem. Physiol. 2019, 154, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, X.Y.; Wang, L.Y.; Lu, X.; Han, L.R.; Zhang, X.; Feng, J.T. A functional analysis of mitochondrial respiratory chain cytochrome bc1 complex in Gaeumannomyces tritici by RNA silencing as a possible target of carabrone. Mol. Plant Pathol. 2020, 21, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Sun, T.; Liu, T.; Jiang, Z.; Xi, P. Effectiveness of volatiles emitted by Streptomyces abikoensis TJGA-19 for managing litchi downy blight disease. Microorganisms 2024, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Munir, E.; Yoon, J.J.; Tokimatsu, T.; Hattori, T.; Shimada, M. A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palustris. Proc. Natl. Acad. Sci. USA 2001, 98, 11126–11130. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.F.; Moomaw, E.W.; Rollins, J.A. Fungal oxalate decarboxylase activity contributes to Sclerotinia sclerotiorum early infection by affecting both compound appressoria development and function. Mol. Plant Pathol. 2015, 16, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K.; Huang, J.S.; Jenkins, S.F. Relationship of mycelial growth and production of oxalic acid and cell wall degrading enzymes to virulence in Sclerotium rolfsii. Can. J. Plant Pathol. 1985, 7, 109–117. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.O.; Qi, Y.; Terrell, E.; Dupre, R.A.; Mattison, C.P. Characterization of levan fructan produced by a Gluconobacter japonicus strain isolated from a sugarcane processing facility. Microorganisms 2024, 12, 107. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, R.; Ren, Y.; Yang, Z.; Li, T.; Huang, H.; Tang, Q.; Li, D.; Jiang, S.; Wu, X.; et al. Transcriptomic, biochemical, and morphological study reveals the mechanism of inhibition of Pseudopestalotiopsis camelliae-sinensis by phenazine-1-carboxylic acid. Front. Microbiol. 2021, 12, 618476. [Google Scholar] [CrossRef]
- Luard, E.J. Effect of osmotic shock on some intracellular solutes in two filamentous fungi. J. Gen. Microbiol. 1982, 128, 2575–2581. [Google Scholar] [CrossRef][Green Version]
- Hocking, A.D.; Norton, R.S. Natural-abundance 13C nuclear magnetic resonance studies on the internal solutes of xerophilic fungi. J. Gen. Microbiol. 1983, 129, 2915–2925. [Google Scholar] [CrossRef][Green Version]
- Gadd, G.M.; Chudek, J.A.; Foster, R.; Reed, R.H. The osmotic responses of Penicillium ochro-chloron: Changes in internal solute levels in response to copper and salt stress. J. Gen. Microbiol. 1984, 130, 1969–1975. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Sun, Y.; Zhang, Y.; Zhang, X.; Feng, J.T. Antifungal activity and biochemical response of cuminic acid against Phytophthora capsici Leonian. Molecules 2016, 21, 756. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Xie, Z.; Guo, E.; Han, L.R.; Zhang, X.; Feng, J.T. Activity and biochemical characteristics of plant extract cuminic acid against Sclerotinia sclerotiorum. Crop Prot. 2017, 101, 76–83. [Google Scholar] [CrossRef]
- Vrba, J.; Doležel, P.; Vičar, J.; Ulrichová, J. Cytotoxic activity of sanguinarine and dihydrosanguinarine in human promyelocytic leukemia HL-60 cells. Toxicol. Vitro 2009, 23, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Schagger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. Eur. Mol. Biol. Organ. J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, K.; Minakami, S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH–ubiquinone reductase preparation. Biochem. J. 1979, 180, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sarewicz, M.; Osyczka, A. Electronic connection between the quinone and cytochrome c redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol. Rev. 2015, 95, 219–243. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.Y.; Han, L.R.; Zhang, X.; Feng, J.T. The effect of carabrone on mitochondrial respiratory chain complexes in Gaeumannomyces graminis. J. Appl. Microbiol. 2017, 123, 1100–1110. [Google Scholar] [CrossRef]
- Cleary, M.R.; Daniel, G.; Stenlid, J. Light and scanning electron microscopy studies of the early infection stages of Hymenoscyphus pseudoalbidus on Fraxinus excelsior. Plant Pathol. 2013, 62, 1294–1301. [Google Scholar] [CrossRef]
- Duan, Y.B.; Ge, C.Y.; Liu, S.M.; Chen, C.J.; Zhou, M.G. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2013, 106, 61–67. [Google Scholar] [CrossRef]
- Rao, P.; Pattabiraman, T.N. Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal. Biochem. 1989, 181, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Mizutani, A.; Yukioka, H.; Miki, N.; Ohba, K.; Masuko, M. Effect of the methoxyiminoacetamide fungicide, SSF129, on respiratory activity in Botrytis cinerea. Pestic. Sci. 1999, 55, 681–686. [Google Scholar] [CrossRef]

| Concentration of Antofine (μg/mL) | Growth Inhibition Rate (%) | Regression Equation | EC30 (μg/mL) | EC50 (μg/mL) | EC70 (μg/mL) | r |
|---|---|---|---|---|---|---|
| 2.5 | 32.61 ± 1.06 e | Y = 4.4047 + 0.8844x | 1.2891 (0.5852–2.8277) 1 | 5.0795 (3.8846–7.4818) | 19.0233 (14.9513–25.5944) | 0.9778 |
| 5 | 57.78 ± 1.32 d | |||||
| 10 | 63.89 ± 1.29 c | |||||
| 20 | 70.00 ± 1.44 b | |||||
| 40 | 87.22 ± 1.67 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, W.; Lu, J.; Huo, Y.; Wang, J. The Effects of Antofine on the Morphological and Physiological Characteristics of Phytophthora capsici. Molecules 2024, 29, 1965. https://doi.org/10.3390/molecules29091965
Wang M, Zhang W, Lu J, Huo Y, Wang J. The Effects of Antofine on the Morphological and Physiological Characteristics of Phytophthora capsici. Molecules. 2024; 29(9):1965. https://doi.org/10.3390/molecules29091965
Chicago/Turabian StyleWang, Mei, Weirong Zhang, Jiaojiao Lu, Yanbo Huo, and Jing Wang. 2024. "The Effects of Antofine on the Morphological and Physiological Characteristics of Phytophthora capsici" Molecules 29, no. 9: 1965. https://doi.org/10.3390/molecules29091965
APA StyleWang, M., Zhang, W., Lu, J., Huo, Y., & Wang, J. (2024). The Effects of Antofine on the Morphological and Physiological Characteristics of Phytophthora capsici. Molecules, 29(9), 1965. https://doi.org/10.3390/molecules29091965