Polymorph Screening of Core-Chlorinated Naphthalene Diimides with Different Fluoroalkyl Side-Chain Lengths
Abstract
1. Introduction
2. Results and Discussion
2.1. Polymorph Screening
2.2. Crystal Structure
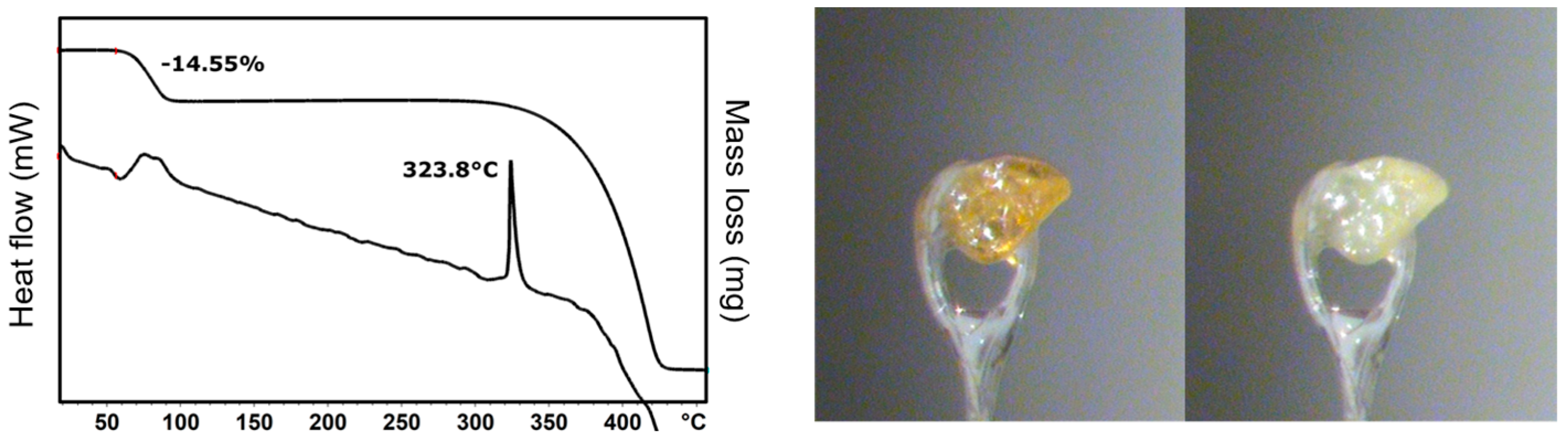
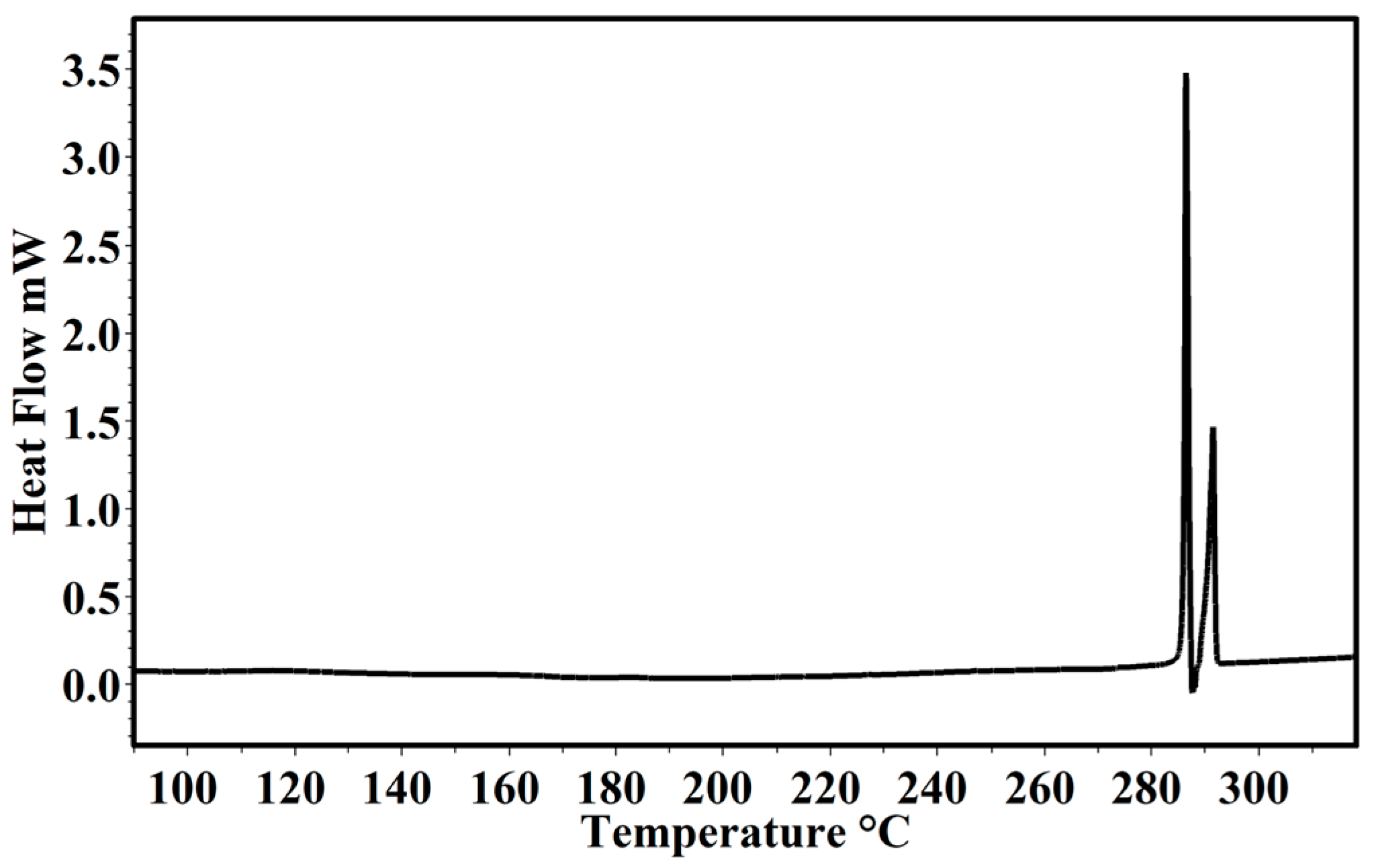
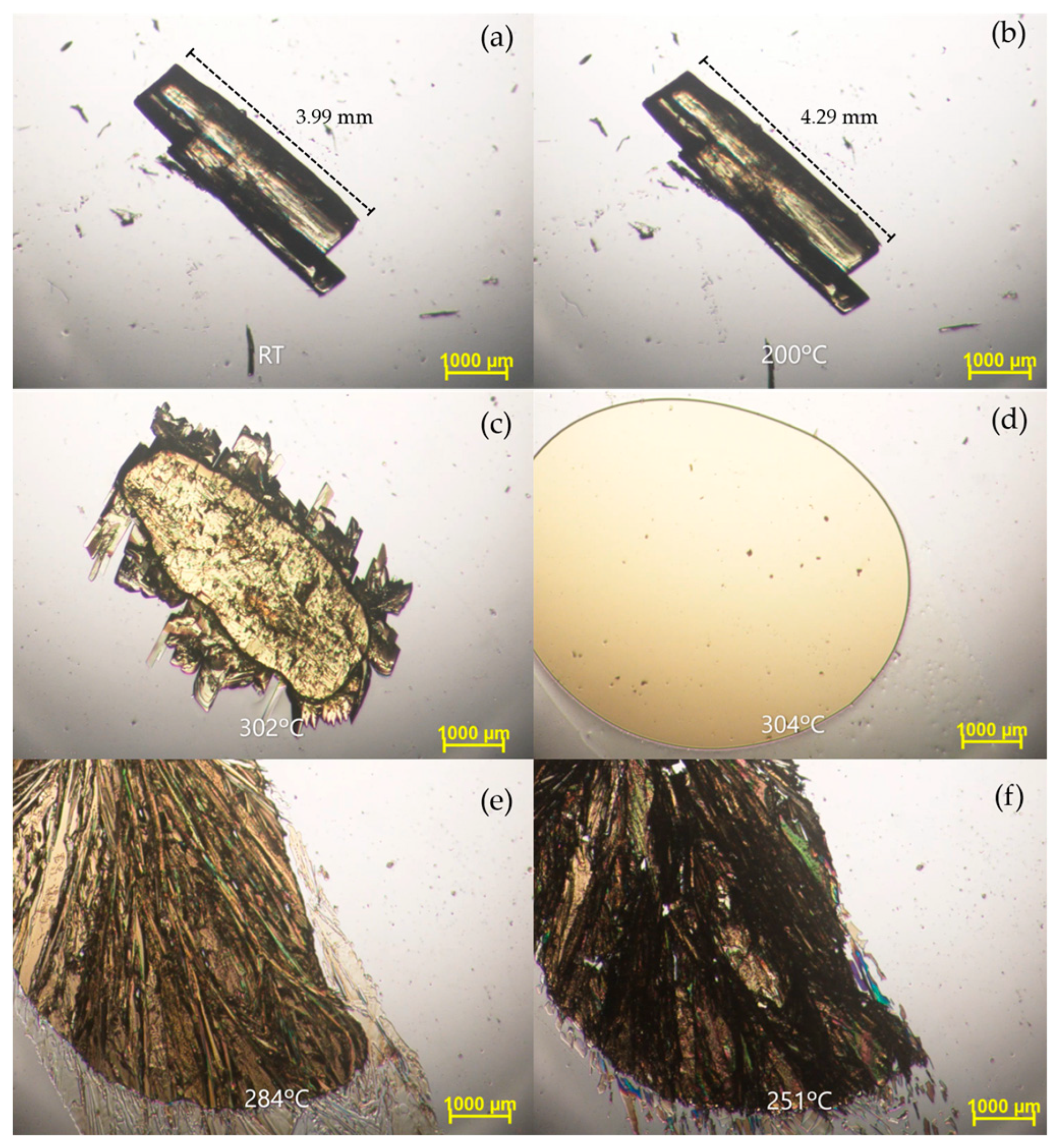
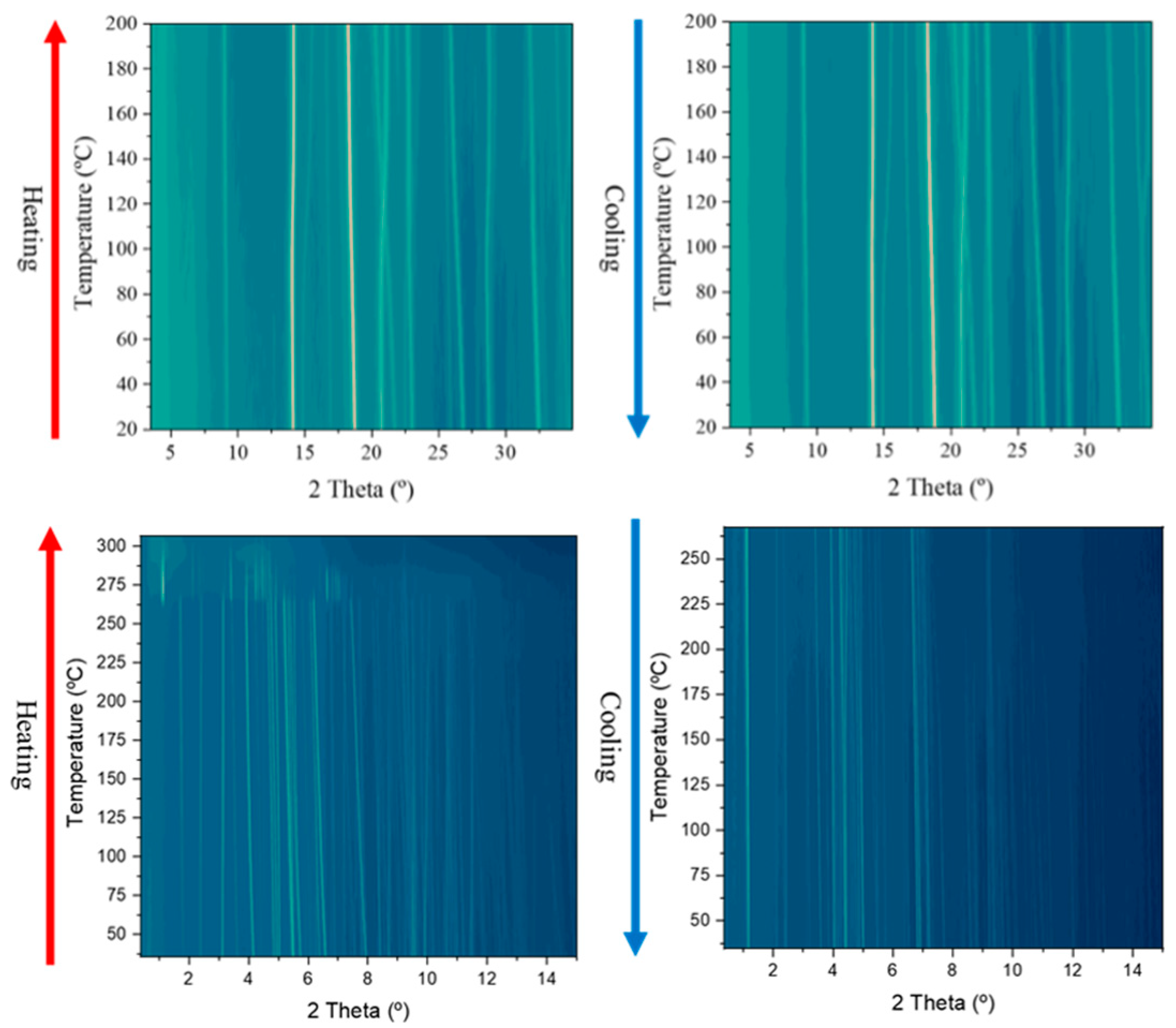
2.3. Thermal Characterization
3. Materials and Methods
3.1. Polymorph Screening
3.2. X-ray Powder Diffraction (XRPD)
3.3. Variable Temperature X-ray Powder Diffraction (VT-XRPD)
3.4. Single-Crystal X-ray Diffraction (SCXRD)
3.5. Differential Scanning Calorimetry (DSC)
3.6. Thermo-Gravimetric Analysis/Evolved Gas Analysis (TGA-EGA)
3.7. Hot Stage Microscopy (HSM)
3.8. Attenuated Total Reflection/Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
3.9. Nuclear Magnetic Resonance (NMR)
3.10. PASCal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.; Zhao, Z. High mobility organic semiconductors for field-effect transistors. Sci. China Chem. 2015, 58, 947–968. [Google Scholar] [CrossRef]
- Zhao, C.; Li, A.; Chen, X.; Ali, M.U.; Meng, H. Hysteresis effect in organic thin film transistors based on naphthalene tetracarboxylic diimide derivatives. Appl. Phys. Lett. 2021, 118, 193302. [Google Scholar] [CrossRef]
- Fukuda, K.; Takeda, Y.; Mizukami, M.; Kumaki, D.; Tokito, S. Fully Solution-Processed Flexible Organic Thin Film Transistor Arrays with High Mobility and Exceptional Uniformity. Sci. Rep. 2014, 4, 3947. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Suraru, S.-L.; Lee, W.-Y.; Könemann, M.; Höffken, H.W.; Röger, C.; Schmidt, R.; Chung, Y.; Chen, W.-C.; Würthner, F.; et al. High-Performance Air-Stable n-Type Organic Transistors Based on Core-Chlorinated Naphthalene Tetracarboxylic Diimides. Adv. Funct. Mater. 2010, 20, 2148–2156. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and Ambipolar Transport in Organic Field-Effect Transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef]
