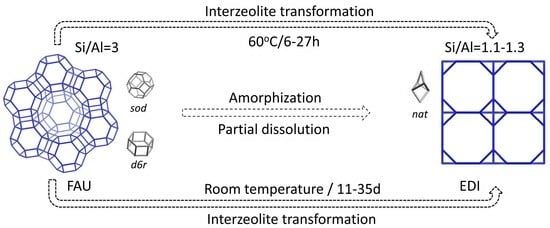
Interzeolite Transformation from FAU-to-EDI Type of Zeolite
Abstract
1. Introduction
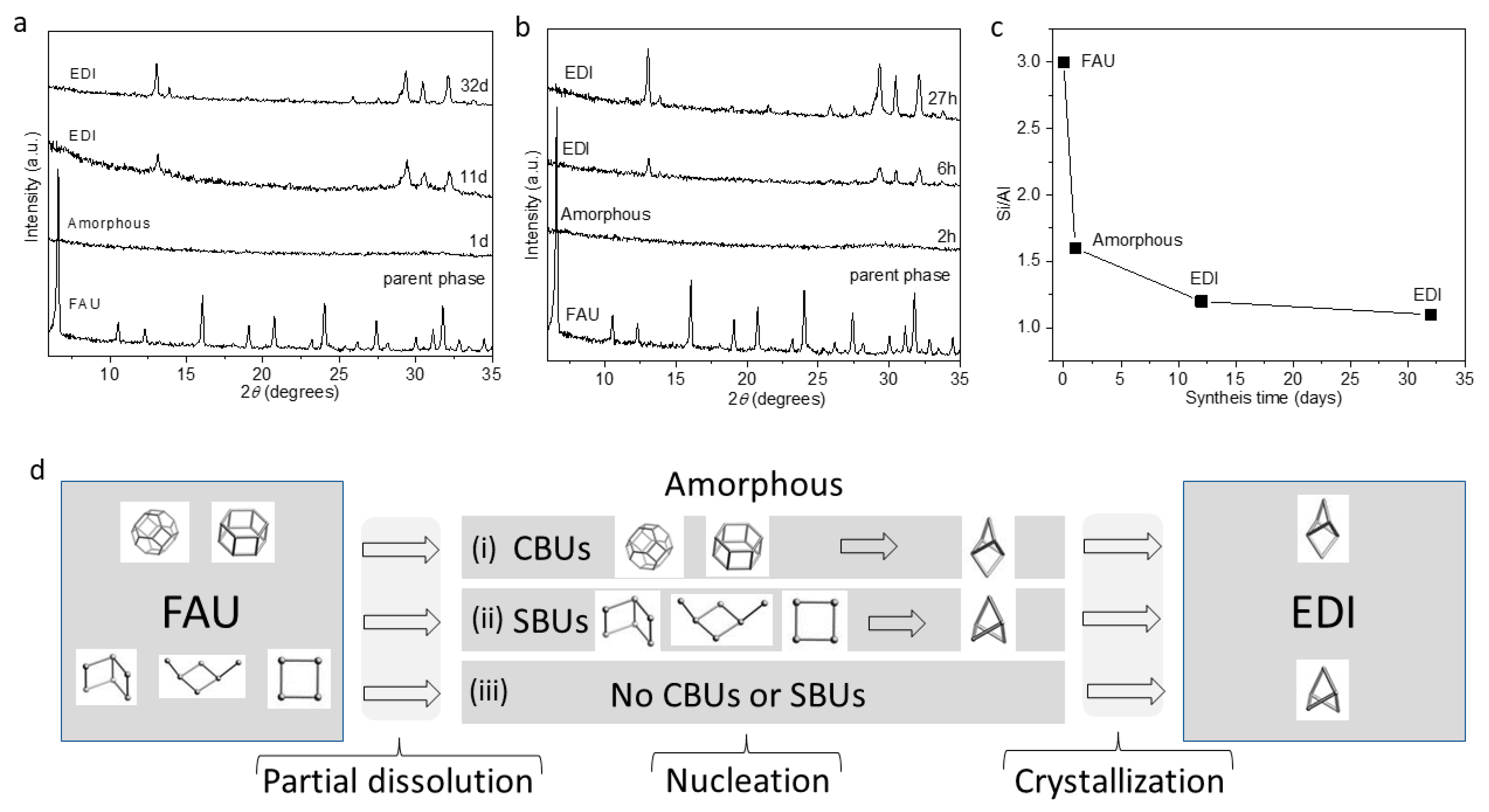
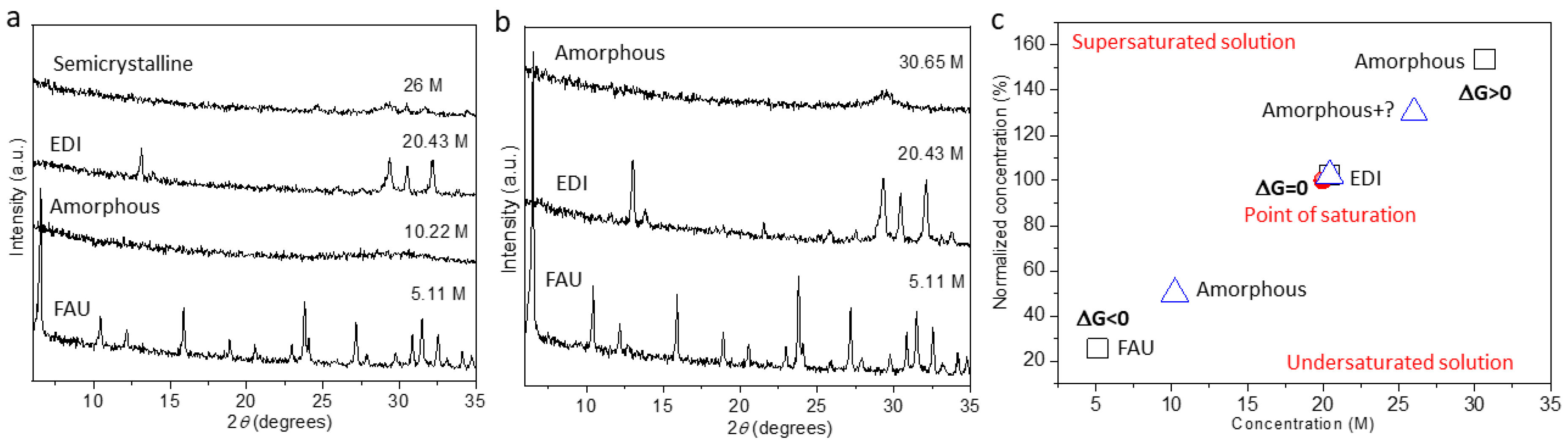
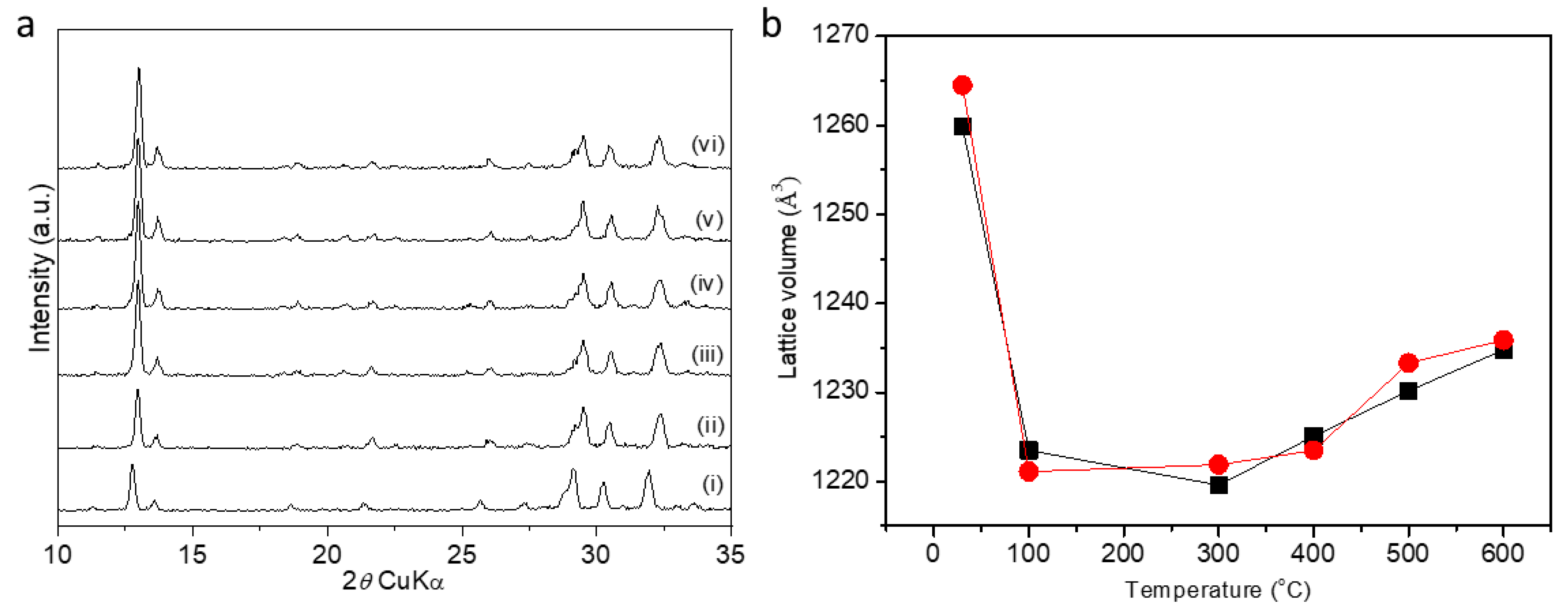
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, R.; Mallette, A.J.; Rimer, J.D. Controlling Nucleation Pathways in Zeolite Crystallization: Seeding Conceptual Methodologies for Advanced Materials Design. J. Am. Chem. Soc. 2021, 143, 21446–21460. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Itakura, M.; Sadakane, M. High Potential of Interzeolite Conversion Method for Zeolite Synthesis. J. Jpn. Pet. Inst. 2013, 56, 183–197. [Google Scholar] [CrossRef]
- Nakazawa, N.; Ikeda, T.; Hiyoshi, N.; Yoshida, Y.; Han, Q.; Inagaki, S.; Kubota, Y. A Microporous Aluminosilicate with 12-, 12-, and 8-Ring Pores and Isolated 8-Ring Channels. J. Am. Chem. Soc. 2017, 139, 7989–7997. [Google Scholar] [CrossRef] [PubMed]
- Khodabandeh, S.; Lee, G.; Davis, M.E. CIT-4: The first synthetic analogue of brewsterite. Microporous Mater. 1997, 11, 87–95. [Google Scholar] [CrossRef]
- Inoue, T.; Itakura, M.; Jon, H.; Oumi, Y.; Takahashi, A.; Fujitani, T.; Sano, T. Synthesis of LEV zeolite by interzeolite conversion method and its catalytic performance in ethanol to olefins reaction. Microporous Mesoporous Mater. 2009, 122, 149–154. [Google Scholar] [CrossRef]
- Devos, J.; Bols, M.L.; Plessers, D.; Goethem, C.V.; Seo, J.W.; Hwang, S.-J.; Sels, B.F.; Dusselier, M. Synthesis–Structure–Activity Relations in Fe-CHA for C–H Activation: Control of Al Distribution by Interzeolite Conversion. Chem. Mater. 2020, 32, 273–285. [Google Scholar] [CrossRef]
- Sonoda, T.; Maruo, T.; Yamasaki, Y.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Synthesis of high-silica AEI zeolites with enhanced thermal stability by hydrothermal conversion of FAU zeolites, and their activity in the selective catalytic reduction of NOx with NH3. J. Mater. Chem. A 2015, 3, 857–865. [Google Scholar] [CrossRef]
- Mendoza-Castro, M.J.; Qie, Z.; Fan, X.; Linares, N.; García-Martínez, J. Tunable hybrid zeolites prepared by partial interconversion. Nat. Commun. 2023, 14, 1256. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Miyake, K.; Hirota, Y.; Nishiyama, N.; Miyamoto, M.; Oumi, Y.; Tanaka, S. Solvent/OSDA-free interzeolite transformation of FAU into CHA zeolite with quantitative yield. Microporous Mesoporous Mater. 2019, 278, 219–224. [Google Scholar] [CrossRef]
