Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Co-Fermentation on the Physicochemical and Flavor Compounds of Huaniu Apple Cider
Abstract
1. Introduction
2. Results and Discussion
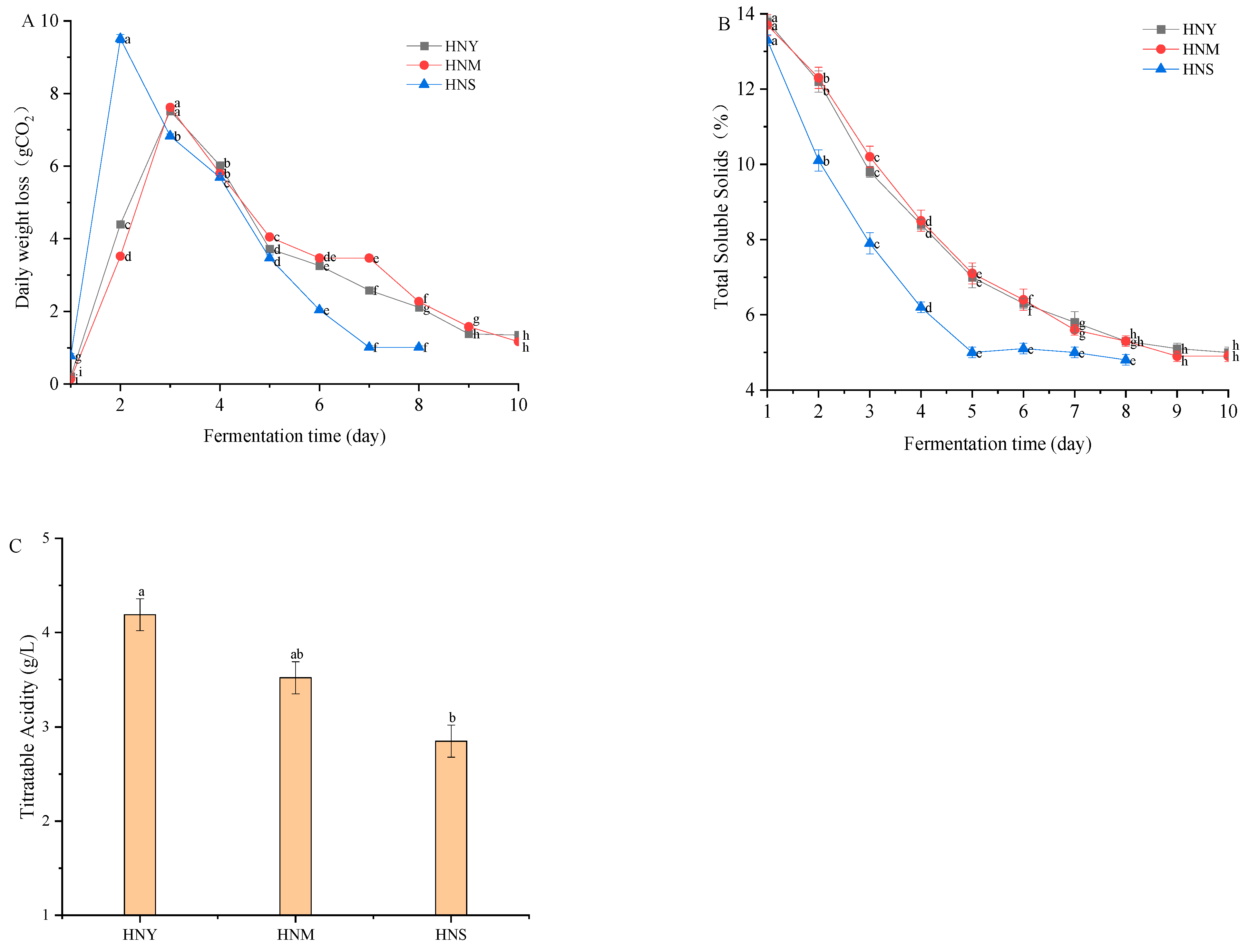
2.1. Effects of Different Inoculation Methods on the Physicochemical Properties of Huaniu Apple Cider
2.2. The Impact of Different Inoculation Methods on the Organic Acid Content in Huaniu Apple Cider
2.3. Analysis of Polyphenol and Flavonoid Contents in Huaniu Apple Cider Prepared via Different Inoculation Methods
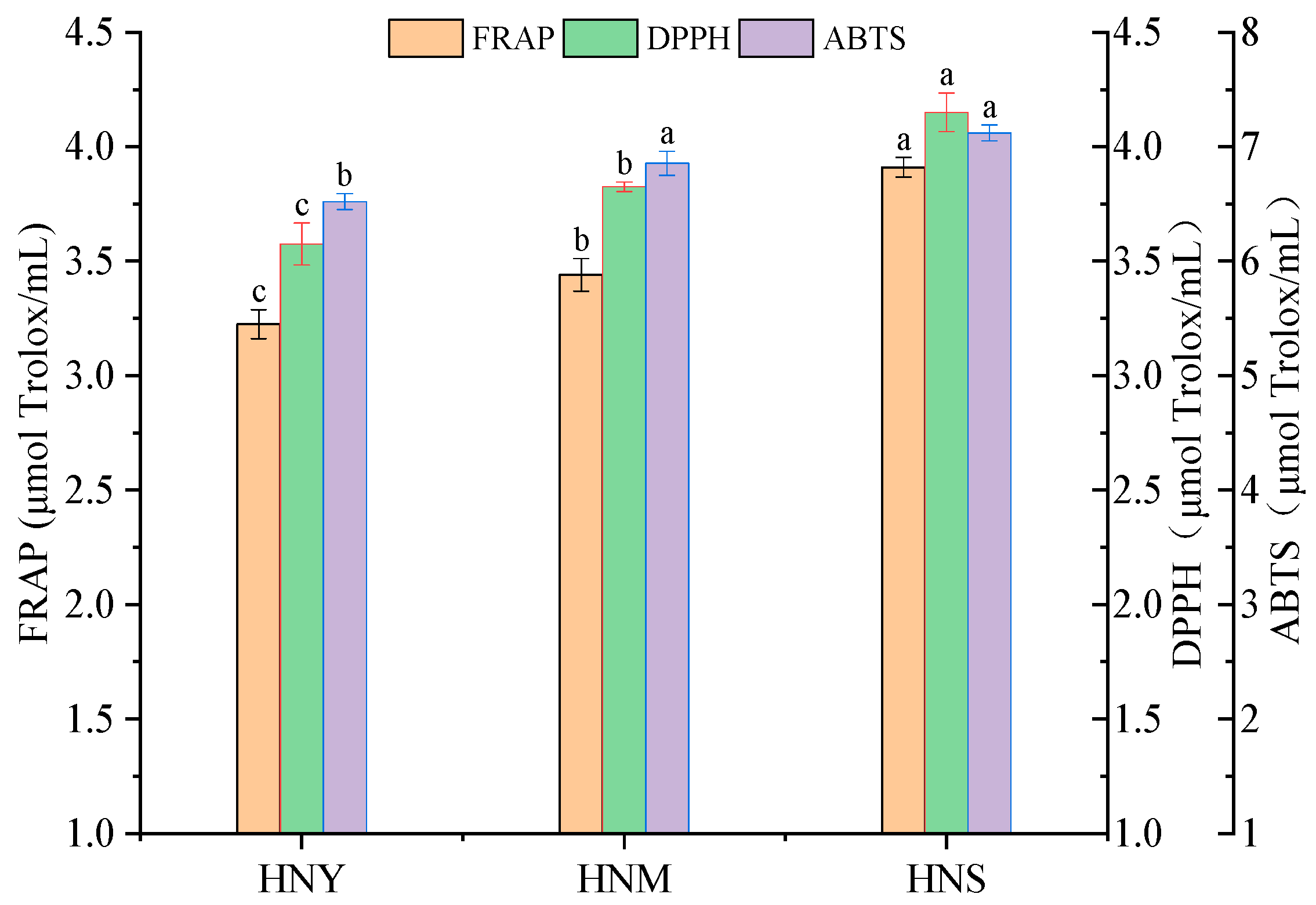
2.4. The Impact of Different Inoculation Methods on the Antioxidant Activity of Huaniu Apple Cider
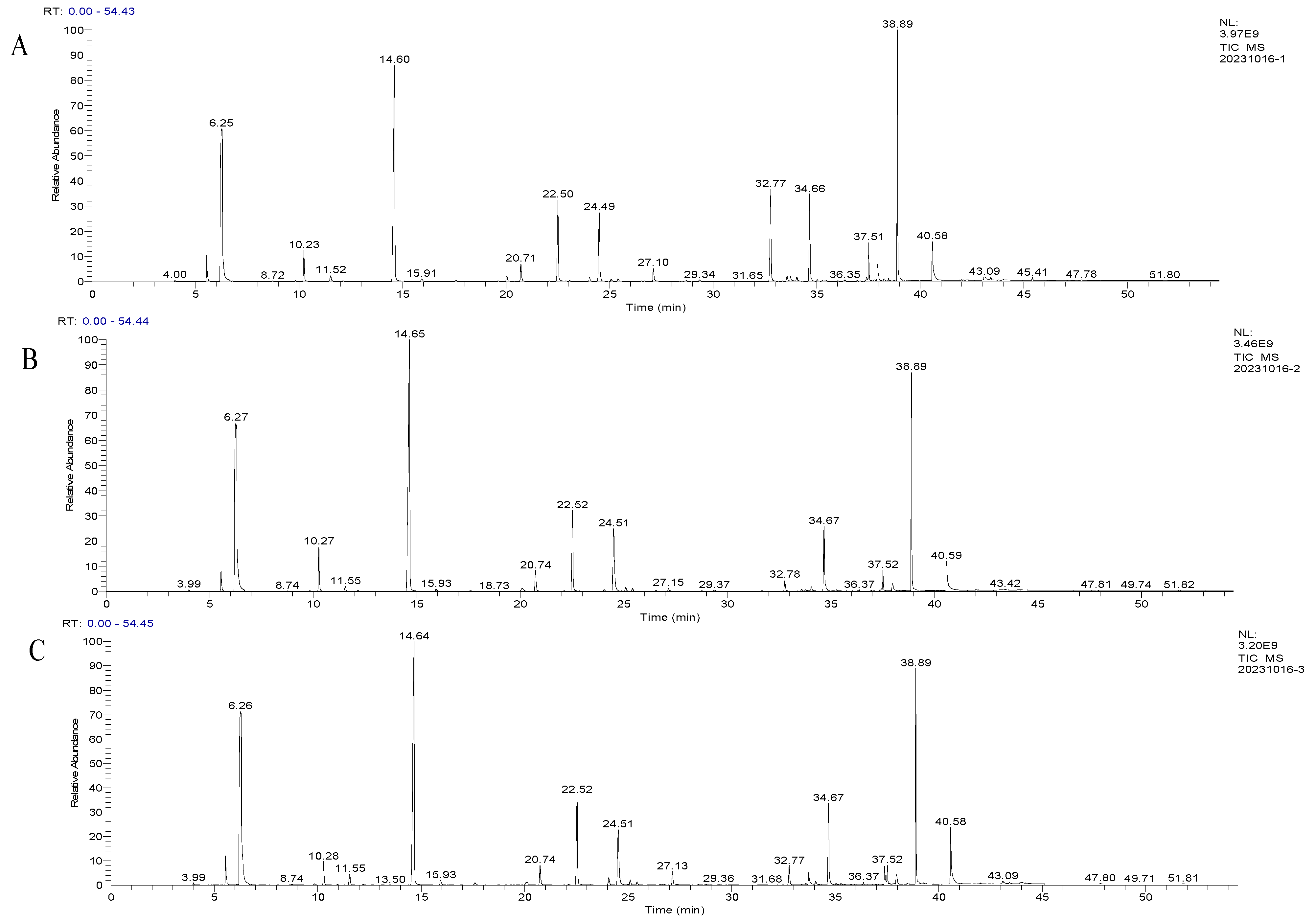
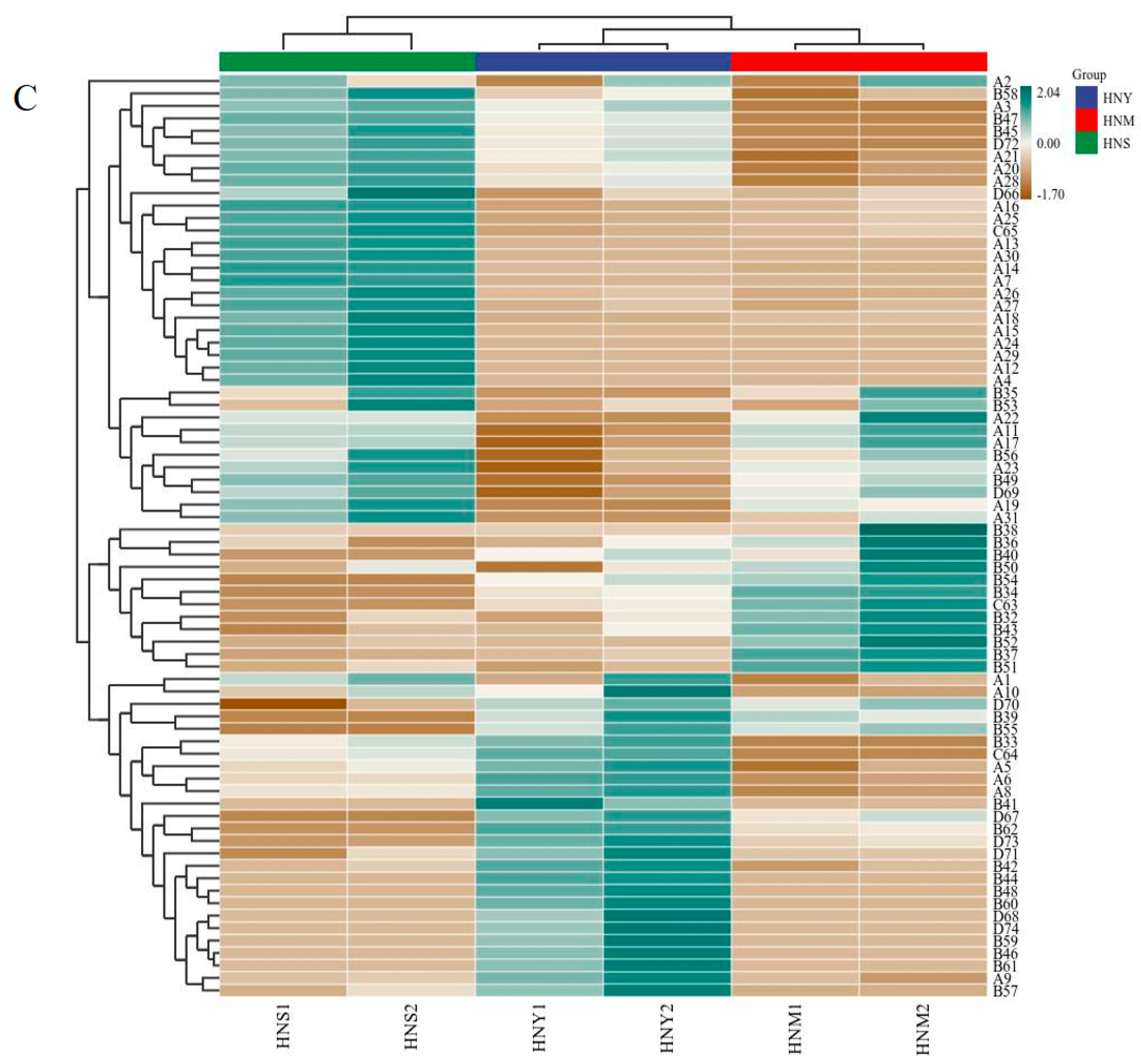
2.5. The Effect of Different Inoculation Methods on the Volatile Aroma Compounds in Huaniu Apple Cider
3. Materials and Methods
3.1. Materials
3.2. Strain Activation
3.3. Huaniu Apple Cider Fermentation
3.4. Measurement of Yeast Ethanol Fermentation Capacity, Soluble Solids Content, and Titratable Acidity
3.5. Organic Acid Analysis
3.6. Phenolic Compound Analysis
3.6.1. Determination of Total Phenolic Content (TPC)
3.6.2. Determination of Total Flavonoid Content (TFC)
3.7. Antioxidant Analysis
3.7.1. DPPH· Radical Scavenging Activity
3.7.2. ABTS Free-Radical Scavenging Assay
3.7.3. Ferric-Ion-Reducing Antioxidant Power (FRAP)
3.8. Volatile Composition Analysis
3.8.1. Extraction of Cider Aroma Components
3.8.2. Chromatographic and Mass Spectrometric Conditions
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Zhao, B.; Xie, M.; Wang, X.; Wu, X.; Chen, B.; Zhang, Y. The physiological differences in peel and pulp of Huaniu apples related to superficial scald. J. Gansu Agri. Univ. 2015, 50, 84–89. [Google Scholar]
- Wu, X.; Xie, M.; Zhao, B.; Wang, X.; Chen, B. Control and mechanism of superficial scald on Huaniu apples with 1-methylcyclopropene treatment. Sci. Technol. Food Ind. 2015, 36, 316–320, 325. [Google Scholar]
- Yang, L.; Yang, G.; Yang, J.; Luo, S.; Xu, A.; Li, Y.; Hu, L. Research on main indexes for evaluating Huaniu apple quality. China Fruits 2019, 5, 29–34. [Google Scholar]
- Xue, X.; Wang, J.; Lu, C.; Zhang, S. The impact of bagging on the fruit quality and storage characteristics of Huaniu apples. Jiangsu Agri. Sci. 2011, 39, 231–233. [Google Scholar]
