Genistein Prevents Apoptosis and Oxidative Stress Induced by Methylglyoxal in Endothelial Cells
Abstract
1. Introduction
2. Results
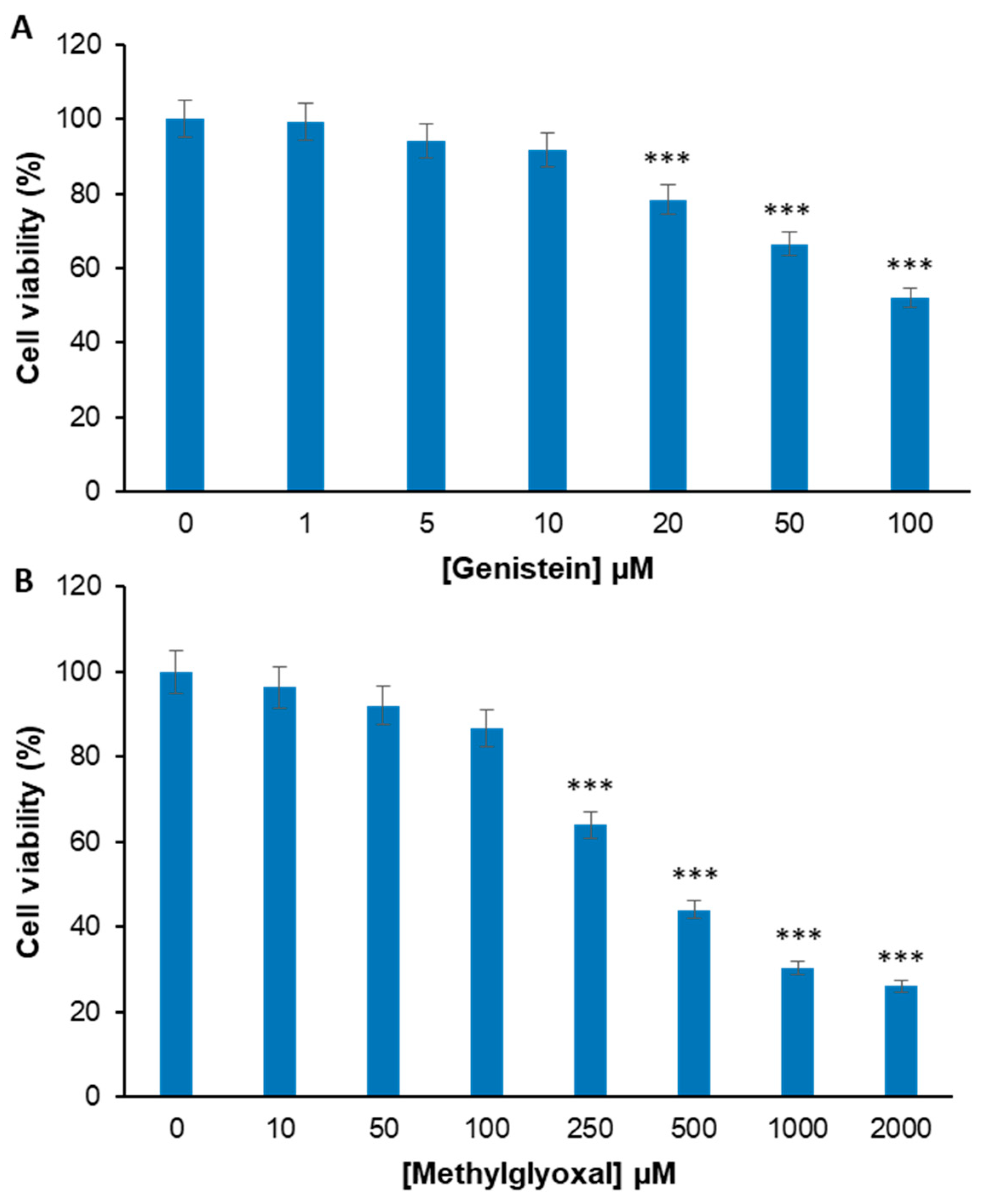
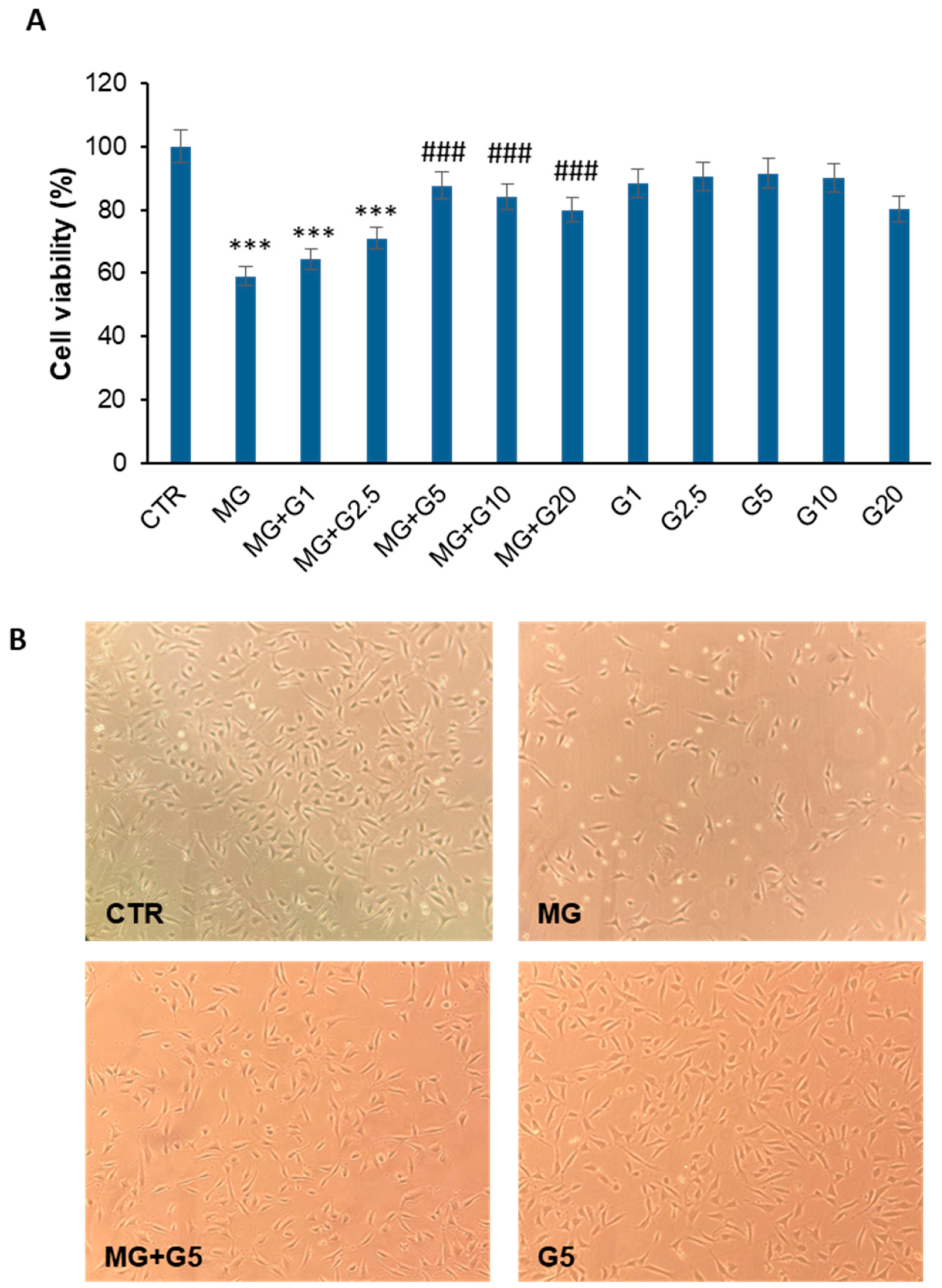
2.1. Genistein Markedly Reduces the MG-Induced Toxicity in EA.HY926 Cells
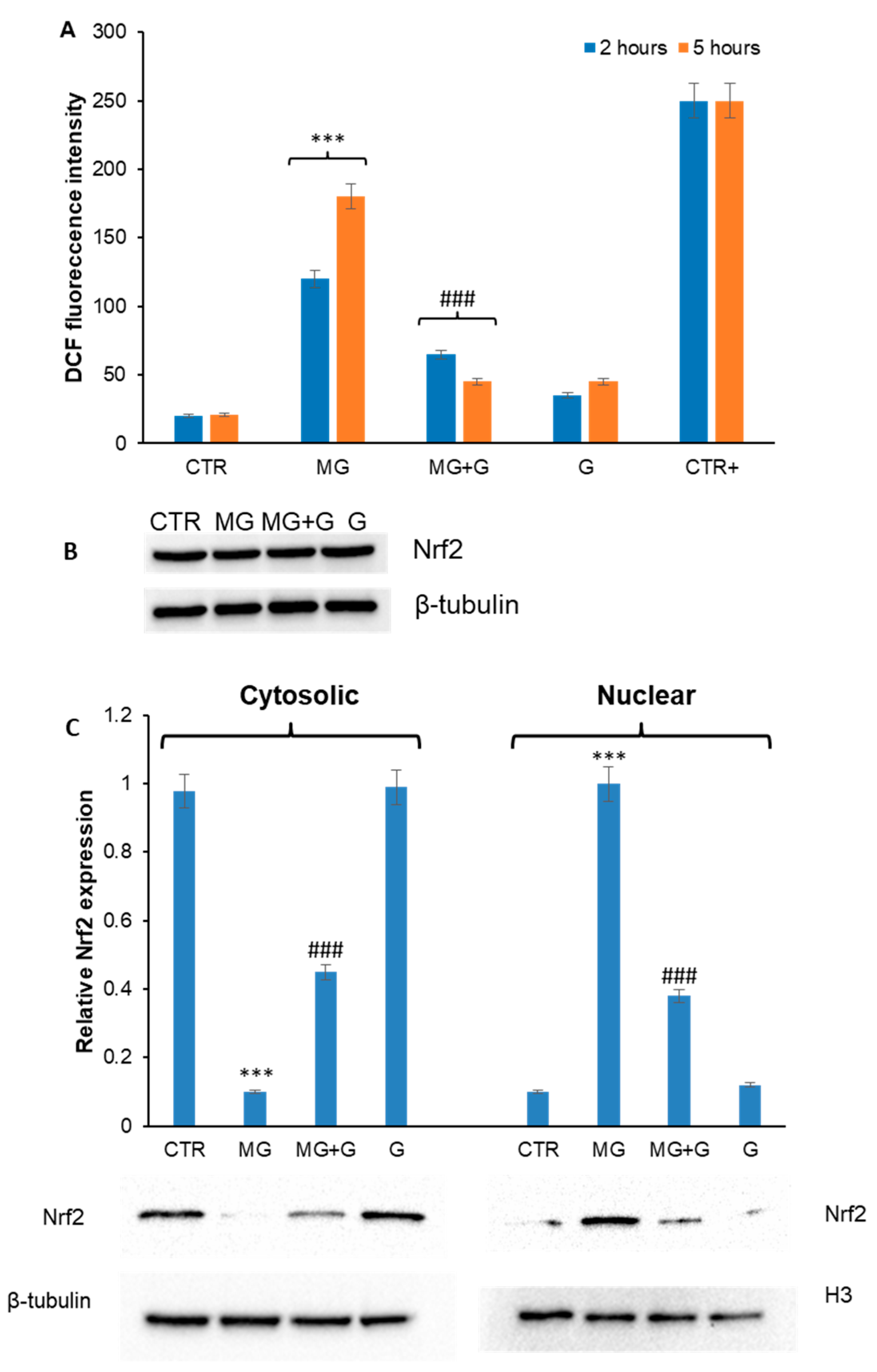
2.2. Genistein Prevents MG-Induced ROS Production and Nrf2 Activation in EA.HY926 Cells
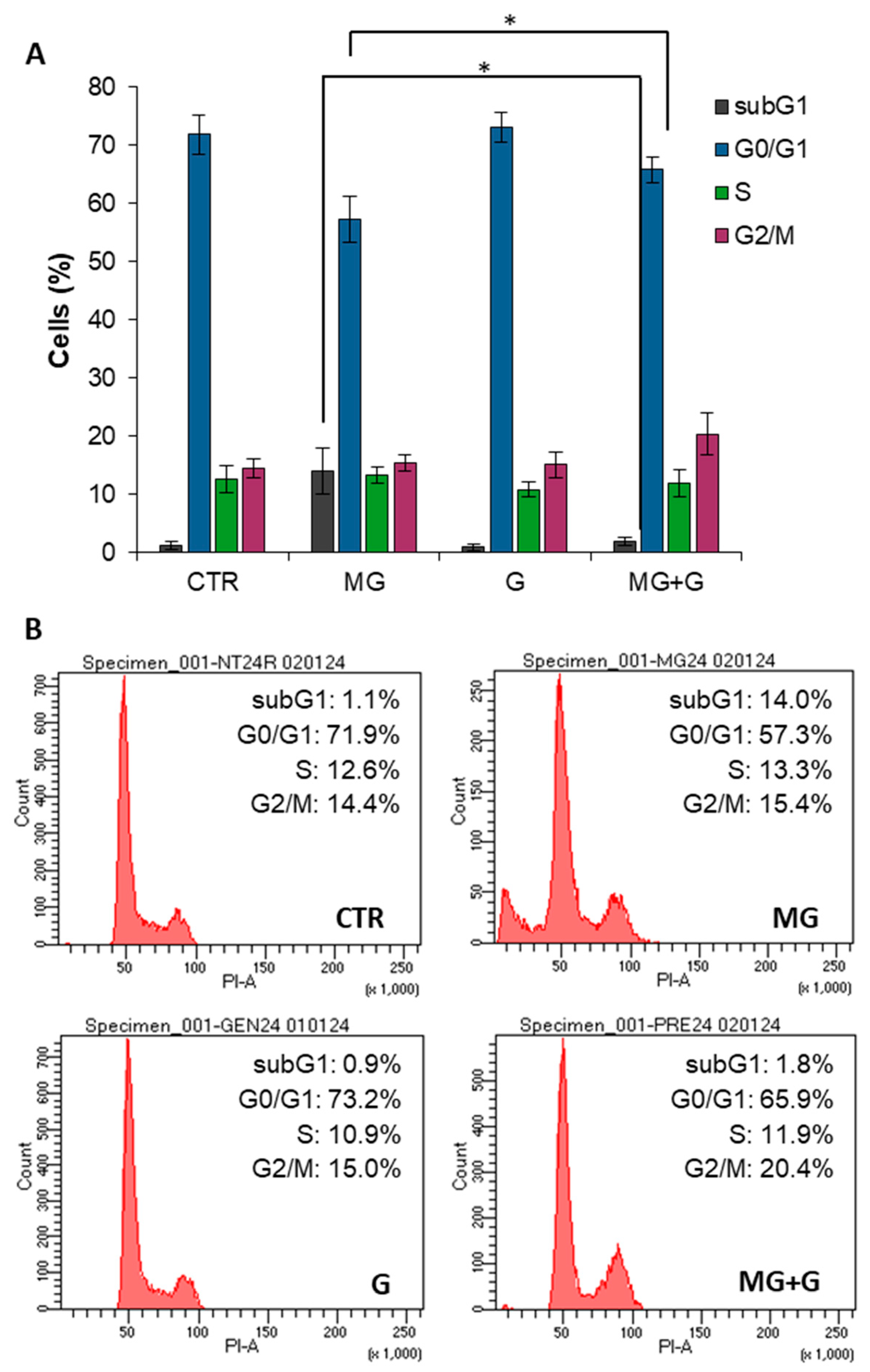
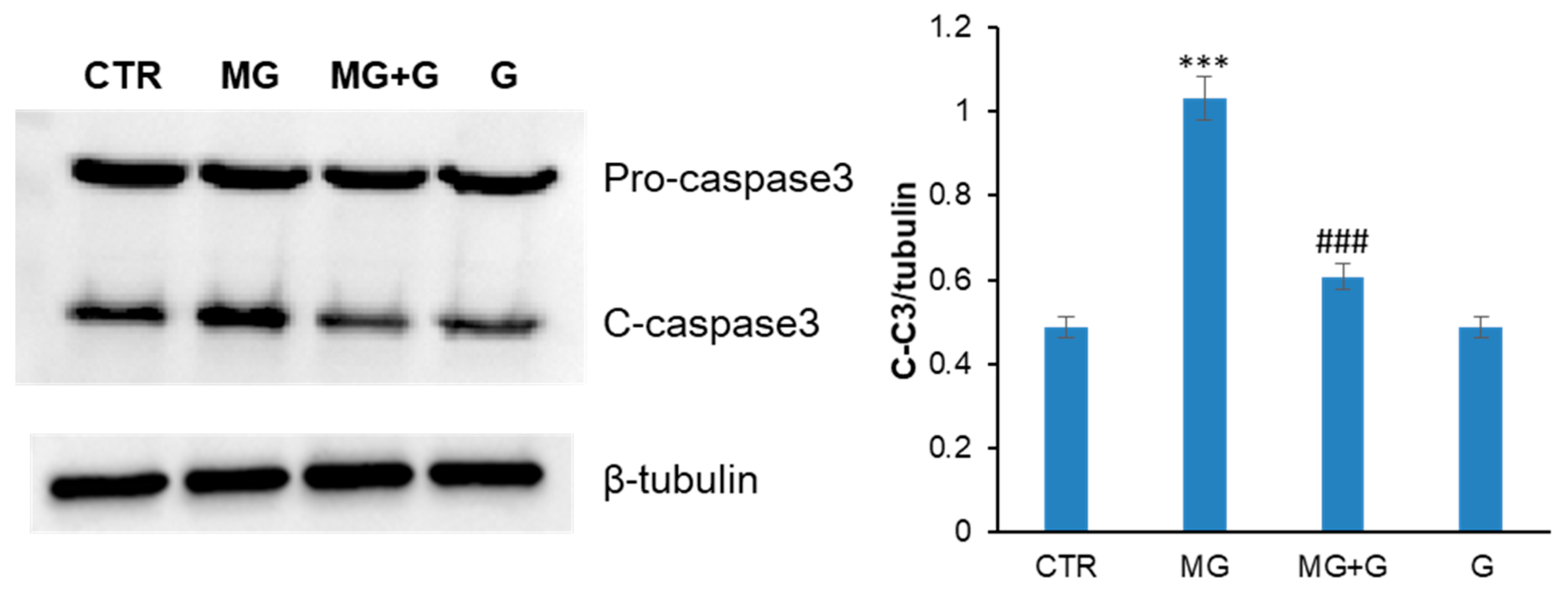
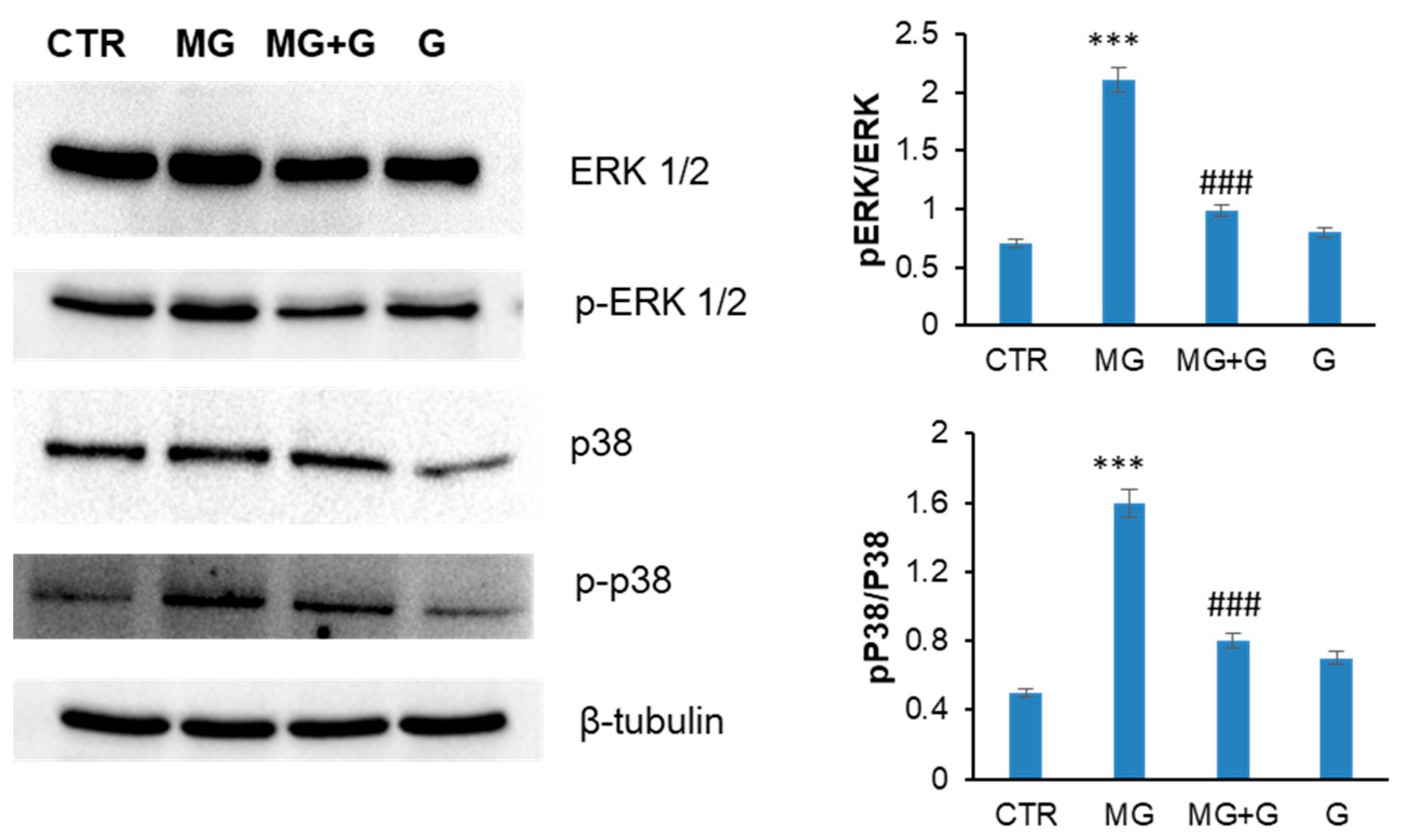
2.3. Genistein Protects Endothelial Cells by the MG-Induced Apoptosis through the MAPK-Mediated Signaling Pathways
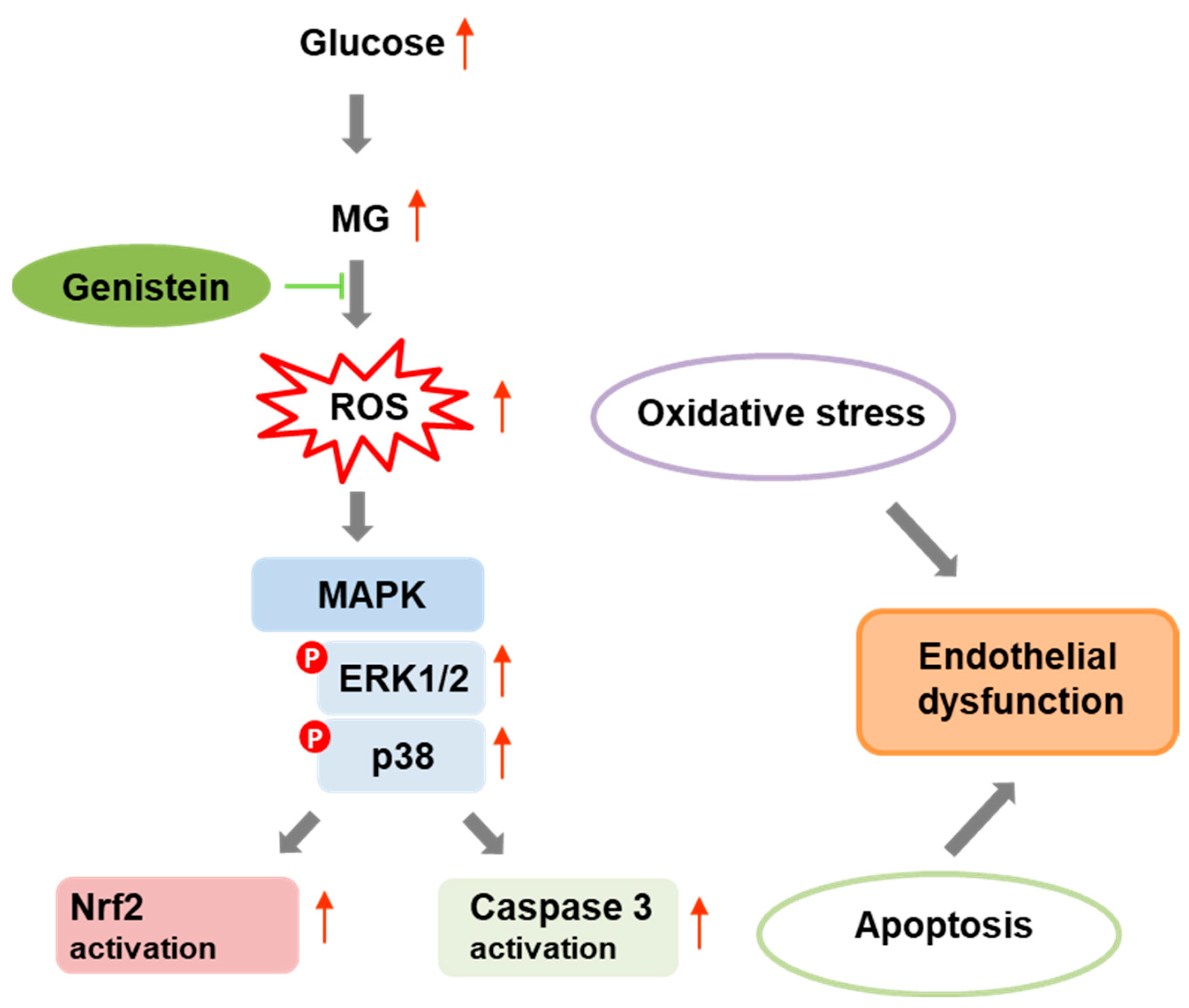
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures and Treatments
4.3. MTT Assay
4.4. Cell Cycle Analysis
4.5. Detection of Intracellular ROS
4.6. Cellular Nuclear Extraction
4.7. Immunoblotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.T.; Lopez Gonzalez, E.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Iannuzzi, C. Understanding the Role of Protein Glycation in the Amyloid Aggregation Process. Int. J. Mol. Sci. 2021, 22, 6609. [Google Scholar] [CrossRef] [PubMed]
- Bellier, J.; Nokin, M.J.; Lardé, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcène, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rahman, T.; Ismail, A.A.; Rashid, A.R. Diabetes-associated macrovasculopathy: Pathophysiology and pathogenesis. Diabetes Obes. Metab. 