Progress and Outlook on Electrochemical Sensing of Lung Cancer Biomarkers
Abstract
1. Introduction
2. Electrochemical Sensing Techniques and Strategies
2.1. Basic Principles of Electrochemical Techniques
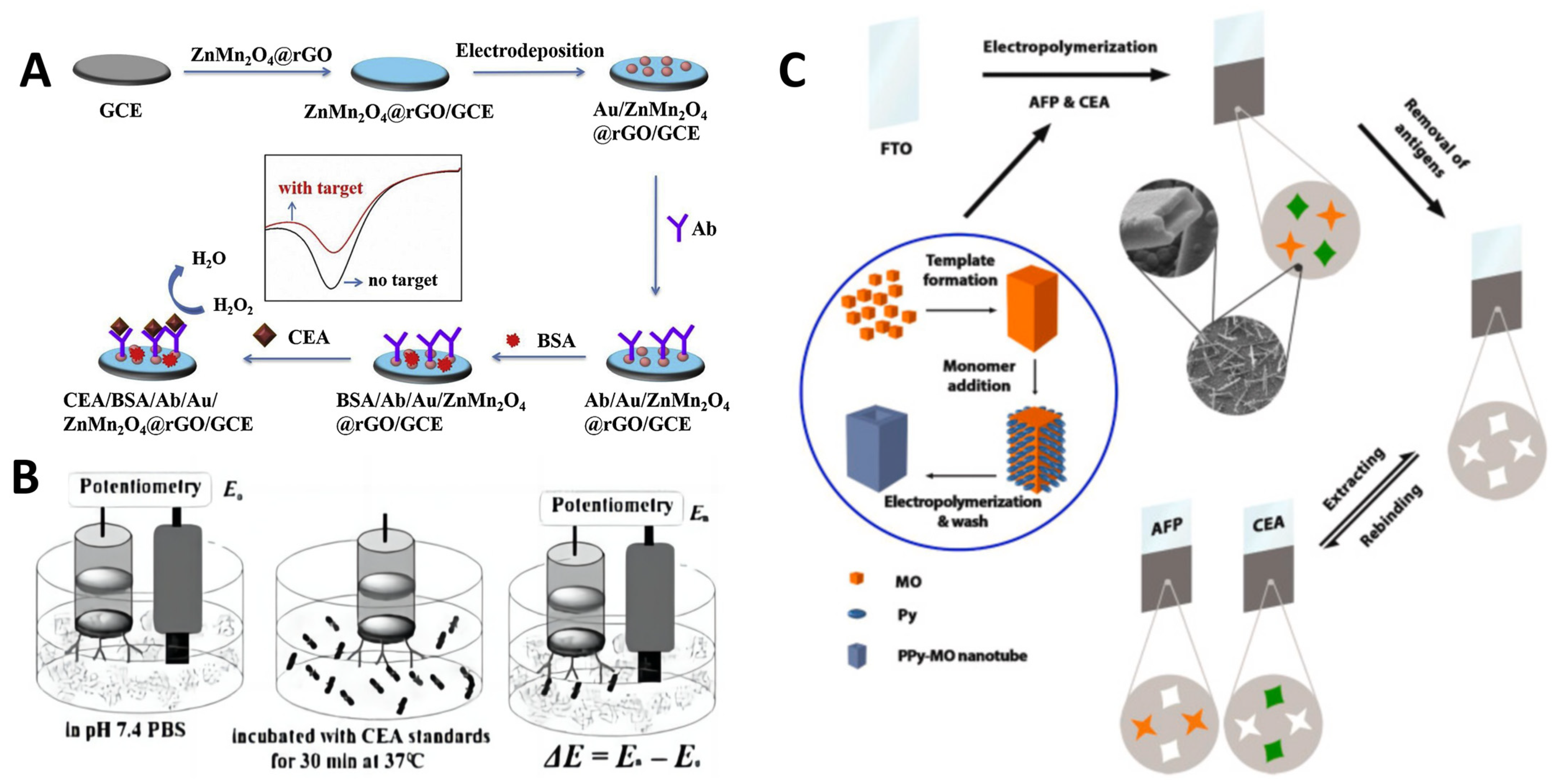
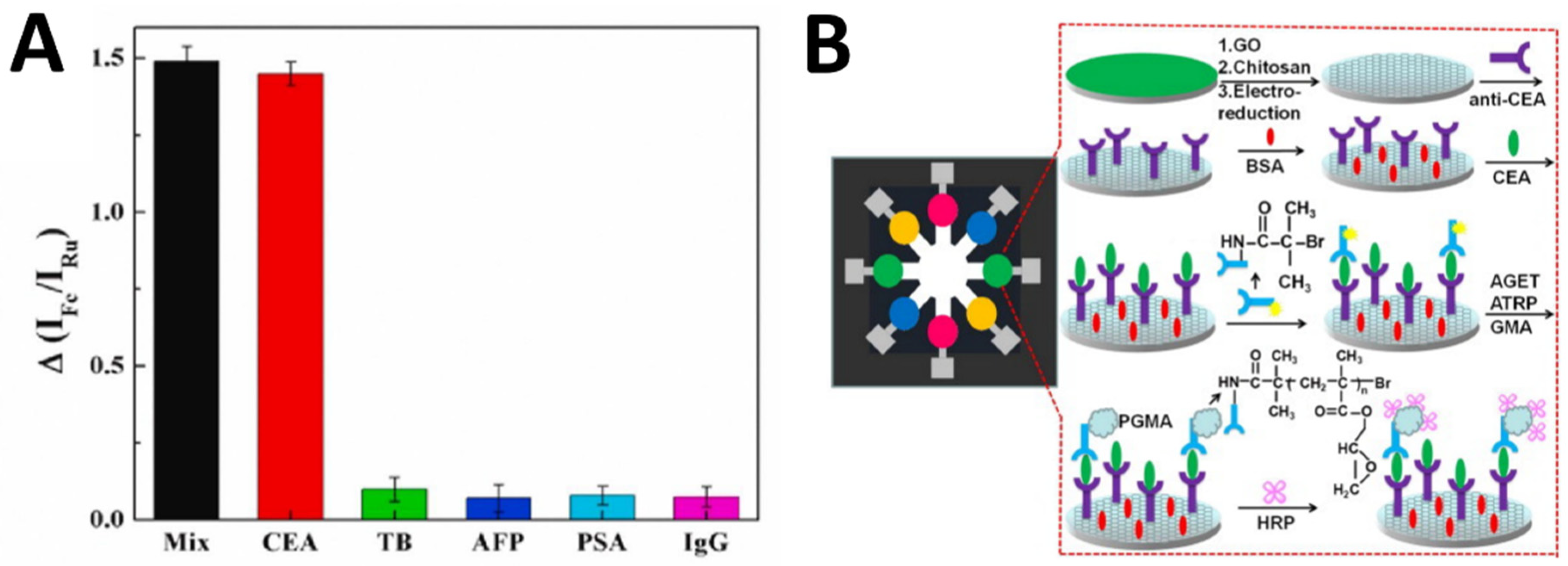
2.2. Electrochemical Immunosensors and Aptasensors
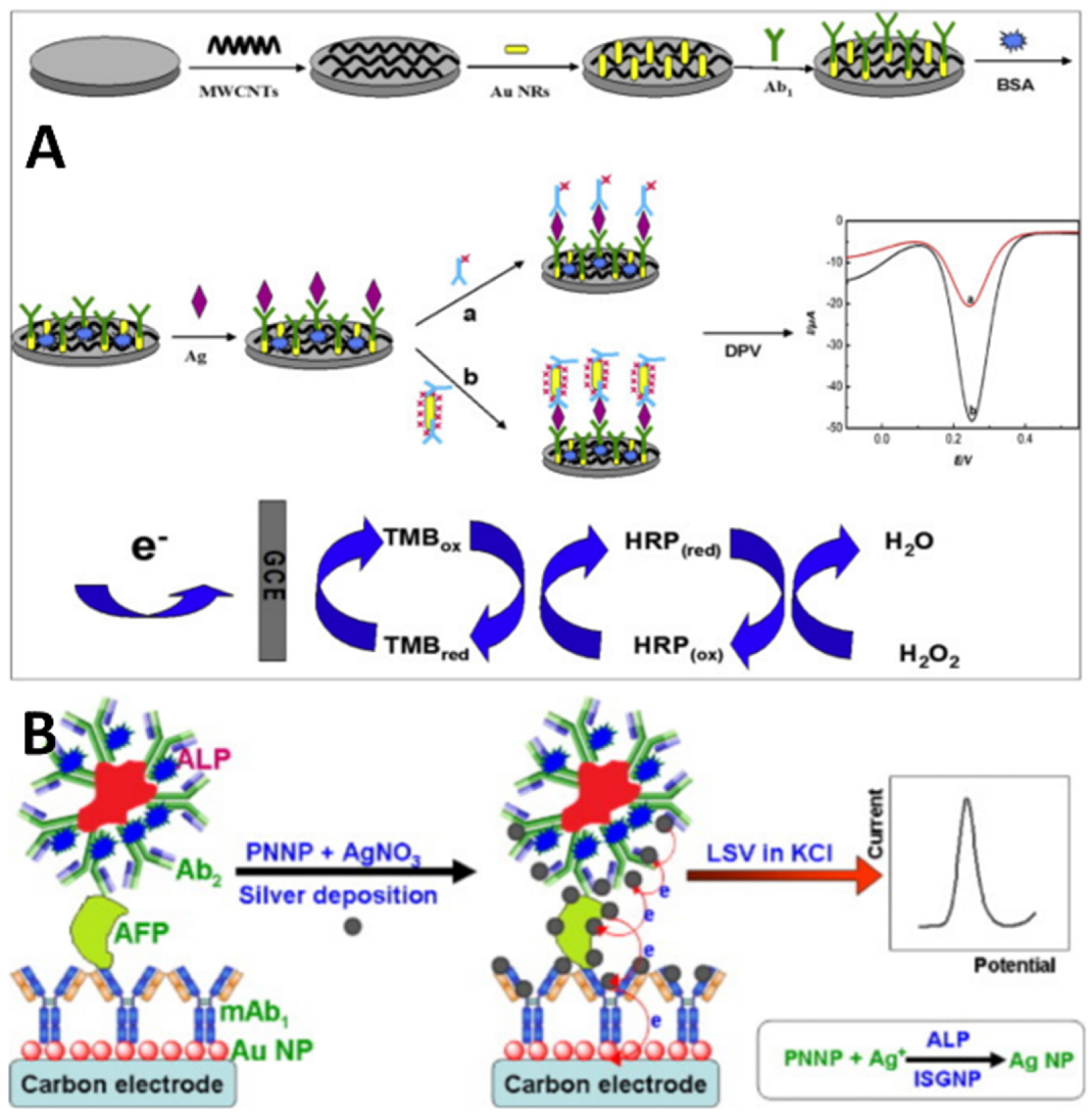
2.3. Signal Amplification Strategies
3. Nanomaterials for Electrochemical Sensing
3.1. Carbon Nanomaterials
3.2. Two-Dimensional Materials
3.3. Metal Nanoparticles
3.4. Conducting Polymers
4. Recent Advances in the Electrochemical Sensing of SCLC Biomarkers
4.1. CEA Sensors
4.2. NSE Sensors
4.3. AFP Sensors
5. Biological Samples and Sensing
6. Sensor Integration and Multiplexed Detection
7. Challenges and Future Outlook
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jafari-Kashi, A.; Rafiee-Pour, H.-A.; Shabani-Nooshabadi, M. A New Strategy to Design Label-Free Electrochemical Biosensor for Ultrasensitive Diagnosis of CYFRA 21–1 as a Biomarker for Detection of Non-Small Cell Lung Cancer. Chemosphere 2022, 301, 134636. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yu, W.; Chen, C.; Guo, S.; Tian, X.; Miao, Y.; Ma, L.; Zhang, X.; Yu, Y.; Huang, L.; et al. A Versatile Electrochemical Biosensor for the Detection of Circulating MicroRNA toward Non-Small Cell Lung Cancer Diagnosis. Small 2022, 18, 2200784. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, H.; Cui, J.; Liu, Y.; Pan, P. Recent Strategies for Electrochemical Sensing Detection of miRNAs in Lung Cancer. Anal. Biochem. 2023, 661, 114986. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- de Jong, C.; Deneer, V.H.M.; Kelder, J.C.; Ruven, H.; Egberts, T.C.G.; Herder, G.J.M. Association between Serum Biomarkers CEA and LDH and Response in Advanced Non-Small Cell Lung Cancer Patients Treated with Platinum-Based Chemotherapy. Thorac. Cancer 2020, 11, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Y.; Wang, L.; Zhou, Y.; Shen, Y.; Xu, F.; Chen, Y. Selective Application of Neuroendocrine Markers in the Diagnosis and Treatment of Small Cell Lung Cancer. Clin. Chim. Acta 2020, 509, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yan, Y.; Wang, T.; Wang, Z.; Cai, J.; Cao, X.; Yang, C.; Zhang, F.; Wu, G.; Shen, B. Identification of ENO1 as a Prognostic Biomarker and Molecular Target among ENOs in Bladder Cancer. J. Transl. Med. 2022, 20, 315. [Google Scholar] [CrossRef]
- Chen, J.M.; Chiu, S.; Chen, K.; Huang, Y.J.; Liao, Y.A.; Yu, C.R. Enolase 1 Differentially Contributes to Cell Transformation in Lung Cancer but Not in Esophageal Cancer. Oncol. Lett. 2020, 19, 3189–3196. [Google Scholar] [CrossRef]
- Liao, C.; Xiao, S.; Wang, X. Bench-to-Bedside: Translational Development Landscape of Biotechnology in Healthcare. Health Sci. Rev. 2023, 7, 100097. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical Sensors Targeting Salivary Biomarkers: A Comprehensive Review. TrAC Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Mamun, M.A.; Sagadevan, S.; Shahnavaz, Z.; Simarani, K.; Johan, M.R. Nucleic Acid-Based Electrochemical Biosensors for Rapid Clinical Diagnosis: Advances, Challenges, and Opportunities. Crit. Rev. Clin. Lab. Sci. 2022, 59, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel Biorecognition Elements against Pathogens in the Design of State-of-the-Art Diagnostics. Biosensors 2021, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Mamun, M.A.; Simarani, K.; Johan, M.R. Nanomaterials Based Electrochemical Nucleic Acid Biosensors for Environmental Monitoring: A Review. Appl. Surf. Sci. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Brito-Rocha, T.; Constâncio, V.; Henrique, R.; Jerónimo, C. Shifting the Cancer Screening Paradigm: The Rising Potential of Blood-Based Multi-Cancer Early Detection Tests. Cells 2023, 12, 935. [Google Scholar] [CrossRef]
- Thenrajan, T.; Alwarappan, S.; Wilson, J. Molecular Diagnosis and Cancer Prognosis—A Concise Review. Diagnostics 2023, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Voitechovič, E.; Pauliukaite, R. Electrochemical Multisensor Systems and Arrays in the Era of Artificial Intelligence. Curr. Opin. Electrochem. 2023, 42, 101411. [Google Scholar] [CrossRef]
- Singh, D. Nanotechnology-Based Assays for the Detection of Cancer through Sputum. Curr. Anal. Chem. 2023, 19, 633–641. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical Sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef]