- Wang, F.; Lu, W.; Yang, L.; Hu, L. Effects of low temperature vs 1-MCP treatment on quality and physiology of Huaniu apple during storage. Food Sci. 2014, 35, 346–349. [Google Scholar]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces cerevisiae and non-Saccharomyces strains on alcoholic fermentation behavior and aroma profile of yellow-fleshed peach wine. LWT-Food Sci. Technol. 2022, 155, 112993. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, X.; Fu, Y.; Zhang, Q.; Wang, X.; Cui, M.; Ma, Y.; Gao, X. Effects of Torulaspora delbrueckii co-fermented with Saccharomyces cerevisiae on physicochemical and aromatic profiles of blueberry fermented beverage. Food Chem. 2023, 409, 135284. [Google Scholar] [CrossRef]
- Vaquero, C.; Escott, C.; Heras, J.; Carrou, F.; Morata, A. Co-inoculations of Lachanceathermotolerans with different Hanseniaspora spp. Acidification, aroma, biocompatibility, and effects of nutrients in wine. Food Res. Int. 2022, 161, 111891. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Z.; Sun, L.; Zhang, Q.; Zhang, J. Effects of mixed fermentation on the aroma compounds of ‘Italian Riesling’ dry white wine in eastern foothill of helanmountain. Fermentation 2023, 9, 303. [Google Scholar] [CrossRef]
- Guerrini, S.; Galli, V.; Barbato, D.; Facchini, G.; Mangani, S.; Pierguidi, L.; Granchi, L. Effects of Saccharomyces cerevisiae and Starmerellabacillaris on the physicochemical and sensory characteristics of sparkling pear cider (Perry). Eur. Food Res. Technol. 2022, 249, 341–352. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT-Food Sci. Technol. 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Yang, B. Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol. 2018, 80, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulasporadelbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microb. Bio. 2014, 31, 277–293. [Google Scholar] [CrossRef]
- Ngqumba, Z.; Ntushelo, N.; Jolly, N.; Ximba, B.; Minnaar, P. Effect of Torulasporadelbrueckii Yeast treatment on flavanols and phenolic acids of chenin blanc wines. S. Afr. J. Enol. Vitic. 2017, 38, 192–200. [Google Scholar]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulasporadelbrueckii or Metschnikowiapulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2014, 240, 999–1012. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulasporadelbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef]
