Chamaecyparis lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle
Abstract
1. Introduction
2. Results
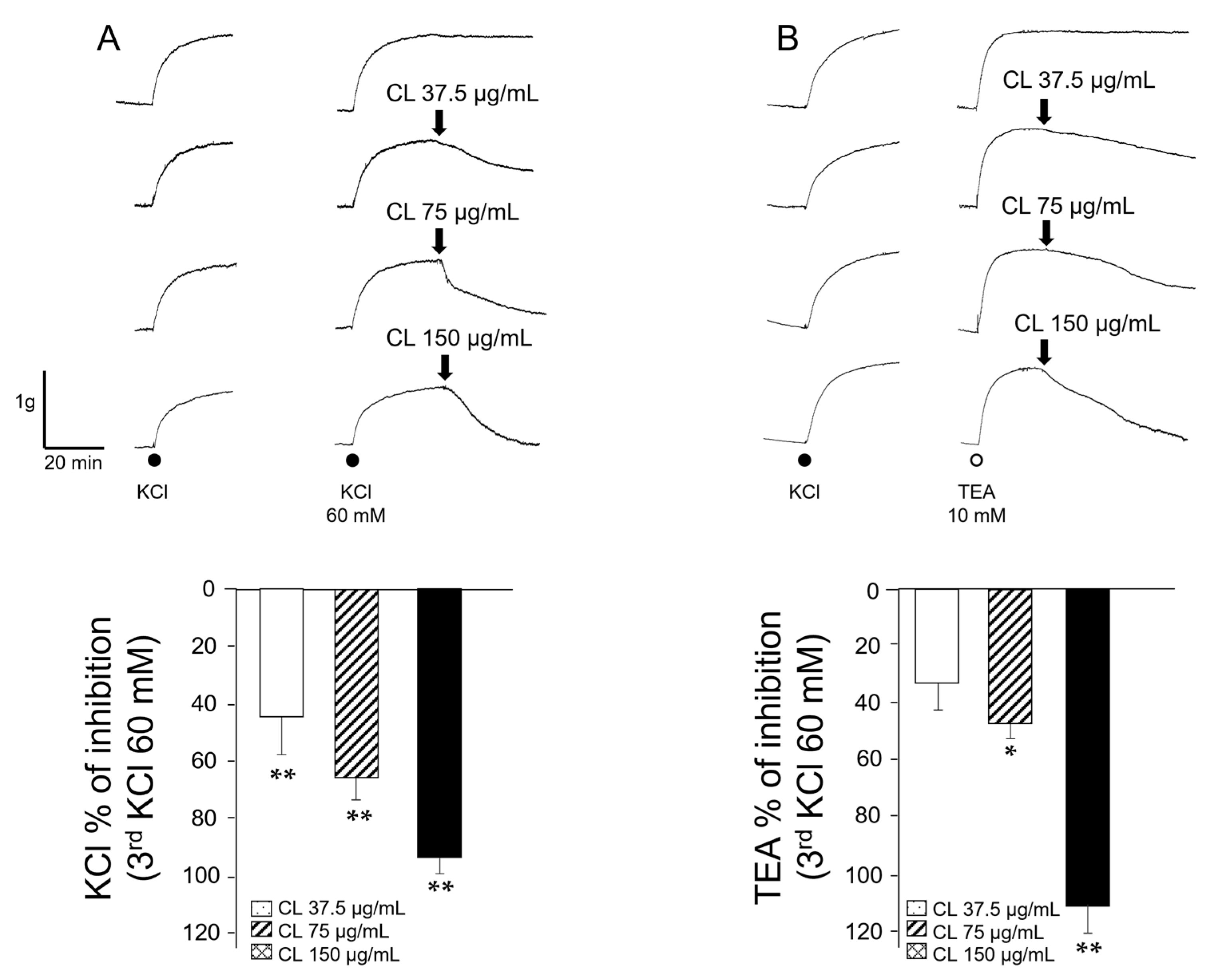
2.1. C. lawsoniana Methanolic (MeOH) Extract Diminishes KCl and TEA-Induced Contractions in Guinea Pig Trachea
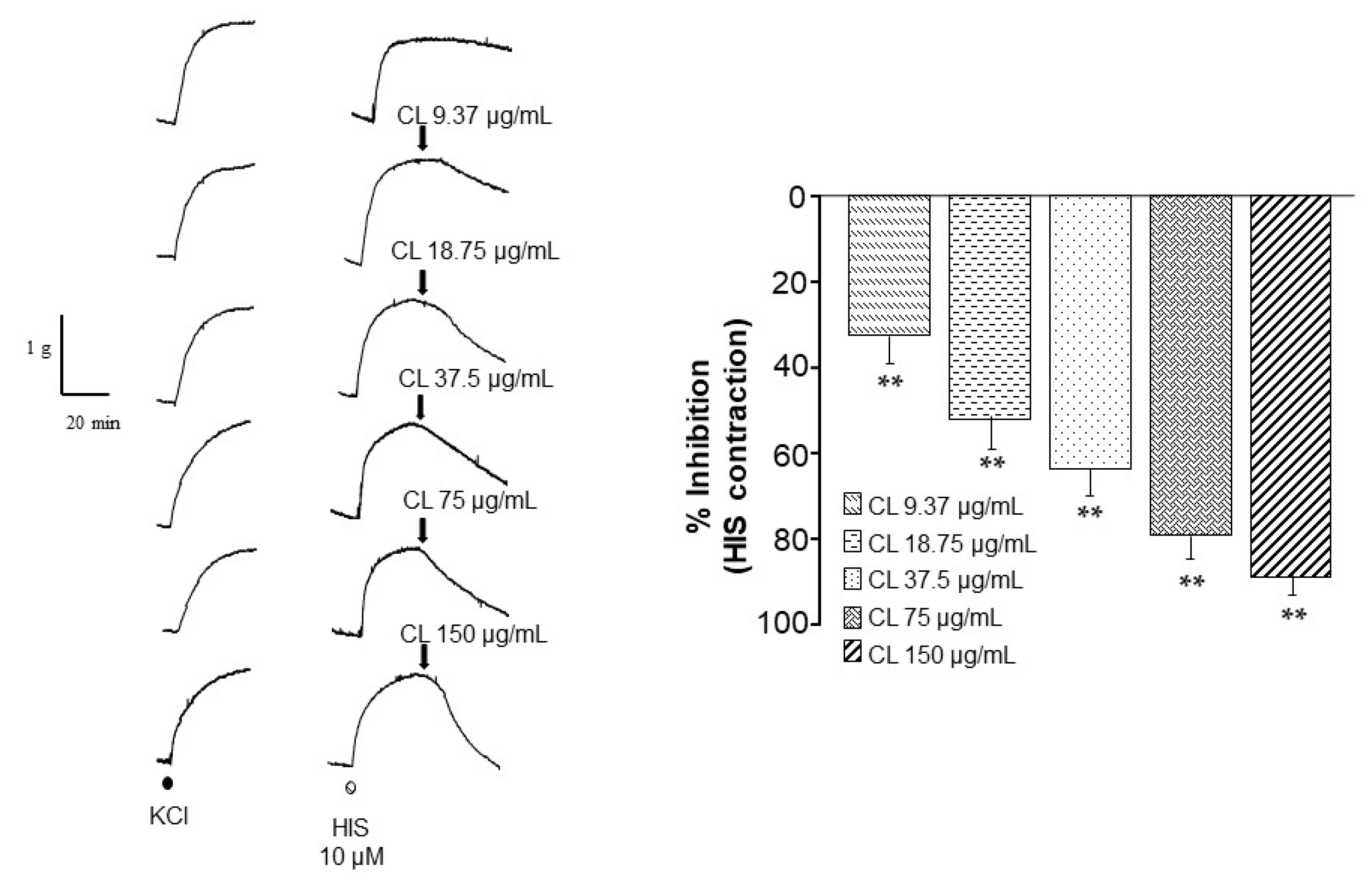
2.2. C. lawsoniana Significantly Lowers Histamine-Induced Contraction in Tracheal Preparations
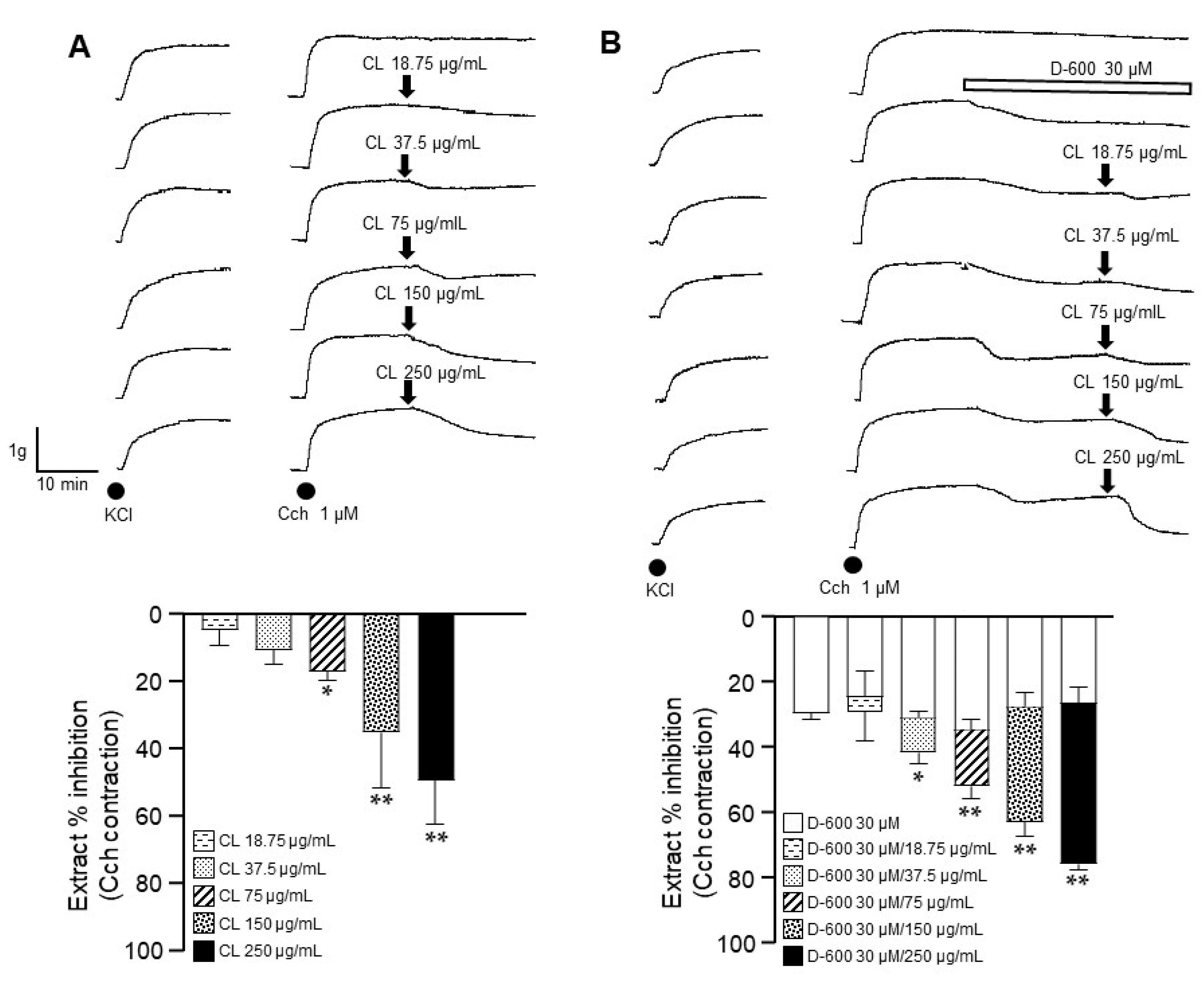
2.3. Store-Operated Ca2+ Channels Are Blocked by C. lawsoniana Methanolic Extract
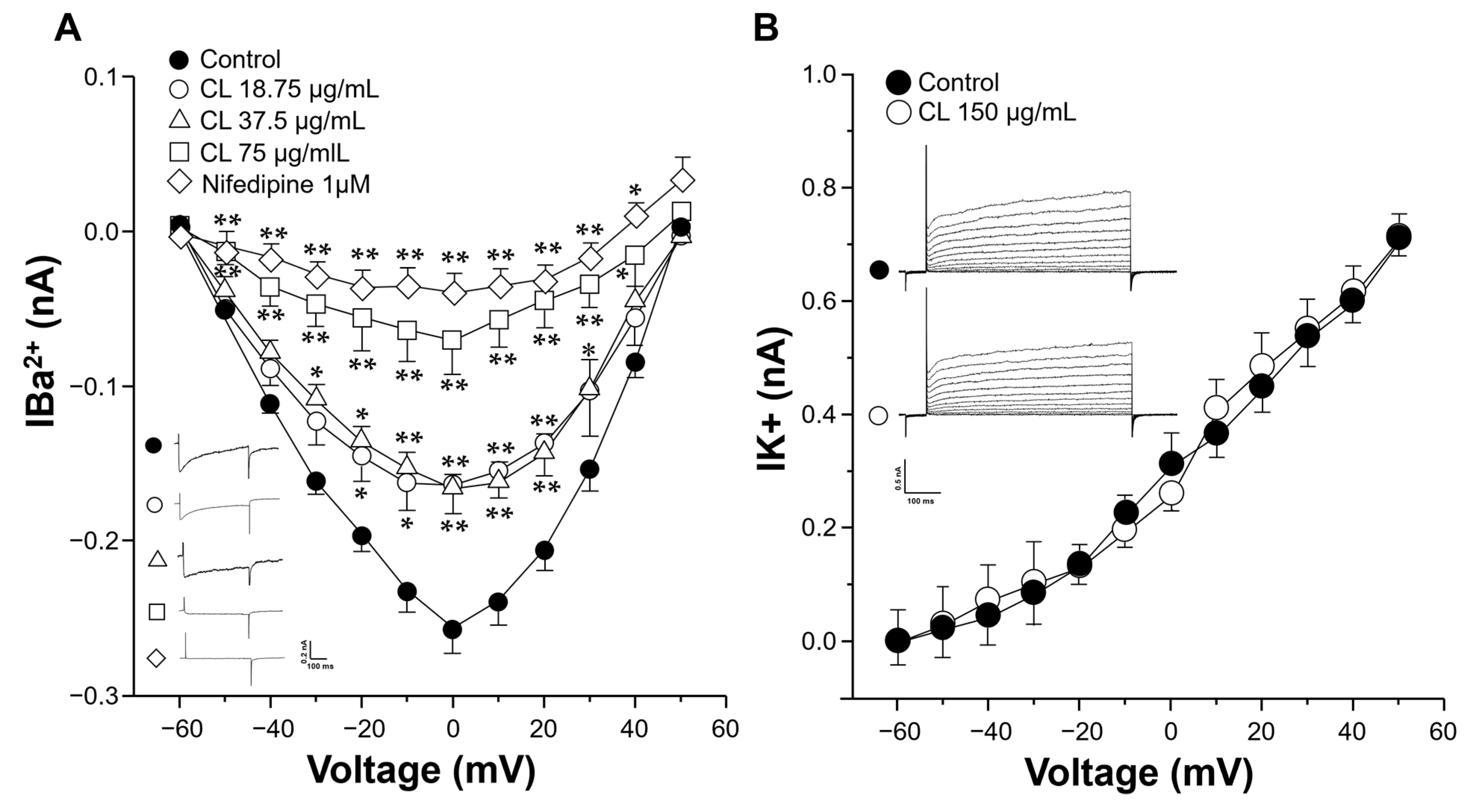
2.4. C. lawsoniana Blocks L-Type Ca2+ Currents but Not K+ Currents in Tracheal Myocytes
2.5. C. lawsoniana Blocks L-Type Voltage-Dependent Ca2+ Channels and Store-Operated Ca2+ Channels Diminishing Intracellular Ca2+ Concentration in Tracheal Myocytes
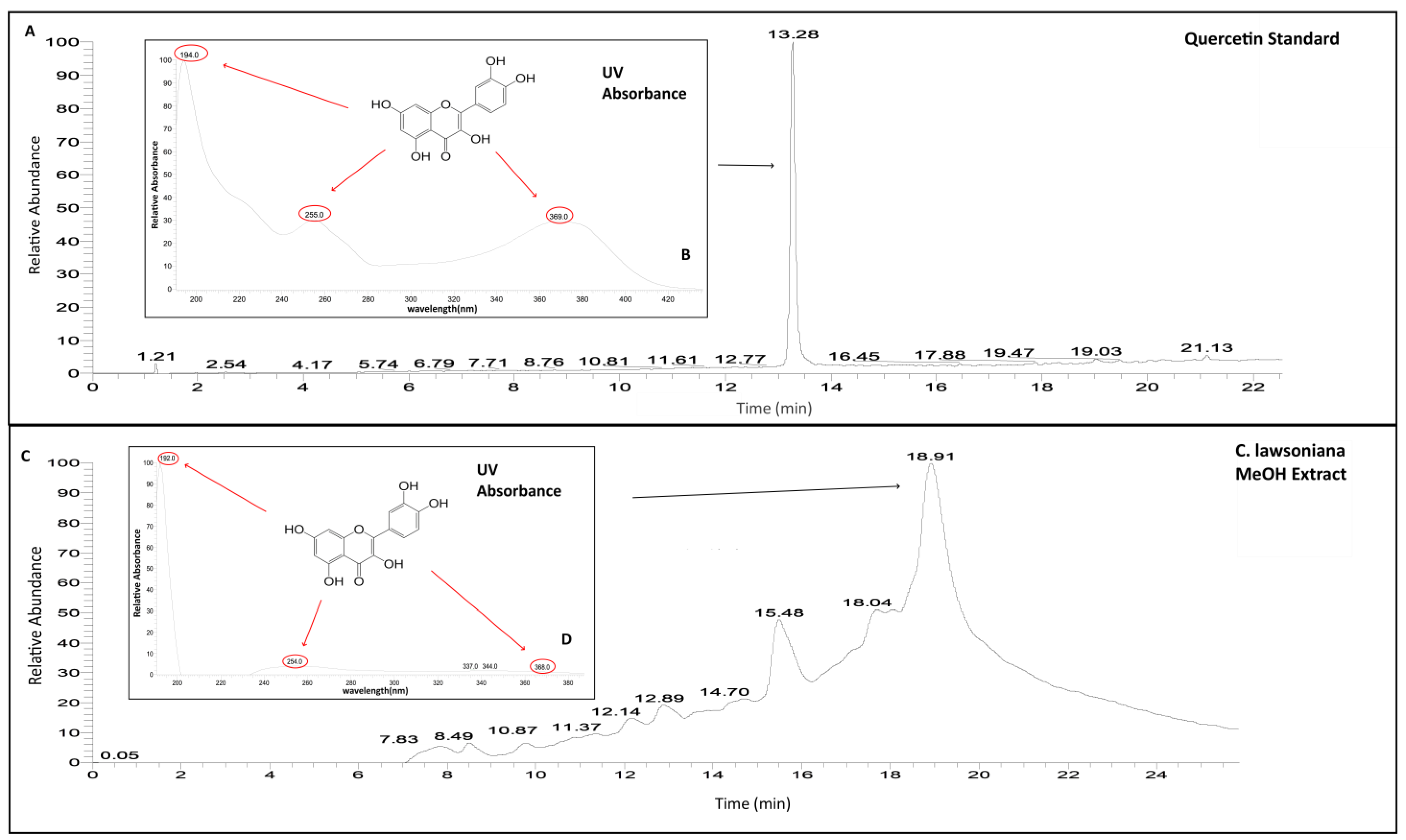
2.6. Phytochemical Composition of C. lawsoniana Methanolic Extract
2.7. Quercetin Inhibits the Contraction of the Airway Smooth Muscle
2.8. Quercetin Blocks the Carbachol-Induced Ca2+ Plateau in Tracheal Myocytes
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Methanolic Extraction Procedure
4.3. Quercetin Identification from C. lawsoniana MeOH Extract
4.4. Animals
4.5. Organ Bath Studies
4.6. Patch Clamp Recordings
4.7. Intracellular Ca2+ Measurements in Tracheal Myocytes
4.8. Drugs and Reagents
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trnjar, K.; Pintarić, S.; Mornar Jelavić, M.; Nesek, V.; Ostojić, J.; Pleština, S.; Šikić, A.; Pintarić, H. Correlation Between Occurrence and Deterioration of Respiratory Diseases and Air Pollution Within the Legally Permissible Limits. Acta Clin. Croat. 