- Katz, H.E.; Lovinger, A.J.; Johnson, J.; Kloc, C.; Siegrist, T.; Li, W.; Lin, Y.-Y.; Dodabalapur, A. A soluble and air-stable organic semiconductor with high electron mobility. Nature 2000, 404, 478–481. [Google Scholar] [CrossRef]
- He, T.; Stolte, M.; Würthner, F. Air-Stable n-Channel Organic Single Crystal Field-Effect Transistors Based on Microribbons of Core-Chlorinated Naphthalene Diimide. Adv. Mater. 2013, 25, 6951–6955. [Google Scholar] [CrossRef]
- Kim, R.; Amegadze, P.S.K.; Kang, I.; Yun, H.-J.; Noh, Y.-Y.; Kwon, S.-K.; Kim, Y.-H. High-Mobility Air-Stable Naphthalene Diimide-Based Copolymer Containing Extended π-Conjugation for n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2013, 23, 5719–5727. [Google Scholar] [CrossRef]
- Gogoi, G.; Bhattacharya, L.; Sahoo, S.R.; Sahu, S.; Sarma, N.S.; Sharma, S. Enhancement of air-stability, π-stacking ability, and charge transport properties of fluoroalkyl side chain engineered n-type naphthalene tetracarboxylic diimide compounds. RSC Adv. 2021, 11, 57–70. [Google Scholar] [CrossRef]
- Kang, M.J.; Doi, I.; Mori, H.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H. Alkylated Dinaphtho[2,3-b:2′,3′-f]Thieno[3,2-b]Thiophenes (Cn-DNTTs): Organic Semiconductors for High-Performance Thin-Film Transistors. Adv. Mater. 2011, 23, 1222–1225. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and Related Diimides for Organic Electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wu, S.-X.; Li, H.-B.; Tang, X.-D.; Wu, Y.; Su, Z.-M.; Liao, Y. A theoretical discussion on the relationships among molecular packings, intermolecular interactions, and electron transport properties for naphthalene tetracarboxylic diimide derivatives. J. Mater. Chem. 2011, 21, 15558–15566. [Google Scholar] [CrossRef]