- Lu, Y.; Chua, J.-Y.; Huang, D.; Lee, P.-R.; Liu, S. Biotransformation of chemical constituents of durian wine with simultaneous alcoholic fermentation by Torulasporadelbrueckii and malolactic fermentation by Oenococcusoeni. Appl. Microbiol. Biotechnol. 2016, 100, 8877–8888. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Liu, W.; Yang, Y.; Zhang, Y.; Jin, C.; Sun, S. Comparison of fermentation behaviors and properties of raspberry wines by spontaneous and controlled alcoholic fermentations. Food Res. Int. 2020, 128, 108801. [Google Scholar] [CrossRef]
- Hou, R.; Zhao, P.; Xu, X.; Zhang, X.; Wang, Y.; Zhang, H. Effect of sequential fermentation with Torulasporadelbrueckii and four Saccharomyces cerevisiaes on volatile aromatic compounds and sensory preferences of strawberry wines. Food Ferment. Ind. 2023, 11, 1–9. [Google Scholar]
- Sottil, C.; Salor-Torregrosa, J.M.; Moreno-Garcia, J.; Peinado, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Using Torulasporadelbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus wine yeasts as starter cultures for fermentation and quality improvement of mead. Eur. Food Res. Technol. 2019, 245, 2705–2714. [Google Scholar] [CrossRef]
- Rêgo, E.; Rosa, C.; Freire, A.; Machado, A.; Gomes, F.; Costa, A.; Mendonça, M.; Hernández- Macdeo, M.; Padilha, F. Cashew wine and volatile compounds produced during fermentation by non-Saccharomyces and Saccharomyces yeast. LWT-Food Sci. Technol. 2020, 126, 109291. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Gao, Z. Evaluation of the quality of fermented kiwi wines made from different kiwifruit cultivars. Food Biosci. 2021, 42, 101051. [Google Scholar] [CrossRef]
- Qin, Z.; Petersen, M.; Bredie, W. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Jolly, N.; Gianvito, D.; Rantsiou, K.; Cocolin, L. Microbial interactions in winemaking: Ecological aspects and effect on wine quality. Trends Food Sci. Tech. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Pham, T.; Varjú, R.; Bujna, E.; Hoschke, Á.; Farkas, C.; Nguyen, T.; Sharma, M.; Pandey, A.; Gupa, V.; Nguyen, Q.; et al. Chemical and volatile composition of Pálinka produced using different commercial yeast strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2022, 381, 109891. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jiang, W.; Zhao, Y. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds and polyphenols in cherry wines. Food Chem. 2011, 127, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Shi, X.; Zhou, J.; Zhu, H. Fermentation condition optimization and antioxidant activity of jujube fruit wine by mixed yeasts. China Brew. 2023, 42, 210–215. [Google Scholar]
