Preparation of Hydrophobic Au Catalyst and Application in One-Step Oxidative Esterification of Methacrolein to Methyl Methacrylate
Abstract
1. Introduction
2. Results
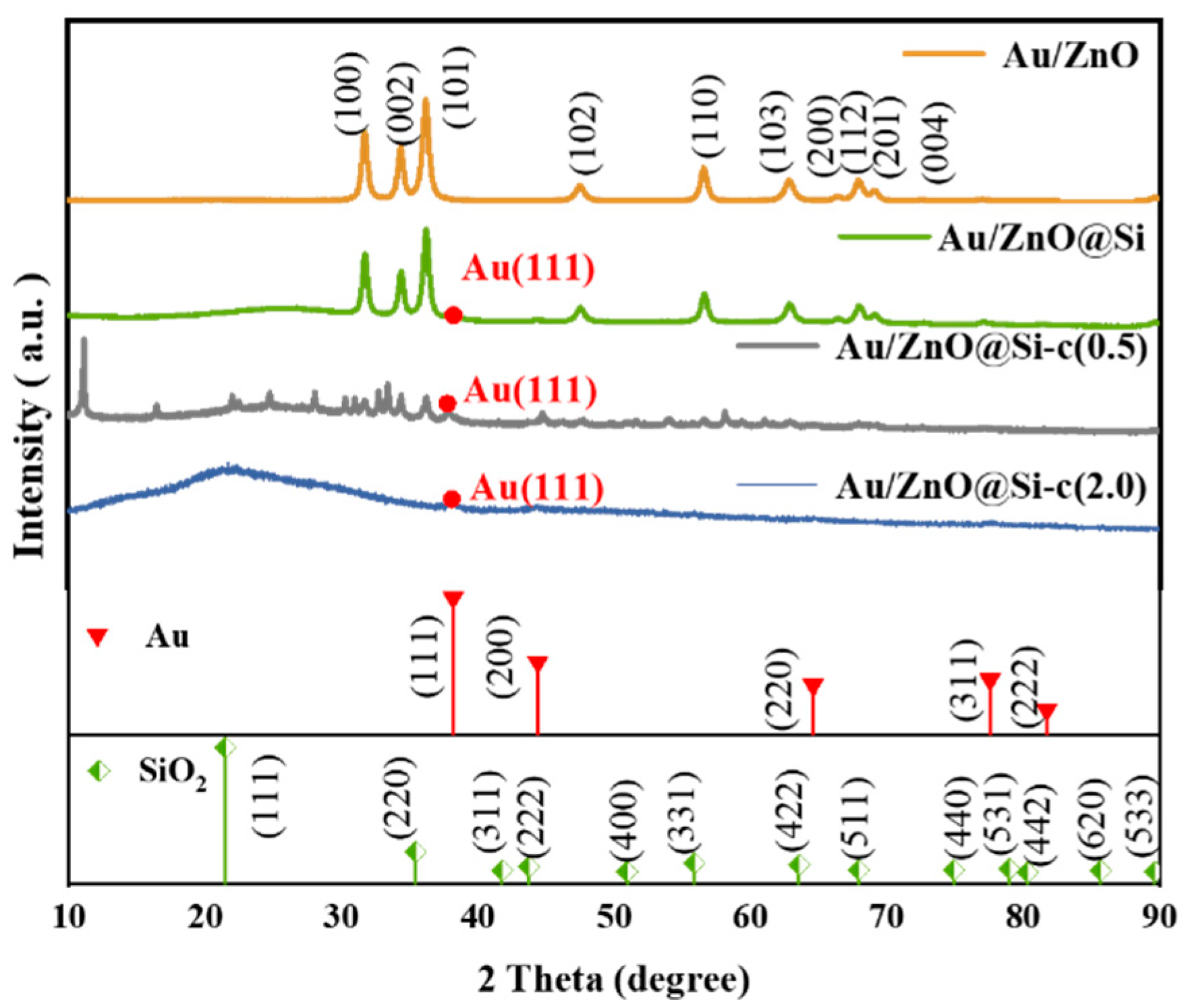
2.1. XRD Analysis
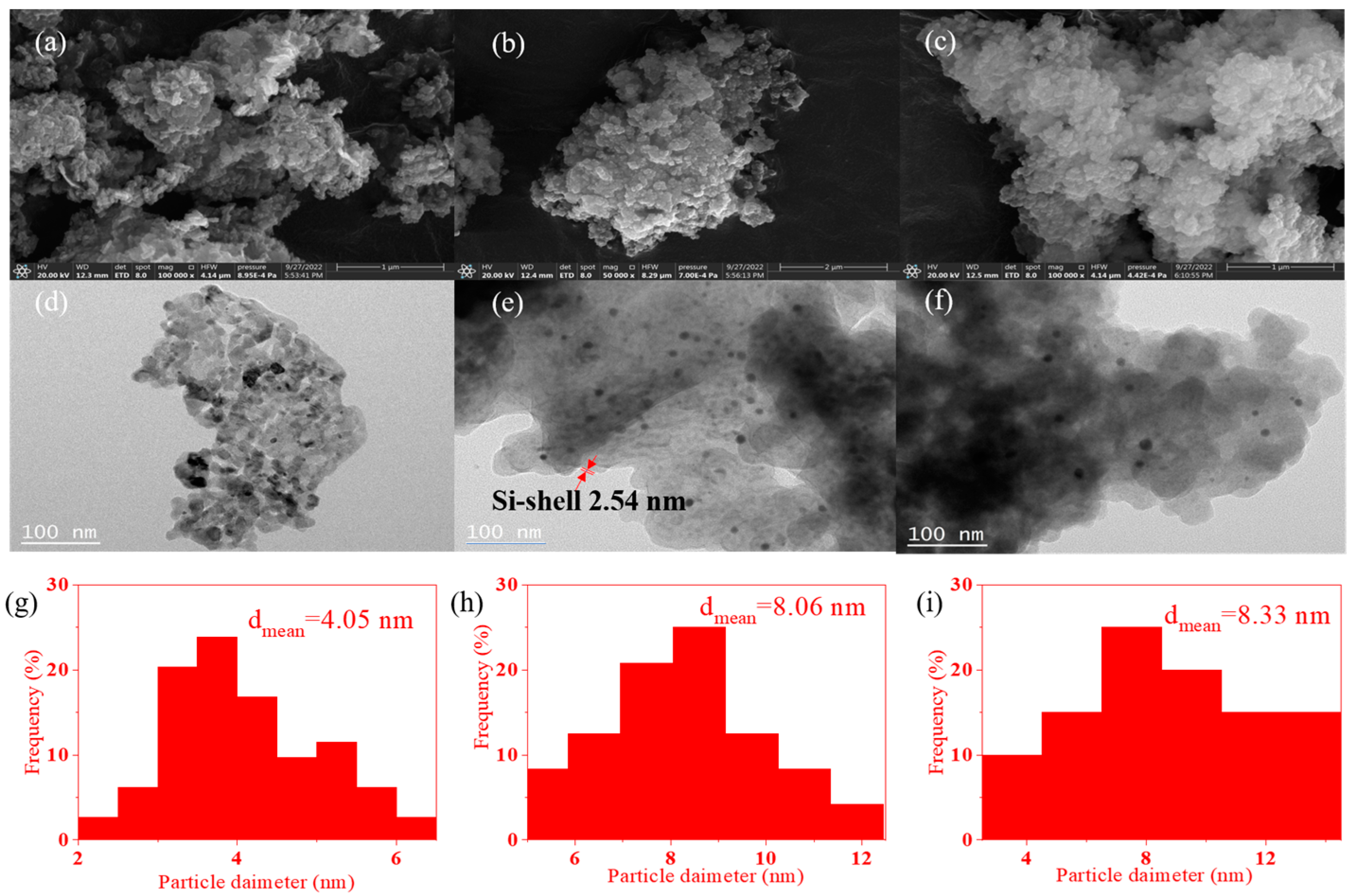
2.2. SEM and TEM Images of the Catalysts
2.3. N2 Physisorption
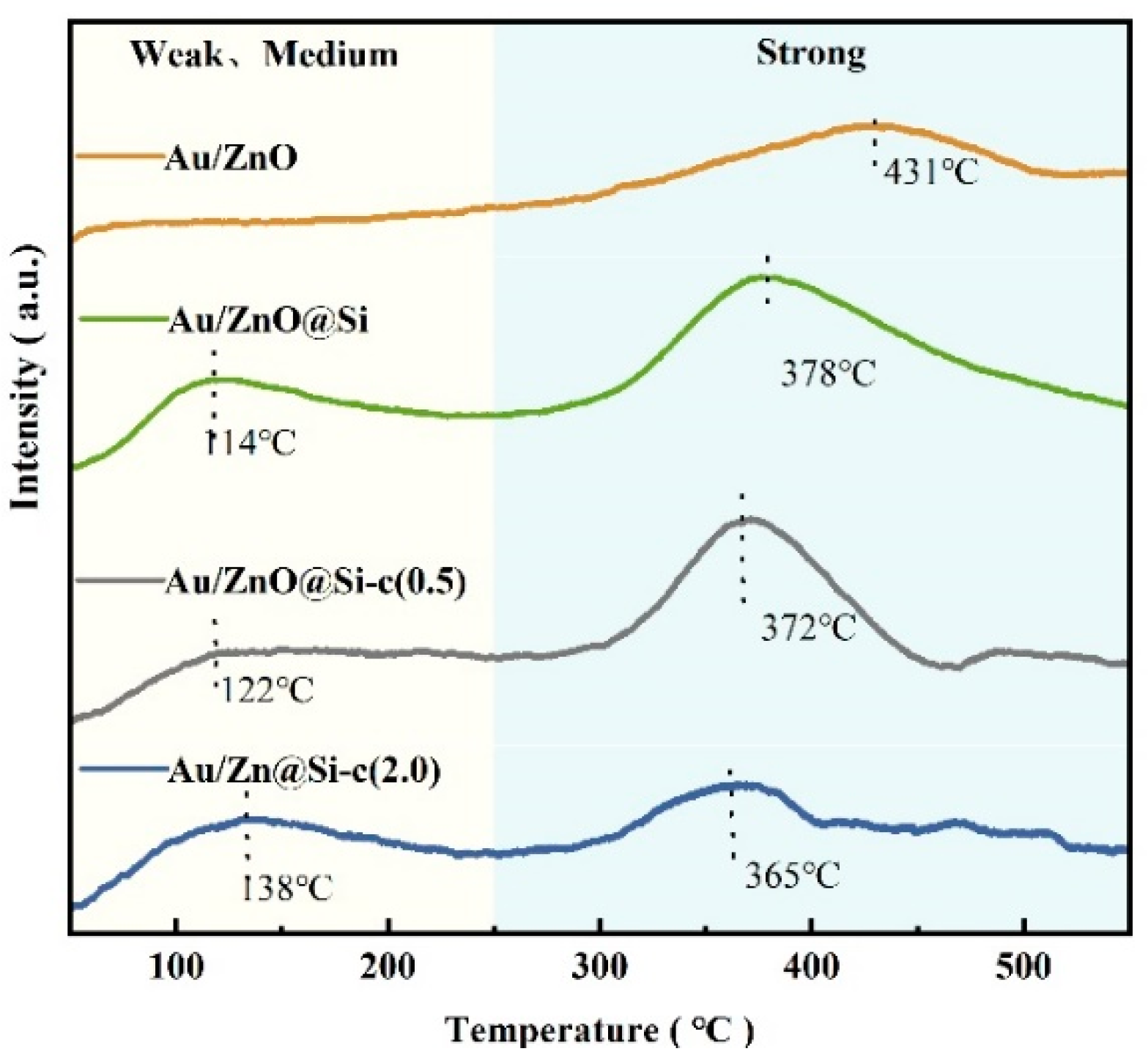
2.4. The CO2-TPD of Catalysts
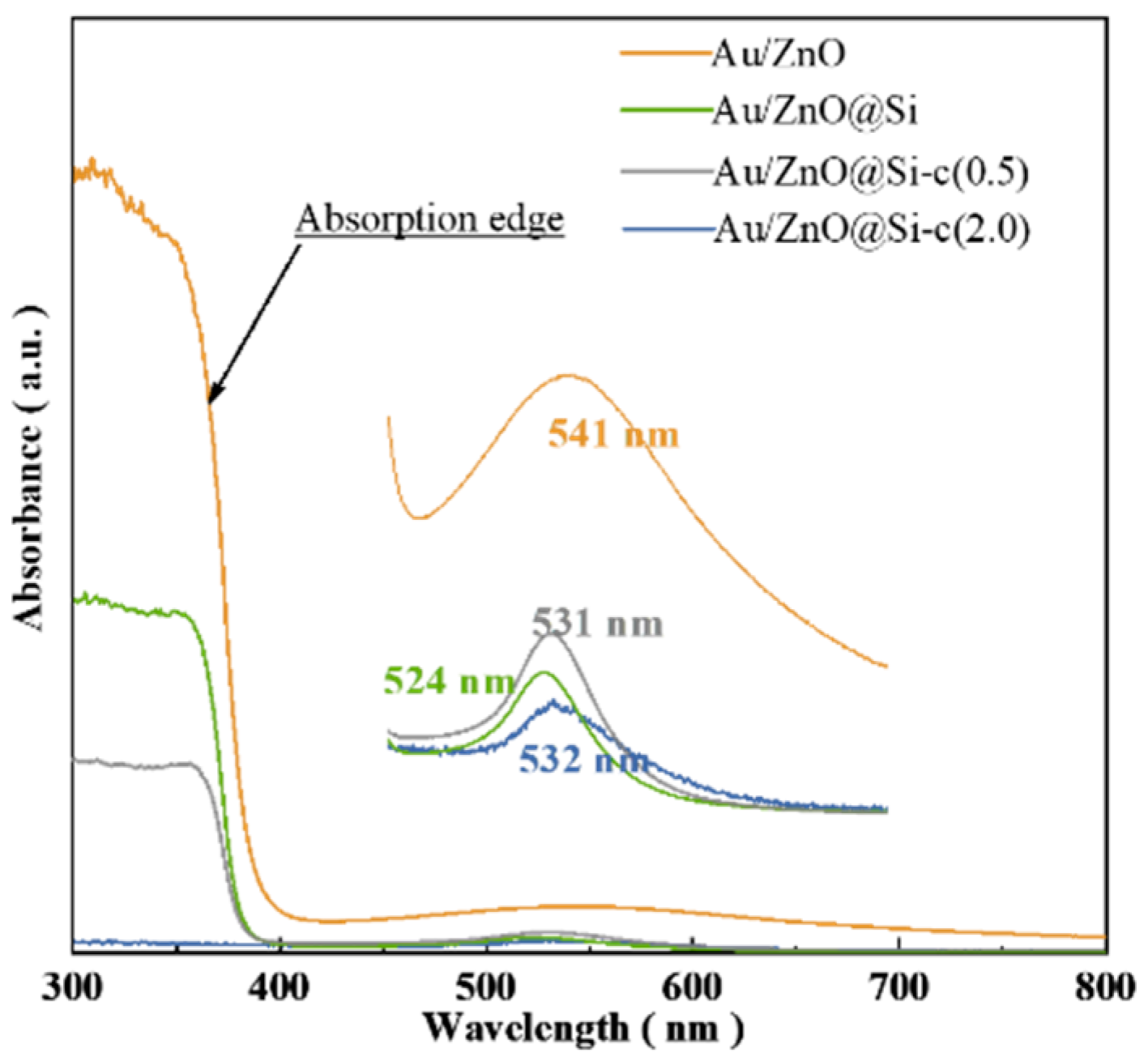
2.5. UV–Vis Characterization of Catalysts
2.6. XPS Analysis
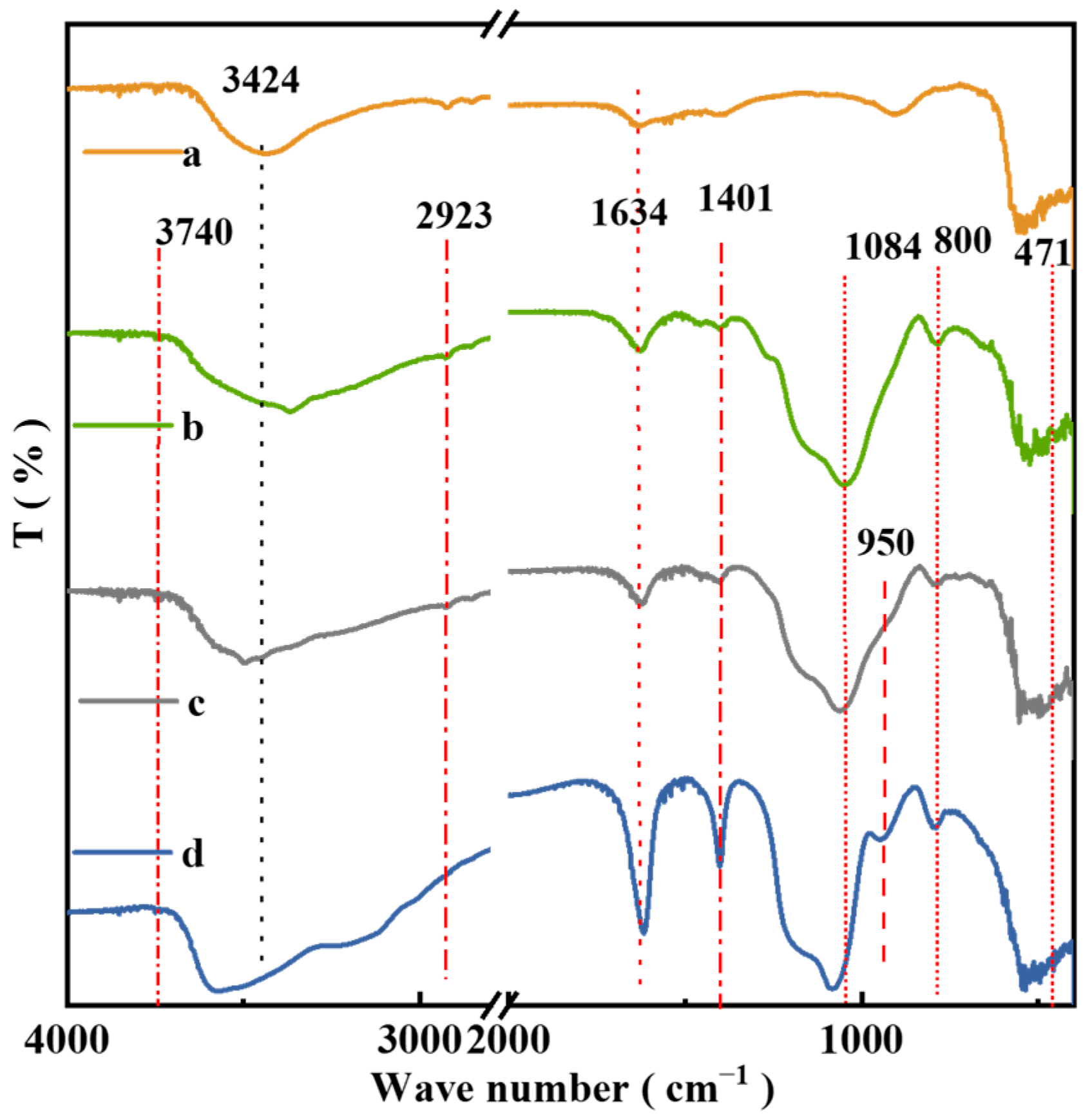
2.7. FT-IR of the Catalysts
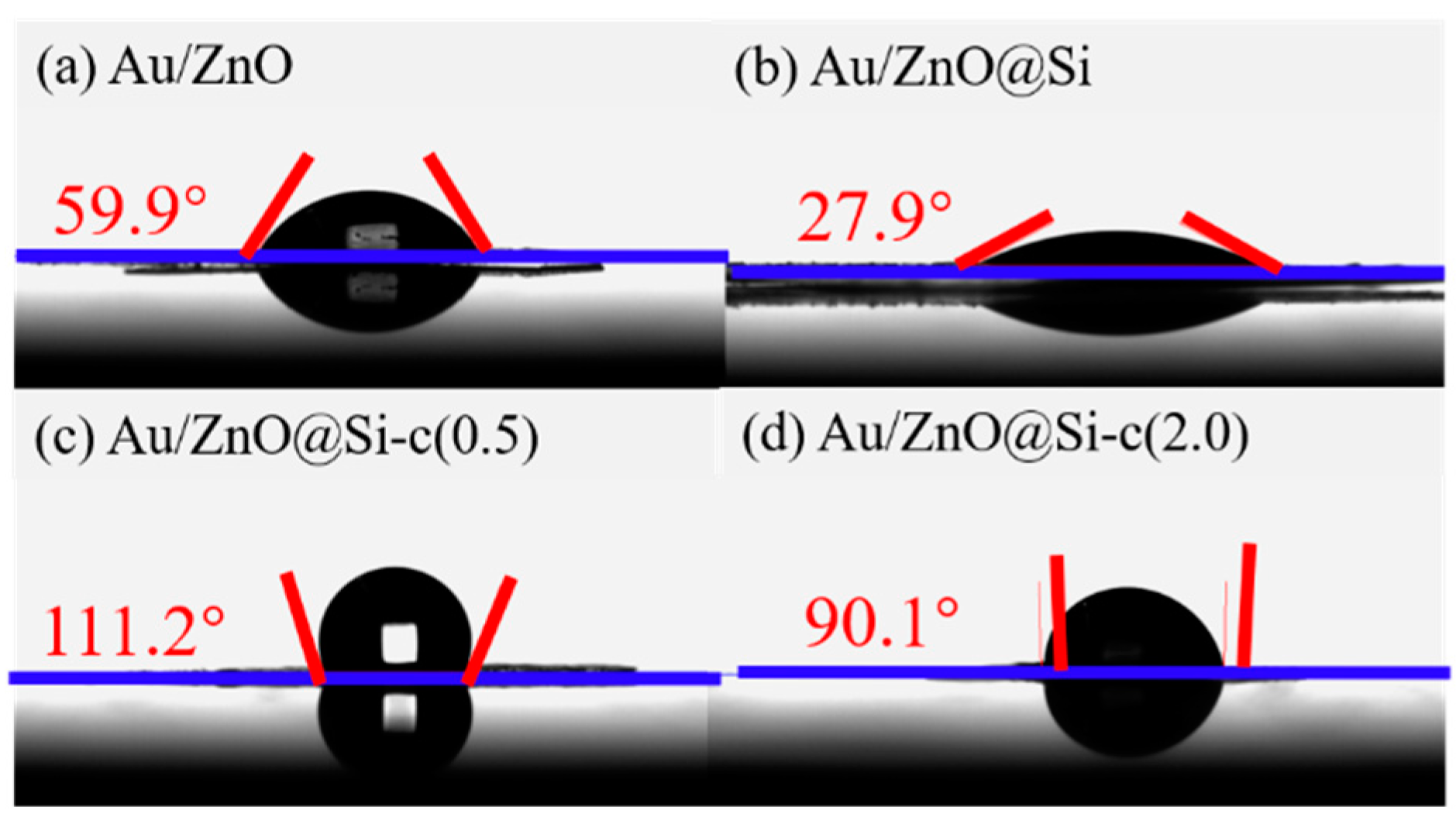
2.8. Water-Droplet Contact Angles of the Catalysts
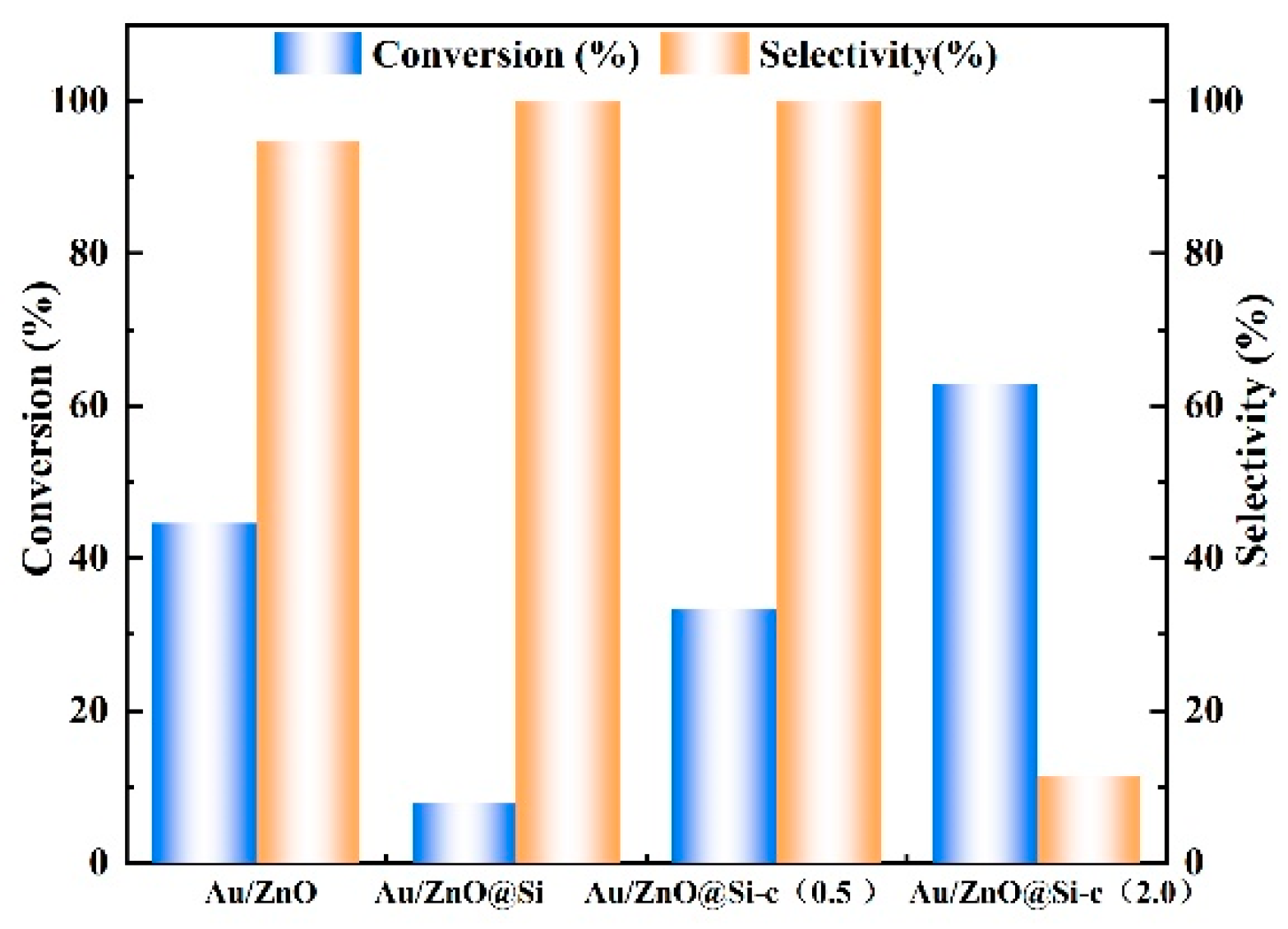
2.9. Catalytic Performance
2.10. Kinetics
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of Hydrophobic Support of ZnO
4.2.2. Synthesis of Au/ZnO@Si
4.2.3. Synthesis of Hydrophobic Catalysts
4.3. Characterization
4.4. Catalytic Activity Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagai, K. New developments in the production of methyl methacrylate. Appl. Catal. A Gen. 2001, 221, 367–377. [Google Scholar] [CrossRef]
- Yamamatsu, S.; Yamaguchi, T.; Yokota, K.; Nagano, O.; Chono, M.; Aoshima, A. Development of catalyst technology for producing methyl methacrylate (MMA) by direct methyl esterification. Catal. Surv. Asia 2010, 14, 124–131. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Dubois, J.L.; Cavani, F.; Rostamizadeh, M.; Patience, G.S. Catalysis for the synthesis of methacrylic acid and methyl methacrylate. Chem. Soc. Rev. 2018, 47, 7703–7738. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Song, K.E.; Xu, H.; Wang, Z.; Ma, Y.; Shang, F.; Kan, Q. Oxidation of isobutane and isobutene to methacrolein over hydrothermally synthesized Mo-V-Te-O mixed oxide catalysts. Catal. Commun. 2009, 10, 528–532. [Google Scholar] [CrossRef]
- Diao, Y.; Yang, P.U.; Yan, R.; Jiang, L.; Wang, L.; Zhang, H.; Li, C.; Li, Z.; Zhang, S. Deactivation and regeneration of the supported bimetallic Pd-Pb catalyst in direct oxidative esterification of methacrolein with methanol. Appl. Catal. B 2013, 142–143, 329–336. [Google Scholar]
- Haruta, M. Nanoparticulate gold catalysts for low-temperature CO oxidation. ChemInform 2004, 35, 48. [Google Scholar] [CrossRef]
- Suzuki, K. Aerobic oxidative esterification of aldehydes with alcohols by gold–nickel oxide nanoparticle catalysts with a core-shell structure. ACS Catal. 2013, 8, 1845–1849. [Google Scholar] [CrossRef]
- Diao, Y.; He, H.; Yang, P.; Wang, L.; Zhang, S. Optimizing the structure of supported Pd catalyst for direct oxidative esterification of methacrolein with methanol. Chem. Eng. Sci. 2015, 135, 128–136. [Google Scholar] [CrossRef]
- Ekoue-Kovi, K.; Wolf, C. One-pot oxidative esterification and amidation of aldehydes. Chem. Eur. J. 2008, 14, 6302–6315. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, G.; Yang, L.; Cao, X.; Zhang, P.; Li, F. Oxidative esterification of methacrolein to methyl methacrylate over gold nanoparticles on hydroxyapatite. ChemCatChem 2017, 9, 1230–1241. [Google Scholar] [CrossRef]