2007, 9, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Westerink, J.; Scheijen, J.L.J.M.; van der Graaf, Y.; Stehouwer, C.D.A.; Schalkwijk, C.G.; SMART Study Group. Higher Plasma Methylglyoxal Levels Are Associated with Incident Cardiovascular Disease and Mortality in Individuals with Type 2 Diabetes. Diabetes Care 2018, 41, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Groener, J.; Oikonomou, D.; Cheko, R.; Kender, Z.; Zemva, J.; Kihm, L.; Muckenthaler, M.; Peters, V.; Fleming, T.; Kopf, S.; et al. Methylglyoxal and Advanced Glycation End Products in Patients with Diabetes—What We Know so Far and the Missing Links. Exp. Clin. Endocrinol. Diabetes 2019, 127, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes. Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Martens, R.J.H.; Broers, N.J.H.; Canaud, B.; Christiaans, M.H.L.; Cornelis, T.; Gauly, A.; Hermans, M.M.H.; Konings, C.J.A.M.; van der Sande, F.M.; Scheijen, J.L.J.M.; et al. Relations of advanced glycation endproducts and dicarbonyls with endothelial dysfunction and low-grade inflammation in individuals with end-stage renal disease in the transition to renal replacement therapy: A cross-sectional observational study. PLoS ONE 2019, 14, e0221058. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Parveen, A.; Do, M.H.; Kang, M.C.; Yumnam, S.; Kim, S.Y. Molecular mechanisms of methylglyoxal-induced aortic endothelial dysfunction in human vascular endothelial cells. Cell Death Dis. 2020, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Figarola, J.L.; Singhal, J.; Rahbar, S.; Awasthi, S.; Singhal, S.S. LR-90 prevents methylglyoxal-induced oxidative stress and apoptosis in human endothelial cells. Apoptosis 2014, 19, 776–788. [Google Scholar] [CrossRef]