- Stolte, M.; Suraru, S.-L.; Würthner, F.; Oh, J.H.; Bao, Z.; Brill, J.; Könemann, M.; Qu, J.; Zschieschang, U.; Klauck, H. Organic n-channel thin film transistors based on dichlorinated naphthalene diimides. Proceedings Volume 7778, Organic Field-Effect Transistors IX. In Proceedings of the SPIE Photonic Devices + Applications, San Diego, CA, USA, 1–5 August 2010; Volume 777804. [Google Scholar]
- Lee, W.-Y.; Oh, J.H.; Suraru, S.-L.; Chen, W.-C.; Würthner, F.; Bao, Z. High-Mobility Air-Stable Solution-Shear-Processed n-Channel Organic Transistors Based on Core-Chlorinated Naphthalene Diimides. Adv. Funct. Mater. 2011, 21, 4173–4181. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; He, Y.; Ali, M.U.; Wu, Y.; Zhao, C.; Wu, P.; Yan, C.; Wudle, F.; Meng, H. Fluoro-alkyl substituted isothianaphthene bisimides as stable n-type semiconductors. Mater. Chem. Front. 2020, 4, 3578–3584. [Google Scholar] [CrossRef]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning Orbital Energetics in Arylene Diimide Semiconductors. Materials Design for Ambient Stability of n-Type Charge Transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-F.; Wang, J.-Y.; Pei, J. F or O, Which One Is the Better Hydrogen Bond (Is It?) Acceptor in C–H⋯X–C (X– = F–, O=) Interactions. Cryst. Growth Des. 2018, 18, 7–15. [Google Scholar]
- Canola, S.; Negri, F. Anisotropy of the n-type charge transport and thermal effects in crystals of a fluoro-alkylated naphthalene diimide: A computational investigation. Phys. Chem. Chem. Phys. 2014, 16, 21550–21558. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Kobaisi, M.A.; Jadhav, R.W.; Morajkar, P.P.; Jones, L.A.; George, S. Naphthalene diimides: Perspectives and promise. Chem. Soc. Rev. 2021, 50, 9845–9998. [Google Scholar] [CrossRef]