- Fan, X.; Deng, D.; Chen, Z.; Qi, J.; Li, Y.; Han, B.; Huan, K.; Luo, L. A Sensitive Amperometric Immunosensor for the Detection of Carcinoembryonic Antigen Using ZnMn2O4@reduced Graphene Oxide Composites as Signal Amplifier. Sens. Actuators B Chem. 2021, 339, 129852. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, W.; Lu, S.; Guo, C.; Wang, P.; Zhang, D.; Ma, W. A Label-Free Electrochemical Immunosensor for CEA Detection on a Novel Signal Amplification Platform of Cu2S/Pd/CuO Nanocomposites. Front. Bioeng. Biotechnol. 2021, 9, 767717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.-K.; Fan, M.-Q.; Liu, A. A Sensitive Label-Free Amperometric CEA Immunosensor Based on Graphene-Nafion Nanocomposite Film as an Enhanced Sensing Platform. Anal. Sci. 2011, 27, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, J.; Li, N.; Wang, L.; Pu, L. Label-Free Electrochemical Immunoassay of Carcinoembryonic Antigen in Human Serum Using Magnetic Nanorods as Sensing Probes. Microchim. Acta 2009, 165, 437–442. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, G.; Yu, S.; Huang, R.; Fan, C. A Potentiometric Aptasensor for Carcinoembryonic Antigen (CEA) on Graphene Oxide Nanosheets Using Catalytic Recycling of DNase I with Signal Amplification. Anal. Methods 2018, 10, 5364–5371. [Google Scholar] [CrossRef]
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H. Dual-Template Rectangular Nanotube Molecularly Imprinted Polypyrrole for Label-Free Impedimetric Sensing of AFP and CEA as Lung Cancer Biomarkers. Talanta 2022, 239, 123146. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Avan, A.A. Nanostructures for Nonlabeled and Labeled Electrochemical Immunosensors: Simultaneous Electrochemical Detection of Cancer Markers: A Review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Multiplexed Electrochemical Immunosensors for Clinical Biomarkers. Sensors 2017, 17, 965. [Google Scholar] [CrossRef]
- Han, J. Preparation of a Highly Sensitive Graphene-Based Sensor to Investigate the Effect of Exercise on Electrolytes in Sweat in Hot and Humid Environment. Carbon Lett. 2023, 33, 1959–1966. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H. Study on Electrochemical Properties of Lead Calcium Tin Anode for Hydrometallurgy. Alex. Eng. J. 2023, 82, 389–395. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical Biosensors for the Detection of Lung Cancer Biomarkers: A Review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Jothi, L.; Arunagirinathan, R.S.; Nageswaran, G. Recent Advances in the Electrochemical Sensing of Lung Cancer Biomarkers. Biosens. Bioelectron. X 2022, 12, 100235. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Zhang, A.; Wang, Y.; Cai, X. Electrochemical Detecting Lung Cancer-Associated Antigen Based on Graphene-Gold Nanocomposite. Molecules 2017, 22, 392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-Free Microfluidic Paper-Based Electrochemical Aptasensor for Ultrasensitive and Simultaneous Multiplexed Detection of Cancer Biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Mo, X.; Wang, Y.; Xiao, Q.; Zhou, X.; Li, H. Conjugated Polymer Sensitized Hyperbranched Titanium Dioxide Based Photoelectrochemical Biosensor for Detecting AFP in Serum. Surf. Interfaces 2021, 24, 101103. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF Modified Glassy Carbon Electrode as Enzyme-Free Electrochemical Sensor Detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Rauf, S.; Beduk, T.; Durmus, C.; Aljedaibi, A.; Timur, S.; Alshareef, H.N.; Amine, A.; Wolfbeis, O.S.; Salama, K.N. Electrochemical Sensors and Biosensors Using Laser-Derived Graphene: A Comprehensive Review. Biosens. Bioelectron. 2020, 168, 112565. [Google Scholar] [CrossRef] [PubMed]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon Nanotube—A Review on Synthesis, Properties and Plethora of Applications in the Field of Biomedical Science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Feng, T.; Wang, Y.; Qiao, X. Recent Advances of Carbon Nanotubes-Based Electrochemical Immunosensors for the Detection of Protein Cancer Biomarkers. Electroanalysis 2017, 29, 662–675. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Wang, J.; Zhao, J.; Guo, Z.; Zhang, Y. Horseradish Peroxidase Functionalized Gold Nanorods as a Label for Sensitive Electrochemical Detection of Alpha-Fetoprotein Antigen. Anal. Biochem. 2015, 491, 58–64. [Google Scholar] [CrossRef]
- Lai, W.; Tang, D.; Que, X.; Zhuang, J.; Fu, L.; Chen, G. Enzyme-Catalyzed Silver Deposition on Irregular-Shaped Gold Nanoparticles for Electrochemical Immunoassay of Alpha-Fetoprotein. Anal. Chim. Acta 2012, 755, 62–68. [Google Scholar] [CrossRef]