- Pang, N.; Chen, T.; Deng, X.; Chen, N.; Li, R.; Ren, M.; Li, Y.; Luo, M.; Hao, H.; Wu, J.; et al. Polydatin Prevents Methylglyoxal-Induced Apoptosis through Reducing Oxidative Stress and Improving Mitochondrial Function in Human Umbilical Vein Endothelial Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7180943. [Google Scholar] [CrossRef]
- Chu, P.; Han, G.; Ahsan, A.; Sun, Z.; Liu, S.; Zhang, Z.; Sun, B.; Song, Y.; Lin, Y.; Peng, J.; et al. Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-κB pathway. Vascul. Pharmacol. 2017, 91, 26–35. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Tannins and vascular complications of Diabetes: An update. Phytomedicine 2019, 56, 229–245. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Huang, H.; Luo, Y.; Wang, Q.; Zhang, Y.; Li, Z.; He, R.; Chen, X.; Dong, Z. Vaccinium as Potential Therapy for Diabetes and Microvascular Complications. Nutrients 2023, 15, 2031. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; De Pascual-Teresa, S.; Ewins, B.A.; Matsugo, S.; Uchida, Y.; Minihane, A.M.; Turner, R.; VafeiAdou, K.; Weinberg, P.D. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 2003, 33, 913–925. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Kulling, S.E. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 310, 108665. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta. Pol. Pharm. 2000, 57, 135–155. [Google Scholar]