- Hong, M.; Li, J.; Chen, Y.; Qi, B.; Huang, Y.; Wu, J.; Yue, H.; Tong, Z.; Liu, Y.; Wang, F. Impact of mixed non-Saccharomyces yeast during fermentation on volatile aroma compounds of Vidal blanc icewine. LWT-Food Sci. Technol. 2021, 145, 111342. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The important contribution of non-Saccharomyces yeasts to the aroma complexity of wine: A review. Foods 2020, 10, 13. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Zhang, X.; Guo, H.; Yuan, Y.; Yue, T. Multi-omics discovery of aroma-active compound formation by Pichia kluyveri during cider production. LWT-Food Sci. Technol. 2022, 159, 113233. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Zhang, C.; Ma, H.; Xiao, D. Regulation of Saccharomyces cerevisiae genetic engineering on the production of acetate esters and higher alcohols during Chinese Baijiu fermentation. J. Ind. Microbiol. Biotechnol. 2017, 44, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-R.; Ong, Y.-L.; Yu, B.; Curran, P.; Liu, S.-Q. Profile of volatile compounds during papaya juice fermentation by a mixed culture of Saccharomyces cerevisiae and Williopsissaturnus. Food Microbiol. 2010, 27, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Mendes, F.; Guedes De Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniasporauvarum and Hanseniasporaguilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Torija, M.-J.; Mas, A.; Beltran, G.; Nanarro, Y. Effect of a multistarteryeast inoculum on ethanol reduction and population dynamics in wine fermentation. Foods 2021, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, L.; Xue, R.; Wang, C.; Chen, M.; Ramos, J.; Zhang, S.; Sun, B. Impact of different oak chips’ aging on the volatile compounds and sensory characteristics of Vitisamurensis wines. Foods 2022, 11, 1126. [Google Scholar] [CrossRef]