- Niu, C.; Lin, X.; Jiang, X.; Guo, F.; Liu, J.; Liu, X.; Huang, H.; Huang, Y. An Electrochemical Aptasensor for Highly Sensitive Detection of CEA Based on Exonuclease III and Hybrid Chain Reaction Dual Signal Amplification. Bioelectrochemistry 2022, 143, 107986. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Ge, J.; Gao, X.; Li, C. Amplified Electrochemiluminescence Detection of CEA Based on Magnetic Fe3O4@Au Nanoparticles-Assembled Ru@SiO2 Nanocomposites Combined with Multiple Cycling Amplification Strategy. Biosens. Bioelectron. 2018, 118, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.-J.; Wang, Q.-L.; Cui, H.-F.; Song, X.; Lv, Q.-Y.; Guo, Y. A DNAzyme-Catalyzed Label-Free Aptasensor Based on Multifunctional Dendrimer-like DNA Assembly for Sensitive Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2021, 194, 113618. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Miranda, G.; Wu, C.; Zhang, Y.; Nörbel, L.; Lo, Y.; Tanner, J.A.; Elling, L.; Offenhäusser, A.; Mayer, D. Polyethylene Glycol-Mediated Blocking and Monolayer Morphology of an Electrochemical Aptasensor for Malaria Biomarker Detection in Human Serum. Bioelectrochemistry 2020, 136, 107589. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, R.; Liu, N.; Gao, F.; Luo, X. Electrochemical Biosensors for the Detection of Carcinoembryonic Antigen with Low Fouling and High Sensitivity Based on Copolymerized Polydopamine and Zwitterionic Polymer. Sens. Actuators B Chem. 2020, 319, 128253. [Google Scholar] [CrossRef]
- Yang, X.; Chen, P.; Zhang, X.; Zhou, H.; Song, Z.; Yang, W.; Luo, X. An Electrochemical Biosensor for HER2 Detection in Complex Biological Media Based on Two Antifouling Materials of Designed Recognizing Peptide and PEG. Anal. Chim. Acta 2023, 1252, 341075. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical Sensing Based on Carbon Nanoparticles: A Review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent Trends in Carbon Nanomaterial-Based Electrochemical Sensors for Biomolecules: A Review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef]
- Bagyalakshmi, S.; Sivakami, A.; Pal, K.; Sarankumar, R.; Mahendran, C. Manufacturing of Electrochemical Sensors via Carbon Nanomaterials Novel Applications: A Systematic Review. J. Nanoparticle Res. 2022, 24, 201. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon Nanomaterials and Their Application to Electrochemical Sensors: A Review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Jothi, L.; Jaganathan, S.K.; Nageswaran, G. An Electrodeposited Au Nanoparticle/Porous Graphene Nanoribbon Composite for Electrochemical Detection of Alpha-Fetoprotein. Mater. Chem. Phys. 2020, 242, 122514. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Wang, W.; Liu, Y.; Yang, H.; Kong, J.; Si, F. Polymer-Functionalized Carbon Nanotubes Prepared via Ring-Opening Polymerization for Electrochemical Detection of Carcinoembryonic Antigen. Sens. Actuators B Chem. 2021, 328, 129031. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Dosekova, E.; Holazova, A.; Paprckova, D.; Vikartovska, A.; Sasinkova, V.; Filip, J.; Kasak, P.; Jerigova, M. Electrochemical Performance of Ti3C2Tx MXene in Aqueous Media: Towards Ultrasensitive H2O2 Sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, N.; Wang, Y.; Xu, Y.; Wu, J.; Jia, G.; Ji, F.; Fang, X.; Chen, F.; Cui, X. Ultrasensitive and Selective Determination of Carcinoembryonic Antigen Using Multifunctional Ultrathin Amino-Functionalized Ti3C2-MXene Nanosheets. Anal. Chem. 2020, 92, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2020, 32, 1903826. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, K.-J.; Wu, X. Recent Advances in Transition-Metal Dichalcogenides Based Electrochemical Biosensors: A Review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Cheng, L.; Feng, X.; Zhang, H.; Han, S. Cobalt-Molybdenum Disulfide Supported on Nitrogen-Doped Graphene towards an Efficient Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2019, 44, 11664–11674. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, M.; Wang, Z.; Chen, K.; Li, X.; Ni, Z. Layer-by-Layer Self-Assembly of MoS2/PDDA Hybrid Film in Microfluidic Chips for Ultrasensitive Electrochemical Immunosensing of Alpha-Fetoprotein. Microchem. J. 2020, 158, 105209. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D Nanomaterials Based Electrochemical Biosensors for Cancer Diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, S. Electrochemical Sensors Based on Metal and Semiconductor Nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Islam, T.; Hasan, M.M.; Awal, A.; Nurunnabi, M.; Ahammad, A.J.S. Metal Nanoparticles for Electrochemical Sensing: Progress and Challenges in the Clinical Transition of Point-of-Care Testing. Molecules 2020, 25, 5787. [Google Scholar] [CrossRef] [PubMed]