- Mielby, J.; Møller, K.H.; Iltsiou, D.; Goodarzi, F.; Enemark-Rasmussen, K.; Kegnæs, S. A shortcut to high-quality gmelinite through steam-assisted interzeolite transformation. Microporous Mesoporous Mater. 2022, 330, 111606. [Google Scholar] [CrossRef]
- Li, Q.; Bing, L.; Li, F.; Liu, J.; Han, D.; Wang, F.; Wang, G. Rapid and facile synthesis of hierarchical nanocrystalline SSZ-13 via the interzeolite transformation of ZSM-5. New J. Chem. 2020, 44, 5501–5507. [Google Scholar] [CrossRef]
- Geng, H.; Li, G.; Liu, D.; Liu, C. Rapid and efficient synthesis of CHA-type zeolite by interzeolite conversion of LTA-type zeolite in the presence of N, N, N-trimethyladamantammonium hydroxide. J. Solid State Chem. 2018, 265, 193–199. [Google Scholar] [CrossRef]
- Mendoza-Castro, M.J.; De Oliveira-Jardim, E.; Ramírez-Marquez, N.-T.; Trujillo, C.-A.; Linares, N.; García-Martínez, J. Hierarchical Catalysts Prepared by Interzeolite Transformation. J. Am. Chem. Soc. 2022, 144, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yuan, Y.; Han, Q.; Dong, L.; Chen, L.; Zhang, X.; Xu, L. High yield synthesis of nanoscale high-silica ZSM-5 zeolites via interzeolite transformation with a new strategy. Catal. Sci. Technol. 2020, 10, 7904–7913. [Google Scholar] [CrossRef]
- Martín, N.; Moliner, M.; Corma, A. High yield synthesis of high-silica chabazite by combining the role of zeolite precursors and tetraethylammonium: SCR of NOx. Chem. Commun. 2015, 51, 9965–9968. [Google Scholar] [CrossRef] [PubMed]
- Zones, S.I. Conversion of faujasites to high-silica chabazite SSZ-13 in the presence of N,N,N-trimethyl-1-adamantammonium iodide. J. Chem. Soc. Faraday Trans. 1991, 87, 3709–3716. [Google Scholar] [CrossRef]
- Møller, K.H.; Debost, M.; Lakiss, L.; Kegnæs, S.; Mintova, S. Interzeolite conversion of a micronsized FAU to a nanosized CHA zeolite free of organic structure directing agent with a high CO2 capacity. RSC Adv. 2020, 10, 42953–42959. [Google Scholar] [CrossRef]
