The Ocean’s Pharmacy: Health Discoveries in Marine Algae
Abstract
1. Introduction
2. Methodology
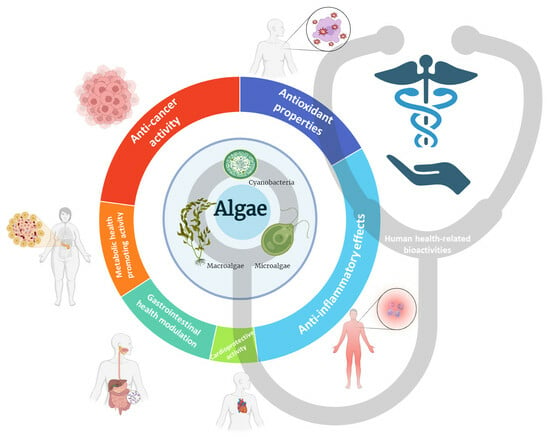
3. Health-Related Bioactivities
3.1. Antioxidant Properties
3.1.1. Antioxidants and Their Role in Oxidative Stress and Disease Development
3.1.2. Potential Health Benefits of Algal Antioxidants
| Complication | Algae Type | Algae Species | Algal Extract or Compound | Cell Line | Oxidative Stress Induced by | Concentrations Tested | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| n.d. | Macroalgae | Ulva pertusa | Ulvan | RAW 264.7 | H2O2 | 200 µg/mL | ↑ antioxidant activity (↑ CAT and SOD); ↑ expression of antioxidant genes (↑ GST, CAT, MnSOD, and GPx mRNA expression) | [34] |
| Undaria pinnatifida | Phlorotannin extract | RAW 264.7 | H2O2 | 10, 20, and 40 µg/mL | ↑ cell survival; ↓ NO production and iNOS protein expression | [37] | ||
| Liver | Macroalgae | Nizamuddinia zanardinii | Fucoidan | HepG2 | H2O2 | 0.1, 0.2, 0.5, and 0.7 µg/mL | Protective effect on H2O2-induced cytotoxicity; ↓ intracellular H2O2-induced ROS production; ↓ H2O2-induced damages | [49] |
| Pyropia haitanensis | Floridoside | L-02 | n.d. | 200 µmol/L | No cytotoxic effect; ↑ SOD and GSH-Px activity; activation of HO-1 expression via upregulation on Nrf2/ARE and p38/ERK MAPK-Nrf2 pathway | [40] | ||
| Lungs | Macroalgae | Gelidiella acerosa | Ethyl acetate extract | A549 | H2O2 | 1.5 mg/mL | ↑ SOD and peroxidase activity | [50] |
| Skeletal muscle | Macroalgae | Ecklonia cava | Phloroglucinol | C2C12 | H2O2 | 10 and 20 µg/mL | ↓ cell toxicity (↓ H2O2-induced cell death); ↓ apoptosis (↓ DNA fragmentation, nuclear fragmentation, and chromatin condensation); ↓ mitochondrial dysfunction; regulation of apoptosis regulatory factors (↑ cytochrome c in the mitochondria, ↑ Bcl-2 expression, and ↑ caspase-3); ↓ ROS H2O2-induced accumulation; upregulation of Nrf2/HO-1 signaling pathway | [38] |
| Macroalgae | Sargassum thunbergii | Indole-6-carboxaldehyde | C2C12 | H2O2 | 400 µM | ↓ cell toxicity (↓ H2O2-induced cell death); ↓ ROS overproduction; ↓ DNA damage; ↓ apoptosis; ↓ mitochondrial dysfunction; regulation of apoptosis regulatory factors (cytochrome c, Bax, Bcl-2, and caspase-3 and -9); downregulation of AMPK signaling pathway | [39] | |
| Skin | Macroalgae | Ecklonia cava | 6,6′-bieckol | HaCaT | UVB radiation | 50 and 100 µM | ↑ cell survival; antioxidant effect (↑ antioxidant enzymes); downregulation of matrix metalloproteinases (MMPs) through MAPK and NF-κB pathways | [46] |
| Fucus spiralis | Ethyl acetate, water, and ethanol extracts | HaCaT | UVB radiation or H2O2 | 1000 µg/mL | ↓ ROS production | [47] | ||
| Symphyocladia latiuscula | Bromophenol bis (2,3,6-tribromo-4,5-dihydroxybenzyl) ether (BTDE) | HaCaT; HUVEC | H2O2 | 5 and 10 µM | ↑ cell survival (↓ apoptosis); reverse oxidative damage induced by H2O2 (↓ ROS generation, ↓ MDA level, ↓ GSSG/GSH, and ↑ SOD activity); upregulation of Nrf2 and decrease in Keap1 expression; activation of AKT signaling pathway | [42] | ||
| n.d. | n.d. | 3-Bromo-4,5-dihydroxybenzaldehyde | HaCaT | H2O2 or UV-B radiation | 30 µM | Protective effect against oxidative stress (↑ cell viability) possibly regulated by ERK and Akt pathways, inducing HO-1 and Nrf2 expression | [43] | |
| Bromophenols | HaCaT | H2O2 | 10 µM | ↑ cell survival (↓ apoptosis); ↓ oxidative cell damage (↓ ROS generation); increased expression of antioxidant proteins (TrxR1 and HO-1) | [44] | |||
| Phloroglucinol | HaCaT | H2O2 | 50 µM | Protected cells from H2O2-induced cytotoxicity (↑ cell viability); upregulation of Nrf2/HO-1 signaling pathway; ↓ oxidative stress (↓ ROS generation and DNA damage); ↓ apoptosis (↓ mitochondrial dysfunction); modulation of apoptosis regulatory genes (↑ Bcl-2, ↑ PARP, ↓ Bax, and ↑ caspase-3 and -9 expression); ↓ release of mitochondrial cytochrome c into the cytoplasm | [45] |
| Complication | Algae Type | Algae Species | Algal Extraction or Compound | Route of Administration | Dosage | Experimental Period | Animal Model (Age) | Oxidative Stress Induced by | n/Group | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n.d. | Macroalgae | Fucus virsoides | Less polar fractions | Incubation with embryo media | 7.5, 15, and 30 µg/mL | 4 d | Zebrafish embryos | H2O2 | 30 | Decreased heartbeat frequency; ↓ ROS formation | [35] |
| Gracilaria lemaneiformis | Agaro-oligosaccharides prepared from the agar | Incubation with embryo media | 25 and 50 µg/mL | 3 d | Zebrafish embryos | H2O2 | n.d. | Increased survival rate (↓ cell death); ↓ heart-beating disorder; ↓ ROS production; ↓ lipid peroxidation | [36] | ||
| Hizikia fusiforme | Fucoidan | Incubation with embryo media | 25 and 50 µg/mL | 2 d | Zebrafish embryos | H2O2 | 15 | Increased survival rate (↓ cell death); ↓ heart-beating disorder; ↓ ROS production; ↓ lipid peroxidation | [51] | ||
| Padina boryana | Ethanol precipitation | Incubation with embryo media | 50 and 100 µg/mL | 3 d | Zebrafish embryos (7–9 hpf) | H2O2 | n.d. | Increased survival rate (↓ cell death); improved heart-beating rates; ↓ intracellular ROS; ↓ lipid peroxidation | [52] | ||
| Pyropia yezoensis | Polyphenols and protein-rich extracts | Incubation with embryo media | 12.5, 25, and 50 µg/mL | 1 d | Zebrafish embryos (7–9 hpf) | AAPH | 15 | Decreased cell death; ↓ ROS production; ↓ lipid peroxidation production | [53] | ||
| Sargassum fulvellum | Polysaccharides | Incubation with embryo media | 50 and 100 µg/mL | 3 d | Zebrafish embryos (7–9 hpf) | AAPH | 15 | Increased survival rate (↓ cell death); improved heart rate; ↓ intracellular ROS; ↓ lipid peroxidation | [54] | ||
| Undaria pinnatifida sporophylls | Fucoidan | Incubation with embryo media | 125 and 250 µg/mL | 7 d | Zebrafish embryos (8 hpf) | AAPH | 15 | Increased survival rate (↓ cell death); ↓ heartbeat rate; ↓ ROS production; ↓ lipid peroxidation | [55] | ||
| Kidney | Macroalgae | Ulva lactuca | Polysaccharide extract | Intragastric | 50 and 300 mg/kg | 10 w | Kunming mice (8 w) | D-gal and ascorbic acid (subcutaneously) | 9 | Protective effect on kidney injury (↓ atrophy, ↓ serum creatinine and serum cystatin C); ↓ oxidative stress in kidney (↓ MDA, protein carbonyl, and 8-OHdG levels, and ↑ SOD, GSH-Px, and T-AOC); ↓ apoptosis (↓ expression of caspase-3 in kidney) | [59] |
| Liver and Kidney | Macroalgae | Halamphora sp. | Methanol extract (80%) | Gastric gavage | 2 mg/kg/day | 3 w | Wistar albino rats (adults) | Lead acetate (i.p.) | 6 | ↓ lipid peroxidation in liver and kidney (↓ MDA); ↑ protection against oxidative stress in liver and kidneys (↑ GPx, SOD, and CAT); improved serum biochemical parameters (↓ AST, ALT, ALP, and LDH, and ↓ creatine and urea) | [58] |
| Skin | Macroalgae | Ecklonia maxima | Sulfated polysaccharides | Incubation with embryo media | 50 and 100 µg/mL | 3 d | Zebrafish embryos (7–9 hpf) | AAPH | 15 | ↑ survival rate (↓ cell death, ↓ apoptosis); improved heart beating disorder; ↓ oxidative stress (↓ ROS generation and ↓ lipid peroxidation) | [60] |
| UVB-exposure | 10 | ↓ intracellular ROS levels; ↓ cell death; ↓ NO production and lipid peroxidation; improved collagen content and inhibition of MMPs | |||||||||
| Sargassum thunbergii | Phenolic-rich extract | Incubation with embryo media | 1.67 µg/mL | 6 d | Zebrafish embryos (2 dpf) | UVB-exposure | 8 to 10 | Repaired skin damage; ↓ intracellular ROS accumulation | [48] |
3.2. Anti-Inflammatory Effects
3.2.1. Inflammation and Its Role in the Onset and Progression of Diseases
3.2.2. Mechanisms of Inflammation Modulation
3.2.3. Algal Applications in Managing Inflammatory Conditions
| Algae Type | Algae Strain | Type of Analyzed Sample (Extract or Pure Compound) | In Vitro Assays Against Pro-Inflammatory Enzymes (IC50 Values in µg/mL Unless Otherwise Stated) | References | ||
|---|---|---|---|---|---|---|
| COX-1 | COX-2 | 5-LOX | ||||
| Macroalgae | Amphiroa fragilíssima (Linnaeus) J.V. Lamouroux | EtOAc-MeOH extracts | 4990 | 5010 | 5020 | [73] |
| Gloeothece sp. | Acetone Ethanol Hexane:isopropanol (3:2) | 120 200 130 | [72] | |||
| Gracilaria canaliculata Sonder | EtOAc-MeOH extracts | 2920 | 2000 | 2010 | [73] | |
| Gracilaria corticata (J. Agardh) J. Agardh | 2990 | 3010 | 3020 | |||
| Gracilaria salicornia | 4′-[10′-[7-hydroxy-2,8-dimethyl-6-(pentyloxy)- 2H-chromen-2-yl]ethyl]-3′,4′-dimethyl-cyclohexanone | 2.46 mM | [68] | |||
| 3′-[10′-(8-hydroxy-5-methoxy-2,6,7-trimethyl-2H-chromen2-yl)ethyl]-3′-methyl-2′-methylene cyclohexyl butyrate | 2.03 mM | |||||
| Gracilaria salicornia | spiro[5.5]undecanes, 3-(hydroxymethyl)-7-(methoxymethyl)-3,11-dimethyl-9-oxospiro[5.5]undec-4-en-10-methylbutanoate | 2.78 mM | [69] | |||
| 4-ethoxy-11,11-dimethyl-7-methylene-8-(propionyloxy)spiro[5.5]undec-2-en-104,106-dihydroxytetrahydro-2H-pyran-10-carboxylate | 1.91 mM | |||||
| Gracilaria salicornia | Methyl-16(13→14)-abeo-7-labdene-(12-oxo) carboxylate | 860 | [70] | |||
| Gracilaria salicornia | EtOAc-MeOH extracts | 1010 | 1020 | 980 | [73] | |
| Halymenia dilatata Zanardini | 3040 | 3000 | 3020 | |||
| Hydropuntia edulis (S.G.Gmelin) Gurgel & Fredericq | 2910 | 3010 | 2980 | |||
| Padina tetrastromatica Hauck | 1230 | 1340 | 1280 | |||
| Palisada pedrochei J.N.Norris | 4040 | 4030 | 4010 | |||
| Portieria hornemannii (Lyngbye) P.C. Silva | 2010 | 1990 | 2030 | |||
| Spyridia filamentosa (Wulfen) Harvey | 3010 | 2980 | 3040 | |||
| Turbinaria decurrens | Decurrencyclic B | 14.0 µM | 3.0 µM | [71] | ||
| Complication | Algae Type | Algae Species | Algal Extraction or Compound | Cell Line | Inflammation Induced by | Concentrations | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| n.d. | Macroalgae | Caulerpa racemosa | Ethanol, hexane, and ethyl acetate carotenoid fractions | RAW 264.7 | LPS | 25 µM | ↑ AMPK expression; ↓ TNF-α expression; ↓ mTOR expression | [84] |
| Cystoseira amentacea | Ethanol or DMSO extract | RAW 264.7 | LPS | 100 µg/mL | ↓ inflammation (↓ IL-1β, IL-6, COX-2, and iNOS expression) | [85] | ||
| Dictyopteris membranacea | Disulfides | RAW 264.7 | LPS | 15.62–31.25 µM | Anti-inflammatory activity (↓ TNF-α, IL-6, and IL-12 production); ↓ NO expression by downregulating iNOS; downregulation of AKT/MAPK/ERK signaling pathway | [86] | ||
| Ecklonia cava and Sargassum horneri | Ethanol extract | RAW 264.7 | LPS | 62.5 µg/mL | No cytotoxic effect; ↓ NO production; ↓ inflammatory response (↓ IL-1β, IL-6, PGE2, and TNF-α expression); downregulation of iNOS and COX-2; downregulation of NF-κB and MAPK pathways | [76] | ||
| Ecklonia cava | Ethanol extract | HGF-1 | LPS | 50 and 100 µg/mL | ↓ PGE2 production and pro-inflammatory enzyme expression; ↓ pro-inflammatory chemokine gene expressions; ↓ ROS production; downregulation of MAPK signaling pathway | [87] | ||
| Himanthalia elongata | Ethyl acetate fraction of a crude acetone extract | RAW 264.7 | LPS | 100 µg/mL | ↓ NO and O2 production regardless of being submitted to a simulated gastrointestinal digestion or not | [78] | ||
| Laurencia majuscula | Sesquiterpene (C17H25BrO3); chamigrane | RAW 264.7 | LPS | 3.7 µM; 3.6 µM | ↓ NO production and no cytostatic activity | [88] | ||
| Padina boryana | Fucosterol | RAW 264.7 | Particulate Matter/LPS | 12.5, 25, and 50 µg/mL | ↓ NO production; ↓ cytokines production (↓ IL-1β, IL-6, TNF-α, and PGE2); ↓ mRNA expression of IL-1β, IL-6, TNF-α, iNOS, and COX-2; downregulation of MAPK and NF-κB phosphorylation; upregulation of Nrf2/HO-1 pathway | [89] | ||
| Porphyra tenera | Water extract | RAW 264.7 | LPS | 1000 µg/mL | ↓ PGE2 and NO production; ↓ COX-2 and iNOS protein expression; ↓ TNF-α and IL-6 production | [90] | ||
| Porphyra sp. | Polydeoxyribonucleotide | RAW 264.7 | LPS | 200 µg/mL | ↓ NO production; ↓ iNOS expression by reducing phosphorylation of p38 MAPK and ERK | [82] | ||
| Rugulopteryx okamurae | Rugukadiol A and ruguloptone A | RAW 264.7 | LPS | 10 µM | ↓ NO production; ↓ Nos2 and IL-1β expression | [91] | ||
| Rugulopteryx okamurae | Okaspatol C Okamurol A | RAW 264.7 | LPS | 10 µM | Decrease in NO production | [92] | ||
| Sargassum autumnale | Fucoidan fractions | RAW 264.7 | LPS | 50, 100, and 200 µg/mL | ↓ NO production (↑ cell viability); ↓ PGE2 production; ↓ pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β); ↓ expression of inducible inflammatory enzymes (iNOS and COX2); downregulation of NF-κB and MAPK pathways | [93] | ||
| Sargassum horneri | Sargachromenol | RAW 264.7 | LPS | 62.5 µg/mL | ↑ antioxidant activity (↓ NO and intracellular ROS production); activation of Nrf2/HO-1 signaling pathway (upregulation of HO-1 expression); ↓ expression of inflammatory cytokines (IL-1β, IL-6, and TNF-α) through the downregulation of iNOS and COX-2 expression; suppression of activation of NF-κB and MAPK signaling pathways | [94] | ||
| Sargassum ilicifolium | Crude lipid extracts | RAW 264.7 | LPS | 50 µg/mL | ↓ NO production in pre-incubated and co-incubated cell culture models | [77] | ||
| Saccharina japonica | Fucoidan | RAW 264.7 | LPS | 100, 150, and 200 µg/mL | ↓ NO production; ↓ inflammation (↓ iNOS and COX-2 expression and ↓ TNF-α, IL-6, and IL-1β production); downregulation of NF-κB, MAPK, and JAK2-STAT1/3 signaling pathways | [95] | ||