- George, J.M.; Antony, A.; Mathew, B. Metal Oxide Nanoparticles in Electrochemical Sensing and Biosensing: A Review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical Sensors Using Conducting Polymer/Noble Metal Nanoparticle Nanocomposites for the Detection of Various Analytes: A Review. J. Nanostruct. Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Raghav, R.; Srivastava, S. Immobilization Strategy for Enhancing Sensitivity of Immunosensors: L-Asparagine–AuNPs as a Promising Alternative of EDC–NHS Activated Citrate–AuNPs for Antibody Immobilization. Biosens. Bioelectron. 2016, 78, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Deng, D.; Luo, L.; He, H.; Wang, Z. Water-Dispersible Graphene/Amphiphilic Pyrene Derivative Nanocomposite: High AuNPs Loading Capacity for CEA Electrochemical Immunosensing. Sens. Actuators B Chem. 2017, 248, 966–972. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Z.; Feng, D.; Guo, J.; Wang, J.; Zhang, Y. Simultaneous Electrochemical Immunosensing of Alpha-Fetoprotein and Prostate Specific Antigen Using a Glassy Carbon Electrode Modified with Gold Nanoparticle-Coated Silica Nanospheres and Decorated with Azure A or Ferrocenecarboxylic Acid. Microchim. Acta 2015, 182, 2435–2442. [Google Scholar] [CrossRef]
- Su, B.; Tang, D.; Li, Q.; Tang, J.; Chen, G. Gold–Silver–Graphene Hybrid Nanosheets-Based Sensors for Sensitive Amperometric Immunoassay of Alpha-Fetoprotein Using Nanogold-Enclosed Titania Nanoparticles as Labels. Anal. Chim. Acta 2011, 692, 116–124. [Google Scholar] [CrossRef]
- Akbari Nakhjavani, S.; Afsharan, H.; Khalilzadeh, B.; Ghahremani, M.H.; Carrara, S.; Omidi, Y. Gold and Silver Bio/Nano-Hybrids-Based Electrochemical Immunosensor for Ultrasensitive Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2019, 141, 111439. [Google Scholar] [CrossRef]
- Shi, B.-J.; Shang, L.; Zhang, W.; Jia, L.-P.; Ma, R.-N.; Xue, Q.-W.; Wang, H.-S. Electrochemical Stripping Chemiluminescent Sensor Based on Copper Nanoclusters for Detection of Carcinoembryonic Antigen. Sens. Actuators B Chem. 2021, 344, 130291. [Google Scholar] [CrossRef]
- Wang, X.; Liao, X.; Mei, L.; Zhang, M.; Chen, S.; Qiao, X.; Hong, C. An Immunosensor Using Functionalized Cu2O/Pt NPs as the Signal Probe for Rapid and Highly Sensitive CEA Detection with Colorimetry and Electrochemistry Dual Modes. Sens. Actuators B Chem. 2021, 341, 130032. [Google Scholar] [CrossRef]
- Song, D.; Zheng, J.; Myung, N.V.; Xu, J.; Zhang, M. Sandwich-Type Electrochemical Immunosensor for CEA Detection Using Magnetic Hollow Ni/C@SiO2 Nanomatrix and Boronic Acid Functionalized CPS@PANI@Au Probe. Talanta 2021, 225, 122006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, P.; Tang, F.; Wang, Y.; Wang, S.; Liu, Q.; Li, Y. Electrochemical Immunosensor Based on Multi-Order Rubik’s Cube-Type Platinum Nickel Nanocubes and Au NPs/cPDA NTs for Detection of CEA. Bioelectrochemistry 2023, 149, 108325. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; He, X.; Zhao, F.; Zhang, Y.; Liu, S.; Xing, R. Dual Labeled Mesoporous Silica Nanospheres Based Electrochemical Immunosensor for Ultrasensitive Detection of Carcinoembryonic Antigen. Anal. Chim. Acta 2020, 1133, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, M.; Song, Z.; Luo, X. An Antifouling Electrochemical Immunosensor for Carcinoembryonic Antigen Based on Hyaluronic Acid Doped Conducting Polymer PEDOT. RSC Adv. 2016, 6, 88411–88416. [Google Scholar] [CrossRef]
- Martínez-Rojas, F.; Castañeda, E.; Armijo, F. Conducting Polymer Applied in a Label-Free Electrochemical Immunosensor for the Detection Prostate-Specific Antigen Using Its Redox Response as an Analytical Signal. J. Electroanal. Chem. 2021, 880, 114877. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Sun, X.; Hui, N.; Luo, X. Reagentless and Label-Free Voltammetric Immunosensor for Carcinoembryonic Antigen Based on Polyaniline Nanowires Grown on Porous Conducting Polymer Composite. Microchim. Acta 2017, 184, 889–896. [Google Scholar] [CrossRef]
- Song, J.; Teng, H.; Xu, Z.; Liu, N.; Xu, L.; Liu, L.; Gao, F.; Luo, X. Free-Standing Electrochemical Biosensor for Carcinoembryonic Antigen Detection Based on Highly Stable and Flexible Conducting Polypyrrole Nanocomposite. Microchim. Acta 2021, 188, 217. [Google Scholar] [CrossRef]