- Tanigawa, T.; Tsunoji, N.; Sadakane, M.; Sano, T. High-quality synthesis of a nanosized CHA zeolite by a combination of a starting FAU zeolite and aluminum sources. Dalton Trans. 2020, 49, 9972–9982. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, P.; Li, J.; Wang, Y.; Xu, S.; Yang, Z.; Liu, X.; Xu, L.; Li, X.; Zhu, X. Inter-zeolite transformation from *MRE to EUO: A new synthesis route for EUO zeolite. Catal. Today 2022, 405–406, 321–328. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Ghorbanpour, A.; Conato, M.T.; McGrail, B.P.; Grabow, L.C.; Motkuri, R.K.; Rimer, J.D. Synthesis Strategies for Ultrastable Zeolite GIS Polymorphs as Sorbents for Selective Separations. Chem. Eur. J. 2016, 22, 16078–16088. [Google Scholar] [CrossRef]
- Chiyoda, O.; Davis, M.E. Hydrothermal conversion of Y-zeolite using alkaline-earth cations. Microporous Mesoporous Mater. 1999, 32, 257–264. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Chu, W.; Yang, C.; Wang, Y.; Zhu, X.; Xin, W.; Liu, Z.; Wang, H.; Liu, S.; et al. N-methyl-2-pyrrolidone-induced conversion of USY into hollow Beta zeolite and its application in the alkylation of benzene with isobutylene. Microporous Mesoporous Mater. 2020, 294, 109944. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Encapsulation of Metal Clusters within MFI via Interzeolite Transformations and Direct Hydrothermal Syntheses and Catalytic Consequences of Their Confinement. J. Am. Chem. Soc. 2014, 136, 15280–15290. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Synthesis of Zeolites via Interzeolite Transformations without Organic Structure-Directing Agents. Chem. Mater. 2015, 27, 2056–2066. [Google Scholar] [CrossRef]
- Van Tendeloo, L.; Gobechiya, E.; Breynaert, E.; Martens, J.A.; Kirschhock, C.E.A. Alkaline cations directing the transformation of FAU zeolites into five different framework types. Chem. Commun. 2013, 49, 11737–11739. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Deimund, M.A.; Bhawe, Y.; Davis, M.E. Organic-Free Synthesis of CHA-Type Zeolite Catalysts for the Methanol-to-Olefins Reaction. ACS Catal. 2015, 5, 4456–4465. [Google Scholar] [CrossRef]
- Qin, W.; Jain, R.; Robles Hernández, F.C.; Rimer, J.D. Organic-Free Interzeolite Transformation in the Absence of Common Building Units. Chem. Eur. J. 2019, 25, 5893–5898. [Google Scholar] [CrossRef]