| Sargassum swartzii | Fucoidan fraction | RAW 264.7 | LPS | 100 and 200 µg/mL | ↓ NO production; ↓ inflammation (↓ PGE2, TNF-α, IL-1β, and IL-6 secretion and expression); ↓ iNOS and COX-2 expression; downregulation of NF-κB and MAPK signaling pathways | [96] | ||
| n.d. | Fucoxanthinol | RAW 264.7 | LPS | 10 and 20 µM | Anti-inflammatory activity (↓ iNOS, IL-6, and TNF-α mRNA expression and ↓ IL-1β, TNF-α, IL-6, and Nitrate production) | [97] | ||
| Microalgae | Phaeodactylum tricornutum | Nonyl8-acetoxy-6-methyloctanoate | RAW 264.7 | LPS | 25 μg/mL | ↓ inflammation (↓ NO, PGE2, IL-1β, and IL-6); downregulation of COX-2 and iNOS | [74] | |
| Tisochrysis lutea | Methanol extract | RAW 264.7 | LPS | 100 µg/mL | Protected cells from cytotoxicity (↓ dendritic structures); ↓ PGE2 production and COX-2 protein expression; ↓ IL-6 and ↑ IL-10 expression; ↓ expression of inflammatory genes (Arg1, SOD2, and NLRP3) | [75] | ||
| Myopathy | Macroalgae | Ishige okamurae | Diphlorethohydroxycarmalol | C2C12 | TNF-α | 3.125, 6.25, and 12.5 µg/mL | ↓ NO and ↓ pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) production; modulation of NF-κB and MAPK signaling pathways | [98] |
| Skin | Macroalgae | Ecklonia cava | Dieckol | HaCaT | Particulate matter | 10 and 30 µM | ↓ PGE2 production; ↓ COX-1 and COX-2 mRNA expression levels; ↓ ROS; ↓ gene expression of enzymes involved in PGE2 synthesis | [99] |
| Halymenia durvillei | Ethyl acetate fraction | HaCaT | UV radiation | 5 µg/mL | ↓ intracellular ROS production; ↓ matrix metalloproteinases; upregulation of mRNA of antioxidant enzymes (SOD, HMOX1, and GSTP1); ↑ procollagen synthesis; activation of Nrf2 pathway | [81] | ||
| Polyopes affinis | Butanol fraction | HaCaT | IFN-γ or TNF-α | 10, 30, and 60 µg/mL | Downregulation of MAPK, STAT1, and NF-κB pathways | [100] | ||
| Polysiphonia morrowii | 3-bromo-4,5-dihydroxybenzaldehyde | HaCaT | IFN-γ or TNF-α | 144 and 288 µM | ↓ inflammatory cytokines (IL-6, IL-8, IL-13, IFN-y, and TNF-α) and chemokine production; downregulation of MAPK and NF-κB signaling pathways; activation of Nrf2/HO-1 signaling; protective activity against deterioration of skin barrier function (preserving skin moisture and tight junction stability) | [80] | ||
| Pyropia yezoensis | Methanol extract | HaCaT | IFN-γ | 40, 200, and 1000 µg/mL | Improvement of atopic dermatitis (↓ mRNA expression and secretion of pro-inflammatory chemokines; inhibition of MAPK activation; downregulation of NF-κB activation) | [101] | ||
| Sargassum confusum | Low-molecular-weight fucoidan | HaCaT | IFN-γ or TNF-α | 15.6, 31.3, and 62.5 µg/mL | ↓ ROS production; ↓ inflammatory cytokines (IL-1β, IL-6, IL-8, IL-13, IFN-y, and TNF-α) and chemokines; downregulation of MAPK and NF-κB signaling pathways; activation of Nrf2/HO-1 signaling | [102] | ||
| Sargassum horneri | (–)-Loliolide | HaCaT | IFN-γ or TNF-α | 15.6, 31.3, and 62.5 µg/mL | ↓ inflammatory cytokines (IL-4, IL-6, IL-13, IFN-y, and TNF-α) and chemokines; downregulation of MAPK and NF-κB signaling pathways; upregulation of Nrf2/HO-1 signaling | [103] | ||
| Sargassum siliquastrum | Low-molecular-weight fucoidan | RAW 264.7 | LPS | 25, 50, and 100 µg/mL | ↓ ROS production; ↓ production of NO and PGE2; ↓ expression of iNOS and COX-2; ↓ inflammatory cytokine expression (IL-1β, IL-6, and TNF-α); downregulation of MAPK and NF-κB signaling pathways; activation of Nrf2/HO-1 signaling; inhibition of the NLRP3 inflammasome protein complex | [104] | ||
| Sargassum horneri | Fucosterol | HDF | IFN-γ or TNF-α | 60 and 120 µM | ↓ ROS production; activation of Nrf2/HO-1 signaling; no effect of cell viability; ↓ mRNA expressions of inflammatory cytokines (IL-6, IL-8, IL-13, IL-33, IL-1β, TNF-α, and IFN-y) and MMPs; downregulation of MAPK and NF-κB signaling pathways | [83] | ||
| Microalgae | Porphyridium cruentum | Sulfated exopolysaccharides | HaCaT | UVA radiation | 12 µg/mL | Protective effect on cells from oxidative damage (↓ ROS formation, ↓ lipid peroxidation, and ↑ intracellular GSH levels); increased wound healing activity | [105] | |
| Phycoerythrin | 10 nM |
| Complication | Algae Type | Algae Species | Algal Extraction or Compound | Route of Administration | Dosage | Experimental Period | Animal Model (Age) | Inflammation Induced by | n/Group | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n.d. | Macroalgae | Codium fragile | Sulfated polysaccharides | n.d. | 50 and 100 µg/mL | 3 d | Zebrafish embryos (7–9 hpf) | LPS (10 µg/mL) | n.d. | ↓ cell death; ↓ NO and ROS generation | [108] |
| Cystoseira crinita (Desf.) Borry | Fucoidan | Intraperitoneally | 25 and 50 mg/kg | 5 h | Wistar Rats | LPS (0.25 mg/kg) | 8 | Decrease in IL-1β production | [109] | ||
| Ecklonia maxima | Ethyl acetate fraction | Incubation with embryo media | 25 and 50 µg/mL | 3 d | Zebrafish embryos (7–9 hpf) | LPS (10 µg/mL) | 15 | Increased survival rate (↓ cell death); improved heart-beating rates; ↓ ROS and NO generation | [113] | ||
| Saccharina japonica | Sulfated polysaccharide | Incubation with embryo media | 50 and 100 µg/mL | 3 d | Zebrafish embryos (8 hpf) | LPS (10 µg/mL) | 15 | ↓ cell death; ↓ NO and ROS generation; protection of phenotypic changes and toxic damages caused by LPS (↓ yolk sack edema, ↓ heart rate, and ↑ survival rate) | [114] | ||
| Saccharina japonica | Fucoidan | Incubation with embryo media | 25 and 50 μg/mL | 3 d | Zebrafish embryos | LPS (10 µg/mL) | n.d. | Increased survival rate (↓ cell death); improved heart-beating rates; ↓ intracellular ROS; ↓ NO generation | [110] | ||
| Sargassum binderi | Polysaccharides | Incubation with embryo media | 25, 50, and 100 µg/mL | 3 d | Zebrafish larvae (7–9 hpf) | LPS (10 µg/mL) | 15 | ↓ LPS-induced cell death; ↓ NO production | [111] | ||
| Sargassum fulvellum | Polysaccharides | Incubation with embryo media | 50 and 100 µg/mL | 3 d | Zebrafish embryos (7–9 hpf) | LPS (10 µg/mL) | 15 | Increased survival rate (↓ cell death); ↓ heartbeat disorder; ↓ ROS; ↓ NO | [112] | ||
| Microalgae | Phaeodactylum tricornutum | Supplements (whole biomass, β-1,3-glucan-rich, or combination thereof) | Oral supplements | 2.3 g biomass powder; 1.8 g of lyophilised supernatant; 2.3 g biomass powder + 1.8 g of lyophilised supernatant | 2 w | Elderly human individuals (67.7 ± 6.5 years) | - | 4 to 5 | No severe reactions, some mild and minimal were reported; decreased inflammatory marker (IL-6); ↑ plasma carotenoids (fucoxanthin); modulation of intestinal permeability (↓ zonulin) | [106] | |
| Tetraselmis sp. | Ethanol extract | Incubation with embryo media | 100 and 200 µg/mL | 7 d | Zebrafish embryos (7–9 hpf) | LPS (10 µg/mL) | 15 | Increased survival rate (↓ cell death); ↓ NO generation | [115] | ||
| Skin | Macroalgae | Sarcodia suiae sp. | Ethyl acetate fraction of ethanol extract | Skin application | 200 µg/day | 18 d | BABL/c mice (8 w) | DNCB (2%) | 6 | ↓ Atopic dermatitis symptoms (↓ inflammation, skin erythema, edema, dryness, and keratinocyte hyperplasia) and ↓ immunoglobulin E upregulation; ↓ swelling of subiliac lymph nodes and spleen; ↑ skin barrier integrity (↑ claudin-1 expression, cell-to-cell connections, and improved dilaggrin deficiency) | [116] |
| Microalgae | Dunaliella salina | Hydrophobic extract | Skin application | 1% | 56 d | Human subjects (aged 35–60, Fitzpatrick skin phototypes II–IV, and with signs of aging) | Intense solar exposure | 25 | Anti-inflammatory activity (↓ skin reactivity to histamine stimulation and red spot count and area); anti-aging effect (↓ wrinkle count and volume) | [107] |
3.3. Cardioprotective Activity
3.3.1. Cardiovascular Diseases and Regulation of Blood Pressure and Blood Lipid Levels
3.3.2. Algal Compounds and Their Potential for Reducing Cardiovascular Risk
3.4. Gastrointestinal Health Modulation
3.4.1. Implications for Digestive Health and Gut-Related Disorders
3.4.2. Algal Compounds and Their Potential for Improving the Impairment of Gastrointestinal Health
| Complication | Algae Type | Algae Species | Algal Extraction or Compound | Route of Administration | Dosage | Experimental Period | Animal Model (Age) | Induced by | n/Group | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatic damage | Macroalgae | Gracilaria caudata | Sulfated polysaccharides | Intraperitoneally | 10 mg/kg/day | 5 d | Swiss mice | Nimesulide (intragastric) | 8 to 10 | ↓ liver weight; improved antioxidant parameter in liver (↓ MPO, MDA, and NO3/NO2 levels, ↑ GSH level); ↓ inflammatory markers (↓ IL-1β and TNF-α levels); enhancement of hepatic function markers (↓ AST, ALT, and GGT levels) | [146] |
| Liver fibrosis | Macroalgae | Caulerpa racemosa | Water extracts | Oral in distilled water | 200 mg/kg/mL | 5 w | Wistar Rats | 40% Carbon tetrachloride (CCl4) intraperitoneally | 7 | Enhanced liver enzymes in serum (↓ ALT, AST, ALP, and LDH) and liver metabolite (↓ total bilirubin and direct bilirubin); improved renal and lipid profile (↓ urea; ↓ creatine); increased hepatic antioxidant enzymes (↑ GSH and CAT; ↓ MDA) | [148] |
| Padina pavonia | 6 | ||||||||||
| NAFLD | Macroalgae | Ishige okamurae | Diphlorethohydroxycarmalol (DPHC) | Incubation with embryo media | 40 µM | 3 d | Transgenic zebrafish embryos (Danio rerio) (3 dpf) | Palmitate | 12 to 15 | ↓ lipogenesis (downregulation of lipogenesis-related genes SREBP1c, ChREBP1 α, and FAS); ↓ liver inflammation (↓ IL-1β, TNF-α, and COX-2); regulation of lipid metabolism (stimulation of AMPK and SIRT1 signaling pathway) | [147] |
| NASH and NAFLD | Macroalgae | A. nodosum and F. vesiculosus | Gdue© | Intragastric gavage | 7.5 mg/kg bw | 12 w for NAFLD; 18 w for NASH | Sprague Dawley rats (4–8 w) | Western diet high-fat diet plus 30% fructose in the drinking water | 10 | ↓ body weight gain; ↓ plasma glucose; reduced liver steatosis (↓ liver TG accumulation); ↓ hepatic inflammation; restored hepatic lipid metabolism (downregulation of lipid droplet forming and fatty acid synthase genes); restored physiological levels of protein expression regulating lipid homeostasis | [145] |
| IBD | Macroalgae | Ulva pertusa | Extract | Oral gavage | 50 mg/kg and 100 mg/kg | 4 d | CD1 mice (4 w) | DNBS injected into the rectum | 10 | ↓ body weight loss; ↑ pain threshold; ↓ DNBS-induced hyperalgesia; ↓ DNBS-induced visceral hypersensitivity; ↓ cell adhesion molecules (↓ ICAM-1 and P-selectin); ↓ gut-inflammation (reduced IL-6, IL-17, and IL-23 levels and enhanced serum IL-10 levels); modulation of innate (↓ CD68+ cells) and adaptive (↓ CD4+ and CD8+ cells) immune system; TLR4 and NLRP3 inflammasome modulation | [149] |
| 11 | ↓ body weight loss; ↓ inflammation (reduced the expression levels of NF-κB and restored the expression of Ikb-α); modulation of pro-inflammatory interleukin production (↓ IL-5, IL-9, and IL-13, and ↑ IL-4); modulation of apoptosis (↓ p-53, caspase-3, -8, and -9, and ↓ ↑ Bcl-2); ↓ oxidative stress (↓ MDA levels, ↑ GSH, CAT, SOD, Mn-SOD, and HO-1); modulation of Nrf2/SIRT1 pathway (↑ Nrf2 and SIRT1 levels) | [150] | |||||||||
| Intestinal Health | Macroalgae | Cymopolia barbata | Cymopol | Oral gavage | 0.1 g/kg and 0.4 g/kg | 3 d | C57BL/6J mice (4 w) | 3% DSS in drinking water | 5 | ↓ inflammatory and oxidant response (downregulation of ERK/MAPK and PI3K/AKT pathways) | [158] |
| Laminaria spp. | Enzymolysis seaweed powder | Feed | 20 g/kg of feed | 4 m | Ragdoll kittens (6 m) | 10 | ↑ growth performance (↑ weight gain); improved immune function (↑ IgG and IgA); improved antioxidant parameters (↑ SOD, ↓ MDA); ↓ inflammation (↓ IL-1β, IL-6, and TNF-α, and ↑ IL-10); more microbiota richness and diversity (↑ relative abundance of Bacteroidetes, Lachnospiraceae, Prevotellaceae, and Faecalibacterium); improved gut mucosal barrier function | [157] | |||
| Porphyra yezoensis | Oligoporphyran | Fish meal | 1% | 8 w | Adult zebrafish (1 m) | n.d. | 25 | Positive effect on digestive enzymes (Protease, Lipase, and Amylase) activity; enhanced lipid content of body composition; enhanced intestinal innate immunity (↑ lysozyme); ↓ inflammation in intestines (↑ IL-10); improvement in gut microbial community | [156] | ||
| Colitis | Macroalgae | Laurencia glandulifera | O11,15-Cyclo-14-Bromo-14,15-Dihydrorogiol-3,11-Diol | Intraperitoneally | 0.25 mg/mouse every 48 h | 5 d | C57BL/6 | DSS in drinking water | 3 to 5 | ↓ Inflammation (↓ IL-1β, TNF-α, and IL-6) | [155] |
| Neorogioldio | |||||||||||
| n.d. | Eckol | Oral gavage | 1 mg/kg | 3 w | C57BL/6J (7–8 w) | DSS | 15 | ↓ body weight loss; attenuation of colitis symptom; improvement in colon shortening; ↓ pro-inflammatory cytokines in colon (TNF-α, IL-1β, and IL-6, ↑ IL-10); downregulation of NF-κB and TLR4 in colons; ↓ apoptosis (↓ caspase-9 protein expression); improved gut microbiota dysbiosis; immunoregulatory effect in colitis (recruitment of dendritic cells to the colonic tissue) | [154] | ||
| UC | Macroalgae | Turbinaria ornate | Methanol fraction from ethanol extract | Oral | 15 mg/kg/ | 6 w | C57BL/6J mice (7 w) | DSS | 6 | ↓ inflammatory response (↓ MPO activity, ↓ COX-2, p-STAT-3, and TNF-α expression levels, ↑ IL-10 and FOXP3 expression levels); upregulation of regulatory T cell activity | [153] |