- Zhang, W.; Weng, P.; Wu, Z. Fermentation efficiency and flavor characteristics of bayberry wine with mixed starter culture of Issatchenkioorientalis and Saccharomyces cerevisiae. Food Sci. 2019, 40, 144–151. [Google Scholar]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Kang, S.; Zhang, J.; Zhang, F.; Zhang, H.; Yuan, J. Effects of fermentation conditions on content of isobutanol, isoamylalcohol and phenylethylalcohol in apple brandy. Modern Food Sci. Technol. 2018, 34, 167–174. [Google Scholar]
- Zeng, C.; Mu, Y.; Yuan, J.; Song, J.; Zhang, H.; Kang, S. Effect of fermentation temperature on physicochemical property and aroma components of cider. China Brew. 2023, 42, 192–197. [Google Scholar]
- GB/T15038-2006; Analytical Methods of Wine and Fruit Wine. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- Hu, L.; Hu, J.; Chen, X.; Lin, M.; Cao, Y.; Xu, T.; Zhao, Z. Effect of mixed-strain fermentation on the quality of compound fruit wine offragrant pear and passion fruit. China Brew. 2023, 42, 122–128. [Google Scholar]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpiniamimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditiselegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef] [PubMed]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteresisora L. and Ceibapentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Crespo, Y.; Naranjo, R.; Quitana, Y.; Sanchez, C.; Sanchez, E. Optimisation and characterisation of bio-oil produced by Acacia mangiumWilld wood pyrolysis. Wood Sci. Technol. 2017, 51, 1155–1171. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]


| Organic Acid | Content (mg/L) | ||
|---|---|---|---|
| HNY | HNM | HNS | |
| Malic Acid | 2934.68 ± 6.35 a | 1468.13 ± 7.99 c | 1919.86 ± 5.11 b |
| Citric Acid | 115.98 ± 2.50 b | 197.03 ± 1.61 a | 195.31 ± 2.05 a |
| Succinic Acid | 730.50 ± 2.56 b | 666.96 ± 2.97 c | 789.03 ± 2.05 a |
| Acetic Acid | 117.12 ± 2.61 b | 139.34 ± 1.56 a | 73.39 ± 2.47 c |
| Fumaric Acid | 6.90 ± 0.48 b | 11.28 ± 0.59 a | 11.53 ± 0.82 a |
| Propionic Acid | 224.76 ± 2.96 c | 439.31 ± 2.50 a | 261.09 ± 2.42 b |
| No. | a RI | Compound | b Concentration (mg/L) | c ID | ||
|---|---|---|---|---|---|---|
| HNY | HNM | HNS | ||||
| A1 | 872 | ethyl acetate | 0.17 ± 0.10 a | 0.09 ± 0.03 a | 0.20 ± 0.03 a | RI/MS |
| A2 | 1094 | isobutyl isobutyrate | 0.16 ± 0.07 a | 0.17 ± 0.08 a | 0.19 ± 0.04 a | RI/MS |
| A3 | 998 | ethyl propionate | 0.47 ± 0.1 a | nd | 0.65 ± 0.07 a | RI/MS |
| A4 | 1051 | ethyl 2,3-epoxybutyrate | nd | nd | 0.06 ± 0.01 a | RI/MS |