- Wang, J.; Hua, X.; Jin, B. Ultrasensitive Detection of Carcinoembryonic Antigen by Chitosan/Polythiophene/CdTe Electrochemical Biosensor. ACS Omega 2022, 7, 45361–45370. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic Antigen (CEA) as Tumor Marker in Lung Cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Harmsma, M.; Schutte, B.; Ramaekers, F.C.S. Serum Markers in Small Cell Lung Cancer: Opportunities for Improvement. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2013, 1836, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Fu, L.; Feng, H.; Namadchian, M. Application of Ag nanoparticles decorated on graphene nanosheets for electrochemical sensing of CEA as an important cancer biomarker. Environ. Res. 2023, 239, 117363. [Google Scholar] [CrossRef]
- Feng, D.; Chen, L.; Zhang, K.; Zhu, S.; Ying, M.; Jiang, P.; Fu, M.; Wei, Y.; Li, L. Highly Sensitive Immunosensing of Carcinoembryonic Antigen Based on Gold Nanoparticles Dotted PB@PANI Core-Shell Nanocubes as a Signal Probe. J. Anal. Methods Chem. 2023, 2023, e7009624. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Guo, J.; Ye, B.; Peng, G.; Zhang, C.; Zou, L. An Ultrasensitive Carcinoembryonic Antigen Electrochemical Aptasensor Based on 3D DNA Nanoprobe and Exo III. Biosens. Bioelectron. 2022, 196, 113741. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Y.; Hou, Q.; Xu, Q.; Ding, C. Magnetic Antifouling Material Based Ratiometric Electrochemical Biosensor for the Accurate Detection of CEA in Clinical Serum. Biosens. Bioelectron. 2022, 208, 114216. [Google Scholar] [CrossRef] [PubMed]
- Shamsazar, A.; Soheili-Moghaddam, M.; Asadi, A. A Novel Electrochemical Immunosensor Based on MWCNT/CuO Nanocomposite for Effectively Detection of Carcinoembryonic Antigen (CEA). Microchem. J. 2024, 196, 109643. [Google Scholar] [CrossRef]
- Chakraborty, B.; Das, A.; Mandal, N.; Samanta, N.; Das, N.; Chaudhuri, C.R. Label Free, Electric Field Mediated Ultrasensitive Electrochemical Point-of-Care Device for CEA Detection. Sci. Rep. 2021, 11, 2962. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, M.; Cheng, Y.; Zhang, Z.; Niu, C.; Liu, X.; Liu, J.; Guo, F.; Huang, H.; Lin, X. A Novel Electrochemical Aptamer Biosensor Based on Tetrahedral DNA Nanostructures and Catalytic Hairpin Assembly for CEA Detection. J. Electroanal. Chem. 2021, 898, 115635. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Pei, J.; Tian, Y.; Liu, J. A Reagentless Electrochemical Immunosensor for Sensitive Detection of Carcinoembryonic Antigen Based on the Interface with Redox Probe-Modified Electron Transfer Wires and Effectively Immobilized Antibody. Front. Chem. 2022, 10, 939736. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, X.; Zhang, M.; Mei, L.; Chen, S.; Qi, Y.; Hong, C. An Immunosensor Based on an Electrochemical-Chemical-Chemical Advanced Redox Cycle Amplification Strategy for the Ultrasensitive Determination of CEA. Anal. Chim. Acta 2021, 1170, 338647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mei, L.; Zhang, L.; Wang, X.; Liao, X.; Qiao, X.; Hong, C. Ti3C2 MXene Anchors CuAu-LDH Multifunctional Two-Dimensional Nanomaterials for Dual-Mode Detection of CEA in Electrochemical Immunosensors. Bioelectrochemistry 2021, 142, 107943. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, X.; Ma, C.; Zhang, L.; Zhao, C.; Chen, S.; Li, K.; Zhang, M.; Mei, L.; Qi, Y.; et al. Enzyme-Free Sandwich-Type Electrochemical Immunosensor for CEA Detection Based on the Cooperation of an Ag/g-C3N4-Modified Electrode and Au@SiO2/Cu2O with Core-Shell Structure. Bioelectrochemistry 2021, 142, 107931. [Google Scholar] [CrossRef] [PubMed]
- Jozghorbani, M.; Fathi, M.; Kazemi, S.H.; Alinejadian, N. Determination of Carcinoembryonic Antigen as a Tumor Marker Using a Novel Graphene-Based Label-Free Electrochemical Immunosensor. Anal. Biochem. 2021, 613, 114017. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Tang, C.; Deng, Y.; Huang, H.; He, Z. Recent Advances in Biosensor for Detection of Lung Cancer Biomarkers. Biosens. Bioelectron. 2019, 141, 111416. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Wang, P.; Yang, Q.; Yu, H.; Pei, F.; Zheng, Y.; Liu, Q.; Dong, Y.; Li, Y. Electrochemical Immunosensors for Sensitive Detection of Neuron-Specific Enolase Based on Small-Size Trimetallic Au@Pd^Pt Nanocubes Functionalized on Ultrathin MnO2 Nanosheets as Signal Labels. ACS Biomater. Sci. Eng. 2020, 6, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, H.; Ma, Z. Conductive Hydrogel Composed of 1,3,5-Benzenetricarboxylic Acid and Fe3+ Used as Enhanced Electrochemical Immunosensing Substrate for Tumor Biomarker. Bioelectrochemistry 2017, 114, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.