| n.d. | Fucoxanthin | Oral | 50 mg/kg/day and 100 mg/kg/day | 2 w | C57BL/6J (8 w) | DSS in drinking water | 10 | ↓ body weight loss; improved colon shortening; ↓ inflammation in colon tissues (prevention of increase in colonic PGE2 production, ↓ COX-2 expression and ↓ NF-κB activation) | [151] | ||
| n.d. | n.d. | Dieckol | Oral gavage | 5, 10, and 15 mg/kg | 11 d | C57BL/6J mice | DSS (3% in drinking water) | 6 | ↓ body weight loss; ↑ colon length; ↓ oxidative stress mediators (↓ MPO and MDA activity) in colon tissue; ↓ inflammation (↓ COX-2, IL-1β, and TNF-α); NF-κB inhibition and upregulation of Nrf2/HO-1 signaling cascade | [152] |
3.5. Metabolic Health-Promoting Activity
3.5.1. Obesity, Diabetes, and Metabolic Health
3.5.2. Algal Compounds in Weight Control and Metabolism Regulation
| Algae Type | Algae Species | Algal Extraction or Compound | Route of Administration | Dosage | Experimental Period | Animal Model (Age) | Induced by | n/Group | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Macroalgae | Caulerpa lentillifera | Biomass | Feed | 5% | 16 w | Wistar rats (8–9 w) | High-carbohydrate, HFD | 12 | ↓ body weight gain; ↓ fat gain (↓ retroperitoneal, epididymal, omental, total abdominal, and visceral fat, and adiposity); ↓ systolic blood pressure; ↓ lipids (↓ TC and liver fat vacuole area); modulation of gut bacteria (↓ Firmicutes to Bacteroidetes ratio) | [183] |
| Caulerpa racemosa | Ethyl acetate extract | Oral-feeding tube | 100 and 200 mg/kg body weight | 24 d | Sprague Dawley rats (8 w) | STZ (i.p.) | 6 | ↓ plasma glucose level; ↓ ALT and AST levels (plasma); ↑ Albumin levels | [185] | |
| Cystoseira compressa | Phlorotannin extracts | Oral | 60 mg/kg | 6 w | Wistar albino rats | STZ (i.p.) | 10 | ↓ blood glucose, ↓ α- amylase and ↓ glucosidase activity; ↓ urea; ↓ creatine; ↓ oxidate stress (↑ GSH and CAT; ↓ MDA) | [186] | |
| Dictyota dichotoma | n-butanol and ethyl acetate extracts | n.d. | 100 and 200 mg/kg | 3 d | Rats | Monohydrate (i.p.) | 6 | Hypoglycemic activity (↓ blood glucose level); activation of AMPK pathway | [191] | |
| Gelidium amansii | Pheophorbide A (PhA) | Oral | 10 mg/kg | 3 w | ICR mice (4 w) | STZ injection | 7 | ↓ blood glucose after 30, 60, and 120 min; ↓ postprandial blood glucose levels | [190] | |
| Ishige okamurae | Diphlorethohydroxycarmalol | Injection | 0.3 µg/g body weight | 90 min | Wild-type zebrafish (adults) | Alloxan (2 mg/mL) and glucose (1%) | 3 | ↓ blood glucose levels; ↑ glucose transport (↑ calcium levels in skeletal myotubes and ↑ Glut4 translocation and ↑ phosphorylation of AMPK); regulation of muscle contraction | [187] | |
| Palmaria palmata | Alcalase/Flavourzyme-produced protein hydrolysate | Oral gavage | 100 mg/kg | 180 min | NIH Swiss mice (10–12 w) | Glucose | 8 | Improved glucose tolerance (↓ blood glucose level) | [192] | |
| Polysiphonia japonica | 5-Bromoprotocatechualdehyde | Incubation with embryo media | 50 µM | 35 h | Zebrafish embryos (3 dpf) | Palmitic acid (0.2 mM); stimulation with glucose | 10 to 12 | Protective effect against PA-induced β-cells dysfunction (↑ insulin secreting cells) | [188] | |
| Rhodomela confervoides | 3,4-Dibromo-5-(2-bromo-6-(ethoxymethyl)-3,4- dihydroxybenzyl)benzene-1,2-diol (BPN) | Oral gavage | 20 mg/kg | 12 w | Wistar Rats | Diet induced obesity; STZ (i.p.) | 10 | ↓ blood glucose | [193] | |
| Sargassum pallidum | Fucoidan | Intragastric | 200 mg/(kg/d) | 8 w | C57BLKS/J db/m and db/db mice (7–8 w)- spontaneous diabetic model | - | 6 | ↓ weight gain; ↓ hyperlipidemia (↓ TG and TC); anti-diabetic activity (↑ glucose tolerance, ↓ insulin resistance, and ↑ insulin sensitivity); ↓ oxidative stress on cardiac tissue (↓ MDA in serum and heart and ↑ SOD, CAT, and GSH/GSSG, ↓ lipid peroxidation); counteracted the repression of AMPK/Nrf2/ARE antioxidant signaling axis in cardiac tissue; ↓ hyperglycemia-associated metabolic cardiac inflammation (↓ activation of NF-κB signaling pathway and ↓ mRNA levels of Il-1β, Il-6, and TNF-α) | [182] | |
| Saccharina japonica | Dietary fibers | Oral | 500 mg/kg/day | 9 w | C57BL/6JGpt (4 w) | HFD | 12 | ↓ body weight; ↑ insulin sensitivity; ameliorated dyslipidemia (↓ TG, LDL-c, and FFA and ↓ visceral fat index); alleviated liver (↑ ALT and AST) and renal (↓ creatine and urea) damage; antioxidant effect on liver (↓ MDA, ↑ CAT, GSH, and SOD); anti-inflammatory potential (↓ TNF-α, IL-6, and MCP-1); improved gut microbiota dysbiosis; modulation of SCFA metabolism (increase in SCFAs production in colonic contents) | [184] | |
| Ulva reticulata | Methanol extract | Oral | 250 mg/kg | 31 d | Wistar albino Rats (2–3 m) | STZ injection | 6 | ↓ cholesterol, ALT, TG, and AST; ↓ blood glucose level; ↓ body weight | [181] | |
| Chloroform fraction | Oral | 10 mg/kg | 17 d | 7 | ↓ cholesterol, ALT, TG, and AST; ↓ blood glucose level; ↓ body weight; ↓ serum insulin | |||||
| n.d. | Fucoidan | Intraperitoneal | 100 mg/kg | 6 w | Wistar albino Rats (3 m) | STZ injection | 9 | ↓ blood glucose; ↓ body weight | [180] | |
| Microalgae | Tetraselmis chui | TetraSOD® | Oral | 17 mg/kg body weight | 16 w | Sprague–Dawley (7 w) | Diet-induced obesity by cafeteria diet | 10 | ↓ oxidative stress (↑ Nox) levels and anti-inflammatory markers (↓ IL-10); ↑ antioxidant enzymes in liver (↑ GSH); modulation of genes involved in antioxidant and anti-inflammatory pathways in the liver, mesenteric white adipose tissue, spleen, and thymus. | [189] |
3.6. Anti-Cancer Activity
3.6.1. Cancer Development and Progression
3.6.2. Oxidative Stress and Inflammation in Cancer Development
3.6.3. Algal Compounds with Anti-Cancer Properties
| Complication | Algae Type | Algae Species | Algal Extract or Compound | Cell Line | IC50 of Cell Viability | Concentrations Tested | Outcomes and Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Brain glioma | Macroalgae | Ecklonia cava | Triphlorethol-A | U251 | 20 µM | ↑ ROS accumulation; ↑ mitochondrial apoptosis (↑ chromatin condensation, fragmented nuclei, and membrane blebbing); ↓ antioxidant enzymes (SOD, CAT, and GSH); ↑ Bax expression and ↓ Bcl-2; ↑ protein expression of cytochrome c and caspase-3 and -9; downregulation on phosphorylated JAK2/STAT3 and MAPK/ERK1/2 pathways | [200] | |
| Breast Cancer | Macroalgae | Caulerpa racemosa | Crude polyphenolic extract | KAIMR C1 | 168.5 µM | [172] | ||
| Chaetomorpha sp. | Ethanol extract | MDA-MB-231 | 225.2 µg/mL | [217] | ||||
| Sargassum myriocystum | Methanol extract | MCF-7 | 66.8 µg/mL | ↓ cell viability (morphological cell changes indicating apoptosis) | [201] | |||
| Microalgae | Nannochloropsis oculata | Methanol extract | MDA-MB-231 | 200, 400, and 600 µg/mL | ↓ cell viability; morphological changes in cancerous cells | [218] | ||
| n.d. | n.d. | Fucoxanthin | MDA-MB-231; MCF-1; SKBR3 | 10 µM | ↓ cell viability tumoral cell lines; (↑ apoptotic cells, ↓ cell proliferation, and ↑ cell damage) when used in combination with known anti-cancer drugs (cisplatin and doxorubicin) | [202] | ||
| Colon Cancer | Macroalgae | Caulerpa racemosa | Crude polyphenolic extract | HCT-8 | 160.0 µM | [172] | ||
| Laurencia synderiae | Methanolic extract | HT-29 | 70.2 µg/mL | 50–100 µg/mL | ↑ cell death of cancerous cells (↓ cell viability); ↑ apoptosis (↑ chromatin condensation, nuclear fragmentation, and DNA fragmentation) | [203] | ||
| Colorectal cancer | Macroalgae | Ecklonia maxima | Fucoidans | HCT-116 | 0.1–0.5 mg/mL | ↓ cell adhesion; ↓ colony formation; ↓ cancer cell sphere formation; ↓ cancerous cell migration 2D and 3D models | [215] | |
| Ecklonia radiata | ||||||||
| Sargassum elegans | ||||||||
| Esophageal adenocarcinoma | Macroalgae | Chondrus armatus | Carrageenans | FLO1 | 100 and 400 µg/mL | ↓ cell viability of cancerous cells | [214] | |
| Gastric cancer | Microalgae | Gloeothece sp. | Hexane–isopropanol extract | AGS | 23.2 µg/mL | anti-proliferative effect (↑ cell death of cancerous cells); not toxic in non-cancerous cells (HCMEC cell line) up to 100 µg/mL | [72] | |
| Hepatic cancer | Macroalgae | Sargassum pallidum | Polysaccharide fractions | HepG2 | 25, 100, and 400 µg/mL | ↓ cell viability of cancerous cells (↑ apoptosis) | [204] | |
| Leukemia | Microalgae | Skeletonema marinoi | Methanol extract | K562 | 0.75 mg/mL | ↓ cell viability; ↑ cell apoptosis (↑ proapoptotic Bax protein expression and ↓ antiapoptotic protein Bcl-2 expression); ↓ oxidative stress (↓ NO production through NOX2 pathway); restored redox status (↑ SOD, CAT, and GPx); ↓ oxidative DNA damage | [205] | |
| Liver cancer | Macroalgae | Dictyotaciliolata | Methanol or aqueous extracts | HepG2 | 0.05–1 mg/mL | ↓ cell viability by inducing apoptosis (↑ caspase 3 and 9 activity) | [206] | |
| Pyropia Yezoensis | Phycoerythrin | HepG2 | 20 and 30 µg/mL | ↓ cell viability (altered cell membrane integrity; ↑ apoptosis) | [207] | |||
| Microalgae | Tisochrysis lutea | Dichloromethane extract | HepG2 | 85.1 µg/mL | Subfractions displayed high selectivity index (S17 vs. HepG2). | [216] | ||
| Lung carcinoma | Macroalgae | Sargassum pallidum | Polysaccharide fractions | A549 | 25, 100, and 400 µg/mL | ↓ cell viability of cancerous cells (↑ apoptosis) | [204] | |
| Udotea flabellum | Hydrolysated protein | A549 | 300.7 mg/mL | [128] | ||||
| Microalgae | Oscillatoria simplicissima | Sulfated polysaccharides | A549 | 100 µg/mL | ↓ cell viability of cancerous cells | [219] | ||
| Melanoma | Microalgae | Isochrysis galbana | Methanol extract and fractions | A2058 | 100 µg/mL | Antiproliferative effect of cancerous cells (↓ cell viability) | [208] | |
| Nasopharyngeal carcinoma | n.d. | n.d. | Fucoxanthin | C666-1 | 25 µM | Cytotoxic effect by inducing autophagy and apoptosis | [209] | |
| Oral cancer | n.d. | n.d. | Fucoidan | Ca9-22 CAL 27 | 800 and 1200 µg/mL | Selectively cytotoxic to cancer cells but not in non-malignant oral cells; ↑ apoptosis in cancerous cells (↑ activation of caspase-8, -9, and -3); ↑ ROS levels in oral cancer cells (downregulation of antioxidant signaling genes NRF2, TXN, and HMOX1); DNA damage-inducible effects in cancer cells | [210] | |
| Ovarian cancer | Macroalgae | Agarum clathratum | Extract | ES2 and OV90 | 25 µg/mL | ↓ cell viability (induced apoptosis; ↓ phosphorylation of ERk1/2 MAPK) | [211] | |
| Pancreatic cancer | Macroalgae | Ecklonia cava | Dieckol | PANC-1 | 20 μM | ↑ apoptosis (↓ Bcl2 expression and ↑ Bax); ↑ ROS generation in cancerous cells; ↓ antioxidant enzymes (SOD, CAT, and GSH); ↓ cell adhesion; anti-inflammatory activity (↓ TNF-α, IL-6, IL-8, and IL-1β) | [212] | |
| Prostate cancer | Microalgae | Skeletonema marinoi | Methanol extract | DU145 LNCaP | 100 µg/mL | ↓ cancerous cell proliferation; ↑ apoptosis; ↓ cell vascular mimicry; downregulation of inflammation- and angiogenesis-associated genes | [213] | |
| Squamous-cell carcinoma | Macroalgae | Chondrus armatus | Carrageenans | KYSE30 | 100 and 400 µg/mL | ↓ cell viability of cancerous cells | [214] |
4. Future Directions and Research Gaps
4.1. Emerging Areas of Research in Algal Bioactivities
4.2. Challenges and Limitations in Studying Algae for Health
5. Conclusions
5.1. Summary of Key Findings on Algal Bioactivities Related to Human Health
5.2. Implications for Future Research and Applications
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rotter, A.; Bacu, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Dalay, M.C.; et al. A New Network for the Advancement of Marine Biotechnology in Europe and Beyond. Front. Mar. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- Johnson, K.; Dalton, G.; Masters, I. Building Industries at Sea: ‘Blue Growth’ and the New Maritime Economy. In Building Industries at Sea: ‘Blue Growth’ and the New Maritime Economy; Johnson, K., Dalton, G., Masters, I., Eds.; River Publishers: Aalborg, Denmark, 2018; pp. 1–516. [Google Scholar]
- van der Westhuyzen, A.E.; Frolova, L.V.; Kornienko, A.; van Otterlo, W.A.L. Chapter Four—The Rigidins: Isolation, Bioactivity, and Total Synthesis—Novel Pyrrolo[2,3-d]Pyrimidine Analogues Using Multicomponent Reactions. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 79, pp. 191–220. [Google Scholar]
- Lu, W.Y.; Li, H.J.; Li, Q.Y.; Wu, Y.C. Application of marine natural products in drug research. Bioorganic Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Chen, T.; Qiang, B.; Liu, N.; Wang, H.; Zhang, L.; Liu, Z. CMNPD: A comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res. 2021, 49, D509–D515. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Dini, I. The Potential of Algae in the Nutricosmetic Sector. Molecules 2023, 28, 4032. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fucinos, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk Dalay, M.; et al. The Essentials of Marine Biotechnology. Front. Mar. Sci. 2021, 8, 629629. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Quiterio, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Richards, N.C.; Gouda, H.N.; Durham, J.; Rampatige, R.; Rodney, A.; Whittaker, M. Disability, noncommunicable disease and health information. Bull. World Health Organ. 2016, 94, 230–232. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 6 December 2023).
