Promising Antileishmanial Activity of Micromeria nervosa Essential Oil: In Vitro and In Silico Studies
Abstract
1. Introduction
2. Results
2.1. Extraction Yield and GC-MS Analysis of M. nervosa EO
2.2. Antioxidant Activity of M. nervosa EO
2.3. Antileishmanial Activity and Cytotoxicity of M. nervosa EO
2.3.1. Antipromastigote Activity
2.3.2. Antiamastigote Activity
2.4. Molecular Mechanism of Action of M. nervosa EO
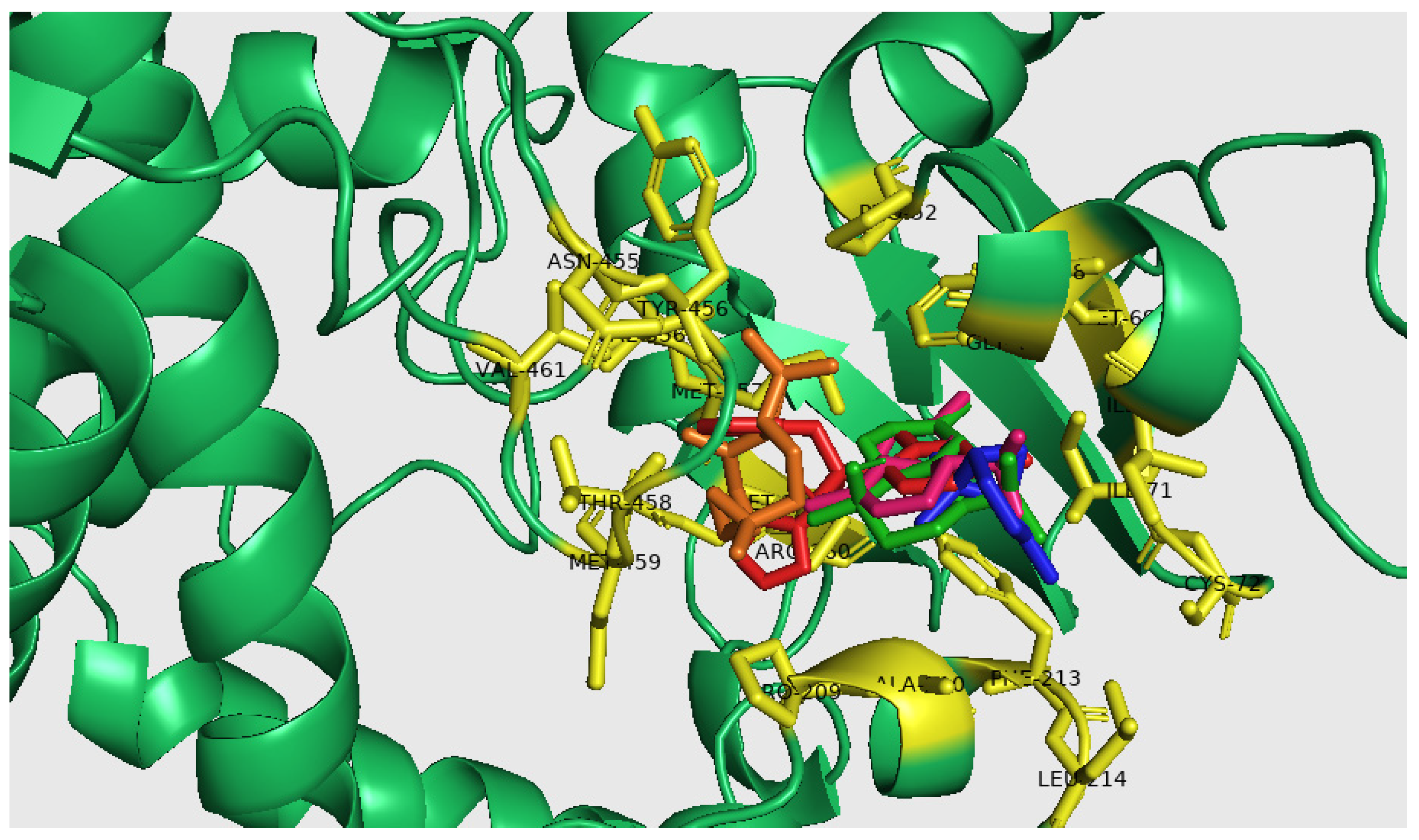
2.5. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Sampling
4.2. Essential oils Extraction
4.3. Chemical Analysis of EO Composition
4.4. Antioxidant Activity
4.4.1. DPPH Radical Scavenging Activity
4.4.2. Beta-Carotene Bleaching Activity
4.5. Antileishmanial Activity
4.5.1. Parasitic Strains
4.5.2. Cultivation of Leishmania Promastigotes
4.5.3. In Vitro Antipromastigote Assay
4.5.4. Antiamastigote Activity
4.6. Assessment of Cytotoxicity
4.7. Quantitative PCR
4.8. Molecular Docking
4.8.1. Preparation of Target Proteins and Ligands
4.8.2. In Silico Study
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagle, A.S.; Khare, S.; Kumar, A.B.; Supek, F.; Buchynskyy, A.; Mathison, C.J.N.; Chennamaneni, N.K.; Pendem, N.; Buckner, F.S.; Gelb, M.H.; et al. Recent developments in drug discovery for Leishmaniasis and human African trypanosomiasis. Chem. Rev. 2014, 114, 11305–11347. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, C.; Giacomin, A.C.; Arossi, K.; Pacheco, L.A.; Hoehne, L.; de Freitas, E.M.; Machado, G.M.d.C.; Cavalheiro, M.M.D.C.; Gnoatto, S.C.B.; Ethur, E.M. Antileishmanial in-vitro activity of essential oil from Myrciaria plinioides, a native species from Southern Brazil. Braz. J. Pharm. Sci. 2019, 55, e17584. [Google Scholar] [CrossRef]
- Cortes, S.; deSousa, C.B.; Morais, T.; Lago, J.; Campino, L. Potential of the natural products against leishmaniasis in Old World—A review of in-vitro studies. Pathog. Glob. Health 2020, 114, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.S.; Amaral, A.C.F.; Lima, E.S.; Silva, J.R.d.A. Chemical composition and biological activities of Bocageopsis multiflora essential oil. J. Essent. Oil Res. 2014, 26, 161–165. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Lajayer, B.A.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Silva, C.E.d.L.d.; Oyama, J.; Ferreira, F.B.P.; Silva, M.P.d.P.; Lordani, T.V.A.; Silva, R.C.d.L.; Monich, M.d.S.T.; Teixeira, J.J.V.; Lonardoni, M.V.C. Effect of essential oils on Leishmania amazonensis: A systematic review. Parasitology 2020, 147, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.; Al-Nuri, M.; Yaghmour, R.M.-R.; Faidi, Y. Antimicrobial activity of Micromeria nervosa from the Palestinian area. J. Ethnopharmacol. 1997, 58, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, F.; Mosaddegh, M.; Motamed, S.M.; Ghorbani, A. Labiatae Family in Folk Medicine in Iran: From Ethnobotany to Pharmacology. Iran. J. Pharm. Res. 2005, 2, 63–79. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie, J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Khdair, I.S.; Soos, I.M.; A Musleh, A.; Isa, B.A.; et al. Traditional knowledge of wilde dibble plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomed. 2008, 4, 13. [Google Scholar] [CrossRef]