- dos Santos, M.B.; Vianna, K.C.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. Studies on the synthesis of ZSM-5 by interzeolite transformation from zeolite Y without using organic structure directing agents. Microporous Mesoporous Mater. 2020, 306, 110413. [Google Scholar] [CrossRef]
- Xie, D. Synthesis of GME Framework Type Zeolites. US. Patent 9,643,853, 19 May 2017. [Google Scholar]
- Itakura, M.; Goto, I.; Takahashi, A.; Fujitani, T.; Ide, Y.; Sadakane, M.; Sano, T. Synthesis of high-silica CHA type zeolite by interzeolite conversion of FAU type zeolite in the presence of seed crystals. Microporous Mesoporous Mater. 2011, 144, 91–96. [Google Scholar] [CrossRef]
- Takata, T.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Nanosized CHA zeolites with high thermal and hydrothermal stability derived from the hydrothermal conversion of FAU zeolite. Microporous Mesoporous Mater. 2016, 225, 524–533. [Google Scholar] [CrossRef]
- Jon, H.; Nakahata, K.; Lu, B.; Oumi, Y.; Sano, T. Hydrothermal conversion of FAU into ∗BEA zeolites. Microporous Mesoporous Mater. 2006, 96, 72–78. [Google Scholar] [CrossRef]
- Sasaki, H.; Jon, H.; Itakura, M.; Inoue, T.; Ikeda, T.; Oumi, Y.; Sano, T. Hydrothermal conversion of FAU zeolite into aluminous MTN zeolite. J. Porous Mater. 2009, 16, 465–471. [Google Scholar] [CrossRef]
- Itakura, M.; Oumi, Y.; Sadakane, M.; Sano, T. Synthesis of high-silica offretite by the interzeolite conversion method. Mater. Res. Bull. 2010, 45, 646–650. [Google Scholar] [CrossRef]
- Jon, H.; Takahashi, S.; Sasaki, H.; Oumi, Y.; Sano, T. Hydrothermal conversion of FAU zeolite into RUT zeolite in TMAOH system. Microporous Mesoporous Mater. 2008, 113, 56–63. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wang, X.; Chen, H.; Li, H.; Sun, C.; Sun, L.; Fan, C.; Wang, C. An efficient route for synthesis of ERI zeolite through conversion of FAU zeolite in the presence of N,N-dimethylpiperidinium hydroxide. Microporous Mesoporous Mater. 2019, 279, 407–415. [Google Scholar] [CrossRef]
- Yoshioka, T.; Liu, Z.; Iyoki, K.; Chokkalingam, A.; Yonezawa, Y.; Hotta, Y.; Ohnishi, R.; Matsuo, T.; Yanaba, Y.; Ohara, K.; et al. Ultrafast and continuous-flow synthesis of AFX zeolite via interzeolite conversion of FAU zeolite. React. Chem. Eng. 2021, 6, 74–81. [Google Scholar] [CrossRef]