| A5 | 1220 | butyl butyrate | 0.94 ± 0.07 a | 0.47 ± 0.10 b | 0.67 ± 0.06 b | RI/MS |
| A6 | 1223 | ethyl 3-methyl-1-butanolate | 9.03 ± 0.17 a | 4.44 ± 0.33 c | 5.83 ± 0.06 b | RI/MS |
| A7 | 1225 | amyl valerate | nd | nd | 0.02 ± 0.00 a | RI/MS |
| A8 | 1232 | ethyl hexanoate | 4.26 ± 0.21 a | 2.08 ± 0.18 c | 2.94 ± 0.04 b | RI/MS |
| A9 | 1603 | hexyl hexanoate | 2.2 ± 0.35 a | 0.76 ± 0.18 b | 0.95 ± 0.06 b | RI/MS |
| A10 | 1294 | ethyl 5-hexen-1-olate | 0.05 ± 0.03 a | 0.01 ± 0.00 a | 0.03 ± 0.01 a | RI/MS |
| A11 | 1440 | ethyl octanoate | 51.16 ± 2.09 b | 65.73 ± 4.24 a | 63.15 ± 0.3 a | RI/MS |
| A12 | 1425 | hexyl isovalerate | nd | nd | 0.26 ± 0.06 a | RI/MS |
| A13 | 1750 | isobutyl decanoate | nd | nd | 1.38 ± 0.1 a | RI/MS |
| A14 | 1648 | ethyl decanoate | 13.01 ± 0.41 b | 8.92 ± 0.64 c | 75.21 ± 0.35 a | RI/MS |
| A15 | 1651 | ethyl undecylenate | nd | nd | 0.08 ± 0.01 a | RI/MS |
| A16 | 1670 | 3-methylbutyl octanoate | 1.20 ± 0.14 b | 1.68 ± 0.31 b | 4.58 ± 0.10 a | RI/MS |
| A17 | 1644 | ethyl benzoate | 3.6 ± 0.20 b | 4.5 ± 0.28 a | 4.35 ± 0.04 a | RI/MS |
| A18 | 1667 | methyl cis-9-decenoate | nd | 0.01 ± 0.00 b | 0.11 ± 0.03 a | RI/MS |
| A19 | 1699 | ethyl cis-2-decenoate | nd | 0.12 ± 0.08 b | 0.22 ± 0.04 a | RI/MS |
| A20 | 1703 | ethyl 9-decenoate | 53.92 ± 1.15 b | 48.85 ± 1.23 c | 60.01 ± 0.88 a | RI/MS |
| A21 | 1705 | ethyl 4-decenoate | 0.78 ± 0.14 a | 0.20 ± 0.14 b | 1.17 ± 0.11 a | RI/MS |
| A22 | 2565 | benzyl benzoate | nd | 0.15 ± 0.08 a | 0.10 ± 0.00 a | RI/MS |
| A23 | 1778 | ethyl phenylacetate | 0.43 ± 0.07 b | 0.58 ± 0.01 ab | 0.66 ± 0.07 a | RI/MS |
| A24 | 1361 | methyl 4-hydroxybutyrate | nd | nd | 0.33 ± 0.06 a | RI/MS |
| A25 | 1779 | ethyl 2-phenylacetate | 8.99 ± 0.34 b | 10.07 ± 0.55 b | 17.84 ± 0.89 a | RI/MS |
| A26 | 1856 | ethyl laurate | 8.69 ± 0.21 b | 8.14 ± 0.1 b | 13.81 ± 0.98 a | RI/MS |
| A27 | 1921 | decyl 3-methylbutyrate | 0.30 ± 0.14 b | 0.21 ± 0.13 b | 2.02 ± 0.18 a | MS |
| A28 | 1932 | isobutyl 2,2,4-trimethyl-1,3-pentanediol ester | 0.82 ± 0.11 b | 0.34 ± 0.08 c | 1.31 ± 0.08 a | MS |
| A29 | 1967 | ethyl 3-hydroxydecanoate | nd | nd | 0.34 ± 0.06 a | MS |
| A30 | 2082 | ethyl 10-undecenoate | nd | nd | 0.62 ± 0.06 a | RI/MS |
| A31 | 2198 | hexyl 2-phenylacetate | nd | 0.17 ± 0.10 b | 0.39 ± 0.08 a | RI/MS |
