The Strength and Fire Properties of Paper Sheets Made of Phosphorylated Cellulose Fibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Freeness, pH, and the Grammage of Different Phosphorylated Cellulose Pulps
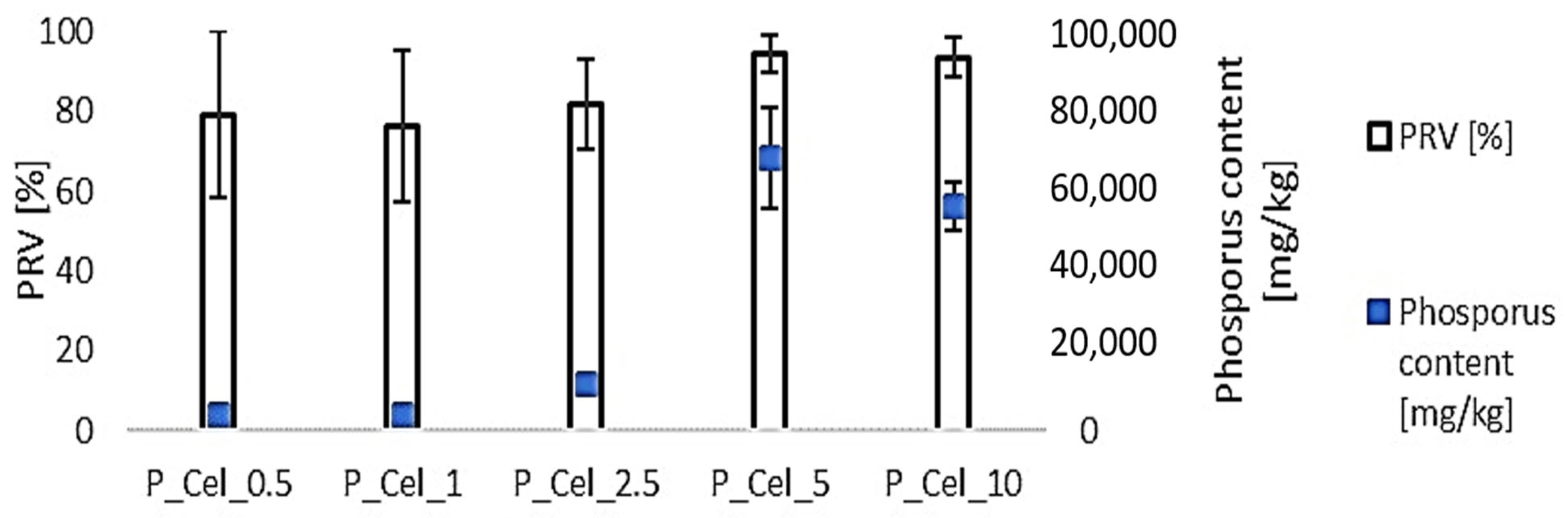
2.2. Phosphorus Content in Pulp
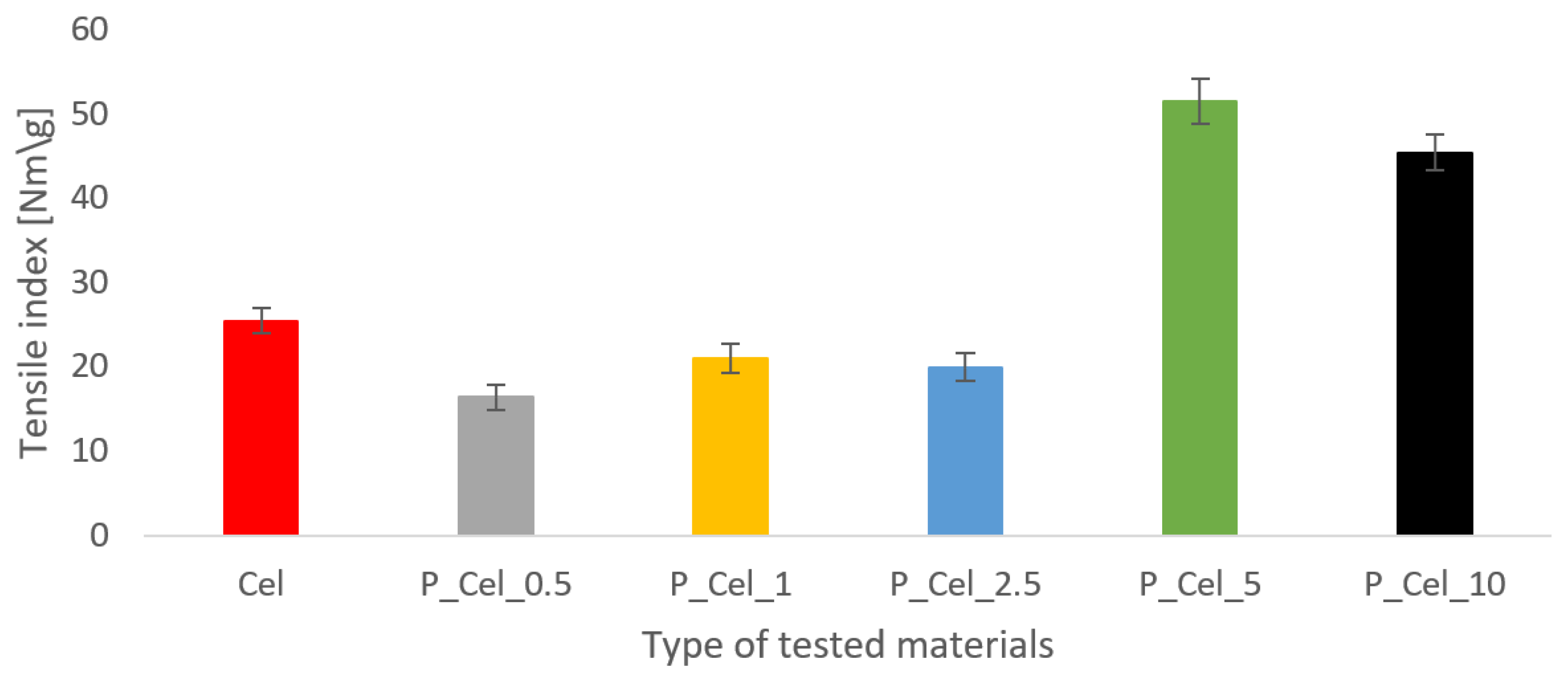
2.3. Tensile Properties of Phosphorylated Cellulose Sheets
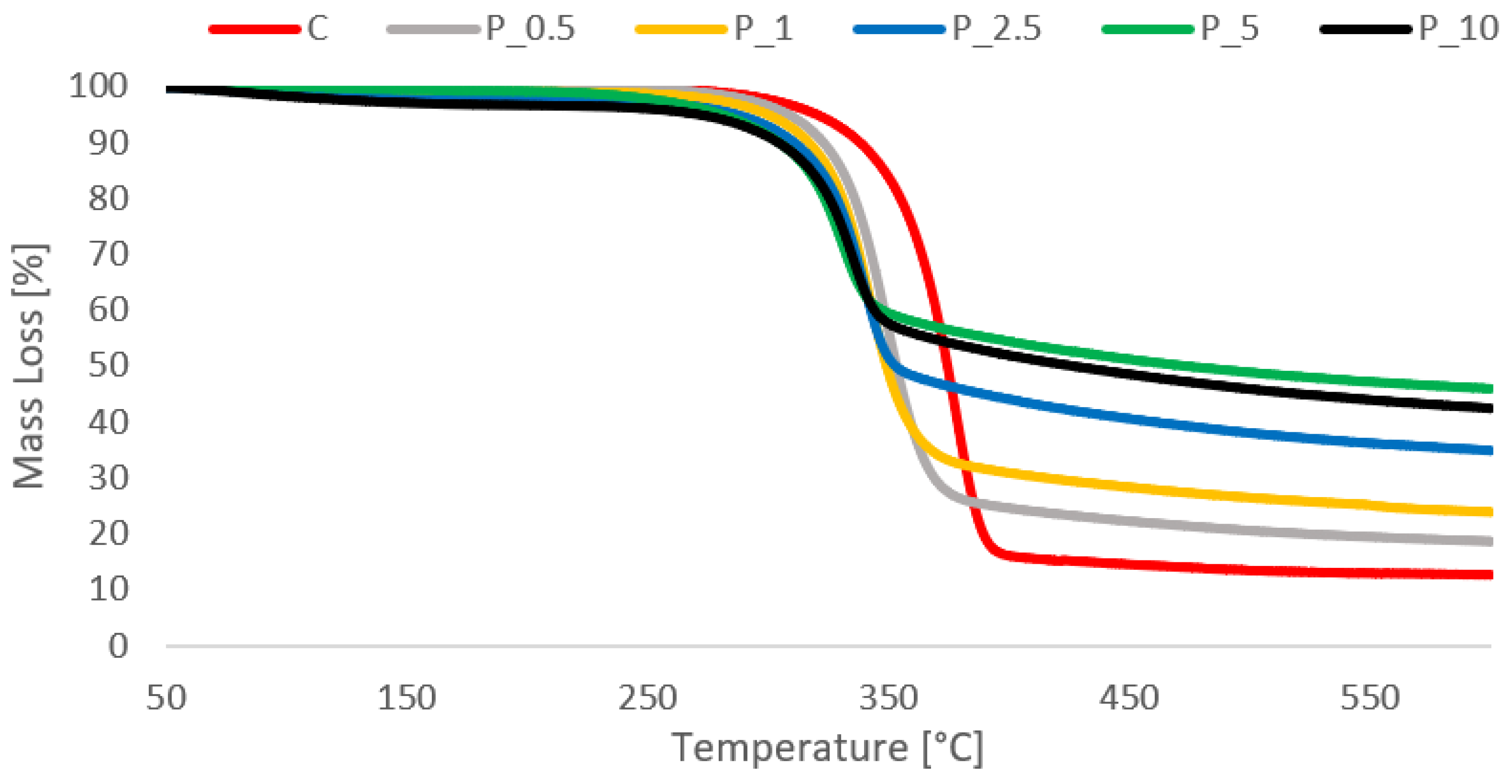
2.4. Fire Properties
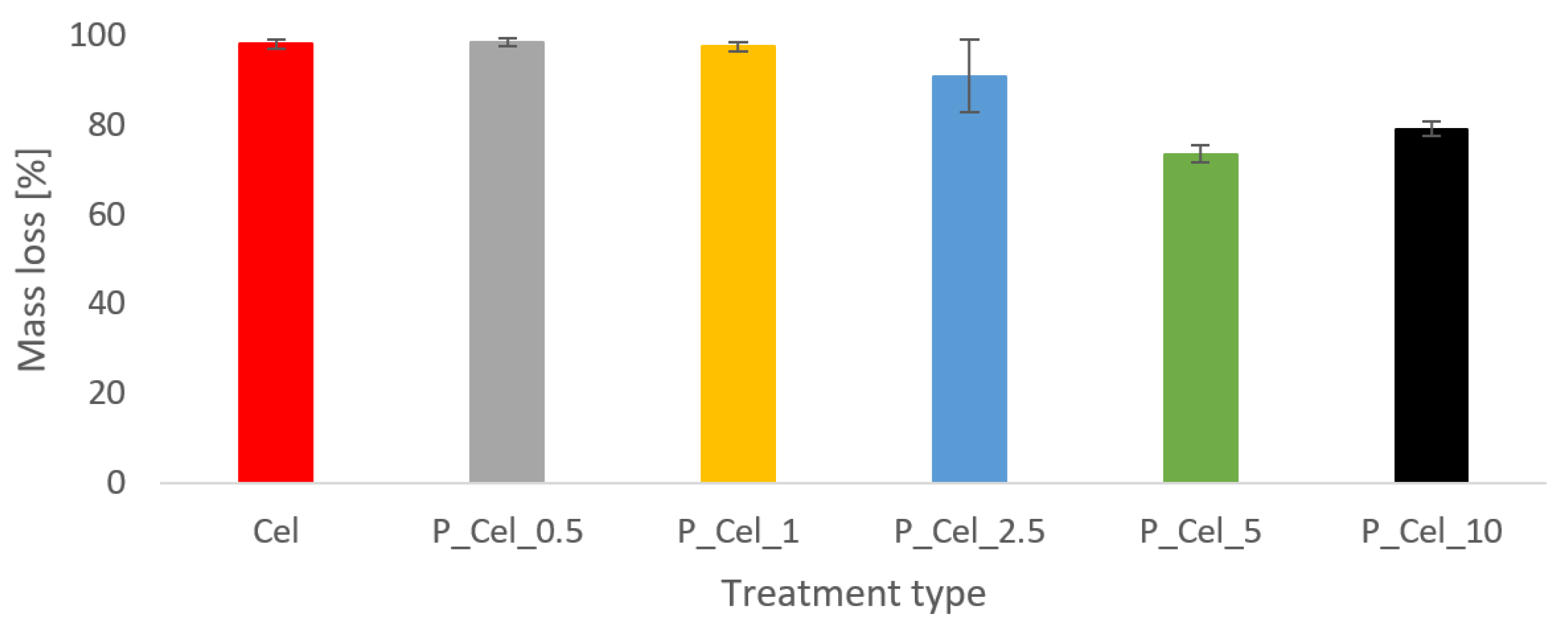
2.4.1. Mini Fire Tube Test
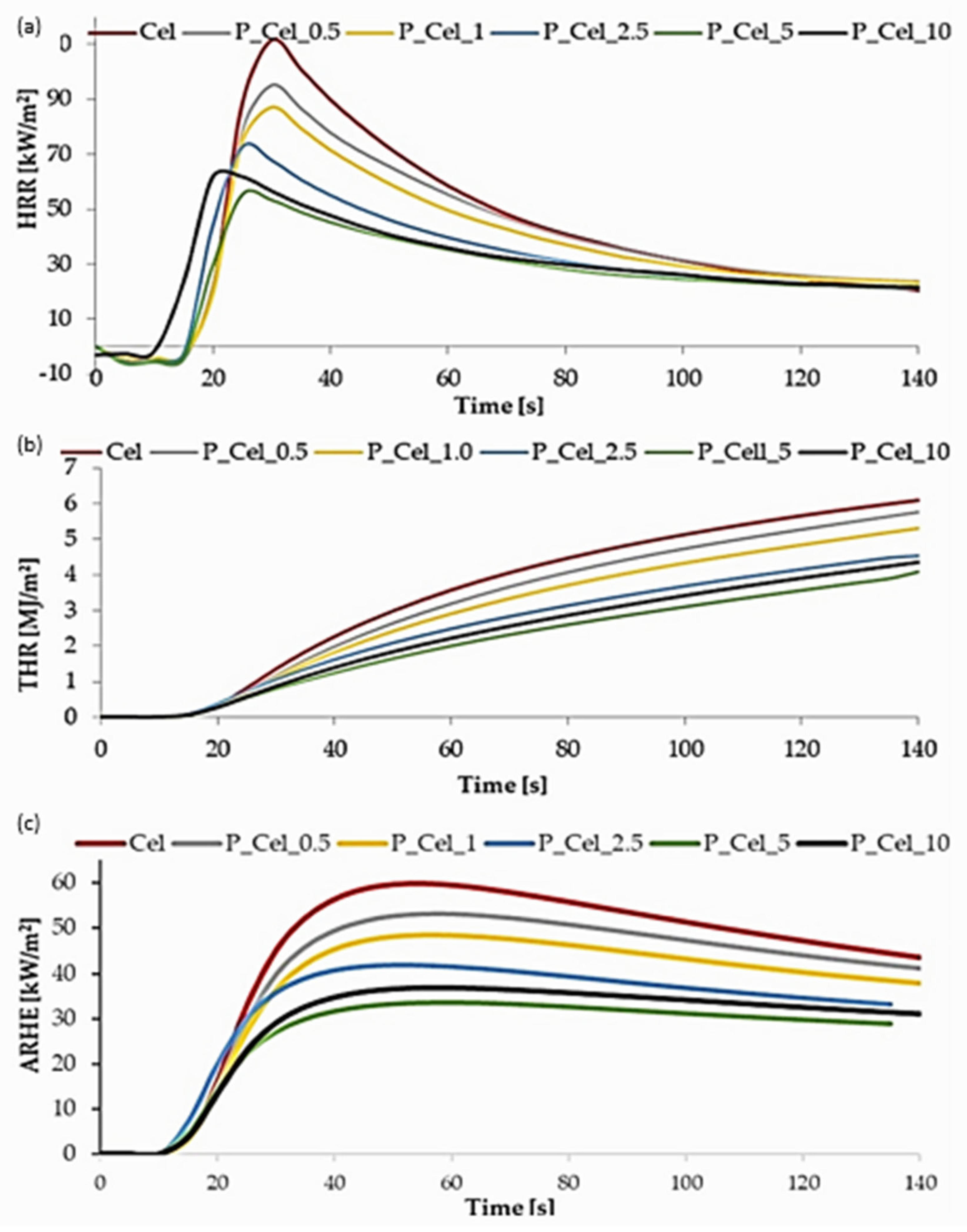
2.4.2. Crucial Results from the MLC Test
2.4.3. Crucial Results from the TG Test
3. Materials and Methods
3.1. Phosphorylation of Cellulosic Suspension
3.2. Preparation of Phosphorylated Cellulose Sheets
3.3. ICP hrOES Analysis of Phosphorus Concentration
- P0—Phosphate content in phosphorylated pulps before the washing process [mg/kg].
- P1—Phosphate content in phosphorylated pulps after the washing process [mg/kg].
3.4. Tensile Strength
3.5. Fire-Proof Evaluation
3.5.1. Mini Fire Tube (MFT)
3.5.2. Mass Loss Calorimeter (MLC)
3.5.3. Thermogravimetric Analysis (TG)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tavakoli, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Mazela, B. Cellulose and Its Nano-Derivatives as a Water-Repellent and Fire-Resistant Surface: A Review. Materials 2021, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.-M. Towards Bio-Based Flame Retardant Polymers; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 3-319-67083-2. [Google Scholar]