- Shelton, C.D.; Byndloss, M.X. Gut Epithelial Metabolism as a Key Driver of Intestinal Dysbiosis Associated with Noncommunicable Diseases. Infect. Immun. 2020, 88, e00939-19. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, F.; Heinken, A.; Thiele, I.; Lindenburg, P.W.; Harms, A.C.; Hankemeier, T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 2021, 13, 1882927. [Google Scholar] [CrossRef] [PubMed]
- Borgoni, S.; Kudryashova, K.S.; Burka, K.; de Magalhaes, J.P. Targeting immune dysfunction in aging. Ageing Res. Rev. 2021, 70, 101410. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Dinu, M. Oxidative Stress and Inflammation as Targets for Novel Preventive and Therapeutic Approaches in Non-Communicable Diseases II. Antioxidants 2022, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Seyedsadjadi, N.; Grant, R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Herath, K.; Sanjeewa, K.K.A.; Jayawardena, T.U. Recent Reports on Bioactive Compounds from Marine Cyanobacteria in Relation to Human Health Applications. Life 2023, 13, 1411. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenco-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal Prospects of Antioxidants From Algal Sources in Cancer Therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Dai, Z.; Zhang, W.; Fan, S.; Liu, H.; Liu, R.; Zhao, T. Antiobesity, Antidiabetic, Antioxidative, and Antihyperlipidemic Activities of Bioactive Seaweed Substances. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 239–253. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer 2017, 8, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cesario, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef]
- Jerkovic, I.; Cikos, A.M.; Babic, S.; Cizmek, L.; Bojanic, K.; Aladic, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Coz-Rakovac, R.; et al. Bioprospecting of Less-Polar Constituents from Endemic Brown Macroalga Fucus virsoides J. Agardh from the Adriatic Sea and Targeted Antioxidant Effects In Vitro and In Vivo (Zebrafish Model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef]
- Wang, L.; Fu, X.; Hyun, J.; Xu, J.; Gao, X.; Jeon, Y.J. In Vitro and In Vivo Protective Effects of Agaro-Oligosaccharides against Hydrogen Peroxide-Stimulated Oxidative Stress. Polymers 2023, 15, 1612. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.J.; Hwangbo, H.; Ji, S.Y.; Kim, D.H.; Kim, M.Y.; Bang, E.; Hong, S.H.; Kim, S.O.; Jeong, S.J.; et al. Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1. Int. J. Mol. Sci. 2023, 24, 4637. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, H.; Park, S.-H.; Hong, S.H.; Song, K.S.; Cha, H.-J.; Kim, G.-Y.; Chang, Y.-C.; Kim, S.; Kim, H.-S.; et al. Indole-6-carboxaldehyde prevents oxidative stress-induced mitochondrial dysfunction, DNA damage and apoptosis in C2C12 skeletal myoblasts by regulating the ROS-AMPK signaling pathway. Mol. Cell. Toxicol. 2020, 16, 455–467. [Google Scholar] [CrossRef]
- Niu, T.; Fu, G.; Zhou, J.; Han, H.; Chen, J.; Wu, W.; Chen, H. Floridoside Exhibits Antioxidant Properties by Activating HO-1 Expression via p38/ERK MAPK Pathway. Mar. Drugs 2020, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, M.; Wang, L.; Liu, Y.; Lu, X.; Stagos, D.; Lin, X.; Liu, M. Bromophenol Bis (2,3,6-Tribromo-4,5-dihydroxybenzyl) Ether Protects HaCaT Skin Cells from Oxidative Damage via Nrf2-Mediated Pathways. Antioxidants 2021, 10, 1436. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Fernando, P.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine Compound 3-bromo-4,5-dihydroxybenzaldehyde Protects Skin Cells against Oxidative Damage via the Nrf2/HO-1 Pathway. Mar. Drugs 2019, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, L.; Guo, M.; Stagos, D.; Giakountis, A.; Trachana, V.; Lin, X.; Liu, Y.; Liu, M. Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. Antioxidants 2022, 11, 786. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective Effect of Phloroglucinol on Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in HaCaT Human Keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef]
- He, Y.L.; Xiao, Z.; Yang, S.; Zhou, C.; Sun, S.; Hong, P.; Qian, Z.J. A Phlorotanin, 6,6′-bieckol from Ecklonia cava, Against Photoaging by Inhibiting MMP-1, -3 and -9 Expression on UVB-induced HaCaT Keratinocytes. Photochem. Photobiol. 2022, 98, 1131–1139. [Google Scholar] [CrossRef]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Goncalves, L.; Petrovski, Z.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef]
- Chen, B.; Chen, H.; Qu, H.; Qiao, K.; Xu, M.; Wu, J.; Su, Y.; Shi, Y.; Liu, Z.; Wang, Q. Photoprotective effects of Sargassum thunbergii on ultraviolet B-induced mouse L929 fibroblasts and zebrafish. BMC Complement. Med. Ther. 2022, 22, 144. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hamzeh, A.; Tabarsa, M.; Cravotto, G. Cellular antioxidant and emulsifying activities of fucoidan extracted from Nizamuddinia zanardinii using different green extraction methods. J. Food Process. Preserv. 2022, 46, e17238. [Google Scholar] [CrossRef]
- Begum, S.; Hemalatha, S. Gelidiella acerosa Compounds Target NFkappaB Cascade in Lung Adenocarcinoma. Appl. Biochem. Biotechnol. 2022, 194, 1566–1579. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, Characterization, and Antioxidant Activity Evaluation of a Fucoidan from an Enzymatic Digest of the Edible Seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-L.; Kim, G.H.; Kang, M.-C.; Jeon, Y.-J. Protective effects of extracts from six local strains of Pyropia yezoensis against oxidative damage in vitro and in zebrafish model. Algae 2020, 35, 189–200. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, E.A.; Kang, S.I.; Yang, H.W.; Ryu, B.; Wang, L.; Lee, J.S.; Jeon, Y.J. Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules 2020, 25, 2361. [Google Scholar] [CrossRef]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting Oxidative Stress with Polyphenols to Fight Liver Diseases. Antioxidants 2023, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Gyuraszova, M.; Gurecka, R.; Babickova, J.; Tothova, L. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, W.; Boukhris, S.; Annabi-Trabelsi, N.; Rebai, T.; Sellami-Kamoun, A.; Aldahmash, W.; Plavan, G.I.; Harrath, A.H.; Ayadi, H. Hyperhalophilic Diatom Extract Protects against Lead-Induced Oxidative Stress in Rats and Human HepG2 and HEK293 Cells. Pharmaceuticals 2023, 16, 875. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, Y.; Fu, S.; Shen, Z.; Zong, W.; Xia, Z.; Zhan, Z.; Jiang, X. Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice. Mar. Drugs 2021, 19, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Jeon, Y.J. The Potential of Sulfated Polysaccharides Isolated from the Brown Seaweed Ecklonia maxima in Cosmetics: Antioxidant, Anti-melanogenesis, and Photoprotective Activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-kappaB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 809952. [Google Scholar] [CrossRef]
- Saha, S.; Muller, D.; Clark, A.G. Mechanosensory feedback loops during chronic inflammation. Front. Cell Dev. Biol. 2023, 11, 1225677. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential anti-inflammatory agents. Nat. Prod. Res. 2020, 34, 3470–3482. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Antony, T. First report of spiro-compounds from marine macroalga Gracilaria salicornia: Prospective natural anti-inflammatory agents attenuate 5-lipoxygenase and cyclooxygenase-2. Nat. Prod. Res. 2021, 35, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. First report of antioxidant abeo-labdane type diterpenoid from intertidal red seaweed Gracilaria salicornia with 5-lipoxygenase inhibitory potential. Nat. Prod. Res. 2020, 34, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Thambi, A.; Chakraborty, K. Anti-inflammatory decurrencyclics A-B, two undescribed nor-dammarane triterpenes from triangular sea bell Turbinaria decurrens. Nat. Prod. Res. 2023, 37, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.M.; Barros, R.; Tavares, T.; Almeida, R.; Pinto, I.S.; Malcata, F.X.; Guedes, A.C. Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents. Mar. Drugs 2021, 19, 623. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. Pharmacological Properties of Seaweeds against Progressive Lifestyle Diseases. J. Aquat. Food Prod. Technol. 2019, 28, 1092–1104. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Ko, J.Y.; Lee, J.H.; Jeon, Y.J. Anti-inflammatory activity of nonyl 8-acetoxy-6-methyloctanoate, isolated from the cultured marine diatom, Phaeodactylum tricornutum: Mediated via suppression of inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J. Natl. Sci. Found. Sri Lanka 2022, 50, 685–693. [Google Scholar] [CrossRef]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Dos Santos Nascimiento, L.B.; Tredici, M.R.; Luceri, C. A Comparative In Vitro Evaluation of the Anti-Inflammatory Effects of a Tisochrysis lutea Extract and Fucoxanthin. Mar. Drugs 2021, 19, 334. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.-Y.; Kim, W.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Ecklonia cava (Laminariales) and Sargassum horneri (Fucales) synergistically inhibit the lipopolysaccharide-induced inflammation via blocking NF-κB and MAPK pathways. Algae 2019, 34, 45–56. [Google Scholar] [CrossRef]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Andarwulan, N. Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia. Mar. Drugs 2021, 19, 252. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Circuncisao, A.R.; Neves, B.; Marcal, C.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Impact of Gastrointestinal Digestion on the Anti-Inflammatory Properties of Phlorotannins from Himanthalia elongata. Antioxidants 2022, 11, 1518. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Han, E.J.; Kirindage, K.; Fernando, I.P.S.; Kim, E.A.; Kim, J.; Jung, K.; Kim, K.N.; Heo, S.J.; Ahn, G. 3-Bromo-4,5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-alpha/IFN-gamma-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes. Mar. Drugs 2022, 20, 563. [Google Scholar] [CrossRef] [PubMed]
- Kraokaew, P.; Manohong, P.; Prasertsuksri, P.; Jattujan, P.; Niamnont, N.; Tamtin, M.; Sobhon, P.; Meemon, K. Ethyl Acetate Extract of Marine Algae, Halymenia durvillei, Provides Photoprotection against UV-Exposure in L929 and HaCaT Cells. Mar. Drugs 2022, 20, 707. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Heo, S.Y.; Han, J.S.; Jung, W.K. Anti-inflammatory effect of polydeoxyribonucleotides (PDRN) extracted from red alga (Porphyra sp.) (Ps-PDRN) in RAW 264.7 macrophages stimulated with Escherichia coli lipopolysaccharides: A comparative study with commercial PDRN. Cell Biochem. Funct. 2023, 41, 889–897. [Google Scholar] [CrossRef]
- Kirindage, K.; Jayasinghe, A.M.K.; Han, E.J.; Jee, Y.; Kim, H.J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-alpha/IFN-gamma-Stimulated Human Dermal Fibroblasts via Regulating Nrf2/HO-1 and NF-kappaB/MAPK Pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfi, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira amentacea var. stricta. Mar. Drugs 2020, 19, 2. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Bafiti, P.; Kikionis, S.; Laskou, M.; Roussis, V.; Ioannou, E.; Kampranis, S.C.; Tsatsanis, C. Disulfides from the Brown Alga Dictyopteris membranacea Suppress M1 Macrophage Activation by Inducing AKT and Suppressing MAPK/ERK Signaling Pathways. Mar. Drugs 2020, 18, 527. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, S.; Baek, S.M.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines. Foods 2021, 10, 1656. [Google Scholar] [CrossRef]