| B32 | 1037 | 1-propanol | 0.13 ± 0.03 ab | 0.22 ± 0.06 a | 0.12 ± 0.06 b | RI/MS |
| B33 | 1327 | 2-heptanol | 0.12 ± 0.01 a | nd | 0.07 ± 0.01 b | RI/MS |
| B34 | 1036 | 2-methyl-1-propanol | 20.89 ± 1.02 b | 29.09 ± 1.20 a | 14.14 ± 0.18 c | RI/MS |
| B35 | 1251 | 2-pentanol | 0.02 ± 0.00 b | 0.04 ± 0.01 a | 0.04 ± 0.01 a | RI/MS |
| B36 | 1125 | 1-butanol | 0.68 ± 0.03 a | 0.76 ± 0.06 a | 0.66 ± 0.03 a | RI/MS |
| B37 | 1202 | 3-methyl-1-butanol | 232.62 ± 3.51 b | 281.91 ± 4.62 a | 224.93 ± 2.53 b | RI/MS |
| B38 | 1251 | 1-pentanol | 0.1 ± 0.00 a | 0.13 ± 0.04 a | 0.1 ± 0.00 a | RI/MS |
| B39 | 1294 | 4-methyl-1-pentanol | 0.11 ± 0.04 a | 0.08 ± 0.01 a | nd | RI/MS |
| B40 | 1464 | (S)-2-heptanol | 0.06 ± 0.01 a | 0.09 ± 0.07 a | nd | RI/MS |
| B41 | 1320 | 3-methyl-1,5-pentanediol | 0.04 ± 0.01 a | nd | nd | RI/MS |
| B42 | 1330 | 3-methyl-1-pentanol | 0.54 ± 0.04 a | 0.19 ± 0.04 b | 0.23 ± 0.03 b | RI/MS |
| B43 | 1361 | 1-hexanol | 14.48 ± 0.51 b | 16.31 ± 0.40 a | 13.85 ± 0.54 b | RI/MS |
| B44 | 1333 | 3-hexen-1-ol | 0.4 ± 0.04 a | nd | nd | RI/MS |
| B45 | 1379 | 2-nonen-1-ol | 0.13 ± 0.03 b | nd | 0.24 ± 0.04 a | RI/MS |
| B46 | 1822 | (E)-2-decen-1-ol | 0.04 ± 0.01 a | nd | nd | RI/MS |
| B47 | 1827 | 3,4,5-trimethyl-4-heptanol | 3.44 ± 0.24 b | nd | 5.95 ± 0.23 a | MS |
| B48 | 1841 | 2,3-dimethyl-3-octanol | 0.08 ± 0.01 a | nd | nd | RI/MS |
| B49 | 1465 | 1-heptanol | 1.69 ± 0.23 c | 2.94 ± 0.27 b | 3.56 ± 0.21 a | RI/MS |
| B50 | 1454 | 6-methyl-5-hepten-2-ol | 2.15 ± 0.11 a | 2.39 ± 0.11 a | 2.21 ± 0.08 a | RI/MS |
| B51 | 1435 | 2-ethyl-1-hexanol | 0.52 ± 0.03 b | 0.78 ± 0.03 a | 0.55 ± 0.04 b | RI/MS |
| B52 | 1524 | 2-nonanol | 0.11 ± 0.00 b | 0.25 ± 0.06 a | 0.11 ± 0.01 b | RI/MS |
| B53 | 1541 | linalool | 0.20 ± 0.01 a | 0.22 ± 0.04 a | 0.24 ± 0.06 a | RI/MS |
| B54 | 1620 | (E)-2-octen-1-ol | 0.22 ± 0.04 a | 0.35 ± 0.10 a | nd | RI/MS |
| B55 | 1672 | 1-nonanol | 0.28 ± 0.10 a | 0.24 ± 0.04 a | nd | RI/MS |
| B56 | 1714 | 3-(methylthio)-1-propanol | 0.67 ± 0.10 c | 0.89 ± 0.11 b | 0.98 ± 0.14 a | RI/MS |
| B57 | 1771 | 1-decanol | 0.27 ± 0.03 a | 0.19 ± 0.00 b | 0.20 ± 0.01 b | RI/MS |
| B58 | 1779 | (R)-3,7-dimethyl-6-decen-1-ol | 0.85 ± 0.07 b | 0.67 ± 0.13 c | 1.16 ± 0.1 a | MS |
| B59 | 1786 | 7-methyl-3-methylene-6-decen-1-ol | 0.07 ± 0.03 a | nd | nd | MS |
| B60 | 1802 | 2-methyl-1-decanol | 0.06 ± 0.01 a | nd | nd | RI/MS |
