Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer
Abstract
1. Introduction
2. Results
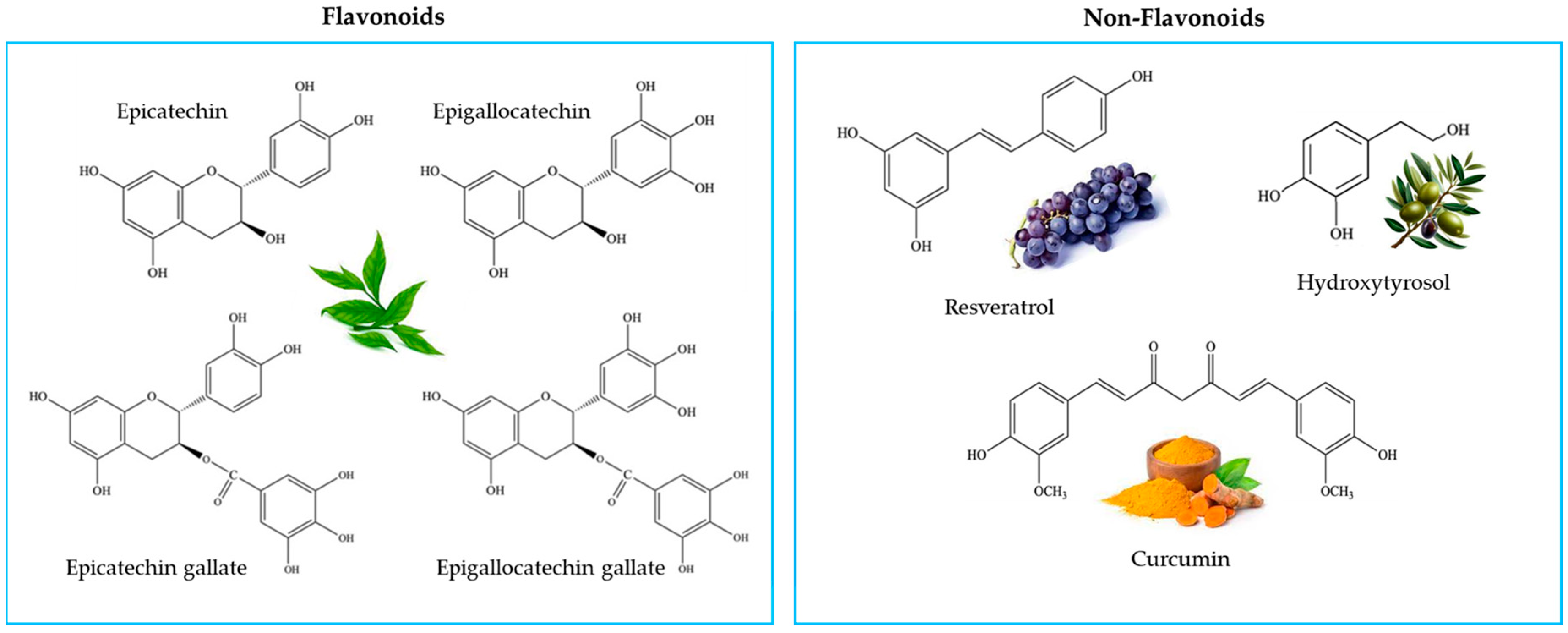
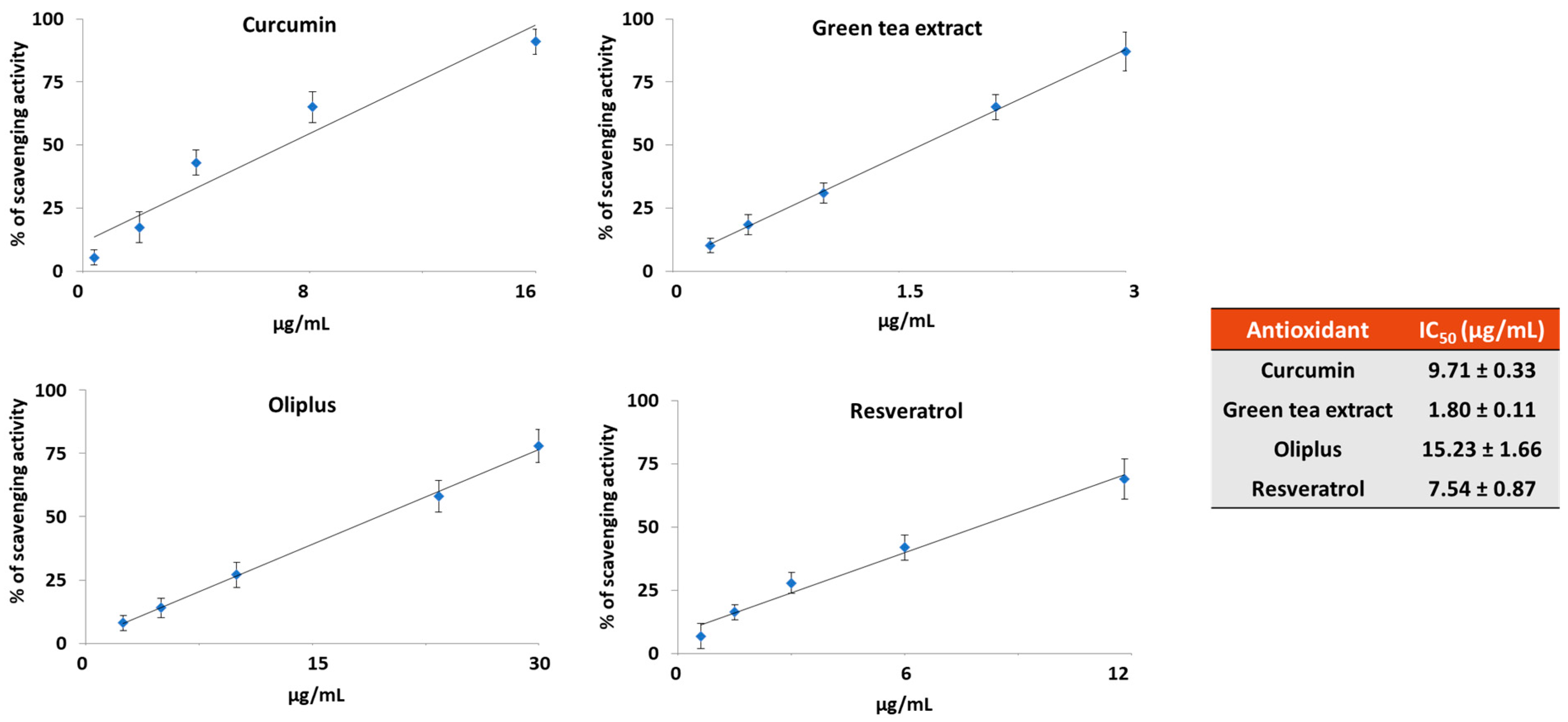
2.1. Antioxidant Activity In Vitro
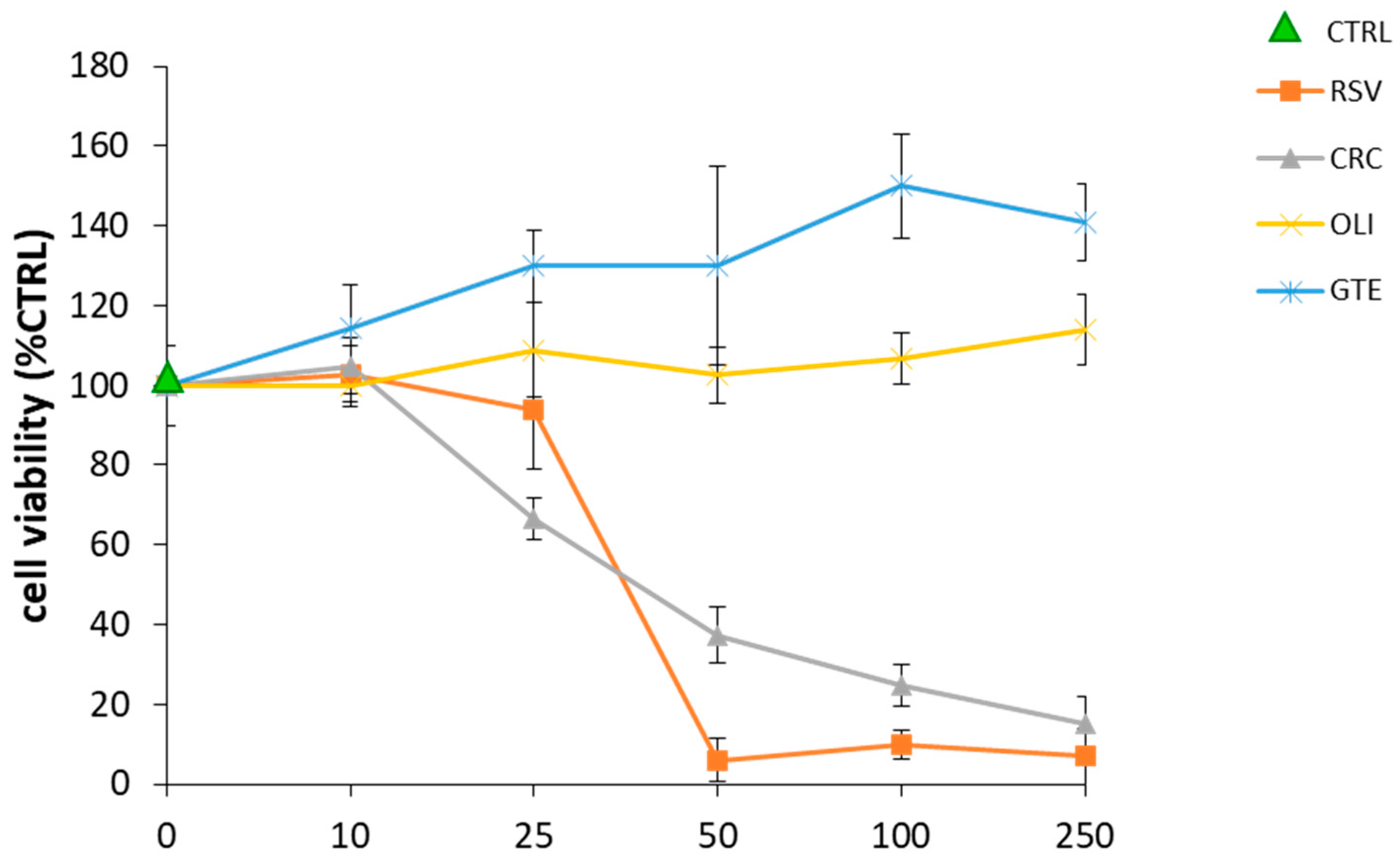
2.2. Effect of Antioxidant Compounds on THP-1 Macrophage Viability
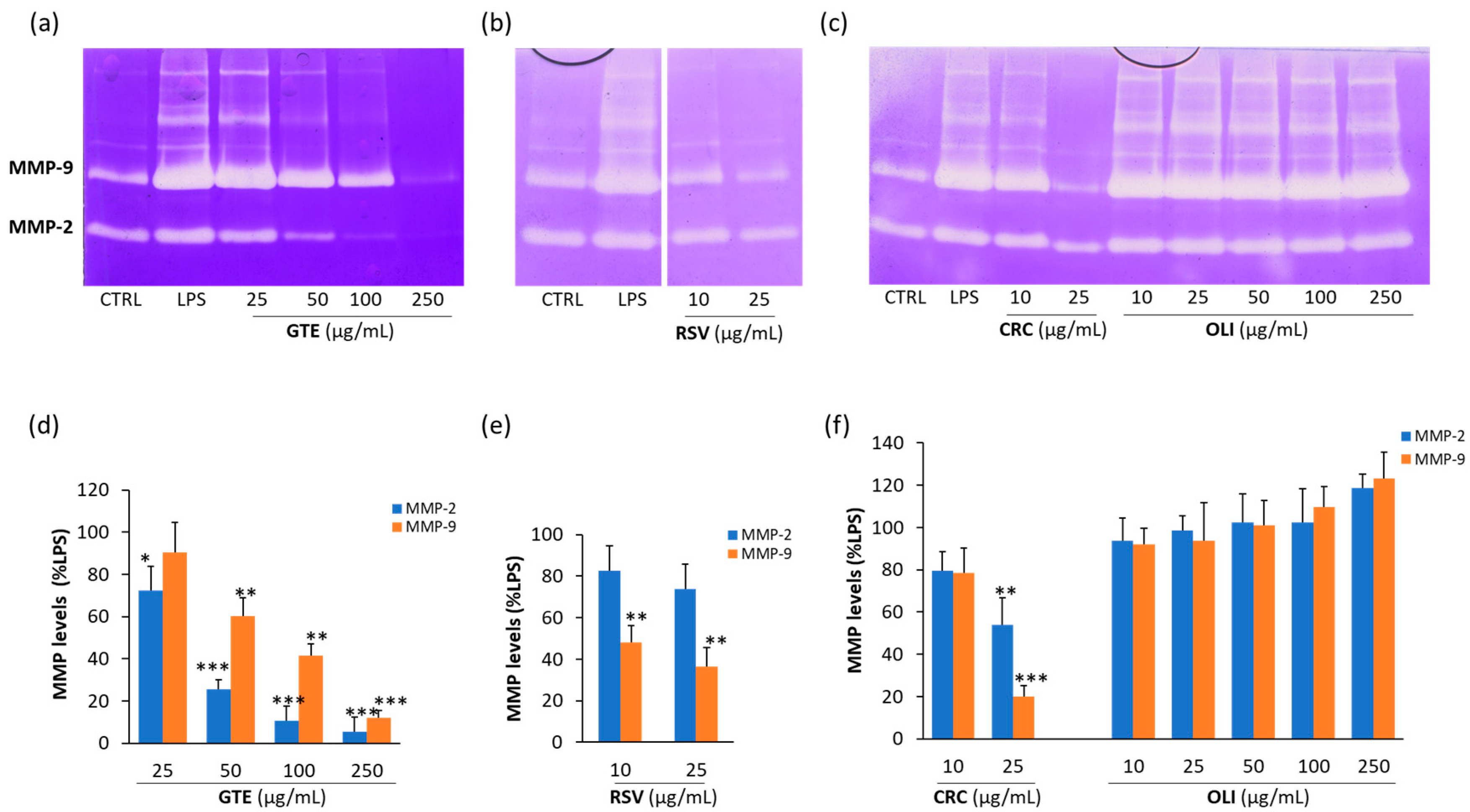
2.3. Effect of Antioxidant Compounds on MMP-2 and MMP-9 Released from LPS-Activated THP-1 Macrophages
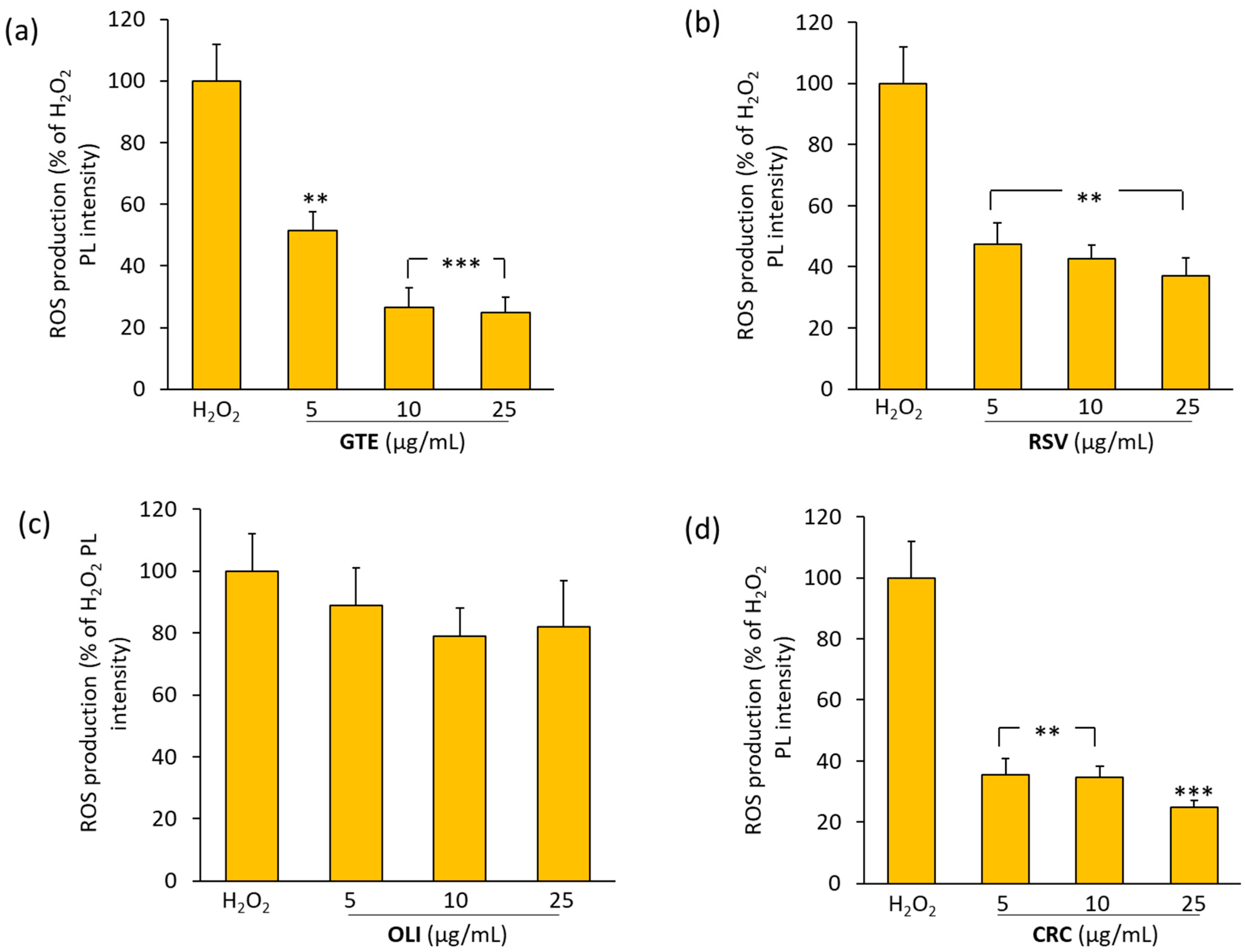
2.4. Effect of Antioxidant Compounds on ROS Production in THP-1 Macrophages Treated with Hydrogen Peroxide
2.5. “In-Gel” Inhibition of MMP-2 and MMP-9 Activity by Antioxidant Compounds
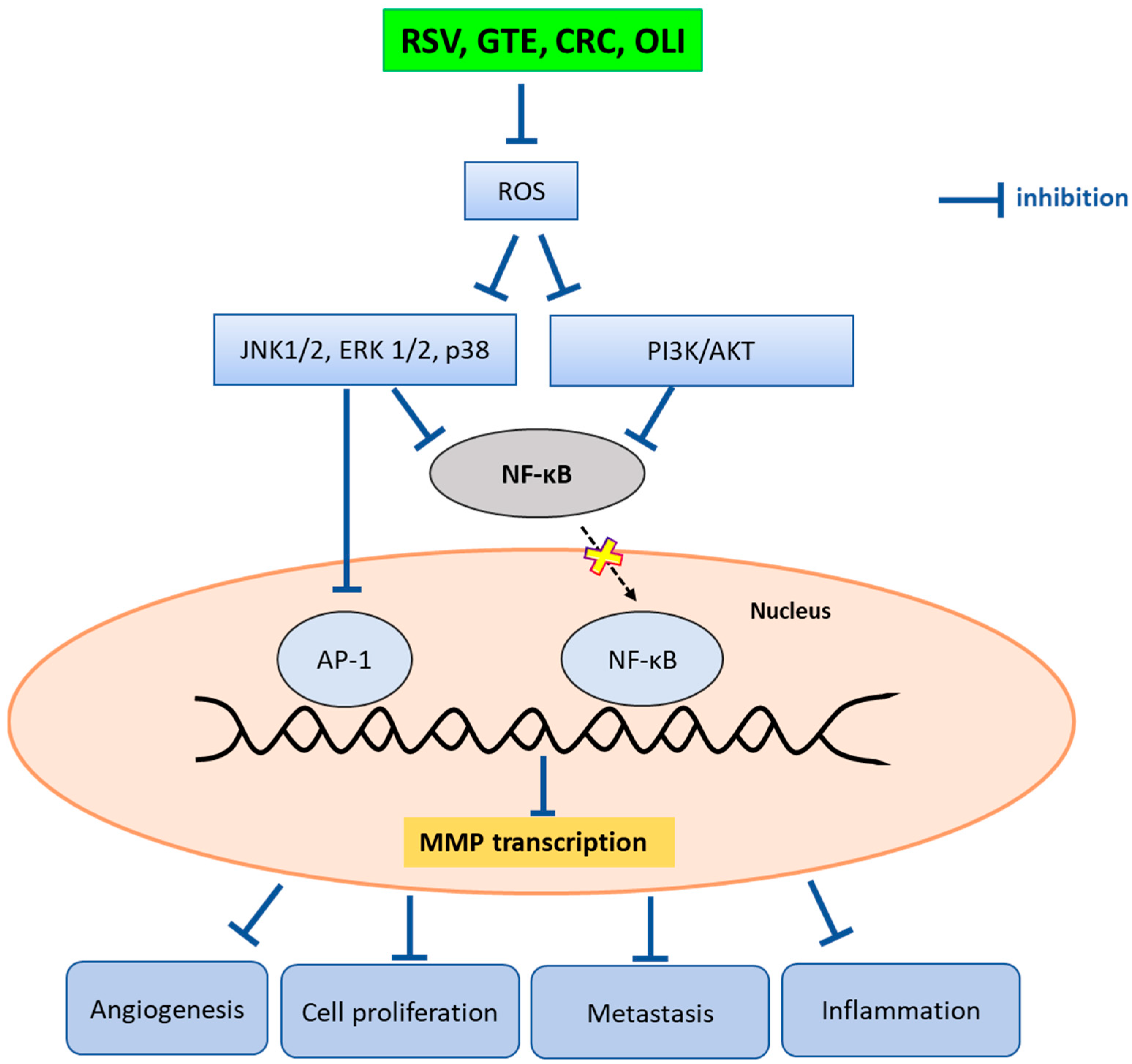
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Antioxidants
4.3. DPPH Radical Scavenging Activity
4.4. THP-1 Differentiation
4.5. Cell Viability
4.6. Treatment of LPS-Activated THP-1 Macrophages with Antioxidant Compounds
4.7. Reactive Oxygen Species Detection
4.8. Detection of MMP-2 and MMP-9 by Zymography
4.9. Determination of Inhibitory Capacity (In-Gel Inhibition) of Dietary Antioxidant on Gelatinases Present in Pool of Sera from Patients with Breast Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. J. Nutr. 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Marzullo, P.; Farinelli, D.; Gennari, A.; Saggia, C.; Riso, S.; Prodam, F. Breast Cancer Diet “BCD”: A Review of Healthy Dietary Patterns to Prevent Breast Cancer Recurrence and Reduce Mortality. J. Nutr. 