- Tammam, M.A.; Daskalaki, M.G.; Tsoureas, N.; Kolliniati, O.; Mahdy, A.; Kampranis, S.C.; Tsatsanis, C.; Roussis, V.; Ioannou, E. Secondary Metabolites with Anti-Inflammatory Activity from Laurencia majuscula Collected in the Red Sea. Mar. Drugs 2023, 21, 79. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Lee, H.G.; Nagahawatta, D.P.; Yang, H.W.; Kang, M.C.; Jeon, Y.J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-kappaB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Ahn, Y.T.; Zhao, R.; Kim, Y.S.; Park, S.M.; Jung, D.H.; Kim, J.K.; Kim, H.W.; Kim, S.C.; An, W.G. Inhibitory Effects of Porphyra tenera Extract on Oxidation and Inflammatory Responses. Evid. Based Complement. Altern. Med. 2021, 2021, 6650037. [Google Scholar] [CrossRef]
- Cuevas, B.; Arroba, A.I.; de Los Reyes, C.; Gomez-Jaramillo, L.; Gonzalez-Montelongo, M.C.; Zubia, E. Diterpenoids from the Brown Alga Rugulopteryx okamurae and Their Anti-Inflammatory Activity. Mar. Drugs 2021, 19, 677. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, B.; Arroba, A.I.; de Los Reyes, C.; Zubia, E. Rugulopteryx-Derived Spatane, Secospatane, Prenylcubebane and Prenylkelsoane Diterpenoids as Inhibitors of Nitric Oxide Production. Mar. Drugs 2023, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.M.; Lee, H.G.; Nagahawatta, D.P.; Jayawardhana, H.; Song, K.M.; Choi, Y.S.; Jeon, Y.J.; Kang, M.C. Fucoidan from Sargassum autumnale Inhibits Potential Inflammatory Responses via NF-kappaB and MAPK Pathway Suppression in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Mar. Drugs 2023, 21, 374. [Google Scholar] [CrossRef]
- Han, E.J.; Jayawardena, T.U.; Jang, J.H.; Fernando, I.P.S.; Jee, Y.; Jeon, Y.J.; Lee, D.S.; Lee, J.M.; Yim, M.J.; Wang, L.; et al. Sargachromenol Purified from Sargassum horneri Inhibits Inflammatory Responses via Activation of Nrf2/HO-1 Signaling in LPS-Stimulated Macrophages. Mar. Drugs 2021, 19, 497. [Google Scholar] [CrossRef]
- Ye, J.; Chen, D.; Ye, Z.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xue, C.; Xiao, M. Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-kappaB, MAPK and JAK-STAT Pathways. Mar. Drugs 2020, 18, 328. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Nagahawatta, D.P.; Lee, H.G.; Lu, Y.A.; Vaas, A.; Abeytunga, D.T.U.; Nanayakkara, C.M.; Lee, D.S.; Jeon, Y.J. Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum Swartzii in Macrophages via Blocking TLR/NF-Kappab Signal Transduction. Mar. Drugs 2020, 18, 601. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Yang, L.; Yi, Z.; Fang, H.; Chen, W.; Hong, Z.; Zhang, Y.; Zhang, G.; Li, L. Anti-Inflammatory Effects of Fucoxanthinol in LPS-Induced RAW264.7 Cells through the NAAA-PEA Pathway. Mar. Drugs 2020, 18, 222. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ahn, G.; Kim, H.S.; Je, J.G.; Kim, K.N.; Jeon, Y.J. Diphlorethohydroxycarmalol (DPHC) Isolated from the Brown Alga Ishige okamurae Acts on Inflammatory Myopathy as an Inhibitory Agent of TNF-alpha. Mar. Drugs 2020, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Lee, W.H.; Kim, J.K.; Jeon, H.K.; Lee, J.; Kim, Y.J. Polyopes affinis Suppressed IFN-gamma- and TNF-alpha-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-kappaB and STAT1 Pathways. Molecules 2022, 27, 1836. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Lee, W.H.; Jeong, J.; Park, M.; Ko, J.Y.; Kwon, O.W.; Lee, J.; Kim, Y.J. Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-kappaB. Nutrients 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Han, E.J.; Oh, G.W.; Jung, W.K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-alpha/IFN-gamma- Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Fernando, I.P.S.; Kim, H.S.; Lee, D.S.; Kim, A.; Je, J.G.; Seo, M.J.; Jee, Y.H.; Jeon, Y.J.; Kim, S.Y.; et al. (-)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-gamma/TNF-alpha-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Kim, K.N.; Oh, J.Y.; Ahn, G. The Anti-Inflammatory Effect of Low Molecular Weight Fucoidan from Sargassum siliquastrum in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via Inhibiting NF-kappaB/MAPK Signaling Pathways. Mar. Drugs 2023, 21, 347. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’Elia, L.; Silva, M.; Barreira, L.; Monti, D.M. Shedding Light on the Hidden Benefit of Porphyridium cruentum Culture. Antioxidants 2023, 12, 337. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Frick, K.; Lehnert, K.; Vetter, W.; Montoya-Arroyo, A.; Frank, J.; Schmid-Staiger, U.; Bischoff, S.C. Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals. Mar. Drugs 2022, 20, 716. [Google Scholar] [CrossRef] [PubMed]
- Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Je, J.G.; Huang, C.; Oh, J.Y.; Fu, X.; Wang, K.; Ahn, G.; Xu, J.; Gao, X.; Jeon, Y.J. Anti-Inflammatory Effect of Sulfated Polysaccharides Isolated from Codium fragile In Vitro in RAW 264.7 Macrophages and In Vivo in Zebrafish. Mar. Drugs 2022, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinie, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural Characterization and In Vivo Anti-Inflammatory Activity of Fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ni, L.; Fu, X.; Wang, L.; Duan, D.; Huang, L.; Xu, J.; Gao, X. Molecular Mechanism of Anti-Inflammatory Activities of a Novel Sulfated Galactofucan from Saccharina japonica. Mar. Drugs 2021, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Je, J.G.; Lee, H.G.; Fernando, K.H.N.; Jeon, Y.J.; Ryu, B. Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.W.; Ahn, G.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.J. In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum. Mar. Drugs 2021, 19, 277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Je, J.G.; An, H.; Baek, K.; Lee, J.M.; Yim, M.J.; Ko, S.C.; Kim, J.Y.; Oh, G.W.; Kang, M.C.; et al. Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa. Mar. Drugs 2022, 20, 471. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-kappaB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Mar. Drugs 2020, 18, 593. [Google Scholar] [CrossRef]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Chen, P.C.; Lo, Y.H.; Huang, S.Y.; Liu, H.L.; Yao, Z.K.; Chang, C.I.; Wen, Z.H. The anti-inflammatory properties of ethyl acetate fraction in ethanol extract from Sarcodia suiae sp. alleviates atopic dermatitis-like lesion in mice. Biosci. Biotechnol. Biochem. 2022, 86, 646–654. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 14 January 2023).
- de Almeida, A.; de Almeida Rezende, M.S.; Dantas, S.H.; de Lima Silva, S.; de Oliveira, J.; de Lourdes Assuncao Araujo de Azevedo, F.; Alves, R.; de Menezes, G.M.S.; Dos Santos, P.F.; Goncalves, T.A.F.; et al. Unveiling the Role of Inflammation and Oxidative Stress on Age-Related Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 1954398. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tan, Z.; Zhou, L.; Yang, M.; Peng, L.; Liu, J.; Cai, J.; Yang, R.; Han, J.; Huang, Y.; et al. Effects of Angiotensin II Receptor Blockers and ACE (Angiotensin-Converting Enzyme) Inhibitors on Virus Infection, Inflammatory Status, and Clinical Outcomes in Patients With COVID-19 and Hypertension: A Single-Center Retrospective Study. Hypertension 2020, 76, 51–58. [Google Scholar] [CrossRef]
- Ko, S.C.; Kim, J.Y.; Lee, J.M.; Yim, M.J.; Kim, H.S.; Oh, G.W.; Kim, C.H.; Kang, N.; Heo, S.J.; Baek, K.; et al. Angiotensin I-Converting Enzyme (ACE) Inhibition and Molecular Docking Study of Meroterpenoids Isolated from Brown Alga, Sargassum macrocarpum. Int. J. Mol. Sci. 2023, 24, 11065. [Google Scholar] [CrossRef]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Windarto, S.; Lee, M.C.; Nursyam, H.; Hsu, J.L. First Report of Screening of Novel Angiotensin-I Converting Enzyme Inhibitory Peptides Derived from the Red Alga Acrochaetium sp. Mar. Biotechnol. 2022, 24, 882–894. [Google Scholar] [CrossRef]
- Caijiao, C.; Leshan, H.; Mengke, Y.; Lei, S.; Miansong, Z.; Yaping, S.; Changheng, L.; Xinfeng, B.; Xue, L.; Xin, L.; et al. Comparative Studies on Antioxidant, Angiotensin-Converting Enzyme Inhibitory and Anticoagulant Activities of the Methanol Extracts from Two Brown Algae (Sargassum horneri and Sargassum thunbergii). Russ. J. Mar. Biol. 2021, 47, 380–387. [Google Scholar] [CrossRef]
- Mousaie, M.; Khodadadi, M.; Tadayoni, M. Hydrolysate protein from brown macroalgae (Sargassum ilicifolium): Antioxidant, antitumor, antibacterial, and ACE inhibitory activities. J. Food Process. Preserv. 2022, 46, e17020. [Google Scholar] [CrossRef]
- Andre, R.; Guedes, L.; Melo, R.; Ascensao, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on In Vitro Bioactivities and Chemical Components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- Pei, Y.; Lui, Y.; Cai, S.; Zhou, C.; Hong, P.; Qian, Z.J. A Novel Peptide Isolated from Microalgae Isochrysis zhanjiangensis Exhibits Anti-apoptosis and Anti-inflammation in Ox-LDL Induced HUVEC to Improve Atherosclerosis. Plant Foods Hum. Nutr. 2022, 77, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Pan, X.; Wang, J.; Li, X.; Yang, S.; Yin, R.; Ma, A.; Zhu, X. Fucoidan Inhibits NLRP3 Inflammasome Activation by Enhancing p62/SQSTM1-Dependent Selective Autophagy to Alleviate Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 3186306. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-J.; Liao, H.-J.; Yang, J.-I. Purification and Identification of an ACE-Inhibitory Peptide from Gracilaria tenuistipitata Protein Hydrolysates. Processes 2022, 10, 1128. [Google Scholar] [CrossRef]
- Barkia, I.; Al-Haj, L.; Abdul Hamid, A.; Zakaria, M.; Saari, N.; Zadjali, F. Indigenous marine diatoms as novel sources of bioactive peptides with antihypertensive and antioxidant properties. Int. J. Food Sci. Technol. 2018, 54, 1514–1522. [Google Scholar] [CrossRef]
- Chang, P.M.; Li, K.L.; Lin, Y.C. Fucoidan(-)Fucoxanthin Ameliorated Cardiac Function via IRS1/GRB2/SOS1, GSK3beta/CREB Pathways and Metabolic Pathways in Senescent Mice. Mar. Drugs 2019, 17, 69. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Tsai, C.H.; Chen, H.Y.; Wang, K.L.; Chang, H.Y.; Huang, Y.J.; Hong, Y.H.; Ali, M.; Shieh, T.M.; Huang, T.C.; et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs 2021, 19, 307. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef] [PubMed]
- Eilam, Y.; Khattib, H.; Pintel, N.; Avni, D. Microalgae—Sustainable Source for Alternative Proteins and Functional Ingredients Promoting Gut and Liver Health. Glob. Chall. 2023, 7, 2200177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sakhare, K.; Bhattacharya, D.; Chattopadhyay, R.; Parikh, P.; Narayan, K.P.; Mukherjee, A. Communication in non-communicable diseases (NCDs) and role of immunomodulatory nutraceuticals in their management. Front. Nutr. 2022, 9, 966152. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Park, H.; Ho, M.; Bhardwaj, K.; Sugimura, N.; Lee, H.W.; Meng, H.; Ebert, M.P.; Chao, K.; et al. Unveiling and harnessing the human gut microbiome in the rising burden of non-communicable diseases during urbanization. Gut Microbes 2023, 15, 2237645. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease? Antioxidants 2023, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- El Rashed, Z.; Lupidi, G.; Grasselli, E.; Canesi, L.; Khalifeh, H.; Demori, I. Antioxidant and Antisteatotic Activities of Fucoidan Fractions from Marine and Terrestrial Sources. Molecules 2021, 26, 4467. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, R.; Zahra, H.A.; Abu Zahra, A.M.; Alfwuaires, M.; Abdulaziz Alamer, S.; Metwally, A.M.; Althnaian, T.A.; Al-Ramadan, S.Y. Protective Effect of Fucoxanthin on Zearalenone-Induced Hepatic Damage through Nrf2 Mediated by PI3K/AKT Signaling. Mar. Drugs 2023, 21, 391. [Google Scholar] [CrossRef]