- Guan, Y.; Ma, H.; Chen, W.; Li, M.; Qian, G. Methyl methacrylate synthesis: Thermodynamic analysis for oxidative esterification of methacrolein and aldol condensation of methyl acetate. Ind. Eng. Chem. Res. 2020, 59, 17408–17416. [Google Scholar] [CrossRef]
- Paul, B.; Khatun, R.; Sharma, S.K.; Adak, S.; Singh, G.; Das, D.; Siddiqui, N.; Bhandari, S.; Joshi, V.; Sasaki, T. Fabrication of Au nanoparticles supported on one-dimensional La2O3 nanorods for selective esterification of methacrolein to methyl methacrylate with molecular oxygen. ACS Sustain. Chem. Eng. 2019, 7, 982–3994. [Google Scholar] [CrossRef]
- Ma, Z.; Dai, S. Design of novel structured gold nanocatalysts. ACS Catal. 2011, 1, 805–818. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, Z.; Overbury, S.H.; Dai, S. Rational design of gold catalysts with enhanced thermal stability: Post modification of Au/TiO2 by amorphous SiO2 decoration. Catal. Lett. 2007, 116, 128–135. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Wang, L.; Fu, Z. Oxidative esterification of methacrolein to methyl methacrylate over supported gold catalysts prepared by colloid deposition. ChemCatChem 2017, 9, 1960–1968. [Google Scholar] [CrossRef]
- Farooq, M.U.; Shi, Y.; Chen, W.; Guan, Y.; Zhou, J.G.; Song, N.; Qian, G.; Zhang, J.; Chen, D.; Zhou, X.; et al. Kinetics insights into the active sites of Au catalysts for the oxidative esterification of methacrolein to methyl methacrylate. Chem. Eng. Sci. 2023, 277, 118840. [Google Scholar] [CrossRef]