- Rasheed, S.; Rehman, K.; Shahid, M.; Suhail, S.; Akash, M.S.H. Therapeutic potentials of genistein: New insights and perspectives. J. Food Biochem. 2022, 46, e14228. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bowen, R.; Cai, Q.; Barnes, S.; Wang, Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc. Soc. Exp. Biol. Med. 1995, 208, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Smith, B.M.; Washnock, C.S. Cardiovascular and renal benefits of dry bean and soybean intake. Am. J. Clin. Nutr. 1999, 70, 464S–474S. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Phytochemical genistein in the regulation of vascular function: New insights. Curr. Med. Chem. 2007, 14, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G. The Antioxidant Role of Soy and Soy Foods in Human Health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Siriviriyakul, P.; Sriko, J.; Somanawat, K.; Chayanupatkul, M.; Klaikeaw, N.; Werawatganon, D. Genistein attenuated oxidative stress, inflammation, and apoptosis in L-arginine induced acute pancreatitis in mice. BMC Complement. Med. Ther. 2022, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Dong, Y.; Zhu, W.; Wu, T.; Chen, L.; Cao, Y.; Yu, X.; Peng, Y.; Wang, L.; Xiao, Y.; et al. Underlying mechanisms and molecular targets of genistein in the management of type 2 diabetes mellitus and related complications. Crit. Rev. Food Sci. Nutr. 2023, 27, 1–13. [Google Scholar] [CrossRef]