2017, 56, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Fokunang, C.; Ndikum, V.; Tabi, O.; Jiofack, R.; Ngameni, B.; Guedje, N.; Tembe-Fokunang, E.; Tomkins, P.; Barkwan, S.; Kechia, F. Traditional Medicine: Past, Present and Future Research and Development Prospects and Integration in the National Health System of Cameroon. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Jules, E.S.; Kauffman, M.J.; Ritts, W.D.; Carroll, A.L. Spread of an invasive pathogen over a variable landscape: A nonnative root rot on port orford cedar. Ecology 2002, 83, 3167–3181. [Google Scholar] [CrossRef]
- Park, Y.; Jung, S.M.; Yoo, S.A.; Kim, W.U.; Cho, C.S.; Park, B.J.; Woo, J.M.; Yoon, C.H. Antinociceptive and anti-inflammatory effects of essential oil extracted from Chamaecyparis obtusa in mice. Int. Immunopharmacol. 2015, 29, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Jang, Y.S.; Yun, J.J.; Song, H. Nanochemicals from Chamaecyparis obtusa, Inhibits Proliferation and Migration of Vascular Smooth Muscle Cells. J. Nanosci. Nanotechnol. 2015, 15, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Williamson, E.M.; Wareham, N.; Kaatz, G.W.; Gibbons, S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry 2007, 68, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Debiaggi, M.; Pagani, L.; Cereda, P.M.; Landini, P.; Romero, E. Antiviral activity of Chamaecyparis lawsoniana extract: Study with herpes simplex virus type 2. Microbiologica 1988, 11, 55–61. [Google Scholar] [PubMed]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Jang, Y.J.; Kim, J.W.; Park, M.J.; Yoo, Y.M.; Jeung, E.B. Anti-asthmatic effects of volatile organic compounds from Chamaecyparis obtusa, Pinus densiflora, Pinus koraiensis, or Larix kaempferi wood panels. J. Physiol. Pharmacol. 