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, R.B.; Tuli, N.Y.; Hanly, E.K.; Rahoma, G.B.; Maniyar, R.; Mittelman, A.; Geliebter, J.; Tiwari, R.K. Macrophage inflammatory factors promote epithelial-mesenchymal transition in breast cancer. Oncotarget 2018, 9, 24272–24282. [Google Scholar] [CrossRef] [PubMed]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-associated macrophages: An effective player of the tumor microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar] [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Kwon, M.J. Matrix metalloproteinases as therapeutic targets in breast cancer. Front. Oncol. 2023, 12, 1108695. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol./Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Nelson, A.R.; Fingleton, B.; Rothenberg, M.L.; Matrisian, L.M. Matrix Metalloproteinases: Biologic Activity and Clinical Implications. J. Clin. Oncol. 2000, 18, 1135. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The impact of plant phytochemicals on the gut microbiota of humans for a balanced life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S. The Role of Nutrition in Chronic Disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent updates on anticancer mechanisms of polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Shah, R.; Ibis, B.; Kashyap, M.; Boussiotis, V.A. The role of ROS in tumor infiltrating immune cells and cancer immunotherapy. Metabolism 2024, 151, 155747. [Google Scholar] [CrossRef]
- Khan, Y.H.; Uttra, A.M.; Qasim, S.; Mallhi, T.H.; Alotaibi, N.H.; Rasheed, M.; Alzarea, A.I.; Iqbal, M.S.; Alruwaili, N.K.; Khan, S.U.D.; et al. Potential role of phytochemicals against matrix metalloproteinase induced breast cancer; An explanatory review. Front. Chem. 2021, 8, 592152. [Google Scholar] [CrossRef]
- Radisky, E.S.; Raeeszadeh-Sarmazdeh, M.; Radisky, D.C. Therapeutic potential of matrix metalloproteinase inhibition in breast cancer. J. Cell. Biochem. 2017, 118, 3531–3548. [Google Scholar] [CrossRef]
- Kumar, G.B.; Nair, B.G.; Perry, J.J.P.; Martin, D.B.C. Recent insights into natural product inhibitors of matrix metalloproteinases. MedChemComm 2019, 10, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Rashid, Z.A.; Bardaweel, S.K. Novel Matrix Metalloproteinase-9 (MMP-9) Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 12133. [Google Scholar] [CrossRef]
- Vandooren, J.; Knoops, S.; Aldinucci Buzzo, J.L.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study. PLoS ONE 2017, 12, e0174853. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Uchida, T.; Yoshie, T.; Mizote, Y.; Ishikawa, F.; Katsuyama, M.; Shibanuma, M. A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J. 2019, 286, 459–478. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Oh, J.H.; Yu, G.H.; Jang, M.S.; Nam, K.H.; Seo, Y.; Kong, C.S. MMP-Inhibitory Effects of Flavonoid Glycosides from Edible Medicinal Halophyte Limonium tetragonum. J. Altern. Complement. Med. 2017, 2017, 6750274. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.M. Resveratrol with antioxidant activity inhibits matrix metalloproteinase via modulation of SIRT1 in human fibrosarcoma cells. J. Life Sci. 2011, 88, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Brossard, M.; Page, M.; Gingras, D.; Beliveau, R. Matrix metalloproteinase inhibition by green tea catechins. BBA 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Park, W.H.; Kim, S.H.; Kim, C.H. A new matrix metalloproteinase-9 inhibitor 3,4-dihydroxycinnamic acid (caffeic acid) from methanol extract of Euonymus alatus: Isolation and structure determination. Toxicology 2005, 207, 383–390. [Google Scholar] [CrossRef]
- Liuzzi, M.G.; Latronico, T.; Branà, M.T.; Gramegna, P.; Coniglio, M.G.; Rossano, R.; Larocca, M.; Riccio, P. Structure-dependent inhibition of gelatinases by dietary antioxidants in rat astrocytes and sera of multiple sclerosis patients. Neurchem. Res. 2011, 36, 518–527. [Google Scholar] [CrossRef]
- Larocca, M.; Di Marsico, M.; Riccio, P.; Rossano, R. The in vitro antioxidant properties of Muscari comosum bulbs and their inhibitory activity on enzymes involved in inflammation, post-prandial hyperglycemia, and cognitive/neuromuscular functions. J. Food Biochem. 2018, 42, e12580. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCa and PKCb1 inhibition. Aterosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Unachukwu, U.J.; Ahmed, S.; Kavalier, A.; Lyles, J.T.; Kennelly, E.J. White and green teas (Camellia sinensis var. sinensis): Variation in phenolic, methylxanthine, and antioxidant profiles. J. Food Sci. 2010, 75, C541–C548. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Košťálová, D.; Bezáková, L.; Fialová, S.; Bauerová, K.; Tóth, J.; Štefek, M.; Vanko, M.; Holková, I.; Obložinský, M. Comparative study of two natural antioxidants, curcumin and Curcuma longa extract. J. Food Nutr. Res. 2009, 48, 148–152. [Google Scholar]
- Oliveira Calil, N.; Senra Gonçalves de Carvalho, G.; Zimmermann Franco, D.C.; da Silva, A.D.; Rezende Barbosa Raposo, N. Antioxidant activity of resveratrol analogs. Lett. Drug Des. Discov. 2012, 9, 8–11. [Google Scholar] [CrossRef]
- Aldini, G.; Piccoli, A.; Beretta, G.; Morazzoni, P.; Riva, A.; Marinello, C.; Maffei Facino, R. Antioxidant activity of polyphenols from solid olive residues of c.v. Coratina. Fitoterapia 2006, 77, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Galli, G.V.; Maschi, O.; Gilardi, F.; Bellosta, S.; Crestani, M.; Bosisio, E.; De Fabiani, E.; Caruso, D. Olive oil phenols modulate the expression of metalloproteinase 9 in THP-1 cells by acting on nuclear factor-kappaB signaling. J. Agric. Food Chem. 2010, 58, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004, 37, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Anti-inflammatory edffects of dietary polyphenols through inhibitory activity against metalloproteinases. Molecules 2023, 28, 5426. [Google Scholar] [CrossRef]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [PubMed]