- Kefi, S.; Essid, R.; Papetti, A.; Abid, G.; Bouslama, L.; Aouani, E.; Tabbene, O.; Limam, F. Antioxidant, antibacterial, and antileishmanial potential of Micromeria nervosa extracts and molecular mechanism of action of the bioactive compound. J. Appl. Microbiol. 2023, 134, lxad007. [Google Scholar] [CrossRef]
- Pandharkar, T.; Zhu, X.; Mathur, R.; Jiang, J.; Schmittgen, T.D.; Shaha, C.; Werbovetz, K.A. Correction for Pandharkar et al., “Studies on the Antileishmanial Mechanism of Action of the Arylimidamide DB766: Azole Interactions and Role of CYP5122A1”. Antimicrob. Agents Chemother. 2017, 62, e01124-17. [Google Scholar] [CrossRef] [PubMed]
- Eser, M.; Çavuş, I. In Vitro and In Silico Evaluations of the Antileishmanial Activities of New Benzimidazole-Triazole Derivatives. Vet. Sci. 2023, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adversere actions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.R.P.; Júnior, W.V.; Lesche, B.; Coimbra, E.S.; deSouza, N.B.; Abramo, C.; Soares, G.L.G.; Kaplan, M.A.C. Essential oil from leaves of Lantanacamara: A potential source of medicine against leishmaniasis. Rev. Bras. Farm. 2012, 22, 1011–1017. [Google Scholar] [CrossRef]
- Pelkonen, O.; Xu, Q.; Fan, T.-P. Why is Research on Herbal Medicinal Products Important and How Can We Improve Its Quality? J. Tradit. Complement. Med. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Slavkovska, V.; Couladis, M.; Bojovic, S.; Tzakou, O.; Pavlovic, M.; Lakusic, B.; Jancic, R. Essential oil and its systematic significance in species of Micromeria Bentham from Serbia & Montenegro. Plant Syst. Evol. 2005, 255, 1–15. [Google Scholar] [CrossRef]
- Marinković, B.; Marin, P.D.; Knezević-Vukcević, J.; Soković, M.D.; Brkić, D. Activity of essential oils of three Micromeria species (Lamiaceae) against micromycetes and bacteria. Phytother. Res. 2002, 16, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Duru, M.E.; Öztürk, M.; Uğur, A.; Ceylan, Ö. The constituents of essential oil and in vitro antimicrobial activity of Micromeriacilicica from Turkey. J. Ethnopharmacol. 2004, 94, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elwahab, M.F. GC/MS and biological evaluations of the essential oil of Micromeria nervosa (desf) Bentham growing in Egypt. Egypt. J. Pharm. Sci. 2010, 51, 135–147. [Google Scholar]
- Yakoubi, M.; Hamini-Kadar, N.; Gherib, M.; Amrouche, A.; Yezli, W.; Benichou, S.L.; Kihal, M. Inhibitory effect of essential oils from Pulicari amauritanica and Micromeria debilis on growth of Alternaria spp., the causal agent of tomato early blight. Environ. Exp. Biol. 2019, 17, 185–191. [Google Scholar] [CrossRef]