- Zuo, C.; Tian, Y.; Zheng, Y.; Wang, L.; Fu, Z.; Jiao, T.; Wang, M.; Huang, H.; Li, Y. One-step oxidative esterification of methacrolein with methanol over Au-CeO2/γ-Al2O3 catalysts. Catal. Commun. 2019, 124, 51–55. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Zheng, Y.; Wang, M.; Zuo, C.; Huang, H.; Yin, D.; Fu, Z.; Tan, J.; Zhou, Z. Nano-Au/MCeOx catalysts for the direct oxidative esterification of methylacrolein to methyl esters. Ind. Eng. Chem. Res. 2019, 58, 19397–19405. [Google Scholar] [CrossRef]
- Tan, H.; Lou, B.; Shen, A.; Ning, C. Experimental and Theoretical Research on One-Step Oxidative Esterification of Methyl Acrolein with Methanol. Ind. Eng. Chem. Res. 2023, 62, 21609–21618. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, L.; Chen, Y.; Yang, Y.; Zuo, C.; An, J.; Wang, Q.; Huang, H.; Li, Y.; Wang, M. Ionic liquid-mediated hexagonally porous ZnO nanocrystal-supported Au catalysts: Highly stable materials for aldehyde oxidative esterification. Catal. Sci. Technol. 2023, 13, 400. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, Y.; Zheng, Y.; Zuo, C.; Ge, T.; Xu, R.; Huang, H.; An, J. Highly Stable Au@CeO2/MnOx core-shell structured catalyst for one-step oxidation esterification of methacrolein to methyl methacrylate. Catal. Lett. 2023, 154, 846–857. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, C.; Li, Y.; Yang, Y.; Xu, R. Review on Performance and Preparation of Catalysts for Oxidative Esterification of Aldehydes or Alcohols to Esters. ChemCatChem 2024, 16, e202300976. [Google Scholar] [CrossRef]