- Rol, F.; Sillard, C.; Bardet, M.; Yarava, J.R.; Emsley, L.; Gablin, C.; Léonard, D.; Belgacem, N.; Bras, J. Cellulose Phosphorylation Comparison and Analysis of Phosphorate Position on Cellulose Fibers. Carbohydr. Polym. 2020, 229, 115294. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Lindström, T.; Flodberg, G.; Sundström, J.; Junel, K.; Runebjörk, A.; Weise, C.F.; Erlandsson, J. Phosphorylated Nanofibrillated Cellulose: Production and Properties. Nord. Pulp Pap. Res. J. 2016, 31, 20–29. [Google Scholar] [CrossRef]
- Aoki, D.; Nishio, Y. Phosphorylated Cellulose Propionate Derivatives as Thermoplastic Flame Resistant/Retardant Materials: Influence of Regioselective Phosphorylation on Their Thermal Degradation Behaviour. Cellulose 2010, 17, 963–976. [Google Scholar] [CrossRef]
- Noguchi, Y.; Homma, I.; Matsubara, Y. Complete Nanofibrillation of Cellulose Prepared by Phosphorylation. Cellulose 2017, 24, 1295–1305. [Google Scholar] [CrossRef]
- Granja, P.L.; Pouységu, L.; Petraud, M.; De Jeso, B.; Baquey, C.; Barbosa, M.A. Cellulose Phosphates as Biomaterials. I. Synthesis and Characterization of Highly Phosphorylated Cellulose Gels. J. Appl. Polym. Sci. 2001, 82, 3341–3353. [Google Scholar] [CrossRef]
