Exploring the Pharmacological Potential of Lithospermum officinale L.: A Review of Phytochemicals and Ethnomedicinal Uses
Abstract
1. Introduction
2. Methods
3. Distribution and Botanical Characterization
4. Historical and Cultural Uses of L. officinale L.
5. Phytochemistry
6. Possible Pharmacological Activities of Compounds
6.1. Alkaloids
6.2. Quinones
6.3. Glucosides
6.4. Phenolics
6.5. Flavonoids and Flavonol Glucosides
6.6. Lipids
7. Pharmacological Effects Studies on L. officinale L.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, D.C.; Xiao, P.G. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.N.F.; Williamson, E.M.; Hawkins, J.A. Which plants used in ethnomedicine are characterized? Phylogenetic patterns in traditional use related to research effort. Front. Plant Sci. 2018, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Saslis-Lagoudakis, C.H.; Hawkins, J.A.; Greenhill, S.J.; Pendry, C.A.; Watson, M.F.; Tuladhar-Douglas, W.; Baral, S.R.; Savolainen, V. The evolution of traditional knowledge: Environment shapes medicinal plant use in Nepal. Proc. R. Soc. B Biol. Sci. 2014, 281, 1780. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gangwar, M.; Kumar, D.; Nath, G.; Kumar, S.A.S.; Tripathi, Y.B. Phytochemical characterization, antimicrobial activity and reducing potential of seed oil, latex, machine oil and presscake of Jatropha curcas. Avicenna J. Phytomed. 2016, 6, 366–375. [Google Scholar] [PubMed]
- Abd-Alla, H.I.; Moharram, F.A.; Naturforsch, C.; Gaara, A.H.; ElSafty, M.M. Phytoconstituents of Jatropha curcas L leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Z. Naturforsch C J. Biosci. 2009, 647, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Coppi, A.; Hilger, H.H.; Selvi, F. Non-monophyly of Buglossoides (Boraginaceae: Lithospermeae): Phylogenetic and morphological evidence for the expansion of Glandora and reappraisal of Aegonychon. Taxon 2014, 63, 1065–1078. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdullah, F.O.; Hassan, A.O.; Galali, Y.; Hassan, R.R.; Rashid, E.Q.; Salih, M.I.; Aziz, K.F. Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules 2022, 27, 8687. [Google Scholar] [CrossRef]
- Cohen, J.I. A phylogenetic analysis of morphological and molecular characters of Lithospermum L. (Boraginaceae) and related taxa: Evolutionary relationships and character evolution. Cladistics 2011, 27, 559–580. [Google Scholar] [CrossRef]
- Lithospermum officinale L.|Plants of the World Online|Kew Science. (n.d.). Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:118163-1 (accessed on 28 February 2024).
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.; Kanthaliya, B.; Joshi, A.; Khan, F.; Arora, J. Biologia futura: Medicinal plants-derived bioactive peptides in functional perspective-a review. Biol. Futur. 2020, 71, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.I.; Chowdhury, A.B.M.A.; Shahjahan, M.; Harun, M.G.D. Traditional healing practices in rural Bangladesh: A qualitative investigation. BMC Complement. Altern. Med. 2018, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Louis, V.R.; Phalkey, R.K.; Saurborn, R. Use of traditional medicines to cope with climate-sensitive diseases in a resource poor setting in Bangladesh. BMC Public. Health. 2014, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, K.; Husen, E. Knowledge and self-reported practice of the local inhabitants on traditional insect repellent plants in Western Hararghe zone, Ethiopia. J. Ethnopharmacol. 2012, 141, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Tugume, P.; Nyakoojo, C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement. Altern. Med. 2019, 19, 353. [Google Scholar] [CrossRef]