- Gao, X.; Hua, Y. Development of n-type organic semiconductors for thin film transistors: A viewpoint of molecular design. J. Mater. Chem. C 2014, 2, 3099–3117. [Google Scholar] [CrossRef]
- Zangoli, M.; Gazzano, M.; Monti, F.; Maini, L.; Gentili, D.; Liscio, A.; Zanelli, A.; Salatelli, E.; Gigli, G.; Baroncini, M.; et al. Thermodynamically versus Kinetically Controlled Self-Assembly of a Naphthalenediimide–Thiophene Derivative: From Crystalline, Fluorescent, n-Type Semiconducting 1D Needles to Nanofibers. ACS Appl. Mater. Interfaces 2019, 11, 16864–16871. [Google Scholar] [CrossRef]
- Cappuccino, C.; Canola, S.; Montanari, G.; Lopez, S.G.; Toffanin, S.; Melucci, M.; Negri, F.; Maini, L. One Molecule, Four Colors: Discovering the Polymorphs of a Thieno(bis)imide Oligomer. Cryst. Growth Des. 2019, 19, 2594–2603. [Google Scholar] [CrossRef]
- Zambianchi, M.; Favaretto, L.; Durso, M.; Bettini, C.; Zanelli, A.; Manet, I.; Gazzano, M.; Maini, L.; Gentili, D.; Toffanin, S.; et al. Synergic effect of unsaturated inner bridges and polymorphism for tuning the optoelectronic properties of 2,3-thieno(bis)imide based materials. J. Mater. Chem. C 2015, 3, 121–131. [Google Scholar] [CrossRef]
- Lovrinčić, R.; Trollmann, J.; Pölking, C.; Schöneboom, J.; Lennartz, C.; Pucci, A. Orientation of Nonplanar Molecules in Polycrystalline Layers from Infrared Spectra: Core-Chlorinated Naphthalene Tetracarboxylic Diimides. J. Phys. Chem. C 2012, 116, 5757–5763. [Google Scholar] [CrossRef]
- Zhen, Y.-G.; Dong, H.-L.; Jiang, L.; Hu, W.-P. Tailoring crystal polymorphs of organic semiconductors towards high-performance field-effect transistors. Chin. Chem. Lett. 2016, 27, 1330–1338. [Google Scholar] [CrossRef]