- Kokol, V.; Božič, M.; Vogrinčič, R.; Mathew, A.P. Characterisation and Properties of Homo-and Heterogenously Phosphorylated Nanocellulose. Carbohydr. Polym. 2015, 125, 301–313. [Google Scholar] [CrossRef]
- Reid, M. Buras, Lnd. Eng. Chem 1949, 41, 2831. [Google Scholar]
- Messa, L.L.; Faez, R.; Hsieh, Y.-L. Phosphorylated Cellulose Nanofibrils from Sugarcane Bagasse with pH Tunable Gelation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100085. [Google Scholar] [CrossRef]
- Davis, F.V.; Findlay, J.; Rogers, E. 52—The Urea-Phosphoric Acid Method of Flameproofing Textiles. J. Text. Inst. Trans. 1949, 40, T839–T854. [Google Scholar] [CrossRef]
- Belosinschi, D.; Benkaddour, A.; Tofanica, B.-M.; Ngo, T. Phosphorylation of Cellulose in the Presence of Urea. Mechanism of Reaction and Reagent Impact. Res. Sq. 2021. preprint (Version 1). [Google Scholar] [CrossRef]
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic Phosphorylation of Cellulose Nanofibers to New Highly-Ions Adsorbing, Flame-Retardant and Hydroxyapatite-Growth Induced Natural Nanoparticles. Cellulose 2014, 21, 2713–2726. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and Their Phosphorylated Derivatives for Selective Adsorption of Ag+, Cu2+ and Fe3+ from Industrial Effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.; Hassinen, J.; Kumar, A.A.; Johansson, L.