- Lin, T.B.; Chung, D.L.; Chang, J.R. Ethyl acetate production from water-containing ethanol catalyzed by supported Pd catalysts: Advantages and disadvantages of hydrophobic supports. Ind. Eng. Chem. Res. 1999, 38, 1271–1276. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Gao, J.; Wang, J.; Ma, G.; Wen, X.; Yang, Y.; Li, Y.; Ding, M. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products. Science 2021, 371, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, H.; Li, R.; Zhang, Z.; Qin, C.; Xu, D.; Fan, H.; Hou, B.; Wang, J.; Gu, X.K.; et al. Insights into the Diffusion Behaviors of Water over Hydrophilic/Hydrophobic Catalysts During the Conversion of Syngas to High-Quality Gasoline. Angew. Chem. Int. Ed. Engl. 2023, 62, 202306786. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, F.-S. The Importance of Catalyst Wettability. ChemCatChem 2014, 6, 3048–3052. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Sun, Q.; Zhu, L.; Meng, X.; Xiao, F.S. Transesterification catalyzed by ionic liquids on superhydrophobic mesoporous polymers: Heterogeneous catalysts that are faster than homogeneous catalysts. J. Am. Chem. Soc. 2012, 134, 16948–16950. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, W.; Zhu, J.; Ran, W.; Chen, S. Pd-Pb/SDB bimetallic catalysts for the direct oxidative esterification of methacrolein to methyl methacrylate. Ind. Eng. Chem. Res. 2012, 51, 15004–15010. [Google Scholar] [CrossRef]