- Maruo, T.; Yamanaka, N.; Tsunoji, N.; Sadakane, M.; Sano, T. Facile Synthesis of AEI Zeolites by Hydrothermal Conversion of FAU Zeolites in the Presence of Tetraethylphosphonium Cations. Chem. Lett. 2013, 43, 302–304. [Google Scholar] [CrossRef]
- Matsuda, K.; Funase, N.; Tsuchiya, K.; Tsunoji, N.; Sadakane, M.; Sano, T. Facile synthesis of highly crystalline EMT zeolite by hydrothermal conversion of FAU zeolite in the presence of 1,1′-(1,4-butanediyl)bis(1-azonia-4-azabicyclo [2,2]octane) dihydroxide. Microporous Mesoporous Mater. 2019, 274, 299–303. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, E.; Gao, X.; Liu, D.; Xie, W.; Zhang, F.; Mu, X.; Shu, X. Topology reconstruction from FAU to MWW structure. Microporous Mesoporous Mater. 2014, 200, 269–278. [Google Scholar] [CrossRef]
- Kweon, S.; An, H.; Son, Y.M.; Park, M.B.; Min, H.-K. Hydrothermal interconversion of FAU-type zeolite in the presence of sodium and tetramethylammonium ions. Microporous Mesoporous Mater. 2021, 317, 111019. [Google Scholar] [CrossRef]
- Inagaki, S.; Tsuboi, Y.; Nishita, Y.; Syahylah, T.; Wakihara, T.; Kubota, Y. Rapid Synthesis of an Aluminum-Rich MSE-Type Zeolite by the Hydrothermal Conversion of an FAU-Type Zeolite. Chem. Eur. J. 2013, 19, 7780–7786. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Meng, X.; Xiao, F.-S. Insights into the Organotemplate-Free Synthesis of Zeolite Catalysts. Engineering 2017, 3, 567–574. [Google Scholar] [CrossRef]
- Barrer, R.M.; Baynham, J.W. 562. The hydrothermal chemistry of the silicates. Part VII. Synthetic potassium aluminosilicates. J. Chem. Soc. 1956, 2882–2891. [Google Scholar] [CrossRef]
- Robson, H.; Lillerud, K.P. (Eds.) Chapter 40—EDI Barrer K-F Si(50), Al(50). In Verified Syntheses of Zeolitic Materials; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 140–141. [Google Scholar]
- Robson, H.; Lillerud, K.P. (Eds.) Chapter 41—EDI Linde Type F Si(50), Al(50). In Verified Syntheses of Zeolitic Materials; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 142–144. [Google Scholar]
- Christensen, A.; Fjellvaag, H. ChemInform Abstract: Crystal Structure Determination of Zeolite N from Synchrotron X-ray Powder Diffraction Data. Cheminform 2010, 29. [Google Scholar] [CrossRef]