-S.; Mäenpää, R.; Pahimanolis, N.; Pradeep, T.; Ikkala, O.; Rojas, O.J. Phosphorylated Cellulose Nanofibers Exhibit Exceptional Capacity for Uranium Capture. Cellulose 2020, 27, 10719–10732. [Google Scholar] [CrossRef]
- Mautner, A.; Maples, H.A.; Kobkeatthawin, T.; Kokol, V.; Karim, Z.; Li, K.; Bismarck, A. Phosphorylated Nanocellulose Papers for Copper Adsorption from Aqueous Solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1861–1872. [Google Scholar] [CrossRef]
- Mucalo, M.R.; Kato, K.; Yokogawa, Y. Phosphorylated, Cellulose-Based Substrates as Potential Adsorbents for Bone Morphogenetic Proteins in Biomedical Applications: A Protein Adsorption Screening Study Using Cytochrome C as a Bone Morphogenetic Protein Mimic. Colloids Surf. B Biointerfaces 2009, 71, 52–58. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Urbaniak, P. Vapor Phosphorylation of Cellulose by Phosphorus Trichlo-Ride: Selective Phosphorylation of 6-Hydroxyl Function—The Synthesis of New Antimicrobial Cellulose 6-Phosphate (III)-Copper Complexes. Antibiotics 2021, 10, 203. [Google Scholar] [CrossRef]
- Suflet, D.M.; Chitanu, G.C.; Popa, V.I. Phosphorylation of Polysaccharides: New Results on Synthesis and Characterisation of Phosphorylated Cellulose. React. Funct. Polym. 2006, 66, 1240–1249. [Google Scholar] [CrossRef]