2018, 69, 933–941. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.-H.; Kim, J.-W.; Park, M.-J.; Lee, S.-S.; Jeung, E.-B. Alleviation Effects of Natural Volatile Organic Compounds from Pinus Densiflora and Chamaecyparis Obtusa on Systemic and Pulmonary Inflammation. Biomedical Reports 2018, 9, 405–414. [Google Scholar] [CrossRef]
- Duringer, J.M.; Swan, L.R.; Walker, D.B.; Craig, A.M. Acute aquatic toxicity of western juniper (Juniperus occidentalis) foliage and Port Orford cedar (Chamaecyparis lawsoniana) heartwood oils. Environ. Monit. Assess. 2010, 170, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamakage, M.; Kohro, S.; Matsuzaki, T.; Tsuchida, H.; Namiki, A. Role of Intracellular Ca2+ stores in the Inhibitory Effect of Halothane on Airway Smooth Muscle Contraction. Anesthesiology 1998, 89, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Montaño, L.M.; Flores-Soto, E.; Reyes-García, J.; Díaz-Hernández, V.; Carbajal-García, A.; Campuzano-González, E.; Ramírez-Salinas, G.L.; Velasco-Velázquez, M.A.; Sommer, B. Testosterone induces hyporesponsiveness by interfering with IP3 receptors in guinea pig airway smooth muscle. Mol. Cell Endocrinol. 2018, 473, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Signal transduction and regulation in smooth muscle. Nature 1994, 372, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Trice, J.; Shinde, P.; Willis, R.E.; Pressley, T.A.; Perez-Zoghbi, J.F. Ca2+ Oscillations, Ca2+ Sensitization, and Contraction Activated by Protein Kinase C in Small Airway Smooth Muscle. J. General. Physiol. 