- Purdum, G.E.; Telesz, N.G.; Jarolimek, K.; Ryno, S.M.; Gessner, T.; Davy, N.C.; Petty II, A.J.; Zhen, Y.; Shu, T.; Facchetti, A.; et al. Presence of Short Intermolecular Contacts Screens for Kinetic Stability in Packing Polymorphs. J. Am. Chem. Soc. 2018, 140, 7519–7525. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Martins, I.; Marin, F.; Modena, E.; Maini, L. On the crystal forms of NDI-C6: Annealing and deposition procedures to access elusive polymorphs. Faraday Discuss. 2022, 235, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Riera-Galindo, S.; Tamayo, A.; Mas-Torrent, M. Role of Polymorphism and Thin-Film Morphology in Organic Semiconductors Processed by Solution Shearing. ACS Omega 2018, 3, 2329–2339. [Google Scholar] [CrossRef]
- Chunga, H.; Diao, Y. Polymorphism as an emerging design strategy for high performance organic electronics. J. Mater. Chem. C 2016, 4, 3915–3933. [Google Scholar] [CrossRef]
- Tam, T.L.D.; Xu, J.W. The role of fluoride in anion–π interaction with naphthalene diimide. Chem. Commun. 2019, 55, 6225–6228. [Google Scholar] [CrossRef]
- Cappuccino, C.; Catalano, L.; Marin, F.; Dushaq, G.; Raj, G.; Rasras, M.; Rezgui, R.; Zambianchi, M.; Melucci, M.; Naumov, P.; et al. Structure-Mechanical Relationships in Polymorphs of an Organic Semiconductor (C4-NT3N). Cryst. Growth Des. 2020, 20, 884–891. [Google Scholar] [CrossRef]
- Karothu, D.P.; Halabi, J.M.; Ahmed, E.; Ferreira, R.; Spackman, P.R.; Spackman, M.A.; Naumov, P. Global Analysis of the Mechanical Properties of Organic Crystals. Angew. Chem. Int. Ed. 2022, 61, e202113988. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Cao, J.; Kampf, J.W. Solid-State Packing of Conjugated Oligomers: From π-Stacks to the Herringbone Structure. J. Am. Chem. Soc. 2004, 126, 4318–4328. [Google Scholar] [CrossRef] [PubMed]