- Wu, H.J.; Chan, W.H. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicol. In Vitro 2007, 21, 335–342. [Google Scholar] [CrossRef]
- Do, M.; Lee, J.H.; Wahedi, H.M.; Pak, C.; Lee, C.H.; Yeo, E.J.; Lim, Y.; Ha, S.K.; Choi, I.; Kim, S.Y. Lespedeza bicolor ameliorates endothelial dysfunction induced by methylglyoxal glucotoxicity. Phytomedicine 2017, 36, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Shao, X.; Chen, H.; Ho, C.T.; Sang, S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem. Res. Toxicol. 2011, 24, 579–586. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Sang, S. Trapping Methylglyoxal by Genistein and Its Metabolites in Mice. Chem. Res. Toxicol. 2016, 29, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Wu, L. Methylglyoxal, oxidative stress, and hypertension. Can. J. Physiol. Pharmacol. 2006, 84, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.M.; Chang, T.; Wang, H.; Banigesh, A.; Dhar, A.; Liu, J.; Untereiner, A.; Wu, L. Oxidative stress and aging: Is methylglyoxal the hidden enemy? Can. J. Physiol. Pharmacol. 2010, 88, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Ki, S.H.; Shin, S.M. Methylglyoxal induces mitochondrial dysfunction and cell death in liver. Toxicol. Res. 2014, 30, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Raina, G.; Camillo, L.; Farruggio, S.; Mary, D.; Veronese, F.; Graziola, F.; Zavattaro, E.; Tiberio, R.; Grossini, E. Anti-oxidative effects of 17 β-estradiol and genistein in human skin fibroblasts and keratinocytes. J. Dermatol. Sci. 2018, 92, 62–77. [Google Scholar] [CrossRef]