2013, 141, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, M.F.; Lei, J.; Peng, Y.B.; Zhao, P.; Xue, L.; Chen, W.; Ma, L.Q.; Liu, Q.H.; Shen, J. Nuciferine Relaxes Tracheal Rings via the Blockade of VDLCC and NSCC Channels. Planta Med. 2018, 84, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Perusquía, M.; Flores-Soto, E.; Sommer, B.; Campuzano-González, E.; Martínez-Villa, I.; Martínez-Banderas, A.I.; Montaño, L.M. Testosterone-induced Relaxation Involves L-type and Store-operated Ca2+ Channels Blockade, and PGE2 in Guinea Pig Airway Smooth Muscle. Pflügers Archiv 2015, 467, 767–777. [Google Scholar] [CrossRef]
- Flores-Soto, E.; Reyes-García, J.; Sommer, B.; Montaño, L.M. Sarcoplasmic reticulum Ca2+ refilling is determined by L-type Ca2+ and store-operated Ca2+ channels in guinea pig airway smooth muscle. Eur J Pharmacol. 2013, 721, 21–28. [Google Scholar] [CrossRef]
- Ay, B.; Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Store-operated Ca2+ entry in porcine airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L909–L917. [Google Scholar] [CrossRef] [PubMed]
- Helli, P.B.; Janssen, L.J. Properties of a Store-operated Nonselective Cation Channel in Airway Smooth Muscle. Eur. Respir. J. 2008, 32, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Pobłocka, L.; El Helab, A.A. Biflavones from Chamaecyparis Obtusa. Z. Naturforsch C J. Biosci. 2005, 60, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Gadek, P.A.; Quinn, C.J. Biflavones of the subfamily cupressoideae, Cupressaceae. Phytochemistry 1985, 24, 267–272. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lin, G.M.; Chang, S.T. Hypoglycemic activity of extracts of Chamaecyparis obtusa var. formosana leaf in rats with hyperglycemia induced by high-fat diets and streptozotocin. J. Tradit. Complement. Med. 2019, 10, 389–395. [Google Scholar] [CrossRef]