- Wang, B.H.; Ran, W.L.; Sun, W.J.; Wang, K. Direct oxidative esterification of aldehyde with alcohol to ester over Pd/styrene-divinyl benzene copolymer catalyst. Ind. Eng. Chem. Res. 2012, 51, 3932–3938. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Zhu, J.; Sun, W.; Chen, S. Preparation and characterization of mono-/multi-metallic hydrophobic catalysts for the oxidative esterification of methacrolein to methyl methacrylate. J. Mol. Catal. A-Chem. 2013, 15, 322–326. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Nie, Y.; Wang, B. The influence of a hydrophobic carrier, reactant and product during H2O adsorption on Pd surface for the oxidative esterification of methacrolein to methyl methacrylate. Phys. Chem. Chem. Phys. 2018, 20, 9965–9974. [Google Scholar] [CrossRef]
- Liu, H.X.; Ma, J.; Wei, D.W.; Wang, B.H.; Zhu, J. Insight into the effect of Pd and Pd3Pb surface structure on activation of reactant O2 and product H2O in direct oxidative esterification. Appl. Surf. Sci. 2020, 500, 143852. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Nie, Y.; Qin, Q.; Ma, J. Insight into the effect of promoter Pb in Pb-Pd catalyst on methyl methacrylate formation via direct oxidative esterification: A DFT study. Appl. Surf. Sci. 2020, 510, 145320. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Ji, S.; Li, Y.T.; Hou, F.; Zhang, H.C.; Zhang, X.W.; Wang, L.; Li, G.Z. Tuning oxygen vacancies on mesoporous ceria nanorods by metal doping: Controllable magnetic property. Appl. Surf. Sci. 2018, 455, 1037–1044. [Google Scholar] [CrossRef]