- Ghorbani, A. Clinical and experimental studies on polyherbal formulations for diabetes: Current status and future prospective. J. Intergr Med. 2014, 12, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Ansari, M.R.; Alipour, A.A.; Ahmadipour, S.H.; Saferi-Faramani, R.; Vaikili, J. A review of medicinal herbs that affects the kidney and bladder dtoned of children and adults in traditional medicine and ethnobotany of Iran. World Appl. Sci. J. 2012, 18, 600–604. [Google Scholar]
- Rabearivony, A.D.; Kuhlman, A.R.; Razafiarison, Z.L.; Raharimalala, F.; Rakotoarivory, F.; Randrianarivory, T.; Rakotoarivelo, N.; Randrianasolo, A.; Bussmann, R.W. Ethnobotanical study of the medicinal plants known by men in Ambalabe, Madagascar. Ethnobot. Res. Appl. 2015, 14, 123–138. [Google Scholar] [CrossRef]
- Tugume, P.; Kakudidi, E.K.; Buyinza, M.; Namaalwa, J.; Kamatenesi, M.; Mucunguzi, P.; Kalema, J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Thiem, B.; Hermosaningtyas, A.A.; Budzianowska, A.; Kikowska, M. Polish contributions in developing medicinal plant in vitro propagation system. Plant Cell Tissue Organ Cult. 2023, 155, 1–28. [Google Scholar] [CrossRef]
- Ahmad, M.; Varela, A.A.; Koletti, A.E.; Rodić, N.; Reichelt, M.; Rödel, P.; Assimopoulou, A.N.; Paun, O.; Declerck, S.; Schneider, C.; et al. Dynamics of alkannin/shikonin biosynthesis in response to jasmonate and salicylic acid in Lithospermum officinale. Sci. Rep. 2022, 12, 17093. [Google Scholar] [CrossRef] [PubMed]
- Tang, C. Exploring the evolutionary process of alkannin/shikonin O-acyltransferases by a reliable Lithospermum erythrorhizon genome. DNA Res. 2021, 28, dsab015. [Google Scholar] [CrossRef] [PubMed]
- Auber, R.P.; Suttiyut, T.; McCoy, R.M.; Ghaste, M.; Crook, J.W.; Pendleton, A.L.; Widhalm, J.R.; Wisecaver, J.H. Hybrid de novo genome assembly of red gromwell (Lithospermum erythrorhizon) reveals evolutionary insight into shikonin biosynthesis. Hortic. Res. 2020, 7, 82. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, C.; He, F.; Li, Z.; Guan, Y.; Hu, Q.; Hu, J. Involvement of LeMDR, an ATP-binding cassette protein gene, in shikonin transport and biosynthesis in Lithospermum erythrorhizon. BMC Plant Biol. 2017, 17, 2017. [Google Scholar] [CrossRef]
- Al-Snai, A.E. Chemical constituents and pharmacological effects of Lithospermum officinale. IOSR J. Pharm. 2019, 9, 12–21. [Google Scholar]
- Flora of Pakistan, Lithospermum officinale. Available online: https://www.efloras.org/florataxon.aspx?flora_id=5&taxon_id=200019088 (accessed on 2 February 2024).
- Fernando, W.G. An International Scientific Open Access Journal to Publish All Facets of Plants, Their Functions and Interactions with the Environment and Other Living Organisms. Plants 2012, 1, 1–5. [Google Scholar] [CrossRef]
- Davidson-Hunt, I. Ecological ethnobotany: Stumbling toward new practices and paradigms. MASA J. 2000, 16, 1–13. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Belhouala, K.; Benarba, B. Medicinal Plants Used by Traditional Healers in Algeria: A Multiregional Ethnobotanical Study. Front. Pharmacol. 2021, 12, 760492. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Sharma, R.; Singh, A.; Attri, S.; Arora, S.; Kaur, S.; Bedi, N. Pharmacological and analytical aspects of alkannin/shikonin and their derivatives: An update from 2008 to 2022. Chin. Herb. Med. 2022, 14, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Baczyńska, B.; Lityńska-Zając, M. Application of Lithospermum officinale L. in early Bronze Age medicine. Veg. Hist. Archaeobot. 2005, 14, 77–80. [Google Scholar] [CrossRef]
- Jiang, H.E.; Li, X.; Liu, C.J.; Wang, Y.F.; Li, C.S. Fruits of Lithospermum officinale L. (Boraginaceae) used as an early plant decoration (2500 years BP) in Xinjiang, China. J. Archaeol. Sci. 2007, 34, 167–170. [Google Scholar] [CrossRef]
- Mollaei, S.; Khanehbarndaz, O.; Gerami-Khashal, Z.; Ebadi, M. Molecular identification and phytochemical screening of endophytic fungi isolated from Lithospermum officinale L. roots: A new source of shikonin. Phytochemistry 2019, 168, 112116. [Google Scholar] [CrossRef] [PubMed]