- Tang, B.; Lee, Y.J.; Lee, Y.R.; Row, K.H. Examination of 1-methylimidazole series ionic liquids in the extraction of flavonoids from Chamaecyparis obtuse leaves using a response surface methodology. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2013, 933, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Brenner, R. Voltage effects on muscarinic acetylcholine receptor-mediated contractions of airway smooth muscle. Physiol. Rep. 2018, 6, e13856. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.J.; Delmotte, P.; Bai, Y.; Perez-Zogbhi, J.F. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc. Am. Thorac. Soc. 2008, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-H.; Zheng, Y.-M.; Liao, B.; Wang, Y.-X. Functional Role of Canonical Transient Receptor Potential 1 and Canonical Transient Receptor Potential 3 in Normal and Asthmatic Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2010, 43, 17–25. [Google Scholar] [CrossRef]
- Sommer, B.; Flores-Soto, E.; Gonzalez-Avila, G. Cellular Na+ Handling Mechanisms Involved in Airway Smooth Muscle Contraction (review). Int. J. Mol. Med. 2017, 40, 3–9. [Google Scholar] [CrossRef]
- Roos, J.; Digregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D. STIM1, an Essential and Conserved Component of Store-operated Ca2+ Channel Function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Peel, S.E.; Liu, B.; Hall, I.P. ORAI and Store-Operated Calcium Influx in Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 744–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dietrich, A.; Chubanov, V.; Kalwa, H.; Rost, B.R.; Gudermann, T. Cation channels of the transient receptor potential superfamily: Their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol. Ther. 2006, 112, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.L.; Brereton, H.M.; Harland, M.L.; Barritt, G.J. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology 2003, 8, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Flores-Soto, E.; Carbajal-García, A.; Sommer, B.; Montaño, L.M. Maintenance of Intracellular Ca2+ Basal Concentration in Airway Smooth Muscle (review). Int. J. Mol. Med. 2018, 42, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Yocum, G.T.; Chen, J.; Choi, C.H.; Townsend, E.A.; Zhang, Y.; Xu, D.; Fu, X.W.; Sanderson, M.J.; Emala, C.W. Role of Transient Receptor Potential Vanilloid 1 in the Modulation of Airway Smooth Muscle Tone and Calcium Handling. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L812–L821. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, H.Y.; Hur, J.; Kim, K.H.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model. Allergy Asthma Immunol. Res. 2018, 10, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Arts, I.C. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Luo, X.; Xue, L.; Xu, H.; Zhao, Q.-Y.; Wang, Q.; She, Y.-S.; Zang, D.-A.; Shen, J.; Peng, Y.-B.; Zhao, P. Polygonum Aviculare L. Extract and Quercetin Attenuate Contraction in Airway Smooth Muscle. Sci. Rep. 2018, 8, 3114. [Google Scholar] [CrossRef]
- Djelili, H.; Arrar, L.; Naline, E.; Devillier, P. Relaxant Effects of Quercetin and Rutin on Human Isolated Bronchus. Chin. Med. 2012, 3, 94–100. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W., Sr. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L396–L403. [Google Scholar] [CrossRef]