- Gabbia, D.; Roverso, M.; Zanotto, I.; Colognesi, M.; Sayaf, K.; Sarcognato, S.; Arcidiacono, D.; Zaramella, A.; Realdon, S.; Ferri, N.; et al. A Nutraceutical Formulation Containing Brown Algae Reduces Hepatic Lipid Accumulation by Modulating Lipid Metabolism and Inflammation in Experimental Models of NAFLD and NASH. Mar. Drugs 2022, 20, 572. [Google Scholar] [CrossRef]
- Júnior, G.J.D.; Lemos, S.I.A.; de Brito, T.V.; Pereira, C.M.C.; da Cruz Júnior, J.S.; dos Santos Ferreira, J.; da Rocha Rodrigues, L.; do Nascimento Lima, J.V.; da Silva Monteiro, C.E.; Franco, A.X.; et al. Macromolecule extracted from Gracilaria caudata reduces inflammation and restores hepatic function in nimesulide-induced hepatic damage. J. Appl. Phycol. 2020, 32, 1511–1520. [Google Scholar] [CrossRef]
- Cha, S.H.; Hwang, Y.; Heo, S.J.; Jun, H.S. Diphlorethohydroxycarmalol Attenuates Palmitate-Induced Hepatic Lipogenesis and Inflammation. Mar. Drugs 2020, 18, 475. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Hira, K.; Qureshi, S.A.; Khatoon, N.; Ara, J.; Ehteshamul-Haque, S. Ameliorative Effect of Marine Macroalgae on Carbon Tetrachloride-Induced Hepatic Fibrosis and Associated Complications in Rats. Turk. J. Pharm. Sci. 2022, 19, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Mannino, D.; Capra, A.P.; Repici, A.; Filippone, A.; Esposito, E.; Campolo, M. New Insights into the Mechanism of Ulva pertusa on Colitis in Mice: Modulation of the Pain and Immune System. Mar. Drugs 2023, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-kappaB/Nrf2/SIRT1 Signaling Pathways. J. Clin. Med. 2022, 11, 4301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Tong, Q.Y.; Zheng, S.H.; Zhou, M.D.; Zeng, Y.M.; Zhou, T.T. Anti-inflammatory effect of fucoxanthin on dextran sulfate sodium-induced colitis in mice. Nat. Prod. Res. 2020, 34, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, Y.; Zhang, Y.; Su, X.; Luo, C.; Alarifi, S.; Yang, H. Dieckol alleviates dextran sulfate sodium-induced colitis via inhibition of inflammatory pathway and activation of Nrf2/HO-1 signaling pathway. Environ. Toxicol. 2021, 36, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Lee, S.M.; Kim, Y.N.; Jeon, Y.J.; Heo, J.D.; Jeong, E.J.; Rho, J.R. Standardized Fraction of Turbinaria ornata Alleviates Dextran Sulfate Sodium-Induced Chronic Colitis in C57BL/6 Mice via Upregulation of FOXP3+ Regulatory T Cells. Biomolecules 2020, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, J.; Hu, X.; Liu, J.; Li, S.; Wang, J. Eckol protects against acute experimental colitis in mice: Possible involvement of Reg3g. J. Funct. Foods 2020, 73, 104088. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and Related Diterpenes from the Red Alga Laurencia Inhibit Inflammatory Bowel Disease in Mice by Suppressing M1 and Promoting M2-Like Macrophage Responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef]
- Wang, X.; Wei, M.; Wang, J.; Liu, J.; Zhang, Q. Effects of oligo-porphyran on immunological parameters related to immunoregulation and growth in RAW264.7 macrophages and zebrafish model. Aquac. Int. 2022, 31, 759–775. [Google Scholar] [CrossRef]
- Zhang, M.; Mo, R.; Li, M.; Qu, Y.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Comparison of the Effects of Enzymolysis Seaweed Powder and Saccharomyces boulardii on Intestinal Health and Microbiota Composition in Kittens. Metabolites 2023, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.S.; Ratnayake, R.; Pope, J.L.; Chen, Q.Y.; Zhu, F.; Chen, S.; Carney, T.J.; Gharaibeh, R.Z.; Jobin, C.; Paul, V.J.; et al. Seaweed natural products modify the host inflammatory response via Nrf2 signaling and alter colon microbiota composition and gene expression. Free Radic. Biol. Med. 2020, 146, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Regufe, V.M.G.; Pinto, C.; Perez, P. Metabolic syndrome in type 2 diabetic patients: A review of current evidence. Porto Biomed. J. 2020, 5, e101. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Le, D.D.; Nguyen, P.H.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Shin, H.M.; Rhee, H.I.; Woo, M.H. PTP1B, alpha-glucosidase, and DPP-IV inhibitory effects for chromene derivatives from the leaves of Smilax china L. Chem. Biol. Interact. 2016, 253, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- Nurkolis, F.; Taslim, N.A.; Qhabibi, F.R.; Kang, S.; Moon, M.; Choi, J.; Choi, M.; Park, M.N.; Mayulu, N.; Kim, B. Ulvophyte Green Algae Caulerpa lentillifera: Metabolites Profile and Antioxidant, Anticancer, Anti-Obesity, and In Vitro Cytotoxicity Properties. Molecules 2023, 28, 1365. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef]
- Antony, T.; Chakraborty, K.; Joy, M. Antioxidative dolabellanes and dolastanes from brown seaweed Padina tetrastromatica as dual inhibitors of starch digestive enzymes. Nat. Prod. Res. 2021, 35, 614–626. [Google Scholar] [CrossRef]
- Thambi, A.; Chakraborty, K. A novel anti-hyperglycemic sulfated pyruvylated polysaccharide from marine macroalga Hydropuntia edulis. Nat. Prod. Res. 2023, 37, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Dhara, S.; Chakraborty, K. Turbinafuranone A–C, new 2-furanone analogues from marine macroalga Turbinaria ornata as prospective anti-hyperglycemic agents attenuate tyrosine phosphatase-1B. Med. Chem. Res. 2021, 30, 1635–1648. [Google Scholar] [CrossRef]
- Jagtap, A.S.; Manohar, C.S.; Ayyapankutty, A.M.T.; Meena, S.N. Antioxidant and Antiglycemic Properties of Macroalgae, an Underutilized Blue Economy Bioresource in India. Russ. J. Mar. Biol. 2022, 47, 489–497. [Google Scholar] [CrossRef]
- Gazali, M.; Jolanda, O.; Husni, A.; Nurjanah; Majid, F.A.A.; Zuriat; Syafitri, R. In Vitro alpha-Amylase and alpha-Glucosidase Inhibitory Activity of Green Seaweed Halimeda tuna Extract from the Coast of Lhok Bubon, Aceh. Plants 2023, 12, 393. [Google Scholar] [CrossRef]
- Suthan, P.; Selva Maleeswaran, P. Secondary Metabolites Screening, in Vitro Antioxidant and Antidiabetic Activity of Marine Red Alga Botryocladia leptopoda (J.Agardh) Kylin. Orient. J. Chem. 2022, 38, 16–27. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Bandaranayake, U.; Keerthirathna, L.R.; Manawadu, C.; Silva, R.M.; Mohamed, B.; Ali, R.; Peiris, D.C. Integration of in vitro and in-silico analysis of Caulerpa racemosa against antioxidant, antidiabetic, and anticancer activities. Sci. Rep. 2022, 12, 20848. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.V.; Parada, J.; Perez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Amarante, S.J.; Catarino, M.D.; Marcal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.L.; Hammouti, B.; Rhazi, L.; et al. Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Gracilaria bursa-pastoris Extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef]
- Deepa, P.K.; Subramanian, A.; Manjusha, W.A. Phytochemical Screening and Evaluation of Antidiabetic Activity of the Marine Microalgae: Nannochloropsis Sp. Int. J. Life Sci. Pharma Res. 2022, 10, L36–L40. [Google Scholar] [CrossRef]
- Vinodkumar, M.; Packirisamy, A.S.B. Exploration of Bioactive Compounds from Sargassum myriocystum; A Novel Approach on Catalytic Inhibition Against Free Radical Formation and Glucose Elevation. Top. Catal. 2023, 67, 60–73. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Chen, H.Y.; Chang, Y.J.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Hsia, S.M. Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, G.; Ersoy, O.; Cerkezkayabekir, A.; Topcu-Tarladacalisir, Y. Sitagliptin and fucoidan prevent apoptosis and reducing ER stress in diabetic rat testes. Andrologia 2021, 53, e13858. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, P.S.; Animish, A.; Madhumitha, G.; Suthindhiran, K.; Jayasri, M.A. Bioactivity Guided Study for the Isolation and Identification of Antidiabetic Compounds from Edible Seaweed—Ulva reticulata. Molecules 2022, 27, 8827. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, F.; Yan, Y.; Jin, J.; Quan, Z.; Tong, H.; Du, J. Fucoidan derived from Sargassum pallidum alleviates metabolism disorders associated with improvement of cardiac injury and oxidative stress in diabetic mice. Phytother. Res. 2023, 37, 4210–4223. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Caulerpa lentillifera (Sea Grapes) Improves Cardiovascular and Metabolic Health of Rats with Diet-Induced Metabolic Syndrome. Metabolites 2020, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, N.; Wang, J.; Zhang, Q. Dietary fibers extracted from Saccharina japonica can improve metabolic syndrome and ameliorate gut microbiota dysbiosis induced by high fat diet. J. Funct. Foods 2021, 85, 104642. [Google Scholar] [CrossRef]
- Qudus, B.A.A.; Abdul Razak, S.; Palaniveloo, K.; Nagappan, T.; Suraiza Nabila Rahmah, N.; Wee Jin, G.; Chellappan, D.K.; Chellian, J.; Kunnath, A.P. Bioprospecting Cultivated Tropical Green Algae, Caulerpa racemosa (Forsskal) J. Agardh: A Perspective on Nutritional Properties, Antioxidative Capacity and Anti-Diabetic Potential. Foods 2020, 9, 1313. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Yang, H.W.; Jiang, Y.F.; Lee, H.G.; Jeon, Y.J.; Ryu, B. Ca2+-Dependent Glucose Transport in Skeletal Muscle by Diphlorethohydroxycarmalol, an Alga Phlorotannin: In Vitro and In Vivo Study. Oxid. Med. Cell. Longev. 2021, 2021, 8893679. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Zhang, C.; Heo, S.J.; Jun, H.S. 5-Bromoprotocatechualdehyde Combats against Palmitate Toxicity by Inhibiting Parkin Degradation and Reducing ROS-Induced Mitochondrial Damage in Pancreatic beta-Cells. Antioxidants 2021, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Del Bas, J.M.; Caimari, A.; Lama, C.; Torres, S.; Mantecon, L.; Infante, C. TetraSOD((R)), a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats. Nutrients 2022, 14, 4028. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.J.; Han, J.S. Pheophorbide A from Gelidium amansii improves postprandial hyperglycemia in diabetic mice through alpha-glucosidase inhibition. Phytother. Res. 2019, 33, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqbal, A.; Badshah, S.L.; Sher, A.A.; Ullah, H.; Ayaz, M.; Mosa, O.F.; Mostafa, N.M.; Daglia, M. Chemical and Nutritional Profiling of the Seaweed Dictyota dichotoma and Evaluation of Its Antioxidant, Antimicrobial and Hypoglycemic Potentials. Mar. Drugs 2023, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.M.; Harnedy-Rothwell, P.A.; Lafferty, R.A.; Sharkey, S.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Macroalgal protein hydrolysates from Palmaria palmata influence the ‘incretin effect’ in vitro via DPP-4 inhibition and upregulation of insulin, GLP-1 and GIP secretion. Eur. J. Nutr. 2021, 60, 4439–4452. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, L.; Chen, D.; Jiang, B. Effects of a natural PTP1B inhibitor from Rhodomela confervoides on the amelioration of fatty acid-induced insulin resistance in hepatocytes and hyperglycaemia in STZ-induced diabetic rats. RSC Adv. 2020, 10, 3429–3437. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 8 December 2023).
- Rehman, F.U.; Al-Waeel, M.; Naz, S.S.; Shah, K.U. Anticancer therapeutics: A brief account on wide refinements. Am. J. Cancer Res. 2020, 10, 3599–3621. [Google Scholar]