- Winterhoff, H. Lithospermum Species. In Adverse Effects of Herbal Drugs; De Smet, P.A.G.M., Keller, K., Hänsel, R., Chandler, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Sci. Bus. Media LLC.: Berlin/Heidelberg, Germany, 2007; p. 380. [Google Scholar]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Cores, Á.; Carmona-Zafra, N.; Clerigué, J.; Villacampa, M.; Menéndez, J.C. Quinones as Neuroprotective Agents. Antioxidants 2023, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Amirkhanova, A.S.; Ustenove, G.O. Review of the current status of study Oxytropis. Asian J. Pharm. Clin. Res. 2018, 11, 50–55. [Google Scholar] [CrossRef]
- Shynykul, Z. Comparative Evaluation of Chemical Carbon Acid Extract of the Ordinary Harmala (Peganum harmala) in Central Asia Region. Asian J. Plant Sci. 2022, 21, 574–581. [Google Scholar] [CrossRef]
- Shegebayev, Z.; Turgumbayeva, A.; Datkhayev, U.; Zhakipbekov, K.; Kalykova, A.; Kartbayeva, E.; Beyatli, A.; Tastambek, K.; Altynbayeva, G.; Dilbarkhanov, B.; et al. Pharmacological Properties of Four Plant Species of the Genus Anabasis, Amaranthaceae. Molecules 2023, 28, 4454. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, S.; Maric, G.; Cvetinovic, N.; Lepojevic-Stefanovic, D.; Bozic Cvijan, B. Anti-Inflammatory Effects of Lipid-Lowering Drugs and Supplements-A Narrative Review. Nutrients 2023, 15, 1517. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, E.; Mousavi, A.; Farhadpour, M.; Ghashghaie, J.; Ghanati, F.; Haghbeen, K. Pyrrolizidine Alkaloids-Free Extract from the Cell Culture of Lithospermum officinale with High Antioxidant Capacity. Appl. Biochem. Biotechnol. 2018, 187, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Dinica, R.M.; Sandu, C.; Botezatu, A.V.D.; Busuioc, A.C.; Balanescu, F.; Mihaila, M.D.I.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from valuable Romanian animal and plant sources with promising anti-inflammatory activity as a nutricosmetic ingredient. Sustainability 2021, 13, 10170. [Google Scholar] [CrossRef]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci. Rep. 2021, 11, 16164. [Google Scholar] [CrossRef] [PubMed]
- Sripathi, S.K.; Lalitha, P. Keratolytic Molecule Aided Inhibition of DNA Damage and Tyrosinase Activity of a Herbal Formulation. Int. J. BioSci. Technol. 2016, 9, 7. [Google Scholar]
- Krenn, L.; Wiedenfeld, H.; Roeder, E. Pyrrolizidine alkaloids from Lithospermum officinale. Phytochemistry 1994, 37, 275–277. [Google Scholar] [CrossRef]
- Ahmad, L.; He, Y.; Semotiuk, A.J.; Liu, Q.R.; Hao, J.C. Survey of pyrrolizidine alkaloids in the tribe Lithospermeae (Boraginaceae) from Pan-Himalaya and their chemotaxonomic significance. Biochem. Syst. Ecol. 2018, 81, 49–57. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, L.; Zheng, Q.; Han, H.; Li, Z.; Zhang, X.; Chen, H. Combined Hepatotoxicity and Toxicity Mechanism of Intermedine and Lycopsamine. Toxins 2022, 14, 633. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Ohno, A.; Saito, Y.; Satake, M. Accelerative effect of shikonin, alkannin and acetylshikonin on the proliferation of granulation tissue in rats. Biol. Pharm. Bull. 1994, 17, 1075–1077. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Yang, L.; Guo, R. Alkannin reverses lipopolysaccharides-induced inflammatory responses by suppressing mitogen-activated protein kinase and nuclear factor kappa-B signalling. Bioengineered 2022, 13, 14936–14946. [Google Scholar] [CrossRef] [PubMed]
- Valipour, M. Recent advances of antitumor shikonin/alkannin derivatives: A comprehensive overview focusing on structural classification, synthetic approaches, and mechanisms of action. Eur. J. Med. Chem. 2022, 235, 114314. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef] [PubMed]