- Hake, A.; Begrow, F.; Spiegler, V.; Symma, N.; Hensel, A.; Düfer, M. Effects of Extracts and Flavonoids from Drosera Rotundifolia L. On Ciliary Beat Frequency and Murine Airway Smooth Muscle. Molecules 2022, 27, 6622. [Google Scholar] [CrossRef]
- Ezeta-Miranda, A.; Vera-Montenegro, Y.; Avila-Acevedo, J.G.; García-Bores, A.M.; Estrella-Parra, E.A.; Francisco-Marquez, G.; Ibarra-Velarde, F. Efficacy of purified fractions of Artemisia ludoviciana Nutt. mexicana and ultraestructural damage to newly excysted juveniles of Fasciola hepatica in vitro. Vet. Parasitol. 2020, 285, 109184. [Google Scholar] [CrossRef] [PubMed]
- García-Bores, A.M.; Álvarez-Santos, N.; López-Villafranco, M.E.; Jácquez-Ríos, M.P.; Aguilar-Rodríguez, S.; Grego-Valencia, D.; Espinosa-González, A.M.; Estrella-Parra, E.A.; Hernández-Delgado, C.T.; Serrano-Parrales, R.; et al. Verbesina crocata: A pharmacognostic study for the treatment of wound healing. Saudi J. Biol. Sci. 2020, 27, 3113–3124. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, D.D.; Tang, L.Y.; Ji, P.X.; Li, X.M.; Guo, Z.F.; Wang, J.; Jia, J.M.; Wang, A.H. Salvirrane A-F. Six undescribed nordrimane sesquiterpene derivatives from Salvia castanea Diels f. tomentosa Stib and their cytotoxic activities. Phytochemistry 2024, 218, 113958. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Santos, N.; Estrella-Parra, E.A.; del Carmen Benitez-Flores, J.; Serrano-Parrales, R.; Villamar-Duque, T.E.; Santiago-Santiago, M.A.; González-Valle, M.; Avila-Acevedo, J.G.; García-Bores, A.M. Asterohyptis stellulata: Phytochemistry and wound healing activity. Food Biosci. 2022, 50, 102150. [Google Scholar] [CrossRef]