- Ruiz-Larrea, B.; Leal, A.; Martín, C.; Martínez, R.; Lacort, M. Effects of estrogens on the redox chemistry of iron: A possible mechanism of the antioxidant action of estrogens. Steroids 1995, 60, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Xiao, W.; Gu, W.T.; Zhang, Z.T.; Xu, S.H.; Chen, Z.Q.; Xu, Y.H.; Zhang, L.Y.; Wang, S.M.; Nie, H. Pterostilbene prevents methylglyoxal-induced cytotoxicity in endothelial cells by regulating glyoxalase, oxidative stress and apoptosis. Food Chem. Toxicol. 2021, 153, 112244. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, D.Y.; Huang, Y.P.; Hsu, S.H.; Kang, L.Y.; Shen, C.M.; Lin, W.W. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J. Cell. Mol. Med. 2016, 20, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Akhand, A.A.; Hossain, K.; Mitsui, H.; Kato, M.; Miyata, T.; Inagi, R.; Du, J.; Takeda, K.; Kawamoto, Y.; Suzuki, H.; et al. Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic. Biol. Med. 2001, 31, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Smolińska, E.; Moskot, M.; Jakóbkiewicz-Banecka, J.; Węgrzyn, G.; Banecki, B.; Szczerkowska-Dobosz, A.; Purzycka-Bohdan, D.; Gabig-Cimińska, M. Molecular action of isoflavone genistein in the human epithelial cell line HaCaT. PLoS ONE 2018, 13, e0192297. [Google Scholar] [CrossRef]