- Taylor, W.H.; Jackson, R. The Structure of Edingtonite. Z. Für Krist.—Cryst. Mater. 1933, 86, 53–64. [Google Scholar] [CrossRef]
- Mackinnon, I.D.R.; Barr, K.; Miller, E.; Hunter, S.; Pinel, T. Nutrient removal from wastewaters using high performance materials. Water Sci. Technol. 2003, 47, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.; Pearce, P.; Parsons, S.A. Ammonium removal from digested sludge liquors using ion exchange. Water Res. 2007, 41, 433–439. [Google Scholar] [CrossRef]
- Thornton, A.; Pearce, P.; Parsons, S.A. Ammonium removal from solution using ion exchange on to MesoLite, an equilibrium study. J. Hazard. Mater. 2007, 147, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Probst, J.; Outram, J.G.; Couperthwaite, S.J.; Millar, G.J.; Kaparaju, P. Sustainable ammonium recovery from wastewater: Improved synthesis and performance of zeolite N made from kaolin. Microporous Mesoporous Mater. 2021, 316, 110918. [Google Scholar] [CrossRef]
- Zwingmann, N.; Singh, B.; Mackinnon, I.D.R.; Gilkes, R.J. Zeolite from alkali modified kaolin increases NH4+ retention by sandy soil: Column experiments. Appl. Clay Sci. 2009, 46, 7–12. [Google Scholar] [CrossRef]
- Mackinnon, I.D.R.; Millar, G.J.; Stolz, W. Hydrothermal syntheses of zeolite N from kaolin. Appl. Clay Sci. 2012, 58, 1–7. [Google Scholar] [CrossRef]
- Barrer, R.M.; Munday, B.M. Cation exchange in the synthetic zeolite K-F. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 2914–2921. [Google Scholar] [CrossRef]
- Wong, S.-F.; Awala, H.; Vincente, A.; Retoux, R.; Ling, T.C.; Mintova, S.; Mukti, R.R.; Ng, E.-P. K-F zeolite nanocrystals synthesized from organic-template-free precursor mixture. Microporous Mesoporous Mater. 2017, 249, 105–110. [Google Scholar] [CrossRef]
- Chawla, A.; Mallette, A.J.; Jain, R.; Le, N.; Robles Hernández, F.C.; Rimer, J.D. Crystallization of potassium-zeolites in organic-free media. Microporous Mesoporous Mater. 2022, 341, 112026. [Google Scholar] [CrossRef]
- Matsumoto, T.; Miyazaki, T.; Goto, Y. Synthesis and characterization of Li-type EDI zeolite. J. Eur. Ceram. Soc. 2006, 26, 455–458. [Google Scholar] [CrossRef]
- Barrer, R.M.; Beaumont, R.; Collela, C. Chemistry of soil minerals. Part XIV. Action of some basic solutions on metakaolinite and kaolinite. J. Chem. Soc. Dalton Trans. 1974, 934–941. [Google Scholar] [CrossRef]