- Lupescu, A.; Bissinger, R.; Jilani, K.; Lang, F. In Vitro induction of Erythrocyte Phosphatidylserine Translocation by the Natural Naphthoquinone Shikonin. Toxins 2014, 6, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, M.; Hao, C.; Yang, W. Shikonin Induces Tumor Apoptosis in Glioma Cells via Endoplasmic Reticulum Stress, and Bax/Bak Mediated Mitochondrial Outer Membrane Permeability. J. Ethnopharmacol. 2020, 263, 113059. [Google Scholar] [CrossRef] [PubMed]
- Haghbeen, K.; Mozaffarian, V.; Ghaffari, F.; Pourazeezi, E.; Saraji, M.; Joupari, M.D. Lithospermum officinale callus produces shikalkin. Biologia 2006, 61, 463–467. [Google Scholar] [CrossRef]
- Ulbrich, B.; Wiesner, W.; Arens, H. Large-Scale Production of Rosmarinic Acid from Plant. In Primary and Secondary Metabolism of Plant Cell Cultures: Part 1: Papers from a Symposium Held in Rauischholzhausen, Germany in 1981; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; p. 293. [Google Scholar]
- Konstantinou, E.K.; Panagiotopoulos, A.A.; Argyri, K.; Panoutsopoulos, G.I.; Dimitriou, M.; Gioxari, A. Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review. Nutrients 2023, 16, 2. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Burczyk, J.; Stolarczyk, A.; Wojtusiak, A.; Bilińska, B. Time- and dose-dependent antigonadotropic activity of oxidation products of gallic acid and pyrogallol on Leydig cells in vitro. Cytobios 1996, 86, 7–16. [Google Scholar] [PubMed]
- Liu, X.; Chen, R.; Shang, Y.; Jiao, B.; Huang, C. Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats. Chem. Biol. Interact. 2008, 176, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 588. [Google Scholar] [CrossRef]
- Cho, Y.C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, Y.; Duan, S.; Sun, D. The Antialgal Mechanism of Luteolin-7-O-Glucuronide on Phaeocystis globosa by Metabolomics Analysis. Int. J. Environ. Res. Public. Health 2019, 16, 3222. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, A.; Ramachandran, J.; Lakshmanan, D.K.; Ravichandran, G.; Thilagar, S. Ethnomedical, phytochemical and pharmacological insights on an Indian medicinal plant: The balloon vine (Cardiospermum halicacabum Linn.). J. Ethnopharmacol. 2022, 291, 115143. [Google Scholar] [CrossRef] [PubMed]
- Adamtsevich, N.Y.; Zakrzheuskaya, Y.I.; Feskova, E.V.; Leontiev, V.N.; Titok, V.V. Development and Validation of a Method to Quantify Flavonoids in Leaves of Lithospermum officinale (Boraginaceae). Dokl. Biol. Sci. 2023, 512, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki, D.S.; Rahbar, S.Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani, J.N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Lyashenko, S.; Yunusova, S.; López-Ruiz, R.; Vasfilova, E.; Kiseleva, O.; Chimitov, D.; Bahanova, M.; Bojko, N.; Guil-Guerrero, J.L. Lipid fractions, fatty acid profiles, and bioactive compounds of Lithospermum officinale L. Seeds. J. Am. Oil Chem. Soc. 2021, 98, 425–437. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Andreoli, M.J.; Nascimento, G.R.; Colquhoun, A. Gamma-Linolenic acid alters migration, proliferation and apoptosis in human and rat glioblastoma cells. Prostaglandins Other Lipid Mediat. 2020, 150, 106452. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Haider, A.; Mahmood, W.; Roome, T.; Abbas, G. Gamma-linolenic acid ameliorated glycation-induced memory impairment in rats. Pharm. Biol. 2017, 55, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.G.; Jebb, S.A.; Calder, P.C. Stearidonic acid as a supplemental source of ω-3 polyunsaturated fatty acids to enhance status for improved human health. Nutrition 2013, 29, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J. Dietary stearidonic acid is a long chain (n−3) polyunsaturated fatty acid with potential health benefits. J. Nutr. 2009, 139, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Belina-Aldemita, M.D.; Schreiner, M.; D’Amico, S. Characterization of phenolic compounds and antioxidative potential of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. J. Food Biochem. 2020, 44, e13102. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): An overview. Food Res. Int. 2011, 44, 1830–1836. [Google Scholar] [CrossRef]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–461. [Google Scholar] [CrossRef]