- Xie, X.; Cong, L.; Liu, S.; Xiang, L.; Fu, X. Genistein alleviates chronic vascular inflammatory response via the miR-21/NF-κB p65 axis in lipopolysaccharide-treated mice. Mol. Med. Rep. 2021, 23, 192. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Hexokinase-2 Glycolytic Overload in Diabetes and Ischemia-Reperfusion Injury. Trends Endocrinol. Metab. 2019, 30, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Fiory, F.; Prevenzano, I.; Nicolò, A.; Mirra, P.; Beguinot, F.; Miele, C. Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging. Cells 2019, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Irshad, Z.; Xue, M.; Ashour, A.; Larkin, J.R.; Thornalley, P.J.; Rabbani, N. Activation of the unfolded protein response in high glucose treated endothelial cells is mediated by methylglyoxal. Sci. Rep. 2019, 9, 7889. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Nakamura, N.; Sotokawauchi, A.; Higashimoto, Y.; Yamagishi, S.I. Methylglyoxal-derived hydroimidazolone-1 evokes inflammatory reactions in endothelial cells via an interaction with receptor for advanced glycation end products. Diab. Vasc. Dis. Res. 2017, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cheng, K.W.; Gong, J.; Li, E.T.S.; Wang, M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Jarisarapurin, W.; Kunchana, K.; Chularojmontri, L.; Wattanapitayakul, S.K. Unripe Carica papaya Protects Methylglyoxal-Invoked Endothelial Cell Inflammation and Apoptosis via the Suppression of Oxidative Stress and Akt/MAPK/NF-κB Signals. Antioxidants 