- Le Nest, G.; Caille, O.; Woudstra, M.; Roche, S.; Guerlesquin, F.; Lexa, D. Zn–polyphenol chelation: Complexes with quercetin, (+)-catechin, and derivatives: I optical and NMR studies. Inorganica Chim. Acta 2004, 357, 775–784. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, M. Zinc-binding sites on selected flavonoids. Biol. Trace Elem. Res. 2014, 161, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as potential metal chelation compounds against Alzheimer’s disease. JAD 2021, 82, S335–S357. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wees, M.A.; Faure, T.D.; Carrillo, C.; Arbiser, J.; Bowen, J.P. The Impact of Ionization States of Matrix MetalloproteinaseInhibitors on Docking-Based Inhibitor Design. ACS Med. Chem. Lett. 2011, 2, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sayed, A.; Ginnebaugh, K.R.; Sharma, V.; Suri, A.; Saraph, A.; Padhye, S.; Sarkar, F.H. Molecular Docking and Inhibition of Matrix Metalloproteinase-2 by Novel Difluorinatedbenzylidene Curcumin Analog. Am. J. Transl. Res. 2015, 7, 298–308. [Google Scholar] [PubMed]
- Chowdhury, A.; Nandy, S.K.; Sarkar, J.; Chakraborti, T.; Chakraborti, S. Inhibition of Pro-/Active MMP-2 by Green Tea Catechins and Prediction of Their Interaction by Molecular Docking Studies. Mol. Cell. Biochem. 2017, 427, 111–122. [Google Scholar] [CrossRef]