- Colella, C.; de Gennaro, M.; Iorio, V. Crystallization of zeolitic aluminosilicates in bicationic systems including lithium. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1986; Volume 28, pp. 263–270. [Google Scholar]
- Barrer, R.M.; Sieber, W. Hydrothermal chemistry of silicates. Part 21. Zeolites from reaction of lithium and caesium ions with tetramethylammonium aluminosilicate solutions. J. Chem. Soc. Dalton Trans. 1977, 1020–1026. [Google Scholar] [CrossRef]
- Tosheva, L.; Garbev, K.; Miller, G.J.; Mihailova, B. Toward the Synthesis of New Zeolite Structures in the Presence of Cesium: Zeolite MMU-1. Cryst. Growth Des. 2023, 23, 3834–3844. [Google Scholar] [CrossRef]
- Sathupunya, M.; Gulari, E.; Wongkasemjit, S. Microwave preparation of Li-zeolite directly from alumatrane and silatrane. Mater. Chem. Phys. 2004, 83, 89–95. [Google Scholar] [CrossRef]
- Kecht, J.; Mintova, S.; Bein, T. Nanosized EDI-type molecular sieve. Microporous Mesoporous Mater. 2008, 116, 258–266. [Google Scholar] [CrossRef]
- Bruter, D.V.; Pavlov, V.S.; Ivanova, I.I. Interzeolite Transformations as a Method for Zeolite Catalyst Synthesis. Pet. Chem. 2021, 61, 251–275. [Google Scholar] [CrossRef]
- Cruciani, G. Zeolites upon heating: Factors governing their thermal stability and structural changes. J. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Leardini, L.; Quartieri, S.; Vezzalini, G.; Arletti, R. Thermal behaviour of siliceous faujasite: Further structural interpretation of negative thermal expansion. Microporous Mesoporous Mater. 2015, 202, 226–233. [Google Scholar] [CrossRef]
- Ferdov, S.; Marques, J.; Tavares, C.J.; Lin, Z.; Mori, S.; Tsunoji, N. UV-light assisted synthesis of high silica faujasite-type zeolite. Microporous Mesoporous Mater. 2022, 336, 111858. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Boronat, M.; Ferri, P.; Xu, Z.; Liu, P.; Shen, B.; Wang, Z.; Yu, J. Organic-Free Synthesis of Zeolite Y with High Si/Al Ratios: Combined Strategy of In Situ Hydroxyl Radical Assistance and Post-Synthesis Treatment. Angew. Chem.—Int. Ed. 2020, 59, 17225–17228. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. EDI—P4¯m2. In Atlas of Zeolite Framework Types, 6th ed.; Baerlocher, C., McCusker, L.B., Olson, D.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 120–121. [Google Scholar]