- Manzoli, M.; Menegazzo, F.; Signoretto, M.; Cruciani, G.; Pinna, F. Effects of synthetic parameters on the catalytic performance of Au/CeO2 for furfural oxidative esterification. J. Catal. 2015, 330, 465–473. [Google Scholar] [CrossRef]
- Xu, B.J.; Liu, X.Y.; Haubrich, J.; Friend, C.M. Vapour-phase gold-surface-mediated coupling of aldehydes with methanol. Nat. Chem. 2010, 2, 61–65. [Google Scholar] [CrossRef]
- Wu, G.D.; Zhao, G.Q.; Sun, J.H. The effect of oxygen vacancies in ZnO at an Au/ZnO interface on its catalytic selective oxidation of glycerol. J. Catal. 2019, 377, 271–282. [Google Scholar] [CrossRef]
- Karpenko, A.; Leppelt, R.; Plzak, V.; Behm, R. The role of cationic Au3+ and nonionic Au0 species in the low-temperature water–gas shift reaction on Au/CeO2 catalysts. J. Catal. 2007, 252, 231–242. [Google Scholar] [CrossRef]
- Sohn, Y.; Pradhan, D.; Radi, A.; Leung, K.T. Interfacial electronic structure of gold nanoparticles on Si (100): Alloying versus quantum size effects. Langmuir 2009, 25, 9557–9563. [Google Scholar] [CrossRef] [PubMed]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]



| Samples | BET Surface Area (m2 g−1) a | Pore Size(nm) a | Au Loading (wt%) b |
|---|---|---|---|
| Au/ZnO | 43.4 | 21.6 | 0.12 |
| Au/ZnO@Si | 20.1 | 14.5 | 0.10 |
| Au/ZnO@Si-c(0.5) | 17.8 | 20.4 | 0.04 |
| Au/ZnO@Si-c(2.0) | 7.6 | 16.9 | 0.04 |
| Catalysts | Desorption of CO2 (mmol·g−1) a | |
|---|---|---|
| Low-Temperature Peak b | High-Temperature Peak c | |
| Au/ZnO | - | 0.9 |
| Au/ZnO@Si | 0.83 | 2.25 |
| Au/ZnO@Si-c(0.5) | 0.41 | 1.0 |
| Au/ZnO@Si-c(2.0) | 0.45 | 0.49 |