- Sarkar, J.; Nandy, S.K.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Inhibition of MMP-9 by Green Tea Catechins and Prediction of Their Interaction by Molecular Docking Analysis. Biomed. Pharmacother. 2016, 84, 340–347. [Google Scholar] [CrossRef]
- Petraglia, T.; Latronico, T.; Fanigliulo, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Antioxidant Activity of Polysaccharides from the Edible Mushroom Pleurotus eryngii. Molecules 2023, 28, 2176. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Pati, I.; Ciavarella, R.; Fasano, A.; Mengoni, F.; Lichtner, M.; Vullo, V.; Mastroianni, C.M.; Liuzzi, G.M. In vitro effect of antiretroviral drugs on cultured primary astrocytes: Analysis of neurotoxicity and matrix metalloproteinase inhibition. J. Neurochem. 2018, 144, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, A.; Rossano, R.; Liuzzi, G.M. Neuroprotective potential of isothiocyanates in an in vitro model of neuroinflammation. Inflammopharmacology 2021, 29, 561–571. [Google Scholar] [CrossRef]
- Rossano, R.; Larocca, M.; Macellaro, M.; Bilancia, D.; Riccio, P. Unveiling a hidden biomarker of inflammation and tumor progression: The 65 kDa isoform of MMP-9 new horizons for therapy. Curr. Issues Mol. Biol. 2022, 44, 105–116. [Google Scholar] [CrossRef]
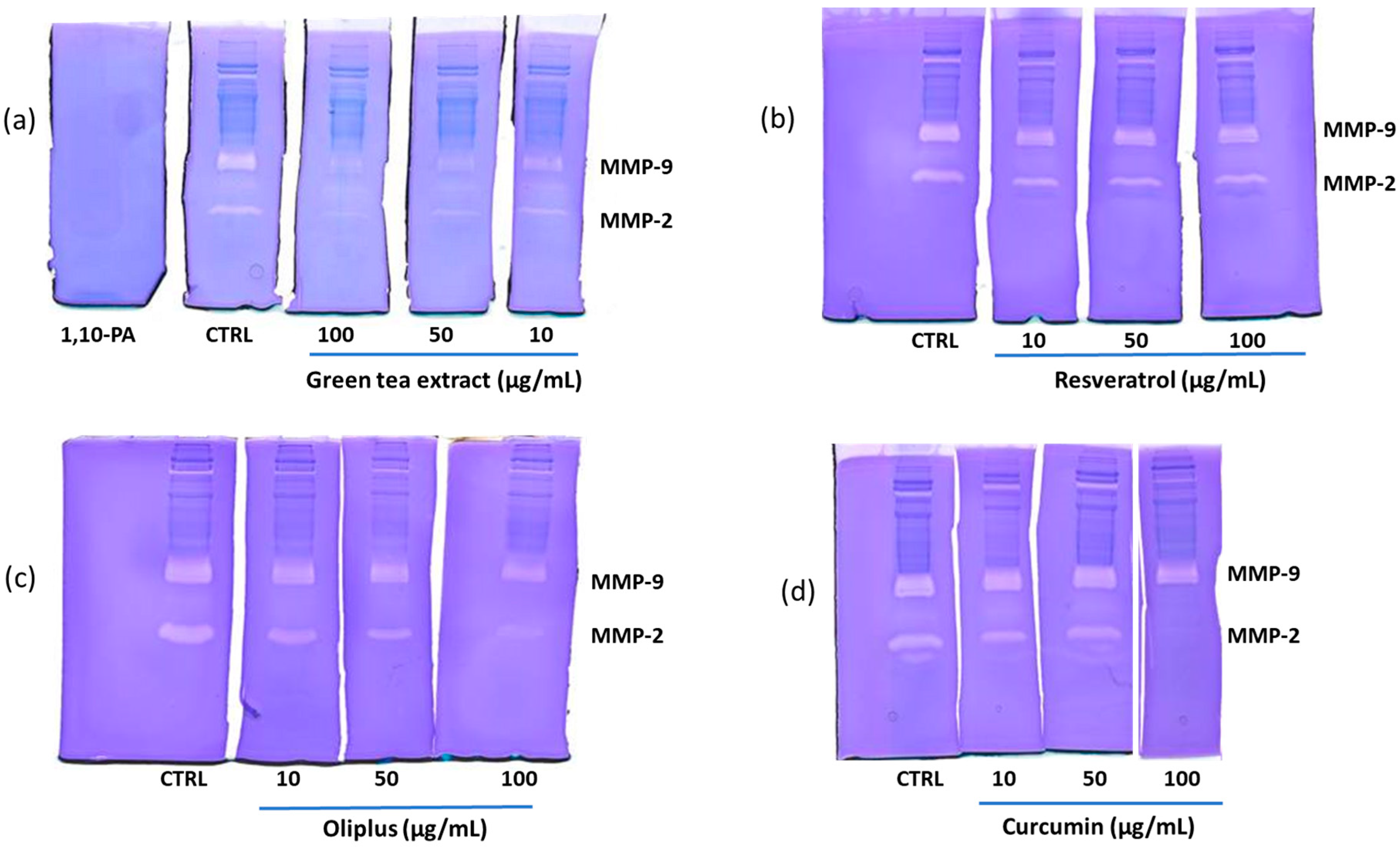
| Inhibition (%) | ||
|---|---|---|
| MMP-2 | MMP-9 | |