- Reid, J.D.; Mazzeno, L.W. Preparation and Properties of Cellulose Phosphates. Ind. Eng. Chem. 1949, 41, 2828–2831. [Google Scholar] [CrossRef]
- Nuessle, A.C.; Ford, F.M.; Hall, W.P.; Lippert, A.L. Some Aspects of the Cellulose-Phosphate-Urea Reaction. Text. Res. J. 1956, 26, 32–39. [Google Scholar] [CrossRef]
- Jani, S.M.; Rushdan, I.; Saad, M.J.; Ibrahim, R. Mechanical Properties of Beating Pulp and Paper from Rice Straw. J. Trop. Agric. Food Sci 2016, 44, 103–109. [Google Scholar]
- Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, K.; Mizuno, H. Flameproofing of Cotton Fabrics with Urea and Phosphoric Acid in Organic Solvent. Sen’i Gakkaishi 1966, 22, 510–514. [Google Scholar] [CrossRef]
- Niu, F.; Wu, N.; Yu, J.; Ma, X. Gelation, Flame Retardancy, and Physical Properties of Phosphorylated Microcrystalline Cellulose Aerogels. Carbohydr. Polym. 2020, 242, 116422. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Homma, I.; Watanabe, T. Properties of Phosphorylated Cellulose Nanofiber Dispersions under Various Conditions. Cellulose 2020, 27, 2029–2040. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Gengembre, L.; Delobel, R. XPS Study of an Intumescent Coating Application to the Ammonium Polyphosphate/Pentaerythritol Fire-Retardant System. Appl. Surf. Sci. 1994, 81, 299–307. [Google Scholar] [CrossRef]