- Bouriah, N.; Bendif, H.; Peron, G.; Miara, M.D.; Dall’acqua, S.; Flamini, G.; Maggi, F. Composition and profiling of essential oil, volatile and crude extract constituents of Micromeria inodora growing in western Algeria. J. Pharm. Biomed. Anal. 2021, 195, 113856. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, G.; Palić, I.; Ursić-Janković, J. Composition and antimicrobial activity of the essential oil of Micromeria cristata and Micromeria juliana. Flavour Fragr. J. 2006, 21, 77–79. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Radnović, D.; Kitić, D.; Zlatković, B.; Ristić, M.; Branković, S. Chemical composition and antimicrobial activity of Sature jahortensis L. essential oil. Cent. Eur. J. Biol. 2009, 4, 411–416. [Google Scholar] [CrossRef]
- Kostadinova, E.; Alipieva, K.; Stefova, M.; Stafilov, T.; Antonova, D.; Evstatieva, L.; Matevski, V.; Kulevanova, S.; Stefkov, G. Chemical composition of the essential oils of three Micromeria species growing in Macedonia and Bulgaria. Maced. J. Chem. Chem. Eng. 2007, 26, 3–7. [Google Scholar]
- Çarikçi, S. The essential oil components of five Micromeria Species grown in Anatolia. BAÜF Bil. Enst. Dergisi Cilt. 2013, 15, 73–79. [Google Scholar]
- Khalid, K.A.; Elsayed, A.A.A.; El-Gohary, A.E.; El-Garf, I.A.; Sabry, R.M. Chemical Composition of Essential Oils Isolated from Aerial Parts of Some Wild Herbs Growing in Arid Regions of Egypt. J. Essent. Oil Bear. Plants. 2021, 24, 1269–1278. [Google Scholar] [CrossRef]
- Baser, K.H.; Kirimer, N.; Tümen, G. Pulegone-Rich Essential Oils of Turkey. J. Essent. Oil Res. 1998, 10, 1–8. [Google Scholar] [CrossRef]
- Alwan, S.; ElOmari, K.; Soufi, H.; Zreika, S.; Sukarieh, I.; Chihib, N.-E.; Jama, C.; Hamze, M. Evaluation of the Antibacterial Activity of Micromeria barbata in Lebanon. J. Essent. Oil Bear. Plants 2016, 19, 321–327. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.F.d.A.; Lacerda, R.d.S.; Correia, J.P.d.A.; de Melo, T.R.; Ferreira, S.B. Potential antibacterial action of α-Pinene. Med. Sci. Forum 2022, 12, 11. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. The rapeutic potential of α-and β-pinene: Amiracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Chien, S.-C.; Wang, S.-Y.; Kuo, Y.-H.; Chang, S.-T. Structure-activity relationships of cadinane-type sesquiterpenederivatives against wood-decay fungi. Holzforschung 2005, 59, 620–627. [Google Scholar] [CrossRef]
- Dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol—A Sesquiterpene Isolated from Casearia sylvestris (Salicaceae)—Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.-H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and anti-acetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crop. Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Rodrigues, K.A.d.F.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; Carvalho, F.A.d.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; García, M.; Pastor, J.; Gil, L.; Scull, R.; Maes, L.; Cos, P.; Gille, L. Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 2014, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Geroldinger, G.; Tonner, M.; Scull, R.; DeSarkar, S.; Bergmann, S.; Bacher, M.; Staniek, K.; Chatterjee, M.; Rosenau, T.; et al. Interaction of ascaridole, carvacrol, and caryophyllene oxide from essential oil of Chenopodium ambrosioides L. with mitochondria in Leishmania and other eukaryotes. Phytother. Res. 2018, 32, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Glumac, M.; Jažo, Z.; Paštar, V.; Golemac, A.; Čulić, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Profiling and Bioactivity Assessment of Helichrysum italicum (Roth) G. Don. Essential Oil: Exploring Pure Compounds and Synergistic Combinations. Molecules 2023, 28, 5299. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.R.; Nakamura, C.V.; Filho, B.P.D.; Ferreira, I.C.P.; Ueda-Nakamura, T. In vitro activity of the essential oil of Cymbopogon citrates and its major component (citral) on Leishmania amazonensis. Parasitol. Res. 2009, 105, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A. Plant-derived compounds in treatment of leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar] [PubMed]
- Jain, S.; Sahu, U.; Kumar, A.; Khare, P. Metabolic Pathways of Leishmania Parasite: Source of Pertinent Drug Targets and Potent Drug Candidates. Pharmaceutics 2022, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Das, S.; Roy, S.; Ghosh, A.K.; Sardar, A.H.; Verma, S.; Saini, S.; Singh, R.; Abhishek, K.; Kumar, A.; et al. Deprivation of L-arginine induces oxidative stress mediated apoptosis in Leishmania donovani promastigotes: Contribution of thepolyamine pathway. PLoS Neglected Trop. Dis. 2016, 10, e0004373. [Google Scholar] [CrossRef] [PubMed]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of Amphotericin B Resistance in Clinical Isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2011, 56, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Abastabar, M.; Keighobadi, M.; Emami, S.; Fakhar, M.; Teshnizi, S.H.; Makimura, K.; Rezaei-Matehkolaei, A.; Mirzaei, H. Promising antileishmanial activity of novel imidazole antifungal drug luliconazole against Leishmania major: In vitro and in silico studies. J. Glob. Antimicrob. Resist. 2018, 14, 260–265. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, J.; Escalona-Arranz, J.C.; Ochoa-Pacheco, A.; Dos Santos, S.G.; González-Fernández, R.; Rojas-Vargas, J.A.; Monzote, L.; Setzer, W.N. Chemical Composition and In Vitro and In Silico Antileishmanial Evaluation of the Essential Oil from Croton linearis Jacq. Stems. Antibiotics 2022, 11, 1712. [Google Scholar] [CrossRef]