- Estrella-Parra, E.A.; Espinosa-González, A.M.; García-Bores, A.M.; Zamora-Salas, S.X.; Benítez-Flores, J.C.; González-Valle, M.R.; Hernández-Delgado, C.T.; Peñalosa-Castro, I.; Avila-Acevedo, J.G. Flavonol glycosides in Dyssodia tagetiflora and its temporal variation, chemoprotective and ameliorating activities. Food Chem. Toxicol. 2019, 124, 411–422. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 13:978-0-309-15400-0. [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Soto, E.; Romero-Martínez, B.S.; Solís-Chagoyán, H.; Estrella-Parra, E.A.; Avila-Acevedo, J.G.; Gomez-Verjan, J.C.; Reyes-García, J.; Casas-Hernández, M.F.; Sommer, B.; Montaño, L.M. Chamaecyparis lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle. Molecules 2024, 29, 2284. https://doi.org/10.3390/molecules29102284
Flores-Soto E, Romero-Martínez BS, Solís-Chagoyán H, Estrella-Parra EA, Avila-Acevedo JG, Gomez-Verjan JC, Reyes-García J, Casas-Hernández MF, Sommer B, Montaño LM. Chamaecyparis lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle. Molecules. 2024; 29(10):2284. https://doi.org/10.3390/molecules29102284
Chicago/Turabian StyleFlores-Soto, Edgar, Bianca S. Romero-Martínez, Héctor Solís-Chagoyán, Edgar A. Estrella-Parra, Jose G. Avila-Acevedo, Juan C. Gomez-Verjan, Jorge Reyes-García, María F. Casas-Hernández, Bettina Sommer, and Luis M. Montaño. 2024. "Chamaecyparis lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle" Molecules 29, no. 10: 2284. https://doi.org/10.3390/molecules29102284
APA StyleFlores-Soto, E., Romero-Martínez, B. S., Solís-Chagoyán, H., Estrella-Parra, E. A., Avila-Acevedo, J. G., Gomez-Verjan, J. C., Reyes-García, J., Casas-Hernández, M. F., Sommer, B., & Montaño, L. M. (2024). Chamaecyparis lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle. Molecules, 29(10), 2284. https://doi.org/10.3390/molecules29102284