| Catalysts | Au0 | Au3+ | ||
|---|---|---|---|---|
| BE (eV) | Fraction (%) | BE (eV) | Fraction (%) | |
| Au/ZnO | 83.21 | 55.50 | 85.54 | 44.50 |
| Au/ZnO@Si | 83.11 | 6.77 | 86.65 | 93.23 |
| Au/ZnO@Si-c(0.5) | 84.10. | 49.90 | 86.70 | 50.10 |
| Au/ZnO@Si-c(2.0) | 83.00 | 36.47 | 88.22 | 63.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Yang, Y.; Li, Y.; Cai, L.; Zhao, X.; Xue, B.; Li, Y.; An, J.; Zhang, J. Preparation of Hydrophobic Au Catalyst and Application in One-Step Oxidative Esterification of Methacrolein to Methyl Methacrylate. Molecules 2024, 29, 1854. https://doi.org/10.3390/molecules29081854
Zheng Y, Yang Y, Li Y, Cai L, Zhao X, Xue B, Li Y, An J, Zhang J. Preparation of Hydrophobic Au Catalyst and Application in One-Step Oxidative Esterification of Methacrolein to Methyl Methacrylate. Molecules. 2024; 29(8):1854. https://doi.org/10.3390/molecules29081854
Chicago/Turabian StyleZheng, Yanxia, Yubo Yang, Yixuan Li, Lu Cai, Xuanjiao Zhao, Bing Xue, Yuchao Li, Jiutao An, and Jialiang Zhang. 2024. "Preparation of Hydrophobic Au Catalyst and Application in One-Step Oxidative Esterification of Methacrolein to Methyl Methacrylate" Molecules 29, no. 8: 1854. https://doi.org/10.3390/molecules29081854
APA StyleZheng, Y., Yang, Y., Li, Y., Cai, L., Zhao, X., Xue, B., Li, Y., An, J., & Zhang, J. (2024). Preparation of Hydrophobic Au Catalyst and Application in One-Step Oxidative Esterification of Methacrolein to Methyl Methacrylate. Molecules, 29(8), 1854. https://doi.org/10.3390/molecules29081854