| No. | Si & Al Source | Conc. (M) | KOH/FAU (wt.) | H2O/FAU (wt.) | Time | T °C | Product | Si/Al |
|---|---|---|---|---|---|---|---|---|
| 1 | FAU | 20.43 | 8.94 | 7.8 | 27 h | 60 | EDI | 1.1 |
| 2 | FAU | 20.43 | 8.94 | 7.8 | 6 h | 60 | EDI | 1.2 |
| 3 | FAU | 20.43 | 8.94 | 7.8 | 35 d | RT | EDI | 1.1 |
| 4 | FAU | 20.43 | 8.94 | 7.8 | 24 h | RT | am. | 1.6 |
| 5 | FAU | 20.43 | 8.94 | 7.8 | 2 h | 60 | am. | 1.4 |
| 6 | FAU | 20.43 | 8.94 | 7.8 | 4 h | 60 | am. + ? | 1.4 |
| 7 | FAU | 20.43 | 8.94 | 7.8 | 12 d | RT | EDI | 1.2 |
| 8 | FAU | 20.43 | 8.94 | 7.8 | 32 d | RT | EDI | 1.1 |
| 9 | FAU | 20.43 | 8.94 | 7.8 | 11 d | RT | EDI | 1.3 |
| 10 | FAU | 5.11 | 2.24 | 7.8 | 12 d | RT | FAU | N/A |
| 11 | FAU | 30.65 | 13.5 | 7.8 | 12 d | RT | am. | N/A |
| 12 | FAU | 10.22 | 4.47 | 7.8 | 6 h | 60 | am. | N/A |
| 13 | FAU | 5.11 | 2.24 | 7.8 | 6 h | 60 | FAU | 2.1 |
| 14 | FAU | 10.22 | 4.47 | 7.8 | 4 h | 60 | am. | N/A |
| 15 | FAU | 26 | 11.38 | 7.8 | 6 h | 60 | am. + ? | N/A |
| 16 | FAU | 5.11 | 2.24 | 7.8 | 9 d | RT | FAU | N/A |
| 17 | SiO2 & NaAlO2 | Gel synthesis | 22 h | 60 | EDI | 1.1 | ||
| 18 | SiO2 & NaAlO2 | Gel synthesis | 32 d | RT | EDI | 1.2 | ||
| Sample No. | K/Al + Si | V (Å3) S.G. I222 | V (Å3) S.G. P-42m |
|---|---|---|---|
| 1 | 0.5 | 1277.9 (1) | 1276.1 (2) |
| 2 | 0.4 | 1281.8 (8) | 1281.8 (5) |
| 3 | 0.7 | 1277.3 (4) | 1274.4 (1) |
| 7 | 0.6 | 1276.6 (1) | 1277.8 (8) |
| 8 | 0.4 | 1276.8 (1) | 1277.6 (2) |
| 9 | 0.4 | 1241.5 (1) | 1276.1 (1) |
| 17 | 0.9 | 1283.0 (4) | 1280.9 (1) |
| 18 | 0.6 | 1276.5 (1) | 1274.2 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdov, S. Interzeolite Transformation from FAU-to-EDI Type of Zeolite. Molecules 2024, 29, 1744. https://doi.org/10.3390/molecules29081744
Ferdov S. Interzeolite Transformation from FAU-to-EDI Type of Zeolite. Molecules. 2024; 29(8):1744. https://doi.org/10.3390/molecules29081744
Chicago/Turabian StyleFerdov, Stanislav. 2024. "Interzeolite Transformation from FAU-to-EDI Type of Zeolite" Molecules 29, no. 8: 1744. https://doi.org/10.3390/molecules29081744
APA StyleFerdov, S. (2024). Interzeolite Transformation from FAU-to-EDI Type of Zeolite. Molecules, 29(8), 1744. https://doi.org/10.3390/molecules29081744