- Azmanova, M.; Pitto-Barry, A. Oxidative Stress in Cancer Therapy: Friend or Enemy? Chembiochem 2022, 23, e202100641. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhang, M.; Li, L.; Yang, M.; Zheng, Q.; Liu, Q.; Ning, R.; Gao, Z.; Deng, X. Research hotspots and trends in discovery of anticancer agents from algae: A 20-year bibliometric and visualized analysis based on Web of Science and CiteSpace. Algal Res. 2023, 74, 103244. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, Z.; Li, W.; Wu, A.; Su, Z.; Jiang, B.; Ganesan, S. Triphlorethol-A attenuates U251 human glioma cancer cell proliferation and ameliorates apoptosis through JAK2/STAT3 and p38 MAPK/ERK signaling pathways. J. Biochem. Mol. Toxicol. 2022, 36, e23138. [Google Scholar] [CrossRef] [PubMed]
- Vinodkumar, M.; Packirisamy, A.S.B. Effective Isolation of Brown Seaweed Flavonoids with Their Potential to Inhibit Free Radicals and Proliferative Cells. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3794–3804. [Google Scholar] [CrossRef]
- Malhao, F.; Macedo, A.C.; Costa, C.; Rocha, E.; Ramos, A.A. Fucoxanthin Holds Potential to Become a Drug Adjuvant in Breast Cancer Treatment: Evidence from 2D and 3D Cell Cultures. Molecules 2021, 26, 4288. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, K.; Zahmatkesh, A. Phytochemical screening, antioxidant potential, and cytotoxic effects of different extracts of red algae (Laurencia snyderiae) on HT29 cells. Res. Pharm. Sci. 2021, 16, 400–413. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Niu, Y.; Ju, H.; Chen, R.; Li, B.; Song, X.; Song, L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules 2021, 26, 7559. [Google Scholar] [CrossRef] [PubMed]
- Ciarcia, R.; Longobardi, C.; Ferrara, G.; Montagnaro, S.; Andretta, E.; Pagnini, F.; Florio, S.; Maruccio, L.; Lauritano, C.; Damiano, S. The Microalga Skeletonema marinoi Induces Apoptosis and DNA Damage in K562 Cell Line by Modulating NADPH Oxidase. Molecules 2022, 27, 8270. [Google Scholar] [CrossRef] [PubMed]
- Kumosani, T.A.; Al-Malki, A.L.; Zeyadi, M.A.; Alghamdi, M.A.; Moselhy, S.S.; Al-balawi, A.A.; Huwait, E.A.; Maddah, M.R. In vitro Study Anti-proliferative Potential of Algae Extract against Cancer Cell Line. J. Pharm. Res. Int. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Ulagesan, S.; Nam, T.J.; Choi, Y.H. Extraction and Purification of R-Phycoerythrin Alpha Subunit from the Marine Red Algae Pyropia yezoensis and Its Biological Activities. Molecules 2021, 26, 6479. [Google Scholar] [CrossRef]
- Riccio, G.; Martinez, K.A.; Ianora, A.; Lauritano, C. De Novo Transcriptome of the Flagellate Isochrysis galbana Identifies Genes Involved in the Metabolism of Antiproliferative Metabolites. Biology 2022, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cao, X.; Zhao, R.; Gong, S.; Jin, L.; Feng, C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ. Toxicol. 2020, 35, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown Algae-Derived Fucoidan Exerts Oxidative Stress-Dependent Antiproliferation on Oral Cancer Cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Hong, T.; Ham, J.; Lim, W. Effects of Agarum clathratum extract on cell death and calcium ion levels of ovarian cancer cell. Mol. Cell. Toxicol. 2022, 19, 303–310. [Google Scholar] [CrossRef]
- Xu, J.W.; Yan, Y.; Wang, L.; Wu, D.; Ye, N.K.; Chen, S.H.; Li, F. Marine bioactive compound dieckol induces apoptosis and inhibits the growth of human pancreatic cancer cells PANC-1. J. Biochem. Mol. Toxicol. 2021, 35, e22648. [Google Scholar] [CrossRef]
- Calabrone, L.; Carlini, V.; Noonan, D.M.; Festa, M.; Ferrario, C.; Morelli, D.; Macis, D.; Fontana, A.; Pistelli, L.; Brunet, C.; et al. Skeletonema marinoi Extracts and Associated Carotenoid Fucoxanthin Downregulate Pro-Angiogenic Mediators on Prostate Cancer and Endothelial Cells. Cells 2023, 12, 1053. [Google Scholar] [CrossRef]
- Cicinskas, E.; Begun, M.A.; Tiasto, V.A.; Belousov, A.S.; Vikhareva, V.V.; Mikhailova, V.A.; Kalitnik, A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. A 2020, 108, 254–266. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Pletschke, B.I.; Edkins, A.L. Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells. Mar. Drugs 2023, 21, 203. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-Hepatocellular Carcinoma (HepG2) Activities of Monoterpene Hydroxy Lactones Isolated from the Marine Microalga Tisochrysis Lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef]
- Wali, A.F.; Al Dhaheri, Y.; Ramakrishna Pillai, J.; Mushtaq, A.; Rao, P.G.M.; Rabbani, S.A.; Firdous, A.; Elshikh, M.S.; Farraj, D.A.A. LC-MS Phytochemical Screening, In Vitro Antioxidant, Antimicrobial and Anticancer Activity of Microalgae Nannochloropsis oculata Extract. Separations 2020, 7, 54. [Google Scholar] [CrossRef]
- Elkomy, R.G.; Ismail, M.M. Crude sulfated polysaccharides extracted from marine cyanobacterium Oscillatoria simplicissima with evaluation antioxidant and cytotoxic activities. Iran. J. Microbiol. 2021, 13, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.E.; Kim, J.E.; Park, S.H.; Lee, S.J.; Choi, Y.J.; Choi, S.I.; Choi, Y.W.; Lee, H.S.; Hong, J.T.; Hwang, D.Y. Anti-tumor effects of an aqueous extract of Ecklonia cava in BALB/cKorl syngeneic mice using colon carcinoma CT26 cells. Oncol. Rep. 2023, 49, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lin, G.; Wu, D.; Liu, D.; You, L.; Högger, P.; Simal-Gandara, J.; Wang, M.; da Costa, J.G.M.; Marunaka, Y.; et al. The algal polysaccharide ulvan suppresses growth of hepatoma cells. Food Front. 2020, 1, 83–101. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Nassef, M.; Bases, E.; Shafay, S.E.; El-Shenody, R. Antitumor immunity and therapeutic properties of marine seaweeds-derived extracts in the treatment of cancer. Cancer Cell Int. 2022, 22, 267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Liu, H.; Lei, Y.; Gao, H.; Alahmadi, T.A.; Peng, H.; Chen, W. Chemopreventive effect of dieckol against 7,12-dimethylbenz(a)anthracene induced skin carcinogenesis model by modulatory influence on biochemical and antioxidant biomarkers. Environ. Toxicol. 2021, 36, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Grubisic, M.; Santek, B.; Zoric, Z.; Cosic, Z.; Vrana, I.; Gasparovic, B.; Coz-Rakovac, R.; Ivancic Santek, M. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterization of Biomass, Pigment, Lipid and Fatty Acid Composition, and Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, A.; Chianese, A.; Zannella, C.; Piccolella, S.; Pacifico, S.; Giugliano, R.; Franci, G.; De Natale, A.; Pollio, A.; Pinto, G.; et al. Galdieria sulphuraria: An Extremophilic Alga as a Source of Antiviral Bioactive Compounds. Mar. Drugs 2023, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- Bamunuarachchi, N.I.; Khan, F.; Kim, Y.M. Bactericidal Activity of Sargassum aquifolium (Turner) C. Agardh against Gram-positive and Gram-negative Biofilm-forming Pathogenic Bacteria. Curr. Pharm. Biotechnol. 2021, 22, 1628–1640. [Google Scholar] [CrossRef]
- Han, E.J.; Zhang, C.; Kim, H.S.; Kim, J.Y.; Park, S.M.; Jung, W.K.; Ahn, G.; Cha, S.H. Sargachromenol Isolated from Sargassum horneri Attenuates Glutamate-Induced Neuronal Cell Death and Oxidative Stress through Inhibition of MAPK/NF-kappaB and Activation of Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 710. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Y.; Neupane, S.; Ptak, S.H.; Romer, R.; Xiong, J.; Ohmes, J.; Seekamp, A.; Frette, X.; Alban, S.; et al. Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization. Mar. Drugs 2021, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, Y.; Liu, S.; Shao, Z.; Chu, X.; Mao, W. Immunomodulatory Activity In Vitro and In Vivo of a Sulfated Polysaccharide with Novel Structure from the Green Alga Ulva conglobata Kjellman. Mar. Drugs 2022, 20, 447. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.F.; Ayoub, H.F.; Abd El-Gawad, E.A.; Abdelghany, M.F.; Abdel-Tawwab, M. Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2021, 119, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Aitouguinane, M.; El Alaoui-Talibi, Z.; Rchid, H.; Fendri, I.; Abdelkafi, S.; El-Hadj, M.D.O.; Boual, Z.; Le Cerf, D.; Rihouey, C.; Gardarin, C.; et al. Elicitor Activity of Low-Molecular-Weight Alginates Obtained by Oxidative Degradation of Alginates Extracted from Sargassum muticum and Cystoseira myriophylloides. Mar. Drugs 2023, 21, 301. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Castellari, M. Algae as Nutritional and Functional Food Sources. Foods 2022, 12, 122. [Google Scholar] [CrossRef]
- Alam, M.A.; Parra-Saldivar, R.; Bilal, M.; Afroze, C.A.; Ahmed, M.N.; Iqbal, H.M.N.; Xu, J. Algae-Derived Bioactive Molecules for the Potential Treatment of SARS-CoV-2. Molecules 2021, 26, 2134. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Rumin, J.; Nicolau, E.; Junior, R.G.O.; Fuentes-Grunewald, C.; Flynn, K.J.; Picot, L. A Bibliometric Analysis of Microalgae Research in the World, Europe, and the European Atlantic Area. Mar. Drugs 2020, 18, 79. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Regal, A.L.; Alves, V.; Gomes, R.; Matos, J.; Bandarra, N.M.; Afonso, C.; Cardoso, C. Drying process, storage conditions, and time alter the biochemical composition and bioactivity of the anti-greenhouse seaweed Asparagopsis taxiformis. Eur. Food Res. Technol. 2020, 246, 781–793. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Oerbekke, A.; Cvijetinovic, S.; Wahlström, N.; Harrysson, H.; Steinhagen, S.; Kinnby, A.; White, J.; Edlund, U.; et al. Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3255–3263. [Google Scholar] [CrossRef]
- Alvarez-Gomez, F.; Korbee, N.; Figueroa, F.L. Effects of UV Radiation on Photosynthesis, Antioxidant Capacity and the Accumulation of Bioactive Compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Kuech, A.; Breuer, M.; Popescu, I. Research for PECH Committee—The Future of the EU Algae Sector. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2023/733114/IPOL_STU(2023)733114_EN.pdf (accessed on 7 January 2024).
- Sobuj, M.K.A.; Islam, M.A.; Islam, M.S.; Islam, M.M.; Mahmud, Y.; Rafiquzzaman, S.M. Effect of solvents on bioactive compounds and antioxidant activity of Padina tetrastromatica and Gracilaria tenuistipitata seaweeds collected from Bangladesh. Sci. Rep. 2021, 11, 19082. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.; Brown, M.T.; Billington, R.A. The cytotoxic activity of extracts of the brown alga Cystoseira tamariscifolia (Hudson) Papenfuss, against cancer cell lines changes seasonally. J. Appl. Phycol. 2020, 32, 2419–2429. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown alga Durvillaea antarctica. Dry. Technol. 2019, 38, 1915–1928. [Google Scholar] [CrossRef]
- Getachew, A.T.; Holdt, S.L.; Meyer, A.S.; Jacobsen, C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Mar. Drugs 2022, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.K.; Lepetit, B.; Kroth, P.G.; Mecking, S. Production of chemicals from microalgae lipids–status and perspectives. Eur. J. Lipid Sci. Technol. 2017, 120, 1700152. [Google Scholar] [CrossRef]
- Zou, Y.; Fu, X.; Liu, N.; Duan, D.; Wang, X.; Xu, J.; Gao, X. The synergistic anti-inflammatory activities of agaro-oligosaccharides with different degrees of polymerization. J. Appl. Phycol. 2019, 31, 2547–2558. [Google Scholar] [CrossRef]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, bioaccessibility and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- European Algae Biomass Association. Algae as Novel Food in Europe. Available online: https://www.algae-novel-food.com/output/algae-novel-food/download.pdf (accessed on 20 March 2024).
- European Algae Biomass Association. Update on the Novel Food Catalogue/EU Novel Food State-of-the-Art -10 Years Later; European Algae Biomass Association: Florence, Italy, 2024. [Google Scholar]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