-L.; Tang, C.; Zhou, H.; Wang, A.-J.; Feng, J.-J.; Cheang, T.Y. Self-Supported PtPdMnCoFe High-Entropy Alloy with Nanochain-like Internetworks for Ultrasensitive Electrochemical Immunoassay of Biomarker. Sens. Actuators B Chem. 2024, 401, 135041. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Selective and Ultrasensitive Electrochemical Immunosensing of NSE Cancer Biomarker in Human Serum Using Epoxy-Substituted Poly(Pyrrole) Polymer Modified Disposable ITO Electrode. Sens. Actuators B Chem. 2020, 306, 127613. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Zhang, S.; Jia, Y.; Xu, Z.; Li, X.; Chen, Z.; Li, Y. Ultrasensitive Electrochemical Detection of Neuron-Specific Enolase Based on Spiny Core-Shell Au/CuxO@CeO2 Nanocubes. Bioelectrochemistry 2021, 138, 107693. [Google Scholar] [CrossRef] [PubMed]
- Acero Sánchez, J.L.; Fragoso, A.; Joda, H.; Suárez, G.; McNeil, C.J.; O’Sullivan, C.K. Site-Directed Introduction of Disulfide Groups on Antibodies for Highly Sensitive Immunosensors. Anal. Bioanal. Chem. 2016, 408, 5337–5346. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, Y.; Zhang, M.; Cui, B.; Hu, Q.; Wang, L. A Novel Electrochemical Strategy Based on Porous 3D Graphene-Starch Architecture and Silver Deposition for Ultrasensitive Detection of Neuron-Specific Enolase. Analyst 2019, 144, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Z. PtCu Nanoprobe-Initiated Cascade Reaction Modulated Iodide-Responsive Sensing Interface for Improved Electrochemical Immunosensor of Neuron-Specific Enolase. Biosens. Bioelectron. 2019, 143, 111612. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, P.; Zhou, K.; Ren, J.; Wang, S.; Tang, F.; Li, Y.; Liu, Q.; Xue, L. Electrochemical Immunosensor Based on Hollow Porous Pt Skin AgPt Alloy/NGR as a Dual Signal Amplification Strategy for Sensitive Detection of Neuron-Specific Enolase. Biosens. Bioelectron. 2022, 197, 113779. [Google Scholar] [CrossRef]
- Huang, X.; Miao, J.; Fang, J.; Xu, X.; Wei, Q.; Cao, W. Ratiometric Electrochemical Immunosensor Based on L-Cysteine Grafted Ferrocene for Detection of Neuron Specific Enolase. Talanta 2022, 239, 123075. [Google Scholar] [CrossRef] [PubMed]
- Karaman, C.; Bölükbaşı, Ö.S.; Yola, B.B.; Karaman, O.; Atar, N.; Yola, M.L. Electrochemical Neuron-Specific Enolase (NSE) Immunosensor Based on CoFe2O4@Ag Nanocomposite and AuNPs@MoS2/rGO. Anal. Chim. Acta 2022, 1200, 339609. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Luo, J.; Gong, Z.; Wang, H.; Ma, H.; Wu, D.; Wei, Q.; Ju, H. Polyacrylic Acid/Polyethylene Glycol Hybrid Antifouling Interface for Photoelectrochemical Immunosensing of NSE Based on ZnO/CdSe. Anal. Chim. Acta 2023, 1254, 341085. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yu, R.; Yan, Y.; Zeng, H.; Luo, S.; Liu, N.; Morrin, A.; Luo, X.; Li, W. A Review of Ratiometric Electrochemical Sensors: From Design Schemes to Future Prospects. Sens. Actuators B Chem. 2018, 274, 501–516. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Chen, Y.; Wang, Z.; Lan, L.; Wang, Y.; Han, B.; Pan, M.; Jiao, J.; Chen, Q. A Simple Immunosensor for Alpha-Fetoprotein Determination Based on Gold Nanoparticles-Dextran-Reduced Graphene Oxide. J. Electroanal. Chem. 2019, 833, 126–132. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Y.; Ye, X.; Wu, K.; Li, C. Fabrication of an Electrochemical Immunosensor for α-Fetoprotein Based on a Poly-L-Lysine-Single-Walled Carbon Nanotubes/Prussian Blue Composite Film Interface. J. Solid State Electrochem. 2016, 20, 2217–2222. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Y.; Wei, Z.; Wang, W. Chemiluminescence Immunoassay Based on Dual Signal Amplification Strategy of Au/Mesoporous Silica and Multienzyme Functionalized Mesoporous Silica. Mater. Sci. Eng. B 2011, 176, 1474–1478. [Google Scholar] [CrossRef]
- Grubisha, D.S.; Lipert, R.J.; Park, H.-Y.; Driskell, J.; Porter, M.D. Femtomolar Detection of Prostate-Specific Antigen: An Immunoassay Based on Surface-Enhanced Raman Scattering and Immunogold Labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, J.; Wang, J.; Zhao, H.; Ren, H.; Li, Z. Dual Signal Amplification Strategy of Au Nanopaticles/ZnO Nanorods Hybridized Reduced Graphene Nanosheet and Multienzyme Functionalized Au@ZnO Composites for Ultrasensitive Electrochemical Detection of Tumor Biomarker. Biosens. Bioelectron. 2017, 97, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wei, S.; Gu, M.; Chen, Z.; Cao, L. A Sandwich-Type Electrochemical Immunosensor Using rGO-TEPA-Thi-Au as Sensitive Platform and CMK-3@AuPtNPs as Signal Probe for AFP Detection. Microchem. J. 2021, 170, 106641. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Li, M.; Li, C.; Qian, L.; Yang, B. Electrochemical Immunosensors with AuPt-Vertical Graphene/Glassy Carbon Electrode for Alpha-Fetoprotein Detection Based on Label-Free and Sandwich-Type Strategies. Biosens. Bioelectron. 2019, 132, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zou, L.; Li, Y.; Guan, Y.; Guan, H.; Zhang, Z.; Zhang, Y.; Gao, H.; Yu, H.; Zhao, F.; et al. An Ultrasensitive Disposable Sandwich-Configuration Electrochemical Immunosensor Based on OMC@AuNPs Composites and AuPt-MB for Alpha-Fetoprotein Detection. Bioelectrochemistry 2021, 141, 107846. [Google Scholar] [CrossRef] [PubMed]
- Bölükbaşi, Ö.S.; Yola, B.B.; Karaman, C.; Atar, N.; Yola, M.L. Electrochemical α-Fetoprotein Immunosensor Based on Fe3O4NPs@covalent Organic Framework Decorated Gold Nanoparticles and Magnetic Nanoparticles Including SiO2@TiO2. Microchim. Acta 2022, 189, 242. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Liang, J.; Lu, C.; Zhang, M.; Hu, F.; Zhou, Z.; Li, G. Electrochemical Aptasensor for Analyzing Alpha-Fetoprotein Using RGO–CS–Fc Nanocomposites Integrated with Gold–Platinum Nanoparticles. Anal. Methods 2020, 12, 4956–4966. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, W.; Tan, Q.; Cui, X.; Dai, Z. Electrochemical Assay of the Alpha Fetoprotein-L3 Isoform Ratio to Improve the Diagnostic Accuracy of Hepatocellular Carcinoma. Anal. Chem. 2018, 90, 13051–13058. [Google Scholar] [CrossRef]
- Sampurno, F.H.H.; Pratiwi, S.D.; Putra, N.P.P. Correlation Between CEA Serum Level on NSCLC Patients with EGFR Mutation from Tissue and Plasma Sample. J. Respirologi Indones. 2022, 42, 97–106. [Google Scholar] [CrossRef]
- Recek, N.; Jaganjac, M.; Kolar, M.; Milkovic, L.; Mozetič, M.; Stana-Kleinschek, K.; Vesel, A. Protein Adsorption on Various Plasma-Treated Polyethylene Terephthalate Substrates. Molecules 2013, 18, 12441–12463. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Tran, H.-V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- Wang, J.; Hui, N. Zwitterionic Poly(Carboxybetaine) Functionalized Conducting Polymer Polyaniline Nanowires for the Electrochemical Detection of Carcinoembryonic Antigen in Undiluted Blood Serum. Bioelectrochemistry 2019, 125, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zha, L.; Wang, H.; Liu, J.; Chen, P.; Zhao, Y.; Jiang, L.; Li, Y.; Ouyang, R.; Miao, Y. A Frogspawn-like Ag@C Core–Shell Structure for an Ultrasensitive Label-Free Electrochemical Immunosensing of Carcinoembryonic Antigen in Blood Plasma. RSC Adv. 2021, 11, 16339–16350. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, P.; Hui, K.M.; Kang, Y. A Paper-Based Microfluidic Electrochemical Immunodevice Integrated with Amplification-by-Polymerization for the Ultrasensitive Multiplexed Detection of Cancer Biomarkers. Biosens. Bioelectron. 2014, 52, 180–187. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, B.; Wang, T.; Li, N.; Zhang, Z.; Zhang, H. Electrochemical Microfluidic Paper-Based Analytical Devices for Tumor Marker Detection. TrAC Trends Anal. Chem. 2022, 157, 116816. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, K.; Liu, Y.; Hao, S.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive Microfluidic Paper-Based Electrochemical/Visual Analytical Device via Signal Amplification of Pd@Hollow Zn/Co Core–Shell ZIF67/ZIF8 Nanoparticles for Prostate-Specific Antigen Detection. Anal. Chem. 2021, 93, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, M.; Muruganand, S.; Parthiban, C. Development of Novel Paper Based Electrochemical Immunosensor with Self-Made Gold Nanoparticle Ink and Quinone Derivate for Highly Sensitive Carcinoembryonic Antigen. Sens. Actuators B Chem. 2018, 257, 496–503. [Google Scholar] [CrossRef]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Zhao, F.; Jiang, Y.; Chen, Z. A Disposable Paper-Based Microfluidic Immunosensor Based on Reduced Graphene Oxide-Tetraethylene Pentamine/Au Nanocomposite Decorated Carbon Screen-Printed Electrodes. Sens. Actuators B Chem. 2017, 252, 44–54. [Google Scholar] [CrossRef]
- Yun, J.W.; Lee, S.; Kim, H.M.; Chun, S.; Engleman, E.G.; Kim, H.C.; Kang, E.-S. A Novel Type of Blood Biomarker: Distinct Changes of Cytokine-Induced Stat Phosphorylation in Blood t Cells between Colorectal Cancer Patients and Healthy Individuals. Cancers 2019, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, X.; Chen, H.; Bai, L.; Xu, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L. Fabrication of Novel Electrochemical Immunosensor by Mussel-Inspired Chemistry and Surface-Initiated PET-ATRP for the Simultaneous Detection of CEA and AFP. React. Funct. Polym. 2020, 154, 104632. [Google Scholar] [CrossRef]