- Council of Europe (COE). European Directorate for the Quality of Medicines, 6th ed.; COE: Strasbourg, France, 2007. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th online ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.; Schmeda-Hirschmann, G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf). J. Agric. Food Chem. 2005, 53, 2511–2517. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Bandyopadhyay, S.; Mandal, C.; Chatterjee, M. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol. Int. 2005, 54, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Essid, R.; Damergi, B.; Fares, N.; Jallouli, S.; Limam, F.; Tabbene, O. Synergistic combination of Cinnamomum verum and Syzygium aromaticum treatment for cutaneous leishmaniasis and investigation of their molecular mechanism of action. Int. J. Environ. Health Res. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Muylder, G.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intra cellular amastigotes: Comparison to a promastigotes screen and identification of a host cell-specific hit. PLoS Neglected Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef] [PubMed]
- Delorenzi, J.C.; Attias, M.; Gattass, C.R.; Andrade, M.; Rezende, C.; Pinto, A.d.C.; Henriques, A.T.; Bou-Habib, D.C.; Saraiva, E.M.B. Antileishmanial activity of an indole alkaloid from Peschiera australis. Antimicrob. Agents Chemother. 2001, 45, 1349–1354. [Google Scholar] [CrossRef] [PubMed]


| N° | Volatils Compounds | Ki* | Ki** | M. nervosa (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 939 | 1032 | 26.44 ± 2.55 |
| 2 | Sabinene | 975 | 1123 | 0.14 ± 0.07 |
| 3 | β-pinene | 980 | 1137 | 1.62 ± 0.53 |
| 4 | β-myrcene | 991 | 1154 | 0.29 ± 0.02 |
| 5 | δ-3-Carene | 1011 | 1159 | 1.47 ± 0.52 |
| 6 | m-cymene | 1027 | 1178 | 0.10 ± 0.03 |
| 7 | p-cymene | 1027 | 1180 | 0.16 ± 0.01 |
| 8 | d-l-limonene | 1031 | 1224 | 3.25 ± 0.92 |
| 9 | ɣ-Terpinene | 1058 | 1266 | 0.10 ± 0.04 |
| 10 | Oxyde de linalool | 1069 | 1425 | 0.10 ± 0.02 |
| 11 | L-Linalool | 1098 | 1553 | 2.08 ± 0.72 |
| 12 | α-Campholenal | 1123 | 1592 | 0.68 ± 0.11 |
| 13 | trans-Pinocarveol | 1139 | 1632 | 0.73 ± 0.42 |
| 14 | cis-Verbenol | 1139 | 1679 | 0.51 ± 0.22 |
| 15 | p-Mentha-1,5-dien-8-ol | 1172 | 1738 | 0.68 ± 0.34 |
| 16 | 4-Terpineol | 1178 | 1740 | 0.68 ± 0.32 |
| 17 | L-α-Terpineol | 1178 | 1742 | 0.41 ± 0.22 |
| 18 | Carveol | 1225 | 1790 | 0.21 ± 0.06 |
| 19 | trans-p-Menth-2-ene-1.8 diol | 1268 | 1737 | 0.88 ± 0.52 |
| 20 | α-Copaene | 1321 | 1500 | 1.09 ± 0.13 |
| 21 | β-Bourbonene | 1380 | 1542 | 0.15 ± 0.08 |
| 22 | trans-Caryophyllene | 1494 | 1583 | 1.19 ± 0.19 |
| 23 | Germacrene D | 1510 | 1732 | 1.69 ± 0.62 |
| 24 | α-Cadinene | 1524 | 1752 | 3.79 ± 0.12 |
| 25 | δ-Cadinene | 1526 | 1757 | 1.54 ± 0.14 |
| 26 | trans-Nerolidol | 1564 | 1961 | 0.97 ± 1.11 |
| 27 | Caryophyllene oxide | 1593 | 2025 | 7.73 ± 1.04 |
| 28 | t-Cadinol | 1641 | 2163 | 26.27 ± 2.82 |
| 29 | α-Bisabolol | 1700 | 2232 | 3.77 ± 0.8 |
| Total | 88.92 ± 0.5 |
| EO | IC50 (μg/mL) ±SD | |
|---|---|---|
| DPPH | β-Carotene | |
| M. nervosa | 926.33 ± 2.4 | 489.45 ± 2.7 |
| BHT | 17.34 ± 0.23 | 70 ± 5.50 |
| EO | IC50 ± SD (μg/mL) | LC50 ± SD (μg/mL) | SI | ||
|---|---|---|---|---|---|
| L. major | L. infantum | Raw 264.7 | L. major | L. infantum | |