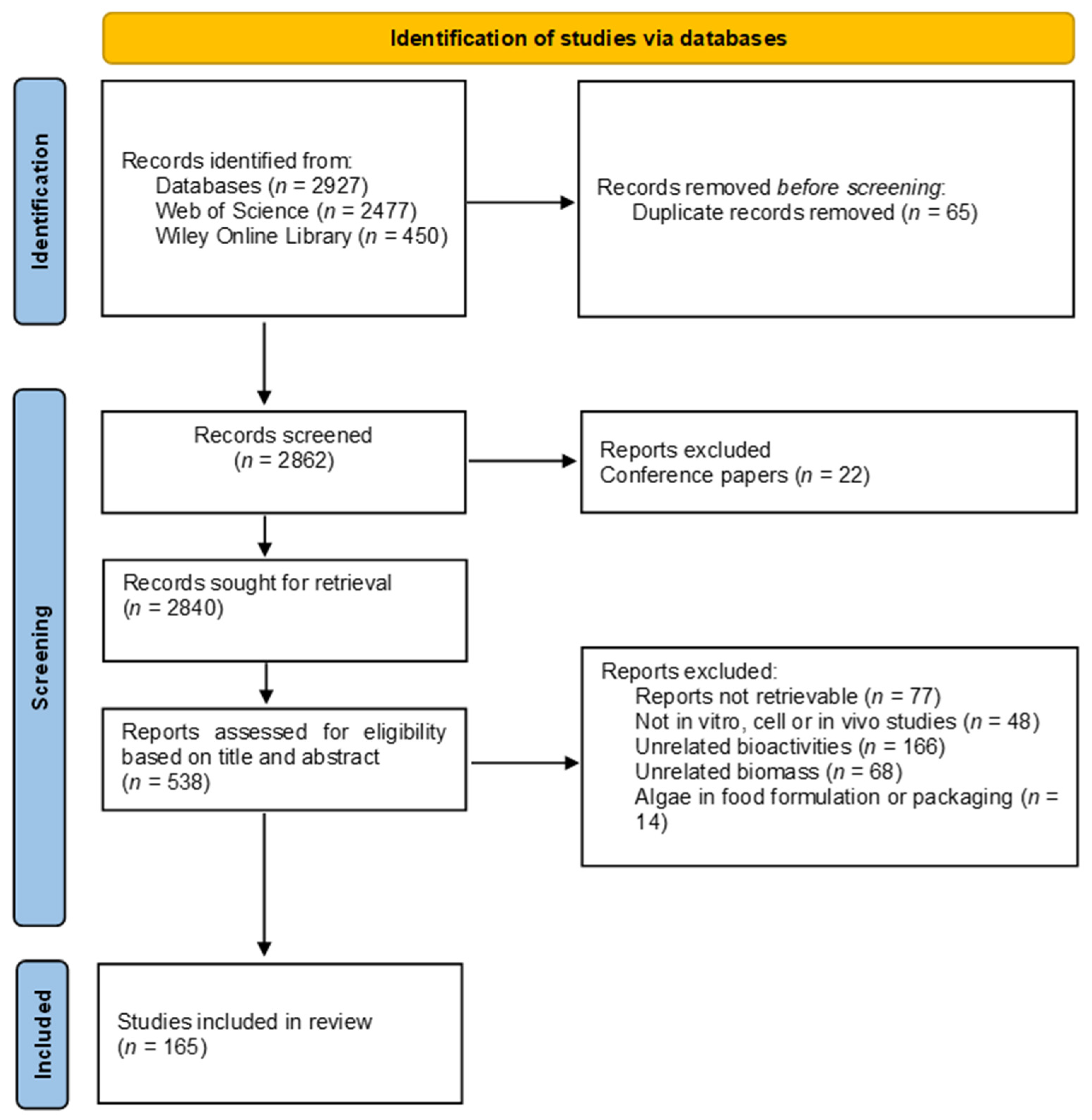
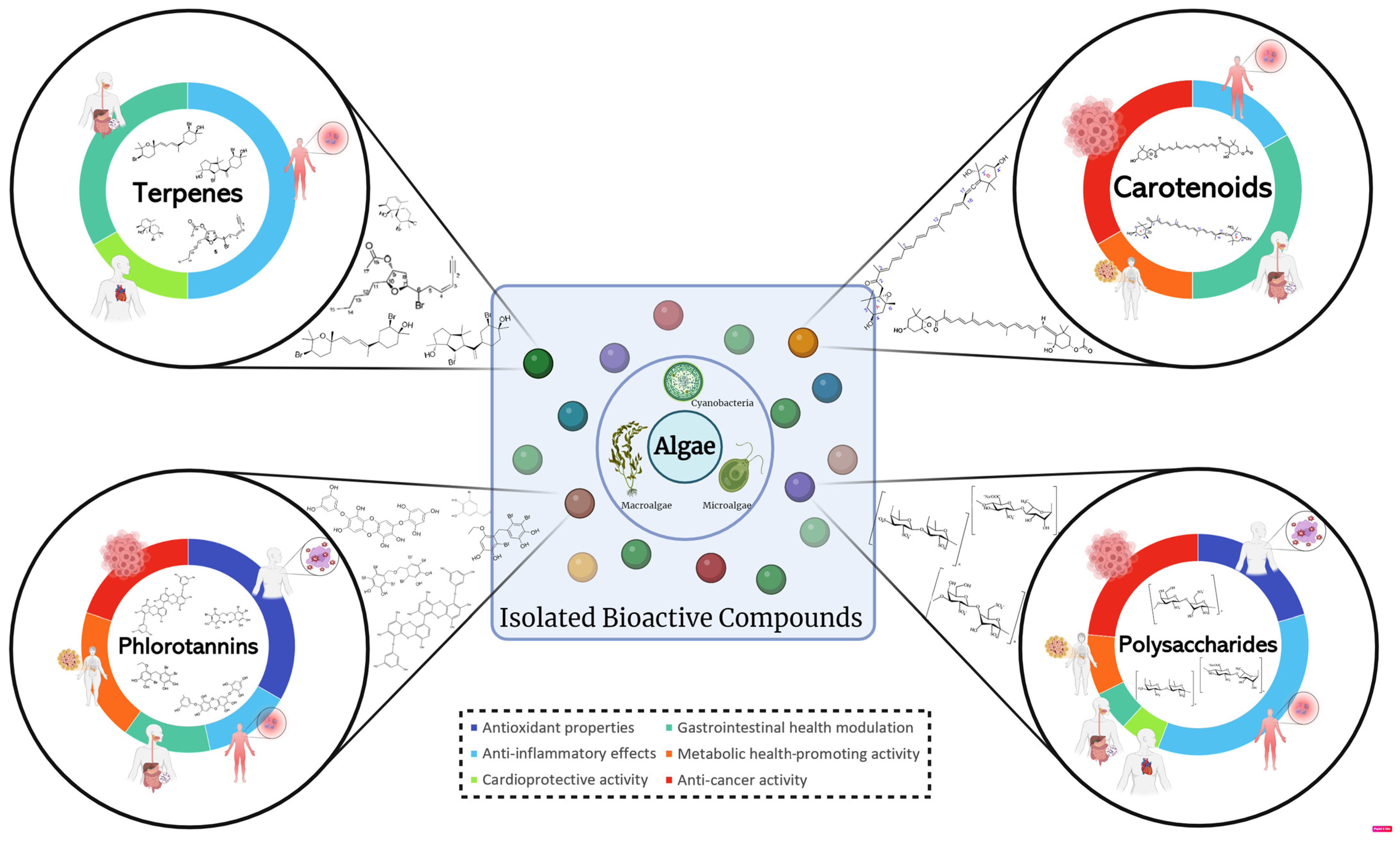
| Algae Type | Algae Strain | Type of Analyzed Sample (Extract or Pure Compound) | ACE Inhibition (IC50 Values in µg/mL Unless Otherwise Stated) | References |
|---|---|---|---|---|
| Macroalgae | Acrochaetium sp. | VGGSDLQAL peptide | 433.1 µM | [126] |
| Amphiroa fragilissima (Linnaeus) J.V. Lamouroux | Ethyl acetate–methanol extracts (pigments were eliminated by means of a first extraction with hexane) | 620 | [73] | |
| Gracilaria canaliculata Sonder | 190 | |||
| Gracilaria corticata (J. Agardh) | 200 | |||
| Gracilaria salicornia | 90 | |||
| Halymenia dilatata Zanardini | 230 | |||
| Hydropuntia edulis (S.G.Gmelin) Gurgel & Fredericq | 180 | |||
| Mazzaella japonica | Protein hydrolysate YRD (sequence YRPY) VSEGLD (sequence DGL) TIMPHPR (sequence PR) GGPAT (sequence GPA) (sequence GP) SSNDYPI (sequence LKYPI) (sequence DY) SRIYNVKSNG (sequence RIY) (sequence IY) (sequence YN) (sequence VK) VDAHY (sequence VDSDVVKG) (sequence HY) YGDPDHY (sequence HY) DFGVPGHEP (sequence DFG) | 262 320 µM 2.1 µM 4.1 µM 405 µM 253 µM 27.1 µM 100 µM 28 µM 2.1 µM 51 µM 13 µM 13.3 µM 26.1 µM 26.1 µM 44.7 µM | [124] | |
| Padina tetrastromatica Hauck | Ethyl acetate–methanol extracts (pigments were eliminated by means of a first extraction with hexane) | 120 | [73] | |
| Palisada pedrochei J.N.Norris | 210 | |||
| Portieria hornemannii (Lyngbye) P.C. Silva | 220 | |||
| Sargassum horneri | Methanol extract | 440 | [127] | |
| Sargassum ilicifolium | Hydrolysated protein by alcalase enzyme | 1280 | [128] | |
| Sargassum macrocarpum | Methanol extract (80%) Hexane fraction Chloroform fraction Ethyl acetate fraction Sargachromenol 7-methyl sargachromenol Sargaquinoic acid | 380 790 180 300 0.44 mM 0.37 mM 0.14 mM | [123] | |
| Spyridia filamentosa (Wulfen) Harvey | Ethyl acetate–methanol extracts (pigments were eliminated by means of a first extraction with hexane) | 240 | [73] | |
| Ulva intestinalis | Unfractionated Trypsin protein hydrolysate MW < 3 kDa 3 kDa < MW < 10 kDa MW > 10 kDa | 1590 1140 2190 2530 | [125] |
| Complication | Algae Type | Algae Species | Algal Extraction or Compound | Route of Administration | Dosage | Experimental Period | Animal Model (Age) | Induced by | n/Group | Outcomes and Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aging | n.d. | n.d. | Low-molecular-weight fucoidan (LMWF) in combination with high-stability fucoxanthin (HSFUCO) | Oral | 500 mg/kg when pure compounds or 250 mg/kg LMWF + 250 mg/kg HSFUCO | 28 d | C57BL/6 mice (2 y) | - | 6 | ↓ senescent deterioration (↓ protein expression levels of SOS1 and GRB2, ↑ GSK3, CREB, and IRS1); ameliorated malfunctions of cardiac system in aging mice (↓ cardiac fibrosis, ↑ ventricular rhythm, and ↓ action potential); better muscular function (↑ strength and ↑ muscle endurance) | [135] |
| Carotid atherosclerotic lesions | n.d. | n.d. | Fucoidan | Intraperitoneal injection | 60 mg/kg/day | 4 w | ApoE C57BL/6 mice (6 w) | HFD and high-cholesterol diet | 12 | ↓ lipid levels (↓ TC, LDL cholesterol, and TG); ↓ unstable carotid atherosclerotic plaque formation and lipid disposition; ↑ selective Autophagy (↑ LC3II/LC3I level and ↓ p62 level); ↓ inflammasome activity (↓ IL-1β, ↓ NLRP3, ASC, and caspase-1) | [131] |
| Calcification of heart valves | Macroalgae | n.d. | Fucoidan—Fuco Pets HeartFight® (Hi-Q Marine Biotech International Ltd., New Taipei City, Taiwan) | Oral | 60 mg/kg | 1.5 y | Dogs with already diagnosed heart disease | - | 26 | In combination with medical treatment, ↓ compensatory cardiac enlargement (decreased vertebral heart size) and recovery in echocardiographic parameters (↓ linkage of the mitral valve and tricuspid valve), showing improved overall function of ventricular contraction and relaxation | [136] |
| Inflammation of heart tissues | Macroalgae | Turbinaria ornata | Neophytadiene | Oral | 50 mg/kg/day | 7 d | Sprague Dawley rats (6–8 w) | LPS 10 mg/kg (intraperitoneal) | 6 | Improved hematological parameters (restored WBC, HCT, and PLT); improved serum markers (↓ AST); ↓ oxidative stress markers (↓ MDA; ↑ SOD); ↓ IL-6, IL-10, and PGE2 expression in heart tissue; ↓ inflammatory protein expression in heart tissue (↓ IL-1β; ↓ TNF-α; ↓ iNOS); downregulation of MAPK and NF-κB signaling pathways | [132] |
| Hypertension | Macroalgae | Gracilaria tenuistipitata | Crude neutrase hydrolysate | Oral | 200 mg/kg | 6 w | Spontaneously hypertensive rats | - | 6 | ↓ systolic blood pressure | [133] |
| Hypertension | Microalgae | Bellerochea malleus | Papain protein hydrolysates | Intraperitoneal injection or oral | 75 mg/kg/day (i.p.) or 400 mg/kg/day (oral) | 2 w | Spontaneously hypertensive rats (11–14 w) | - | 4 | ↓ systolic blood pressure | [134] |
| Algae Type | Algae Strain | Type of Sample (Extract and/or Purified Compound) | Anti-Diabetic Activity (IC50 Values in µg/mL Unless Otherwise Stated) | References | |||
|---|---|---|---|---|---|---|---|
| α-amylase Inhibition Activity | α-glucosidase Inhibition Activity | DPP-IV Inhibition | PTP-1B Inhibition | ||||
| Macroalgae | Amphiroa fragilíssima (Linnaeus) J.V. Lamouroux | Ethyl acetate–methanol extracts | 870 | 730 | 50 | [73] | |
| Botryocladia leptopoda | Ethanol extract | 95.8 | 27.3 | [171] | |||
| Caulerpa racemosa | Ethanol extract/fraction Hexane fraction Ethyl acetate fraction | 69.1 88.2 85.8 | 64.2 52.8 80.6 | [84] | |||
| Crude polyphenolic extract | 202.5 | 399.1 | [172] | ||||
| Durvillaea antarctica | Ethanol extract | 473.4 | [173] | ||||
| Acetone extract | 466.0 | ||||||
| Fucus vesiculosus | Conventional extraction | 1.73 | [174] | ||||
| Acetone (67%) extract | 28.8 | 4.5 | [165] | ||||
| Ethyl acetate fraction from extract | 2.8 | 0.82 | |||||
| Gracilaria bursa-pastoris | Methanol extract (Soxhlet) | 800 | 400 | [175] | |||
| Aqueous extract (Maceration) | 900 | 300 | |||||
| Gracilaria canaliculate Sonder | Ethyl acetate–methanol extract | 700 | 650 | 19 | [73] | ||
| Gracilaria corticata (J. Agardh) | 540 | 530 | 60 | ||||
| Gracilaria edulis (Gmelin) Silva | Crude methanol extract Hexane fraction Chloroform fraction Ethyl acetate fraction Aqueous fraction | 349.6 393.1 22.7 279.5 376.5 | 102.2 163.9 122.7 87.9 148.6 | [176] | |||
| Gracilaria salicornia | Ethyl acetate–methanol extracts | 500 | 450 | 30 | [73] | ||
| Halimeda tuna | Ethyl acetate fraction from methanol extract | 870 | 10 | [170] | |||
| Halymenia dilatata Zanardini | Ethyl acetate–methanol extracts | 950 | 820 | 170 | [73] | ||
| Hydropuntia edulis | [“3)-4,6-O-(1-carboxyethylidene)-b-D-galp-(2SO3-)-(1”4)-3,6-a-LAnGalp-(2OMe)-(1”] | 4.44 µM | [167] | ||||
| Hydropuntia edulis (S.G.Gmelin) Gurgel & Fredericq | Ethyl acetate–methanol extracts | 790 | 630 | 60 | [73] | ||
| Lessonia spicata | Ethanol extract Acetone extract | 5317.6 479.2 | [173] | ||||
| Nannochloropsis sp. | Ethyl acetate extract | 122.0 | 178.5 | [177] | |||
| Padina tetrastromatica | 6-methoxy-dolabella-8(17),12-diene-10b,18-diol | 180 | 150 | [166] | |||
| 3-methoxy-dolabella-12(18)-ene-4b-ol | 210 | 200 | |||||
| 3-methoxydolabella- 10,18(19)-diene-5a,8b-diol | 160 | 140 | |||||
| 2,7-dimethoxy-14a-hydroxy-dolasta-1(15),9-diene | 220 | 210 | |||||
| 4,7-dimethoxy-9b,14a-dihydroxy-dolasta-1-ene | 130 | 110 | |||||
| Padina tetrastromatica Hauck | Ethyl acetate–methanol extracts | 450 | 400 | 20 | [73] | ||
| Palisada pedrochei J.N.Norris | 610 | 640 | 80 | ||||
| Portieria hornemannii (Lyngbye) P.C. Silva | 810 | 830 | 80 | ||||
| Pterocladia capillacea | Water extract (Soxhlet) | 62 | [169] | ||||
| Sargassum myriocystum | Methanol (80%) (Soxhlet) | 11.5 | [178] | ||||
| Sphacelaria rigidula | Water extract (Soxhlet) | 13 | [169] | ||||
| Spyridia filamentosa (Wulfen) Harvey | Ethyl acetate–methanol extracts | 770 | 730 | 50 | [73] | ||
| Stoechospermum marginatum | Water extract (Soxhlet) | 151 | [169] | ||||
| Turbinaria ornata | 6, 7-dihydroxy-8-methyl-3-(5′-methyloct-4′-en-1′-yl)-hexahydrocyclooct-1-en-[1, 2-c]furan-11-one (turbinafuranone A) | 0.39 mM | 0.34 mM | 2.58 mM | [168] | ||
| 4-hydroxy-3-isopropyl-7, 8-dimethyl-6-(pentan-2′-acetate)-hexahydrocycloocta-1-en-[1, 2-c]furan 11-one (turbinafuranone B) | 0.31 mM | 0.27 mM | 2.42 mM | ||||
| 6-acetoxy-8-ethyl-5-methoxy-3-(2′-methylhex-4′-en-1′-yl)-pentahydrocycloocta-1, 7-dien-[1, 2-c]furan-11-one (turbinafuranone C) | 0.48 mM | 0.44 mM | 2.77 mM | ||||
| Complication | Algae Type | Algae Species | Algal Extraction or Compound | Route of Administration | Dosage | Experimental Period | Animal Model (Age) | Induced Way | n/Group | Outcomes and Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colon carcinoma | Macroalgae | Ecklonia cava | Ethanol extract | Oral | 250, 500, and 1000 mg/mL | 37 d | BALB/cKorl syngeneic mice (7 w) | 4 × 105 CT26 cells were injected subcutaneously | 7 | ↓ tumor growth (↓ volume and weight); suppression of tumor proliferation; ↑ apoptosis (↑ phosphorylation of members of the MAPK signaling pathway and Bax/Bcl2 signaling pathway); ↓ migration ability of tumor cells; tumor suppressing activity (downregulation of the NF-ΚB signaling pathway) | [220] |
| Ehrlich ascites carcinoma | Macroalgae | Jania rubens | Methanol extract | i.p. injection | 2.3 µg/mouse and 1.2 µg/mouse | 14 d | Swiss albino mice (6–8 w) | 0.25 × 106 EAC cells were i.p. implanted into naïve female Swiss albino mice | 11 | ↓ tumor growth; anti-tumor immunity (↑ immunological response in cancer; immunostimulant of the immune system); ↑ tumor apoptosis (↑ cancerous cells apoptosis, cancerous cell cycle arrest–prevention of cancer progression); ↑ leucocytes (↓ leukocytosis by tumor inoculation); ↑ organ health (restored liver function and integrity, hepaprotective role, ↓ initiation and progression of nephrocellular injury) | [222] |
| Padina pavonica | 2.5 µg/mouse and 1.3 µg/mouse | 10 | |||||||||
| Hepatoma | Macroalgae | Ulva lactuca | Polysaccharide | Oral | 150 and 300 mg/kg | 7 d | Kunming mice (6 w) | H22 cell (108/mL) injection | 9 | Anti-tumor activity (↓ tumor weight); downregulating the expressions of PI3K/Akt and mTOR, and promoting BAX/Bcl-2 ratio; ↓ tumorigenesis (↑ p53, ↓ NF-κB, and ↑ IKKα); direct killing effect on tumor cells (↓ TRAF2/TNF-α); inhibition of tumor proliferation by inhibiting angiogenesis | [221] |
| Skin cancer | n.d. | n.d. | Dieckol | Gavage | 30 mg/kg | 25 w | Swiss albino mice (6–8 w) | DMBA | 6 | Improved body and liver weight; ↓ tumor incidence, volume, number, and burden; ↓ pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α); ↑ antioxidant enzymes (SOD, CAT, GPx, and GSH); ↑ expression of pro-apoptotic protein (p53, Bax, and caspase-3 and -9); inhibition of the NF-ƙB pathway | [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Avni, D.; Varela, J.; Barreira, L. The Ocean’s Pharmacy: Health Discoveries in Marine Algae. Molecules 2024, 29, 1900. https://doi.org/10.3390/molecules29081900
Silva M, Avni D, Varela J, Barreira L. The Ocean’s Pharmacy: Health Discoveries in Marine Algae. Molecules. 2024; 29(8):1900. https://doi.org/10.3390/molecules29081900
Chicago/Turabian StyleSilva, Mélanie, Dorit Avni, João Varela, and Luísa Barreira. 2024. "The Ocean’s Pharmacy: Health Discoveries in Marine Algae" Molecules 29, no. 8: 1900. https://doi.org/10.3390/molecules29081900
APA StyleSilva, M., Avni, D., Varela, J., & Barreira, L. (2024). The Ocean’s Pharmacy: Health Discoveries in Marine Algae. Molecules, 29(8), 1900. https://doi.org/10.3390/molecules29081900