- Kuchta, K.; Schmidt, M. Safety of medicinal comfrey cream preparations (Symphytum officinale s.l.): The pyrrolizidine alkaloid lycopsamine is poorly absorbed through human skin. Regul. Toxicol. Pharmacol. 2020, 118, 104784. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Johkura, K.; Ogiwara, N.; Zhao, X.; Cui, L.; Iida, I.; Okouchi, Y.; Asanuma, K.; Sasaki, K. Morphological analysis of leucocyte transmigration in the pleural cavity. J. Anat. 2003, 203, 391–404. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.H.; Meng, Q.Q.; Li, S.S.; Zhou, W.; Xiao, S. Advance in Anti-tumor Mechanisms of Shikonin, Alkannin and their Derivatives. Mini Rev. Med. Chem. 2018, 18, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Sosa, A.; Winternitz, F.; Wylde, R.; Pavia, A. Structure of a cyanoglucoside of Lithospermum purpureo-caeruleum. Phytochemistry 1977, 16, 707–709. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kheyrollah, M.; Farhadpour, M.; Sabouni, F.; Haghbeen, K. Neuroprotective effect of Lithospermum officinale callus extract on inflamed primary microglial cells. Curr. Pharm. Biotechnol. 2023, 25, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Mohtasham, A.Z.; Tanideh, N.; Seddighi, A.; Mokhtari, M.; Amini, M.; Shakouri, P.A.; Manafi, A.; Hashemi, S.S.; Mehrabani, D. The Effect of Lithospermum officinale, Silver Sulfadiazine and Alpha Ointments in Healing of Burn Wound Injuries in Rat. World J. Plast. Surg. 2017, 6, 313–318. [Google Scholar]
- Winterhoff, H.; Sourgens, H.; Kemper, F.H. Antihormonal effects of plant extracts. Pharmacodynamic effects of lithospermum officinale on the thyroid gland of rats; comparison with the effects of iodide. Horm. Metab. Res. 1983, 15, 503–507. [Google Scholar] [CrossRef]
| Keywords | Number of Searches | |
|---|---|---|
| Google Scholar | PubMed | |
| L. officinale L. | 13,100 | 48 |
| L. officinale L. compounds | 3210 | 3 |
| L. officinale L. phytochemicals | 1560 | 2 |
| L. officinale L. pharmacological | 2840 | 6 |
| L. officinale L. traditional uses | 4910 | — |
| Country | Plant Part | Ethnomedicinal Use(s) | Findings | References |
|---|---|---|---|---|
| Poland | fruit | nuts of L. officinale L. were utilized as an antiseptic | 1. from about 1750 to 1600 B.C.; 2. a plaster made of tar with the fruit of L. officinale L. | [34] |
| China | fruit | 1. a treatment for urogenital tract disorders and as a medication for relaxing spasms; 2. utilized as an ancient form of plant adornment | 1. 2500 years BP; 2. fruit from L. officinale L. were found adhered to two wooden tubs in the Yanghai Tombs of Xinjiang, China. | [35,36] |
| North America (by Indians) | root | 1. antidiarrhoeal drug; 2. oral contraceptives made from cold water extracts of the root. | saline extract of root | [37,38] |
| India, Spain | leaves | used as sedative | ||
| India | seeds | used as diuretic and lithotriptic | ||
| India | roots and twigs | utilized as a remedy, a decoction of roots and twigs was administered as a syrup for eruptive diseases like smallpox and measles. |
| Chemical Class | Compound | Structure | Known Pharmacological Activities |
|---|---|---|---|
| Alkaloids (pyrrolizidine alkaloids) | allantoin [48] | 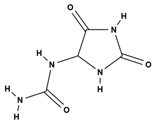 | antinociceptive and anti-inflammatory [49], wound healing [50], keratolytic [51] |
| lithosenine [52] | 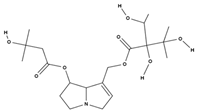 | — | |