- Inagaki, N.; Nakamura, S.; Asai, H.; Katsuura, K. Phosphorylation of Cellulose with Phosphorous Acid and Thermal Degradation of the Product. J. Appl. Polym. Sci. 1976, 20, 2829–2836. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, N.; Meyer, V.; Petit-Conil, M.; Bras, J. Production of Fire-Retardant Phosphorylated Cellulose Fibrils by Twin-Screw Extrusion with Low Energy Consumption. Cellulose 2019, 26, 5635–5651. [Google Scholar] [CrossRef]
- Khakalo, A.; Jaiswal, A.K.; Kumar, V.; Gestranius, M.; Kangas, H.; Tammelin, T. Production of High-Solid-Content Fire-Retardant Phosphorylated Cellulose Microfibrils. ACS Sustain. Chem. Eng. 2021, 9, 12365–12375. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hasa, T.; Ahola, J.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Phosphonated Nanocelluloses from Sequential Oxidative–Reductive Treatment—Physicochemical Characteristics and Thermal Properties. Carbohydr. Polym. 2015, 133, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Babrauskas, V. Book Review: SFPE Handbook of Fire Protection Engineering; SAGE Publications Sage UK: London, UK, 2016; ISBN 1-4939-2564-4. [Google Scholar]
- Grześkowiak, W.Ł. Effectiveness of New Wood Fire Retardants Using a Cone Calorimeter. J. Fire Sci. 2017, 35, 565–576. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Blasio, A.D.