| M. nervosa | 6.79 ± 0.97 | 5.24 ± 1.64 | 80.26 ± 3.54 | 11.82 | 15.31 |
| AMB | 0.97 ± 0.08 | 0.64 ± 0.24 | 10.62 ± 0.58 | 10.94 | 16.59 |
| EO | IC50 ±SD (μg/mL) | SP | ||
|---|---|---|---|---|
| L. major | L. infantum | L. major | L. infantum | |
| M. nervosa | 8.04 ± 0.5 | 7.32 ± 0.87 | 0.84 | 0.71 |
| AmpB | 0.72 ±0.08 | 0.43 ±0.05 | 1.34 | 1.48 |
| Receptors | Ligands | Binding Energy AG(Kcal/Mol) | Active Site Amino Acids | Interaction Type |
|---|---|---|---|---|
| t-Cadinol | −7.50 | A/ILE. 45, A/PHE. 48, A/MET. 69, A/ILE. 71, A/PRO. 209, A/PHE. 213, A/MET. 357 | Alkyl | |
| A/PHE. 48, A/ILE. 71, A/PHE. 213, A/MET. 357 | Pi-Alkyl | |||
| A/ALA. 210 | Van der waals | |||
| α-Cadinene | −7.30 | A/ILE. 45, A/PHE. 48, A/MET. 69, A/ILE. 71, A/ILE. 76, A/PRO. 209, A/PHE. 213 | Alkyl | |
| A/PHE. 48, A/ILE. 71 | Pi-Alkyl | |||
| A/PHE. 48 | Pi-Segma | |||
| A/GLY. 49 | Van der waals | |||
| Caryophyllene Oxide | −7.00 | A/VAL. 356, A/MET. 357, A/ASN. 455 | Conventional Hydrogen Bond | |
| A/PHE. 48, A/PRO. 52, A/PRO. 209, A/VAL. 356, A/TYR. 456, A/VAL. 461 | Alkyl | |||
| A/PRO. 209, A/VAL. 356 | Pi-Alkyl | |||
| A/THR. 458, A/MET. 459 | Van der waals | |||
| α-Pinene | −5.50 | A/ILE. 45, A/PHE. 48, A/ILE. 71, A/CYS. 72, A/PHE. 213, A/LEU. 214, | Alkyl | |
| A/ILE. 45, A/PHE. 48, A/PHE. 213 | Pi-Alkyl | |||
| A/GLY. 49, A/ALA. 210 | Van der waals | |||
| Fluconazole | −6.90 | A/ARG. 360, A/VAL. 461 | Conventional Hydrogen Bond | |
| A/MET. 357 | Carbon Hydrogen Bond | |||
| A/ILE. 71, A/PRO. 209, A/VAL. 212, A/VAL. 356 | Alkyl | |||
| A/MET. 359 | Pi-Segma | |||
| A/PHE. 48 | Pi-Pi T-shaped | |||
| A/ILE. 45, A/ILE. 76, A/PHE. 213, A/TYR. 456 | Van der waals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essid, R.; Kefi, S.; Damergi, B.; Abid, G.; Fares, N.; Jallouli, S.; Abid, I.; Hussein, D.; Tabbene, O.; Limam, F. Promising Antileishmanial Activity of Micromeria nervosa Essential Oil: In Vitro and In Silico Studies. Molecules 2024, 29, 1876. https://doi.org/10.3390/molecules29081876
Essid R, Kefi S, Damergi B, Abid G, Fares N, Jallouli S, Abid I, Hussein D, Tabbene O, Limam F. Promising Antileishmanial Activity of Micromeria nervosa Essential Oil: In Vitro and In Silico Studies. Molecules. 2024; 29(8):1876. https://doi.org/10.3390/molecules29081876
Chicago/Turabian StyleEssid, Rym, Sarra Kefi, Bilel Damergi, Ghassen Abid, Nadia Fares, Selim Jallouli, Islem Abid, Dina Hussein, Olfa Tabbene, and Ferid Limam. 2024. "Promising Antileishmanial Activity of Micromeria nervosa Essential Oil: In Vitro and In Silico Studies" Molecules 29, no. 8: 1876. https://doi.org/10.3390/molecules29081876
APA StyleEssid, R., Kefi, S., Damergi, B., Abid, G., Fares, N., Jallouli, S., Abid, I., Hussein, D., Tabbene, O., & Limam, F. (2024). Promising Antileishmanial Activity of Micromeria nervosa Essential Oil: In Vitro and In Silico Studies. Molecules, 29(8), 1876. https://doi.org/10.3390/molecules29081876