- Milita, S.; Liscio, F.; Cowen, L.; Cavallini, M.; Drain, B.A.; Degousée, T.; Luong, S.; Fenwick, O.; Guagliardi, A.; Schroeder, B.C.; et al. Polymorphism in N,N′-dialkyl-naphthalene diimides. J. Mater. Chem. C 2020, 8, 3097–3112. [Google Scholar] [CrossRef]
- Pandey, P.; Demitri, N.; Gigli, L.; James, A.M.; Devaux, F.; Geerts, Y.H.; Modena, E.; Maini, L. Discovering Crystal Forms of the Novel Molecular Semiconductor OEG-BTBT. Cryst. Growth Des. 2022, 22, 1680–1690. [Google Scholar] [CrossRef]
- Courté, M.; Ye, J.; Jiang, H.; Ganguly, R.; Tang, S.; Klocc, C.; Fichou, D. Tuning the π–π overlap and charge transport in single crystals of an organic semiconductor via solvation and polymorphism. Phys. Chem. Chem. Phys. 2020, 22, 19855–19863. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Tombolesi, S.; Salzillo, T.; Yaffe, O.; Maini, L. Thorough investigation on the high-temperature polymorphism of dipentyl-perylenediimide: Thermal expansion vs. polymorphic transition. J. Mater. Chem. C 2022, 10, 8089–8100. [Google Scholar] [CrossRef]
- Goodwin, A.L.; Calleja, M.; Conterio, M.J.; Dove, M.T.; Evans, J.S.O.; Keen, D.A.; Peters, L.; Tucker, M.G. Colossal Positive and Negative Thermal Expansion in the Framework Material Ag3[Co(CN)6]. Science 2008, 319, 794–797. [Google Scholar] [CrossRef]
- van der Lee, A.; Dumitrescu, D.G. Thermal expansion properties of organic crystals: A CSD study. Chem. Sci. 2021, 12, 8537–8547. [Google Scholar] [CrossRef]
- Juneja, N.; Unruh, D.K.; Hutchins, K.M. Engineering Colossal Anisotropic Thermal Expansion into Organic Materials through Dimensionality Control. Chem. Mater. 2023, 35, 7292–7300. [Google Scholar] [CrossRef]
- van der Lee, A.; Roche, G.H.; Wantz, G.; Moreau, J.J.E.; Dautel, O.J.; Filhol, J.-S. Experimental and theoretical evidence of a supercritical-like transition in an organic semiconductor presenting colossal uniaxial negative thermal expansion. Chem. Sci. 2018, 9, 3948–3956. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Q.; Chen, J.; Deng, J.; Lina, K.; Xing, X. Negative thermal expansion in molecular materials. Chem. Commun. 2018, 54, 5164–5176. [Google Scholar] [CrossRef] [PubMed]