| 1,10 PA | 100 | 100 |
| CTRL | 0 | 0 |
| Antioxidant (μg/mL) | ||
| Green tea extract | ||
| 10 | 50.4 ± 3.7 | 56.4 ± 2.1 |
| 50 | 65.7 ± 2.5 | 53.6 ± 5.5 |
| 100 | 85.8 ± 6.1 | 89.9 ± 2.8 |
| Resveratrol | ||
| 10 | 20.1 ± 1.2 | 3.2 ± 0.2 |
| 50 | 22.6 ± 1.9 | 2.6 ± 0.5 |
| 100 | 21.4 ± 1.1 | 2.1 ± 0.4 |
| Oliplus | ||
| 10 | 34.9 ± 2.9 | 10.3 ± 2.3 |
| 50 | 59.0 ± 8.3 | 15.4 ± 3.3 |
| 100 | 74.5 ± 3.4 | 28.4 ± 4.1 |
| Curcumin | ||
| 10 | 20.2 ± 1.3 | 1.3 ± 0.2 |
| 50 | 18.6 ± 6.3 | 2.2 ± 0.8 |
| 100 | 100 | 18.2 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latronico, T.; Petraglia, T.; Sileo, C.; Bilancia, D.; Rossano, R.; Liuzzi, G.M. Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer. Molecules 2024, 29, 1718. https://doi.org/10.3390/molecules29081718
Latronico T, Petraglia T, Sileo C, Bilancia D, Rossano R, Liuzzi GM. Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer. Molecules. 2024; 29(8):1718. https://doi.org/10.3390/molecules29081718
Chicago/Turabian StyleLatronico, Tiziana, Tania Petraglia, Carmela Sileo, Domenico Bilancia, Rocco Rossano, and Grazia Maria Liuzzi. 2024. "Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer" Molecules 29, no. 8: 1718. https://doi.org/10.3390/molecules29081718
APA StyleLatronico, T., Petraglia, T., Sileo, C., Bilancia, D., Rossano, R., & Liuzzi, G. M. (2024). Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer. Molecules, 29(8), 1718. https://doi.org/10.3390/molecules29081718