| B61 | 1880 | benzyl alcohol | 0.12 ± 0.04 a | nd | nd | RI/MS |
| B62 | 1915 | phenylethanol | 125.68 ± 1.26 a | 101.52 ± 1.98 b | 86.83 ± 0.51 c | RI/MS |
| C63 | 1580 | isobutyric acid | 0.24 ± 0.04 b | 0.62 ± 0.11 a | nd | RI/MS |
| C64 | 2281 | decanoic acid | 6.87 ± 0.21 a | nd | 3.6 ± 0.71 b | RI/MS |
| C65 | 2347 | 9-decenoic acid | 0.46 ± 0.07 c | 0.61 ± 0.08 b | 1.74 ± 0.14 a | RI/MS |
| D66 | 915 | 3-methylbutanal | 0.1 ± 0.03 b | 0.11 ± 0.01 b | 0.21 ± 0.06 a | RI/MS |
| D67 | 1241 | 3-octanone | 0.09 ± 0.01 a | 0.05 ± 0.01 a | nd | RI/MS |
| D68 | 1320 | 1-decen-3-one | 0.03 ± 0.01 a | nd | nd | RI/MS |
| D69 | 1425 | 6-methyl-5-hepten-2-one | 0.21 ± 0.01 b | 0.26 ± 0.01 a | 0.27 ± 0.01 a | RI/MS |
| D70 | 1505 | dihydro-2-methyl-3(2H)-thiophenone | 0.28 ± 0.01 a | 0.27 ± 0.01 ab | 0.21 ± 0.03 b | RI/MS |
| D71 | 1681 | 2,4,4-trimethyl-3-(3-methylbutyl)cyclohex-2-enone | 0.41 ± 0.03 a | 0.33 ± 0.01 b | 0.32 ± 0.03 b | MS |
| D72 | 1814 | β-damascenone | 0.40 ± 0.02 b | nd | 0.79 ± 0.10 a | RI/MS |
| D73 | 1830 | 2,5-dimethylbenzaldehyde | 1.06 ± 0.07 a | 0.75 ± 0.03 b | 0.62 ± 0.01 b | MS |
| D74 | 1953 | 6,10-dimethyl-5,9-undecadien-2-one | 0.16 ± 0.07 a | nd | nd | MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Mu, Y.; Yuan, J.; Zhang, H.; Song, J.; Kang, S. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Co-Fermentation on the Physicochemical and Flavor Compounds of Huaniu Apple Cider. Molecules 2024, 29, 1750. https://doi.org/10.3390/molecules29081750
Zeng C, Mu Y, Yuan J, Zhang H, Song J, Kang S. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Co-Fermentation on the Physicochemical and Flavor Compounds of Huaniu Apple Cider. Molecules. 2024; 29(8):1750. https://doi.org/10.3390/molecules29081750
Chicago/Turabian StyleZeng, Chaozhen, Yuwen Mu, Jing Yuan, Haiyan Zhang, Juan Song, and Sanjiang Kang. 2024. "Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Co-Fermentation on the Physicochemical and Flavor Compounds of Huaniu Apple Cider" Molecules 29, no. 8: 1750. https://doi.org/10.3390/molecules29081750
APA StyleZeng, C., Mu, Y., Yuan, J., Zhang, H., Song, J., & Kang, S. (2024). Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Co-Fermentation on the Physicochemical and Flavor Compounds of Huaniu Apple Cider. Molecules, 29(8), 1750. https://doi.org/10.3390/molecules29081750







