Enhancing the Quality of Low-Alcohol Navel Orange Wine through Simultaneous Co-Fermentation Using Saccharomyces cerevisiae SC-125, Angel Yeast SY, and Lactiplantibacillus plantarum BC114
Abstract
1. Introduction
2. Results and Discussion
2.1. Changes in Fundamental Parameters during the Fermentation Process
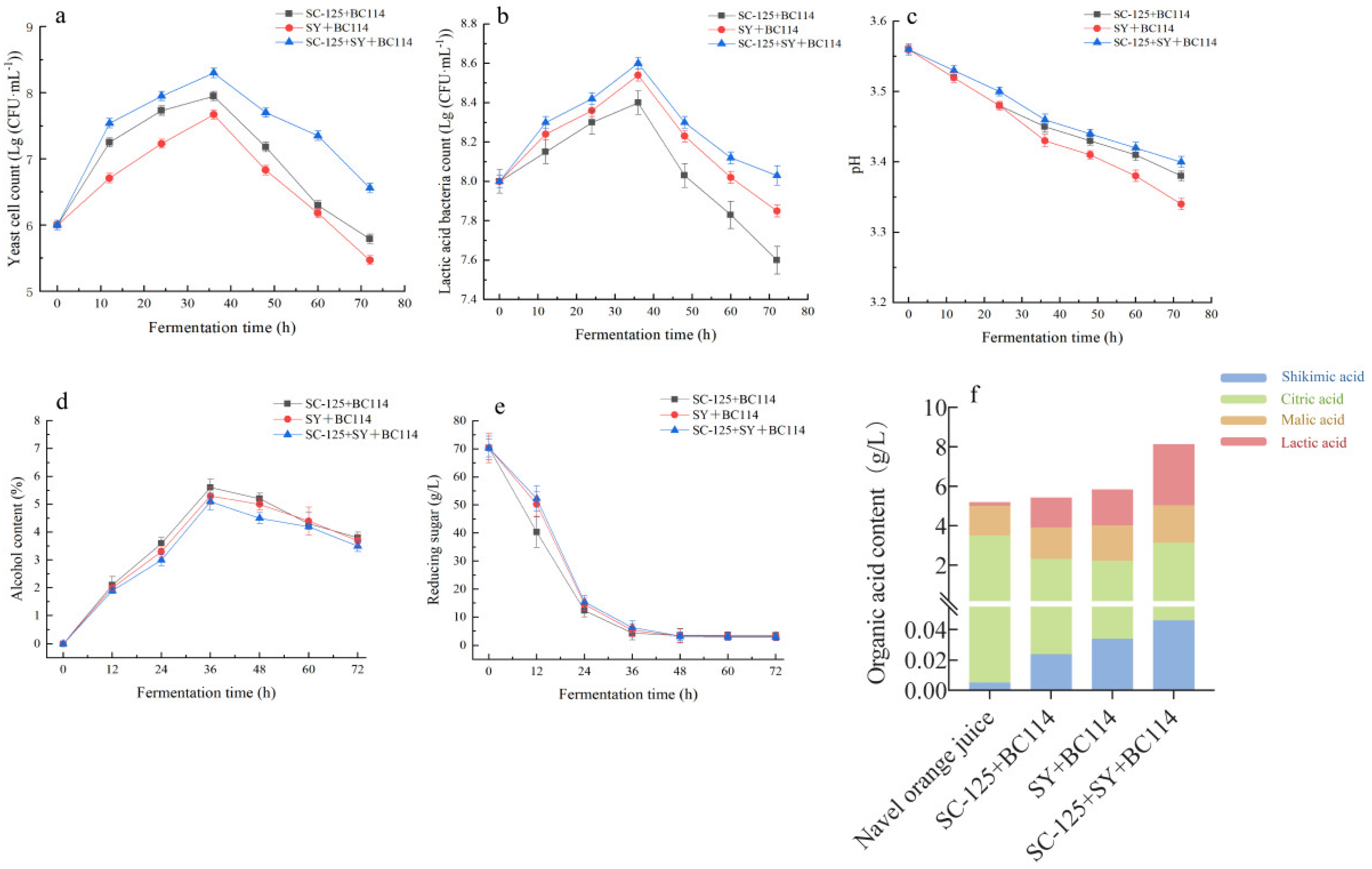
2.1.1. Variations in Cell Count and pH during the Fermentation Process
2.1.2. The Changes in Reducing Sugars and Alcohol Content during Fermentation
2.1.3. The Content of Organic Acids
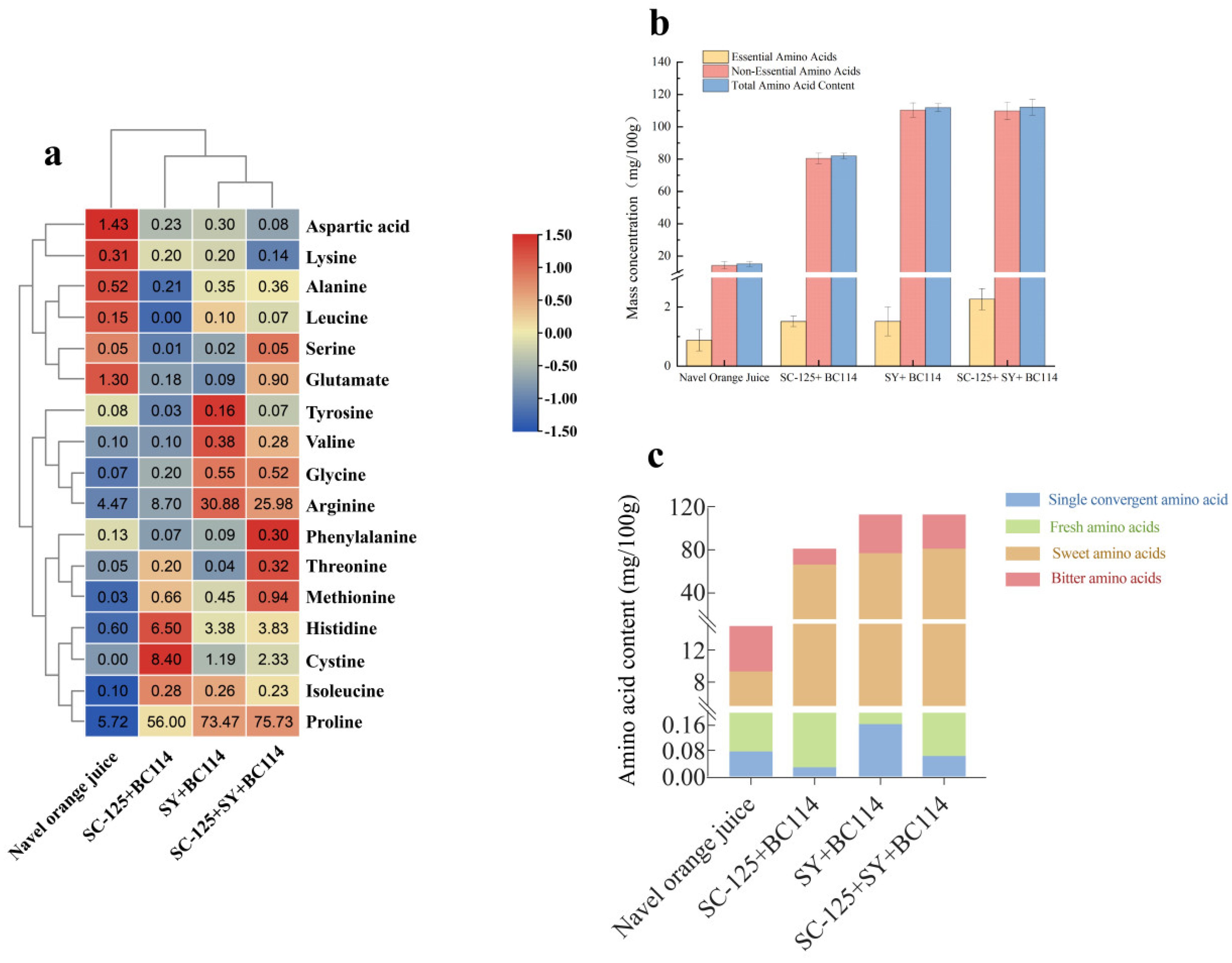
2.2. The Changes in Amino Acids before and after Fermentation
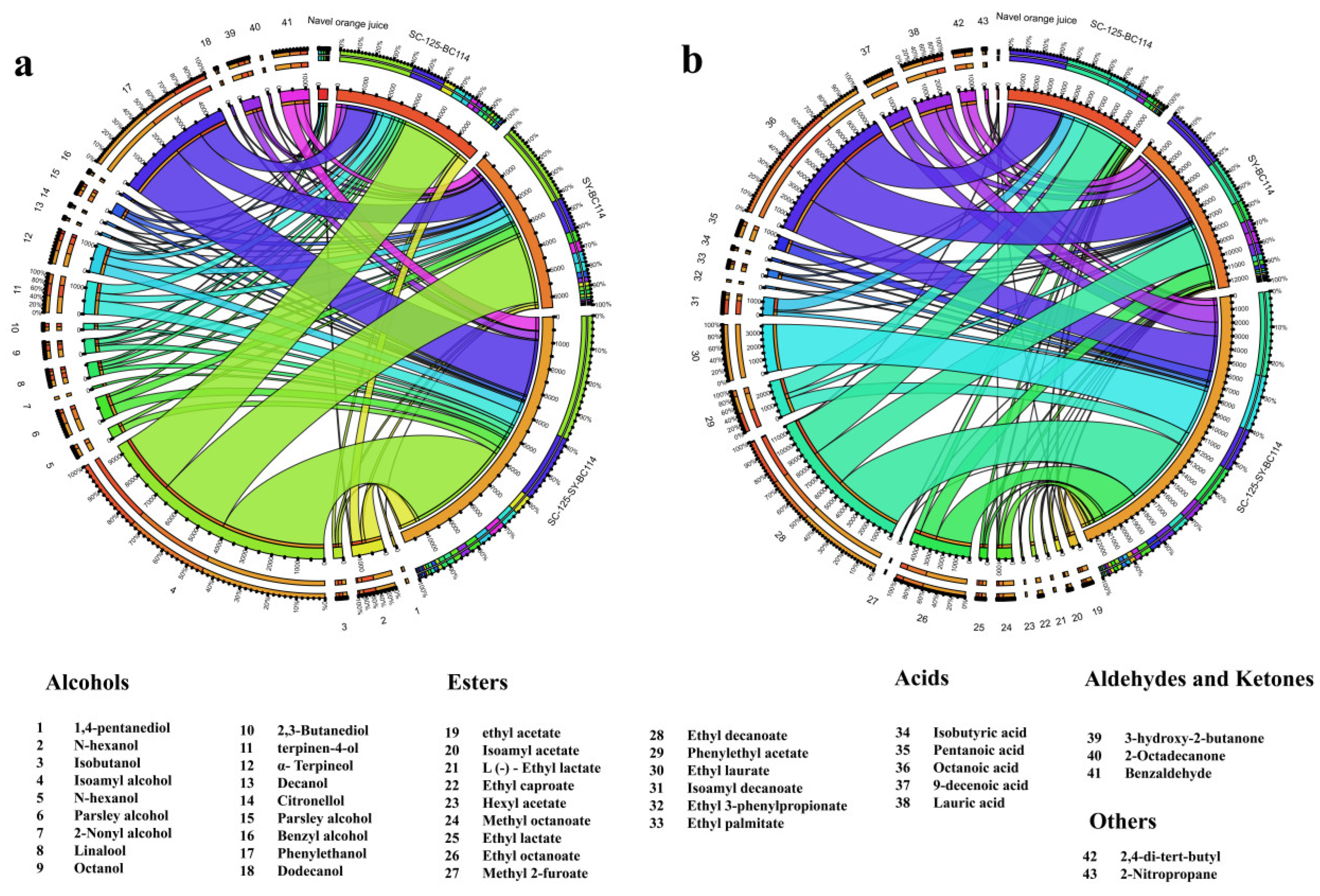
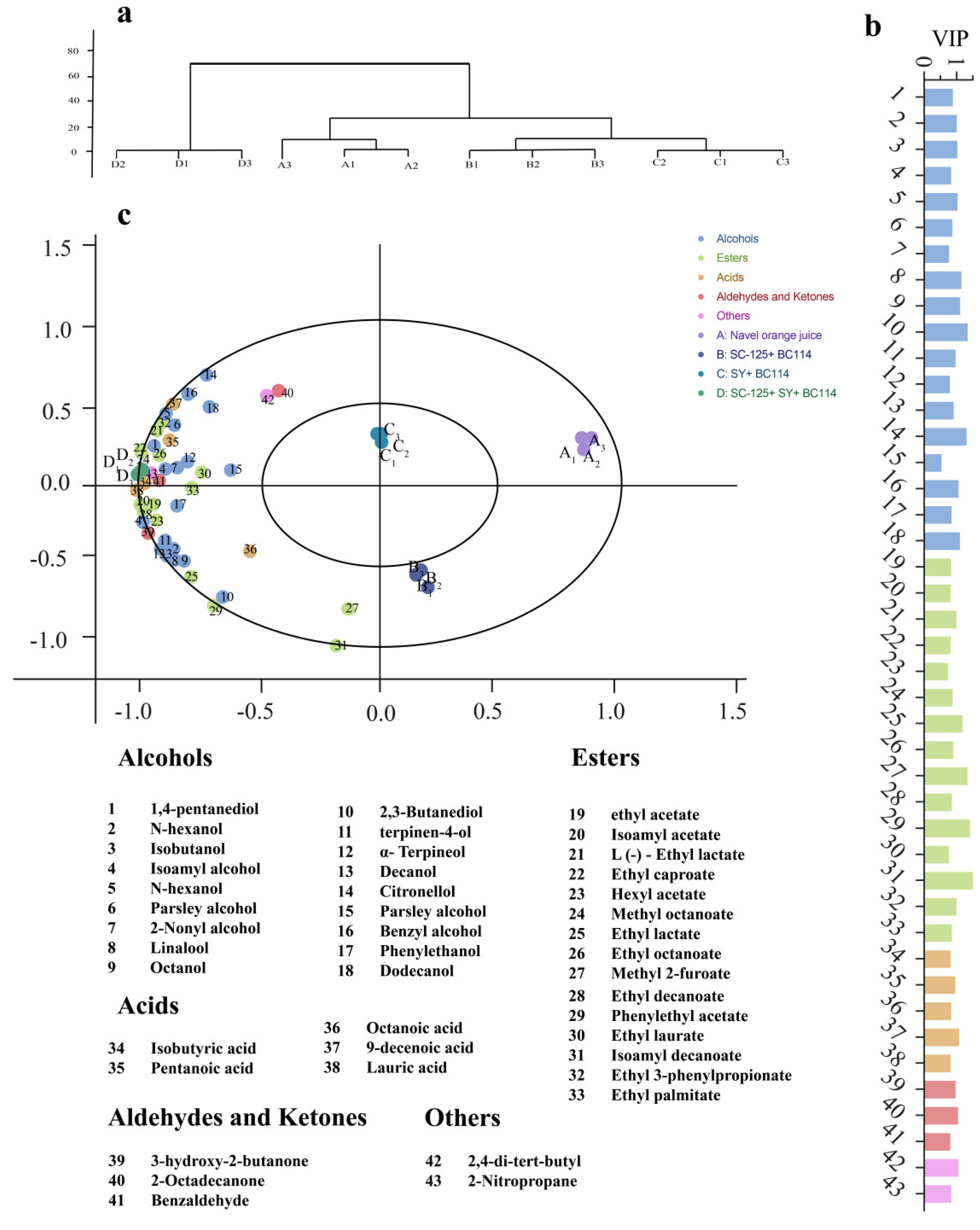
2.3. The Content of Volatile Compounds
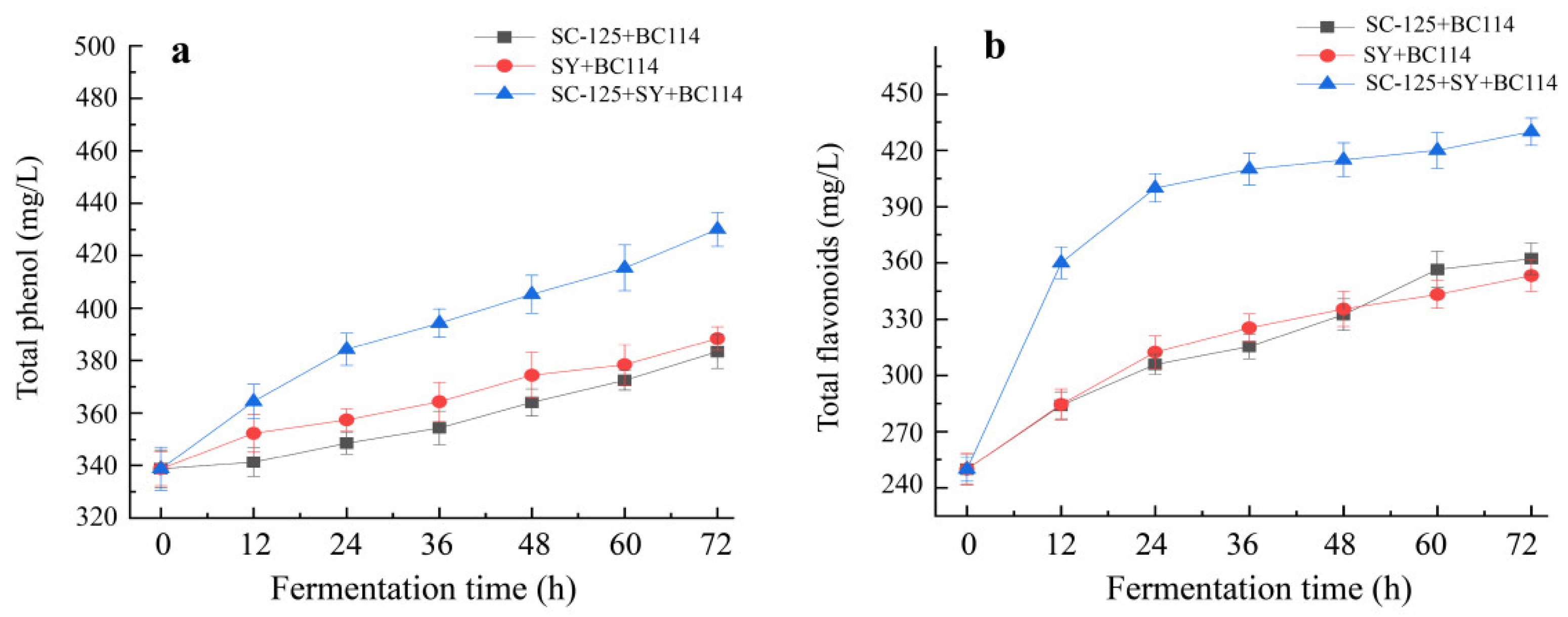
2.4. Changes in Total Phenol and Total Flavonoid Contents during the Fermentation Process
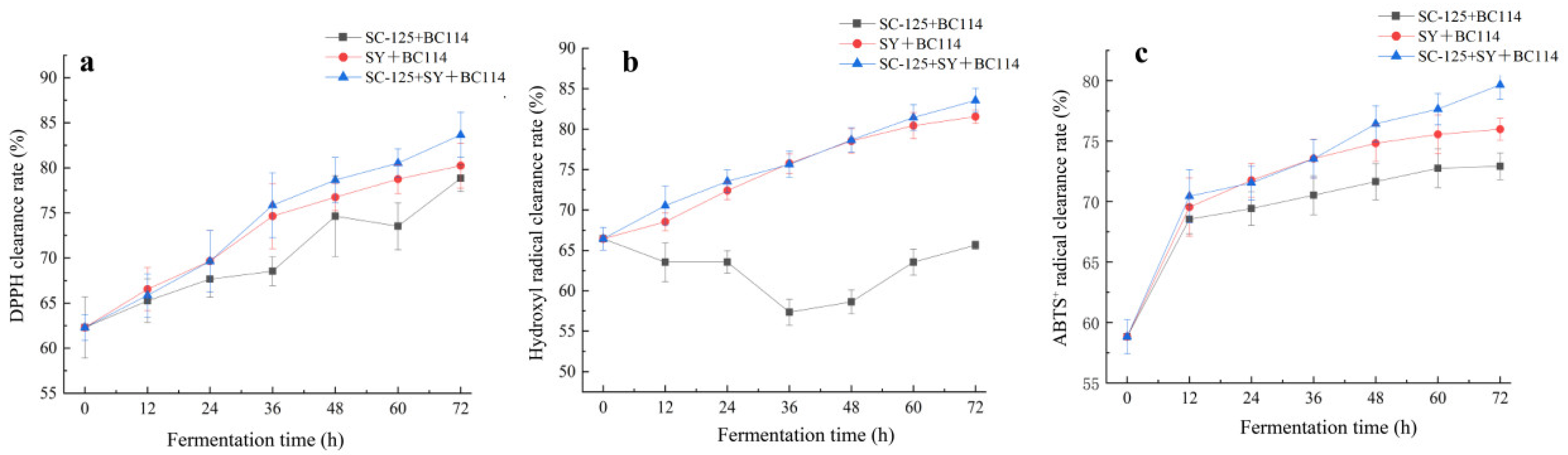
2.5. Changes in Free Radical Scavenging Activity during the Fermentation Process
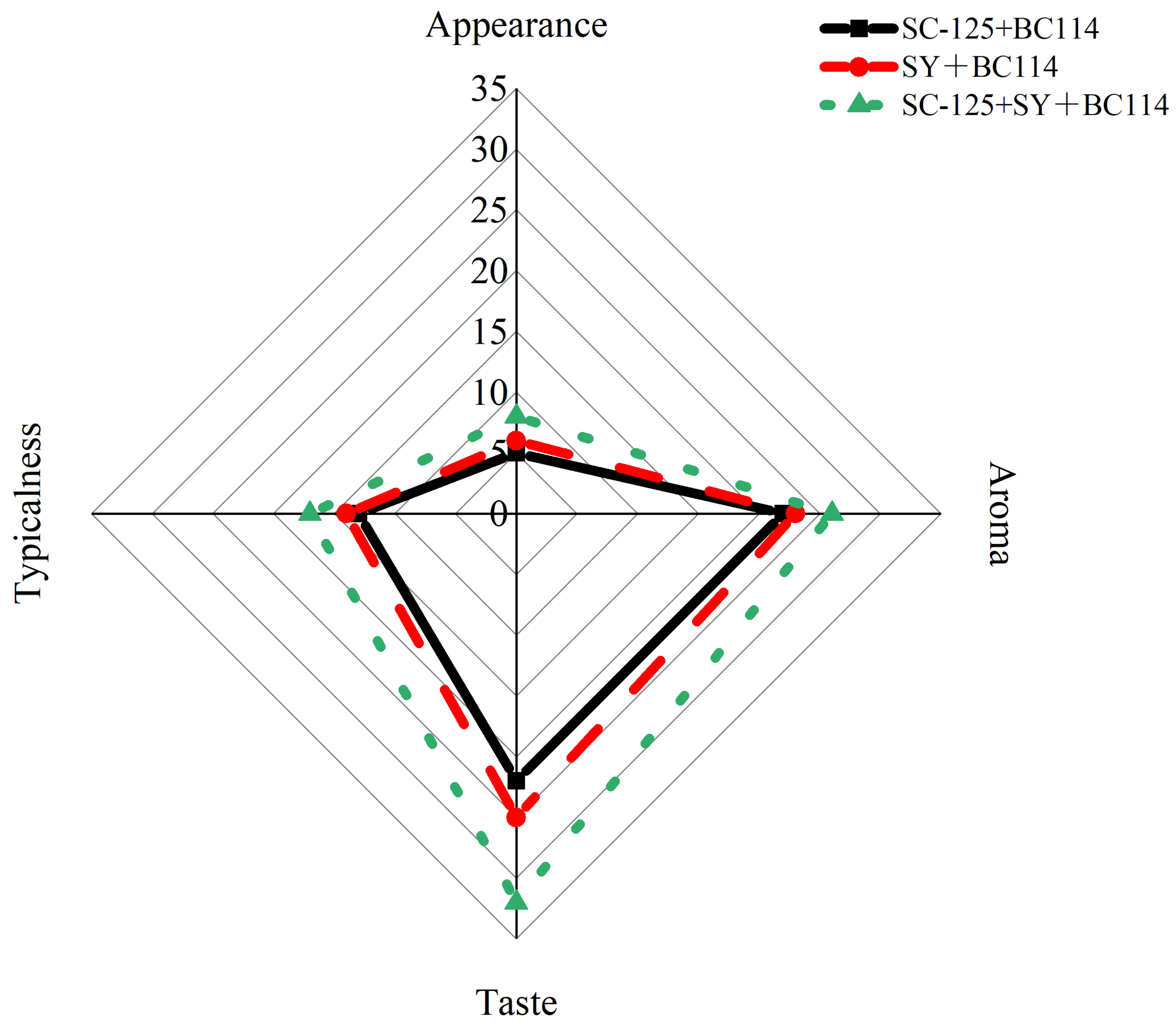
2.6. Sensory Evaluation Analysis
3. Materials and Methods
3.1. Strains and Materials
3.2. Determination of Colony Counts and pH during the Fermentation Process