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and Hydrophobins as Novel Green Flame Retardants for Cotton Fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Cuttica, F.; Alongi, J.; Malucelli, G. Flame Retardancy of Polyester and Polyester–Cotton Blends Treated with Caseins. Ind. Eng. Chem. Res. 2014, 53, 3917–3923. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of Phosphorus and Nitrogen on Flame Retardant Cellulose: A Study of Phosphorus Compounds. J. Anal. Appl. Pyrolysis 2007, 78, 371–377. [Google Scholar] [CrossRef]
- Shi, Y.; Belosinschi, D.; Brouillette, F.; Belfkira, A.; Chabot, B. The Properties of Phosphorylated Kraft Fibers. Bioresources 2015, 10, 4375–4390. [Google Scholar] [CrossRef]
- SIST PN-EN ISO 5267-1:2000/AC:2003; Pulps—Determination of Drainability—Part 1: Schopper-Riegler Method (ISO 5267-1:1999/Cor.1:2001). ISO: Geneva, Switzerland, 2001.
- ASTM E69-22; Standard Test Method for Combustible Properties of Treated Wood by the Fire-Tube Apparatus. ASTM International: West Conshohocken, PA, USA, 2022.
- Zeinali, D.; Kolaitis, D.; Schmid, J.; Barrio, A.; Pfriem, A.; Galan, A.; Donmez Cavdar, A.; Bedon, C.; Hopkin, D.; Barber, D. Guide for Obtaining Data from Reaction to Fire Tests; ArTS Università Degli Studi di Trieste: Trieste, Italy, 2019. [Google Scholar]
- EN ISO 13927:2015; Plastics—Simple Heat Release Test Using a Conical Radiant Heater and a Thermopile Detector. European Committee for Standardization: Brussels, Belgium, 2015.