| acetyllithosenine [52] | 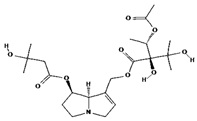 | — | |
| lycopsamine [53] | 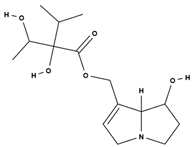 | toxic and potentially carcinogenic due to its hepatotoxicity [54] | |
| echimidine [53] | 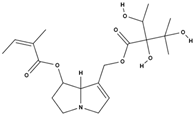 | toxic and potentially carcinogenic due to its hepatotoxicity [54] | |
| Quinones (naphthoquinones) | alkannin [55] | 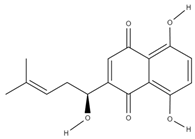 | anti-inflammatory, antimicrobial, antioxidant, wound healing, anticancer [56] |
| shikonin [57] | 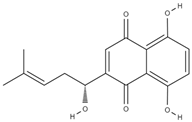 | anti-inflammatory, antioxidant, antimicrobial, wound healing, anticancer, antidiabetic, antiallergic [58,59,60] | |
| Phenolics (phenolic acids) | rosmarinic acid [48,61,62] | 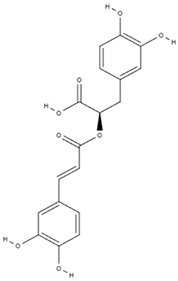 | anticancer [63], anti-inflammation, antioxidation, antidiabetes, antivirus, antitumor, neuroprotection, hepatoprotection [64] |
| lithospermic acid [65] | 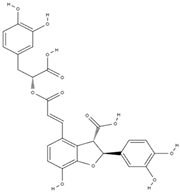 | antioxidant, anti-inflammatory, antimicrobial, anticancer, cardioprotective, neuroprotective [66] | |
| caffeic acid [38] | 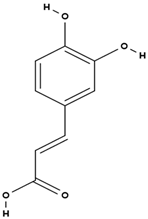 | antioxidant, anti-inflammatory, antimicrobial, anticancer (inhibits), neuroprotective, cardioprotective [67] | |
| chlorogenic acid [38] | 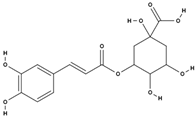 | antioxidant, anti-inflammatory, weight loss, antidiabetic, neuroprotective, cardioprotective [67] | |
| Flavonoids | luteolin-7 beta-glucuronide [38] | 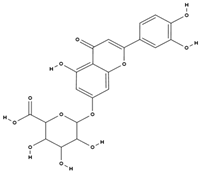 | antioxidant, anti-inflammatory, antiallergic, neuroprotective, cardioprotective [68,69,70] |
| Flavonoids | rutin [71] | 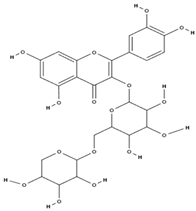 | antioxidant, anti-inflammatory, antidiabetic, vasoprotective, neuroprotective, cardioprotective [72] |
| Lipids (fatty acids) | γ-linolenic acid [73] | 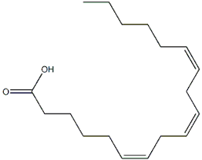 | anti-inflammatory, skin health, hormonal balance, immune system support [74,75,76] |
| stearidonic acid [73] | 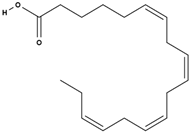 | anti-inflammatory, skin health, hormonal balance, immune system support [77,78] | |
| Δ5-avenasterol [73] | 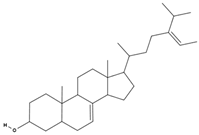 | cholesterol-lowering, anti-inflammatory, immune modulation, anticancer, skin health [79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkizatova, G.; Turgumbayeva, A.; Zhakipbekov, K.; Bekesheva, K.; Arystanov, Z.; Arystanova, T.; Kayupova, F.; Zhumalina, K.; Toxanbayeva, Z.; Ibragimova, A.; et al. Exploring the Pharmacological Potential of Lithospermum officinale L.: A Review of Phytochemicals and Ethnomedicinal Uses. Molecules 2024, 29, 1856. https://doi.org/10.3390/molecules29081856
Barkizatova G, Turgumbayeva A, Zhakipbekov K, Bekesheva K, Arystanov Z, Arystanova T, Kayupova F, Zhumalina K, Toxanbayeva Z, Ibragimova A, et al. Exploring the Pharmacological Potential of Lithospermum officinale L.: A Review of Phytochemicals and Ethnomedicinal Uses. Molecules. 2024; 29(8):1856. https://doi.org/10.3390/molecules29081856
Chicago/Turabian StyleBarkizatova, Gulzhanat, Aknur Turgumbayeva, Kairat Zhakipbekov, Kuralay Bekesheva, Zhalgaskali Arystanov, Tanagul Arystanova, Farida Kayupova, Klara Zhumalina, Zhanat Toxanbayeva, Aigul Ibragimova, and et al. 2024. "Exploring the Pharmacological Potential of Lithospermum officinale L.: A Review of Phytochemicals and Ethnomedicinal Uses" Molecules 29, no. 8: 1856. https://doi.org/10.3390/molecules29081856
APA StyleBarkizatova, G., Turgumbayeva, A., Zhakipbekov, K., Bekesheva, K., Arystanov, Z., Arystanova, T., Kayupova, F., Zhumalina, K., Toxanbayeva, Z., Ibragimova, A., Blinova, O., Utegenova, G., Iztileu, N., & Shynykul, Z. (2024). Exploring the Pharmacological Potential of Lithospermum officinale L.: A Review of Phytochemicals and Ethnomedicinal Uses. Molecules, 29(8), 1856. https://doi.org/10.3390/molecules29081856