3.3. Determination of Reducing Sugars, Alcohol, Organic Acids, Amino Acids, and Volatile Compounds during the Fermentation Process
3.4. Determination of Total Phenol and Total Flavonoid Contents and Antioxidant Activity during the Fermentation Process
3.5. Sensory Evaluation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, J.X.; Liang, L.; Wang, Y.X. Volatile composition changes in navel orange at different growth stages by HS-SPME–GC–MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wan, Y.; Chen, Y.R.; Li, M.X.; Liu, N.; Luo, H.B.; Huang, D.; Peng, H.; Fu, G.M. Effects of Torulaspora delbrueckii on physicochemical properties and volatile flavor compounds of navel orange wine. J. Food Compos. Anal. 2023, 121, 105328. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ye, H.; Zou, Y.T.; He, Z.H.; Xu, B.T.; Wang, S.; Peng, C.N.; Zhou, X.R.; Zhang, Q.; Xiang, W.L.; et al. Flavor characteristics of navel orange wine fermented by Saccharomyces cerevisiae SC-125 and Angel Yeast SY. Fermentation 2023, 9, 872. [Google Scholar] [CrossRef]
- Einfalt, D. Barley-sorghum craft beer production with Saccharomyces cerevisiae, Torulaspora delbrueckii and Metschnikowia pulcherrima yeast strains. Eur. Food Res. Technol. 2021, 247, 385–393. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. The life of Saccharomyces and non-Saccharomyces Yeasts in drinking wine. Microorganisms 2022, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, D.P.C.; Rempel, A.; Lanzini, J.R.; Silva, A.C.M.; Lazzari, T.; Barbizan, L.D.; Briao, V.B.; Colla, L.M.; Treichel, H. Fruit residues as biomass for bioethanol production using enzymatic hydrolysis as pretreatment. World J. Microbiol. Biotechnol. 2023, 39, 144. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Tristezza, M.; Giordano, L.; Rampino, P.; Perrotta, C.; Baruzzi, F.; Capozzi, V.; Mita, G.; Grieco, F. Exploitation of Prunus mahaleb fruit by fermentation with selected strains of Lactobacillus plantarum and Saccharomyces cerevisiae. Food Microbiol. 2019, 84, 103262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.P.; Wei, Z.H.; Yin, B.X.; Man, C.X.; Jiang, Y.J. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT Food Sci. Technol. 2020, 139, 110590. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Zhao, K.; Wang, X.L.; Wang, X.; Zhao, X.H.; Yu, B.; Wang, H.X. Co-inoculation of yeast and lactic acid bacteria to improve cherry wines sensory quality. Int. J. Food Sci. Technol. 2013, 48, 1783–1790. [Google Scholar] [CrossRef]
- Li, Y.; Nguyen, T.T.H.; Jin, J.H.; Lim, J.; Lee, J.; Piao, M.Z.; Mok, I.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Samaniego-Sánchez, C.; Marín-García, G.; Quesada-GranadosA, J.J. A new fermented beverage from sugarcane (Saccharum officinarum L.) molasses: Analysis of physicochemical properties and antioxidant capacity, and comparison with other industrial alcohol products. LWT Food Sci. Technol. 2020, 128, 109505. [Google Scholar] [CrossRef]
- Li, H.C.; Huang, J.T.; Wang, Y.Q.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Schieber., A.; Ganzle., M.G. Formation of tasteactive amino acids, amino acid derivatives and peptides in food fermentations-A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Antalick, G.; Suklje, K.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M. Influence of grape composition on red wine ester profile: Comparison between Cabernet Sauvignon and Shiraz cultivars from Australian warm climate. J. Agric. Food Chem. 2015, 63, 4664–4672. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Hu, K.; Zhang, J.X.; Zhu, X.L.; Tao, Y.S. Aroma modulation of Cabernet Gernischt dry red wine by optimal enzyme treatment strategy in winemaking. Food Chem. 2018, 245, 1248–1256. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Song, J.X.; Bi, J.F.; Meng, X.J.; Wu, X.Y. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose—ScienceDirect. Food Res. Int. 2018, 32, 132–135. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.K.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Bai, L.; Maimaitiviming, R.; Wang, L. Effects of four individual lactic acid bacteria on the physical and chemical and antioxidant properties of Kuqa apple juice during fermentation. Food Process. 2021, 45, 2411–2502. [Google Scholar] [CrossRef]
- Johnson, M.H.; Lucius, A.; Meyer, T.; de Mejia, E.G. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase by highbush blueberry (Vaccinium corombosum). J. Agric. Food Chem. 2011, 59, 8923–8930. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjärv, R.; Yang, B. Impact of lactic acid fermentation on acids, sugars, and phenolic compounds in black chokeberry and sea buckthorn juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Tan, X.; Zhang, S.M.; Zeng, L.; Tang, J.; Xiang, W.L. Characterization of γ-aminobutyric acid (GABA)-producing Saccharomyces cerevisiae and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 2019, 129, 447–453. [Google Scholar] [CrossRef]
- Stupak, M.; Kocourek, V.; Kolouchova, I.; Hajslova, J. Rapid approach for the determination of alcoholic strength and overall quality check of various spirit drinks and wines using GC–MS. Food Control 2017, 80, 307–313. [Google Scholar] [CrossRef]
- Gomis, D.B.; Gutierrez, M.J.M.; Alvarez, M.D.G. High-performance liquid chromatographic determination of major organic acids in apple juices and ciders. Chromatographia 1987, 24, 347–350. [Google Scholar] [CrossRef]
- Meng, Q.; Zhou, J.W.; Gao, D.; Xu, E.B.; Guo, M.M.; Liu, D.H. Desorption of nutrients and flavor compounds formation during the cooking of bone soup. Food Control 2022, 132, 108408. [Google Scholar] [CrossRef]
- Rossi, L.; Foschi, M.; Biancolillo, A.; Maggi, M.A.; D’Archivio, A.A. Optimization of HS-SPME-GC/MS analysis of wine volatiles supported by chemometrics for the aroma profiling of Trebbiano d’Abruzzo and Pecorino white wines produced in Abruzzo (Italy). Molecules 2022, 28, 1534. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Fatima, A.; Busquets, R.; Khan, M.R.; Ashraf, S.; Khan, M.S.; Oz, F. Effect of the media in the Folin-Ciocalteu assay for the analysis of the total phenolic content of olive product. Food Anal. Methods 2023, 16, 1627–1634. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Tian, Y.G.; Li, X.C.; Xie, H.; Wang, X.Z.; Xie, Y.L.; Chen, C.B.; Chen, D.F. Protective mechanism of the antioxidant baicalein toward hydroxyl Radical-Treated bone marrow-derived mesenchymal stem cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.A.; Park, G.G.; Jang, J.K.; Park, Y.S. Semi-continuous fermentation of onion vinegar and its functional properties. Molecules 2017, 22, 1313. [Google Scholar] [CrossRef]
- Liang, Z.C.; Lin, X.Z.; He, Z.G.; Su, H.; Li, W.X.; Ren, X.Y. Amino acid and microbial community dynamics during the fermentation of Hong Qu glutinous rice wine. Food Microbiol. 2020, 90, 103467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Zhang, Y.; Wang, W.; Ye, H.; Zhang, Q. Enhancing the Quality of Low-Alcohol Navel Orange Wine through Simultaneous Co-Fermentation Using Saccharomyces cerevisiae SC-125, Angel Yeast SY, and Lactiplantibacillus plantarum BC114. Molecules 2024, 29, 1781. https://doi.org/10.3390/molecules29081781
Xiong H, Zhang Y, Wang W, Ye H, Zhang Q. Enhancing the Quality of Low-Alcohol Navel Orange Wine through Simultaneous Co-Fermentation Using Saccharomyces cerevisiae SC-125, Angel Yeast SY, and Lactiplantibacillus plantarum BC114. Molecules. 2024; 29(8):1781. https://doi.org/10.3390/molecules29081781
Chicago/Turabian StyleXiong, Hua, Yingyue Zhang, Wanting Wang, Hong Ye, and Qing Zhang. 2024. "Enhancing the Quality of Low-Alcohol Navel Orange Wine through Simultaneous Co-Fermentation Using Saccharomyces cerevisiae SC-125, Angel Yeast SY, and Lactiplantibacillus plantarum BC114" Molecules 29, no. 8: 1781. https://doi.org/10.3390/molecules29081781
APA StyleXiong, H., Zhang, Y., Wang, W., Ye, H., & Zhang, Q. (2024). Enhancing the Quality of Low-Alcohol Navel Orange Wine through Simultaneous Co-Fermentation Using Saccharomyces cerevisiae SC-125, Angel Yeast SY, and Lactiplantibacillus plantarum BC114. Molecules, 29(8), 1781. https://doi.org/10.3390/molecules29081781