- Fauth, F.; Peral, I.; Popescu, C.; Knapp, M. The new Material Science Powder Diffraction beamline at ALBA Synchrotron. Powder Diffr. 2013, 28, S360–S370. [Google Scholar] [CrossRef]
- Fauth, F.; Boer, R.; Gil-Ortiz, F.; Popescu, C.; Vallcorba, O.; Peral, I.; Fullà, D.; Benach, J.; Juanhuix, J. The crystallography stations at the Alba synchrotron. Eur. Phys. J. Plus 2015, 130, 160. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Goodwin, A.L. PASCal: A principal axis strain calculator for thermal expansion and compressibility determination. J. Appl. Cryst. 2012, 45, 1321–1329. [Google Scholar] [CrossRef]


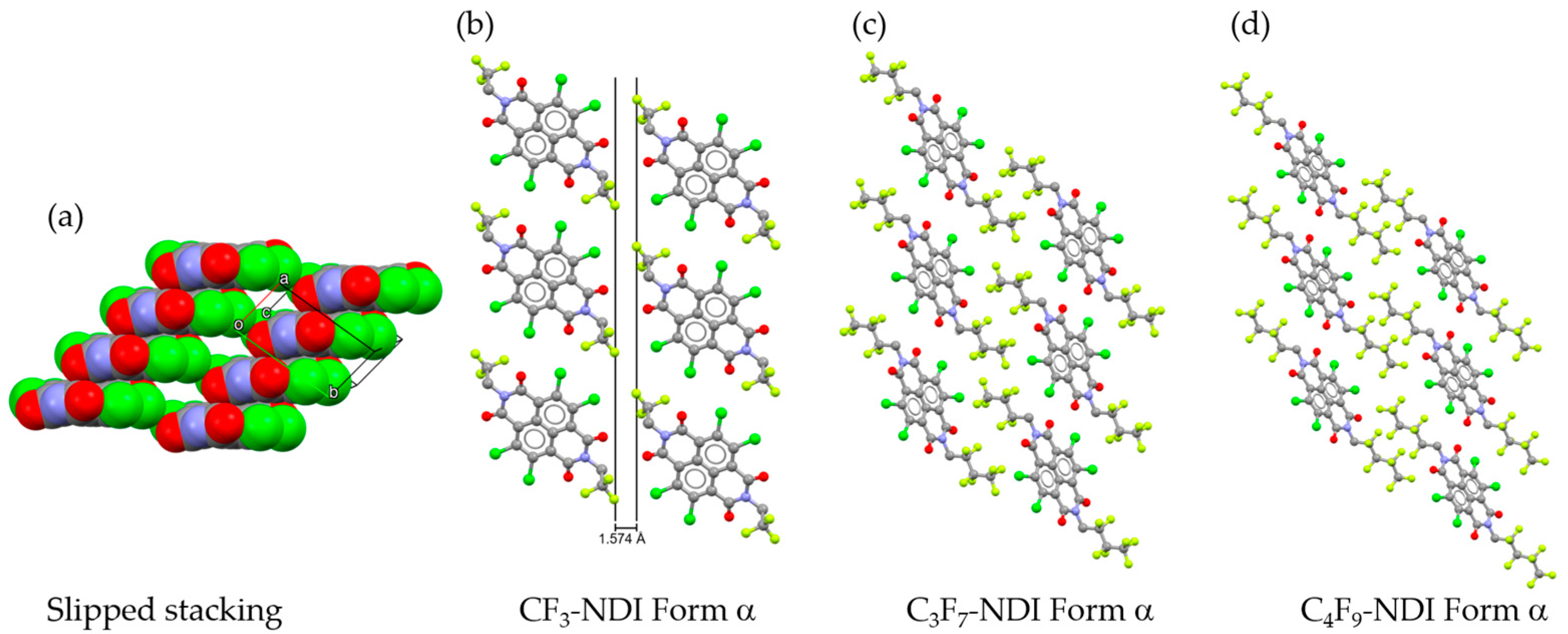

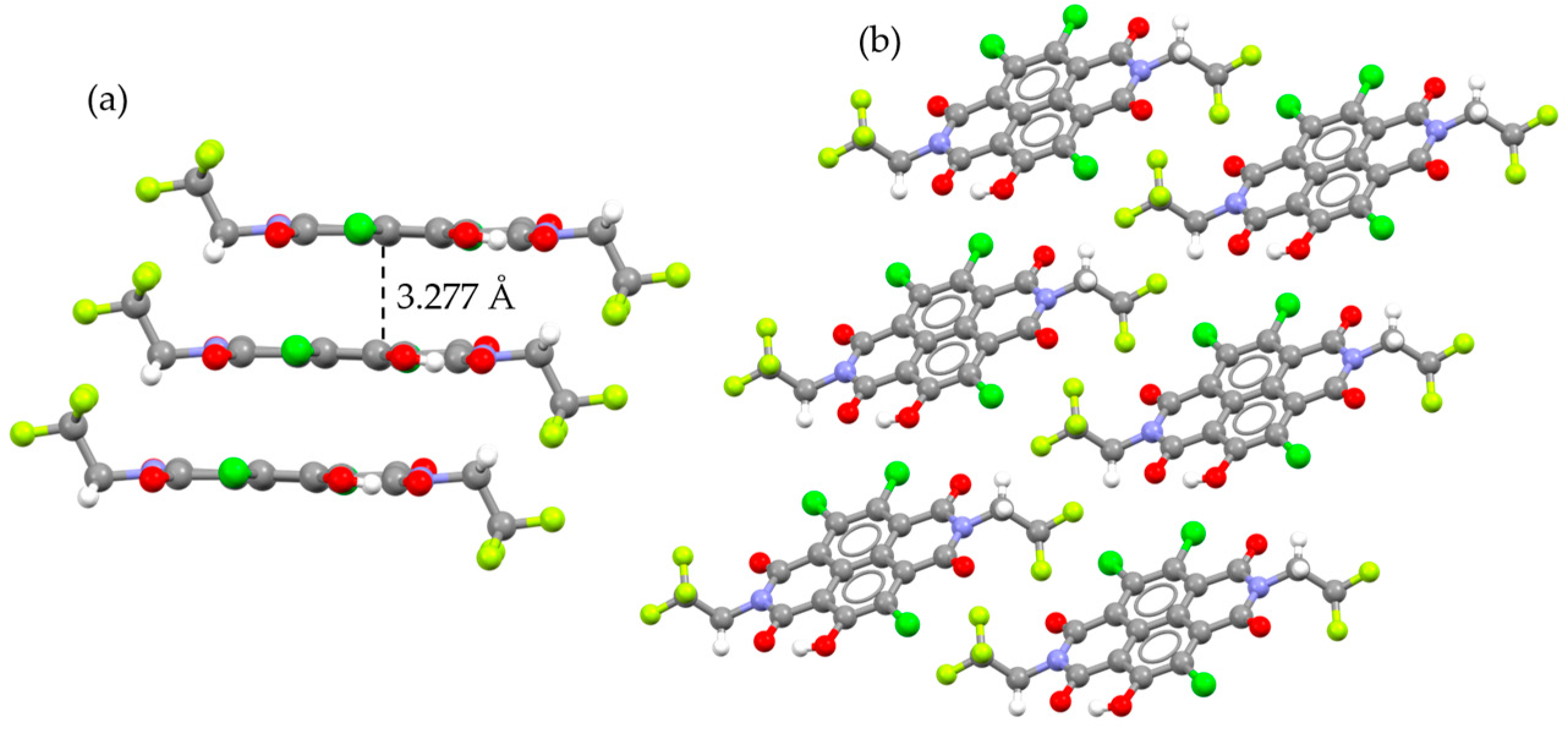

| CF3-NDI Form α | CF3-NDI·SS | CF3-NDI·PXY | C3F7-NDI Form α | C4F9-NDI Form α | |
|---|---|---|---|---|---|
| CCDC number | 2373444 | 2373442 | 2373443 | BUYJIP [4] | BUYJEL [4] |
| Temperature (K) | 160.15 | 160.15 | RT | 100 | 100 |
| Formula | C18H4Cl4F6N2O4 | C18H4.8Cl3.2F6N2O4.8 | C18H4Cl4F6N2O4, C8H10 | C22H4Cl4F14N2O4 | C24H4Cl4F18N2O4 |
| Molecular weight | 568.03 | 553.46 | 674.19 | 768.07 | 868.08 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P1 | ||||
| ) | 4.8722(2) | 5.83510(10) | 7.7798(7) | 5.1039(19) | 5.5055(7) |
| ) | 8.8027(3) | 8.09990(10) | 8.1543(9) | 10.358(4) | 9.8095(11) |
| ) | 11.5608(2) | 9.7935(2) | 10.6415(7) | 12.395(5) | 12.7073(15) |
| α (°) | 75.075(2) | 97.9620(10) | 91.60(7) | 111.077(19) | 87.786(6) |
| β (°) | 89.777(2) | 95.3400(10) | 101.96(7) | 90.07(2) | 81.088(7) |
| γ (°) | 82.792(3) | 102.323(2) | 90.58(8) | 96.67(2) | 87.289(7) |
| ) | 475.103 | 444.242 | 660.08 | 606.645 | 676.888 |