2021, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Cheon, J.; Yoon, H.; Jun, H.S. Cudrania tricuspidata Root Extract Prevents Methylglyoxal-Induced Inflammation and Oxidative Stress via Regulation of the PKC-NOX4 Pathway in Human Kidney Cells. Oxid. Med. Cell. Longev. 2021, 2021, 5511881. [Google Scholar] [CrossRef]
- Choudhary, D.; Chandra, D.; Kale, R.K. Influence of methylglyoxal on antioxidant enzymes and oxidative damage. Toxicol. Lett. 1997, 93, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liccardo, M.; Sapio, L.; Perrella, S.; Sirangelo, I.; Iannuzzi, C. Genistein Prevents Apoptosis and Oxidative Stress Induced by Methylglyoxal in Endothelial Cells. Molecules 2024, 29, 1712. https://doi.org/10.3390/molecules29081712
Liccardo M, Sapio L, Perrella S, Sirangelo I, Iannuzzi C. Genistein Prevents Apoptosis and Oxidative Stress Induced by Methylglyoxal in Endothelial Cells. Molecules. 2024; 29(8):1712. https://doi.org/10.3390/molecules29081712
Chicago/Turabian StyleLiccardo, Maria, Luigi Sapio, Shana Perrella, Ivana Sirangelo, and Clara Iannuzzi. 2024. "Genistein Prevents Apoptosis and Oxidative Stress Induced by Methylglyoxal in Endothelial Cells" Molecules 29, no. 8: 1712. https://doi.org/10.3390/molecules29081712
APA StyleLiccardo, M., Sapio, L., Perrella, S., Sirangelo, I., & Iannuzzi, C. (2024). Genistein Prevents Apoptosis and Oxidative Stress Induced by Methylglyoxal in Endothelial Cells. Molecules, 29(8), 1712. https://doi.org/10.3390/molecules29081712