| Treatment Type | Freeness (°SR) Cellulose Pulp | pH of Pulp before Washing | pH of Pulp after Leaching | Grammage of Cellulose Sheets (g/m2) |
|---|---|---|---|---|
| Cel | 14 | - | - | 308.4 |
| P_Cel_0.5 | 10 | 6.82 | 7.73 | 301.9 |
| P_Cel_1 | 8.5 | 6.81 | 7.53 | 297.7 |
| P_Cel_2.5 | 16.5 | 6.71 | 7.02 | 296.8 |
| P_Cel_5 | 38 | 5.62 | 6.11 | 297.4 |
| P_Cel_10 | 64.5 | 5.79 | 6.20 | 292.9 |
| Treatment Type | Maximum Temp. of Combustion (°C) | Time to Reach the Max. Combustion Temperature (s) |
|---|---|---|
| Cel | 567.2 ± 24.2 | 15.0 ± 1.1 |
| P_Cel_0.5 | 395.7 ± 45.7 | 17.0 ± 1.9 |
| P_Cel_1 | 276.8 ± 49.5 | 16.8 ± 4.1 |
| P_Cel_2.5 | 289.1 ± 42.1 | 15.0 ± 1.7 |
| P_Cel_5 | 255.3 ± 31.7 | 15.0 ± 2.3 |
| P_Cel_10 | 286.1 ± 20.5 | 17.0 ± 3.9 |
| Parameters (Time to Maximum Value (s)) | Treatment Type | |||||
|---|---|---|---|---|---|---|
| Cel | P_Cel_0.5 | P_Cel_1 | P_Cel_2.5 | P_Cel_5 | P_Cel_10 | |
| Peak of Heat Release Rate (pHRR) (kW/m2) | 111.5 (25 *) | 87.6 (22 *) | 76.9 (22 *) | 40.3 (17 * 6.67) | 43.90 (15) | 61.6 (20 *) |
| Effective heat of combustion (EHC) (MJ/kg) | 34.7 (28 *) | 6.3 (21 *) | 1.5 (21 *) | 16.8 (16 *) | 1.5 (15 *) | 6.3 (20 *) |
| Time to ignition (s) | 10.0 | 10 | 10 | 9 | 9 | 10 |
| Time to flameout (s) | 34 | 24 | 24 | 19 | 19 | 21 |
| Treatment Type | Thermolysis Temp. Area (°C) | Max. Temp. of Decomposition Speed (°C) | Mass Loss (%) | |
|---|---|---|---|---|
| In Thermolysis Area | Whole to 600 °C | |||
| Cel | 289.9–404.3 | 340–396.8 | 82.67 | 87.4 |
| P_Cel_0.5 | 261.2–397.2 | 314.5–379 | 74.6 | 81.42 |
| P_Cel_1 | 273.83–390.7 | 311.2–379.8 | 66.35 | 76.15 |
| P_Cel_2.5 | 254.2–375.5 | 318.3–353.8 | 41.8 | 57.61 |
| P_Cel_5 | 249.2–374 | 317–340.17 | 41.29 | 54.11 |
| P_Cel_10 | 262.5–373.83 | 320.2–362.83 | 50.27 | 65.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, M.; Mazela, B.; Grześkowiak, W.; Proch, J.; Mleczek, M.; Perdoch, W. The Strength and Fire Properties of Paper Sheets Made of Phosphorylated Cellulose Fibers. Molecules 2024, 29, 133. https://doi.org/10.3390/molecules29010133
Tavakoli M, Mazela B, Grześkowiak W, Proch J, Mleczek M, Perdoch W. The Strength and Fire Properties of Paper Sheets Made of Phosphorylated Cellulose Fibers. Molecules. 2024; 29(1):133. https://doi.org/10.3390/molecules29010133
Chicago/Turabian StyleTavakoli, Mehrnoosh, Bartłomiej Mazela, Wojciech Grześkowiak, Jędrzej Proch, Mirosław Mleczek, and Waldemar Perdoch. 2024. "The Strength and Fire Properties of Paper Sheets Made of Phosphorylated Cellulose Fibers" Molecules 29, no. 1: 133. https://doi.org/10.3390/molecules29010133
APA StyleTavakoli, M., Mazela, B., Grześkowiak, W., Proch, J., Mleczek, M., & Perdoch, W. (2024). The Strength and Fire Properties of Paper Sheets Made of Phosphorylated Cellulose Fibers. Molecules, 29(1), 133. https://doi.org/10.3390/molecules29010133