| Z/Z’ | 1/0.5 | 1/1 | 1/0.5 | 1/0.5 | 1/0.5 |
| Density (g·cm−3) | 1.985 | 2.069 | 1.696 | 2.102 | 2.13 |
| F(000) | 280 | 274 | 338 | ||
| GOF on F2 | 1.156 | 1.100 | 1.039 | 1.076 | 1.030 |
| R1 (on F, I > 2σ(I)/Rex) | 0.0306 | 0.0695 | 0.0477 | 0.0918 | 0.0397 |
| WR2 (F2 all data) Rwp | 0.0856 | 0.2323 | 0.1049 | 0.2512 | 0.1027 |
| Compound | Χ (deg) | Ψ (deg) | Stacking Vector (Å) | d (π-π) (Å) | P (deg) | R (deg) |
|---|---|---|---|---|---|---|
| CF3-NDI | 74.42 | 49.37 | 4.872 | 3.449 | 20.78 | 42.61 |
| C3F7-NDI | 69.89 | 48.44 | 5.104 | 3.463 | 26.87 | 44.36 |
| C4F9-NDI | 78.39 | 40.88 | 5.506 | 3.438 | 17.86 | 50.45 |
| CF3-NDI·SS | 75.08 | 38.39 | 5.835 | 3.316 | 24.37 | 54.06 |
| CF3-NDI | 74.42 | 49.37 | 4.872 | 3.449 | 20.78 | 42.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Martins, I.; Marchini, M.; Maini, L.; Modena, E. Polymorph Screening of Core-Chlorinated Naphthalene Diimides with Different Fluoroalkyl Side-Chain Lengths. Molecules 2024, 29, 4376. https://doi.org/10.3390/molecules29184376
de Oliveira Martins I, Marchini M, Maini L, Modena E. Polymorph Screening of Core-Chlorinated Naphthalene Diimides with Different Fluoroalkyl Side-Chain Lengths. Molecules. 2024; 29(18):4376. https://doi.org/10.3390/molecules29184376
Chicago/Turabian Stylede Oliveira Martins, Inês, Marianna Marchini, Lucia Maini, and Enrico Modena. 2024. "Polymorph Screening of Core-Chlorinated Naphthalene Diimides with Different Fluoroalkyl Side-Chain Lengths" Molecules 29, no. 18: 4376. https://doi.org/10.3390/molecules29184376
APA Stylede Oliveira Martins, I., Marchini, M., Maini, L., & Modena, E. (2024). Polymorph Screening of Core-Chlorinated Naphthalene Diimides with Different Fluoroalkyl Side-Chain Lengths. Molecules, 29(18), 4376. https://doi.org/10.3390/molecules29184376