- Yang, H.; Bao, J.; Huo, D.; Zeng, Y.; Wang, X.; Samalo, M.; Zhao, J.; Zhang, S.; Shen, C.; Hou, C. Au Doped Poly-Thionine and Poly-m-Cresol Purple: Synthesis and Their Application in Simultaneously Electrochemical Detection of Two Lung Cancer Markers CEA and CYFRA21-1. Talanta 2021, 224, 121816. [Google Scholar] [CrossRef] [PubMed]
- Dhanapala, L.; Krause, C.E.; Jones, A.L.; Rusling, J.F. Printed Electrodes in Microfluidic Arrays for Cancer Biomarker Protein Detection. Biosensors 2020, 10, 115. [Google Scholar] [CrossRef]
- Dhanapala, L.; Jones, A.L.; Czarnecki, P.; Rusling, J.F. Sub-Zeptomole Detection of Biomarker Proteins Using a Microfluidic Immunoarray with Nanostructured Sensors. Anal. Chem. 2020, 92, 8021–8025. [Google Scholar] [CrossRef]

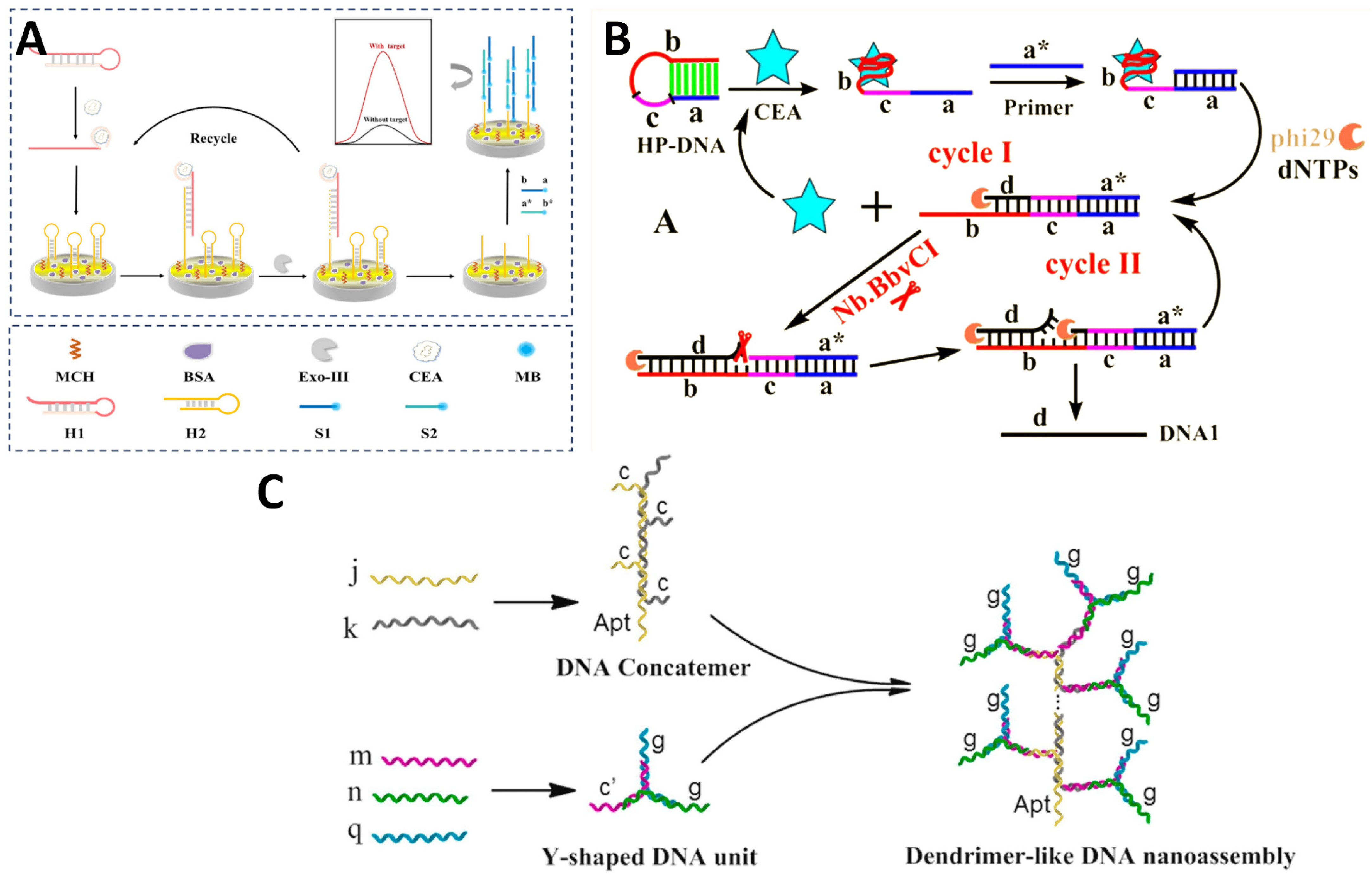
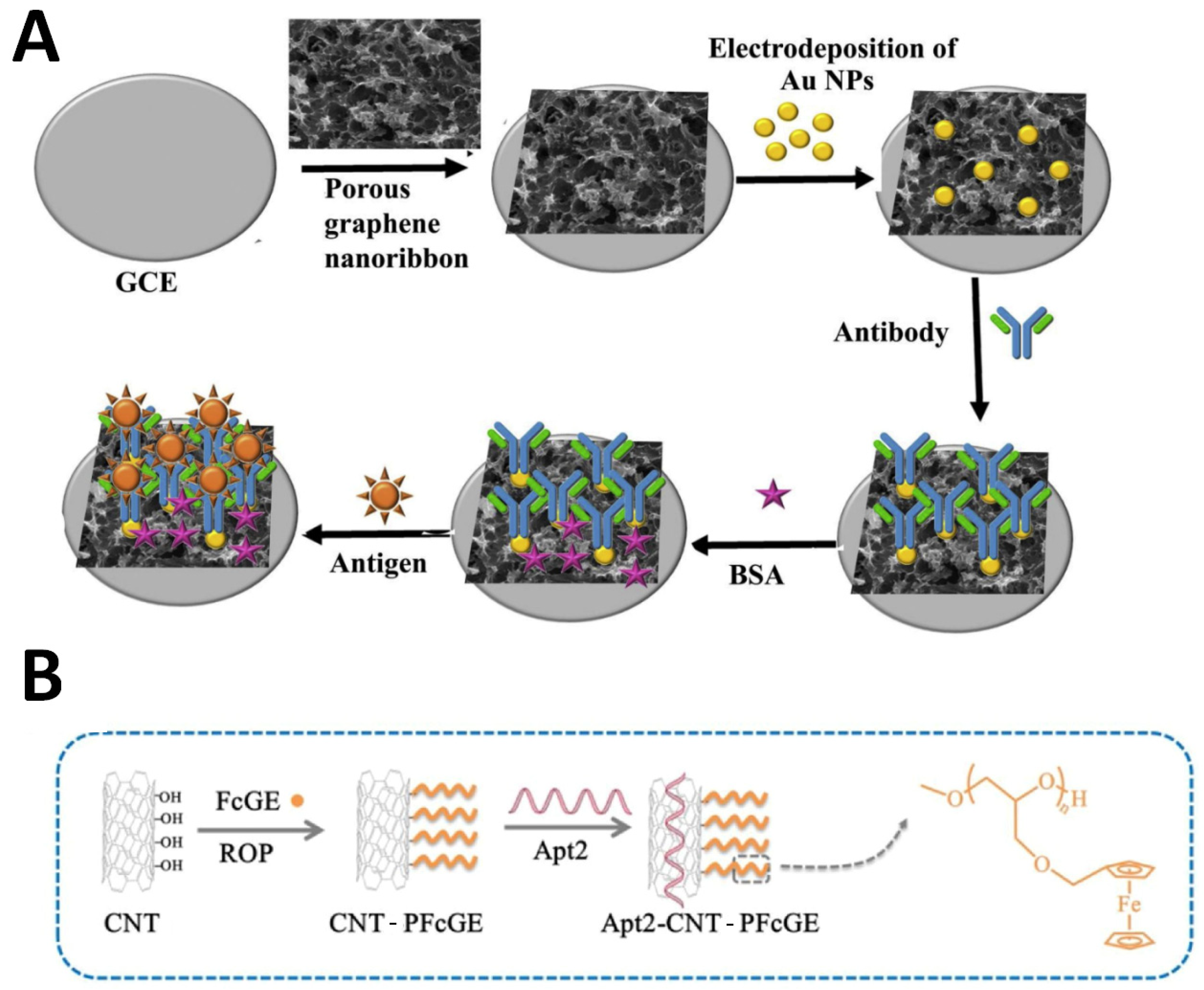

| Sensing Strategy | Technology | Linear Detection Range | Limit of Detection | Real Sample | Ref. |
|---|---|---|---|---|---|
| Self-assembled, label-free 3D DNA nanoprobe and exonuclease III-assisted signal amplification | DPV | 10 fg/mL to 50 ng/mL | 4.88 fg/mL | Serum | [86] |
| Ratiometric electrochemical detection using an aptamer and an internal standard | DPV | 1 pg/mL to 1 μg/mL | 0.62 pg/mL | Serum | [87] |
| Sandwich-type assay using primary anti-CEA antibody immobilized on MWCNT/CuO nanocomposite-modified electrode, CEA antigen, and secondary anti-CEA antibody conjugated to Fe3O4 nanoparticles | DPV | 0.005 ng/mL to 4 ng/mL | 1.9 pg/mL | Serum | [88] |
| Sensing strategy exonuclease III and hybrid chain reaction dual signal amplification | I-T | 10 pg/mL to 100 ng/mL | 0.84 pg/mL | Serum | [41] |
| Label-free, electric field-mediated electrochemical detection using a graphene–ZnO nanorod heterostructure | EIS | 0.001 pg/mL to 10 pg/mL | 1 fg/mL | - | [89] |
| Electrochemical aptamer biosensor based on tetrahedral DNA nanostructures and catalytic hairpin assembly | DPV | 1 pg/mL to 30,000 pg/mL | 0.04567 pg/mL | Serum | [90] |
| Electrochemical immunosensor based on redox probe-modified electron transfer wires and an immobilized antibody | DPV | 10 pg/mL to 100 ng/mL | 0.6 pg/mL | Serum | [91] |
| Electrochemical immunosensor with RCA | DPV | 0.01 pg/mL to 80 ng/mL | 0.0037 pg/mL | Serum | [92] |
| Sandwich-type electrochemical immunosensor using magnetic hollow Ni/C@SiO2 nanomatrix and a boronic acid-functionalized CPS@PANI@Au probe | DPV | 0.006–12.00 ng/mL | 1.56 pg/mL | Serum | [73] |
| Electrochemical immunosensor using Ti3C2 MXene-anchored CuAu-LDH as signal enhancer | I-T/DPV | 0.0001–80 ng/mL | 33.6 fg/mL | Serum | [93] |
| Enzyme-free sandwich-type electrochemical immunosensor using a Ag/g-C3N4-modified electrode and a Au@SiO2/Cu2O signal probe | I-T | 0.01 pg/mL to 80 ng/mL | 0.0038 pg/mL | Serum | [94] |
| Label-free electrochemical immunosensor based on graphene oxide | EIS | 0.1 to 5 ng/mL | 0.05 ng/mL | Serum | [95] |
| Sensing Strategy | Technology | Linear Detection Range | Limit of Detection | Real Sample | Ref. |
|---|---|---|---|---|---|
| Label-free electrochemical immunosensor using PtPdMnCoFe HEAINN as signal amplifier | DPV | 0.1 pg/mL to 200 ng/mL | 0.0036 pg/mL | Serum | [99] |
| Label-free electrochemical impedimetric immunosensor using an epoxy-substituted polypyrrole (P(Pyr-Epx)) polymer-modified disposable ITO electrode | EIS | 0.02 pg/mL to 7.5 pg/mL | 6.1 fg/mL | Serum | [100] |
| Label-free electrochemical immunoassay based on anti-NSE antibodies immobilized on a AuNP-modified conductive hydrogel film | DPV | 1 pg/mL to 200 ng/mL | 0.26 pg/mL | - | [98] |
| Sandwich-type electrochemical immunosensor using Au/Cu x O@CeO2 as label material and AuPt NSNs as substrate | I-T | 50 fg/mL to 100 ng/mL | 31.3 fg/mL | Serum | [101] |
| Sandwich immunoassay using anti-NSE21 antibody modified with disulfide groups via carbohydrate residues as the capture antibody and anti-NSE17-HRP conjugate as the reporter antibody | DPV | 0–25 ng/mL | 4.6 ng/mL | - | [102] |
| A 3D graphene–starch-modified immunoelectrode to capture antigens, AuNP-loaded antibody tags to catalyze silver deposition, and direct detection of AgNPs using stripping voltammetry for signal amplification | LSV | 0.02 pg/mL to 35 ng/mL | 0.008 pg/mL | Serum | [103] |
| PtCu nanoprobe-initiated cascade reaction and iodide-responsive sensing interface | SWV | 0.0001 to 100 ng/mL | 52.14 fg/mL | Serum | [104] |
| Sandwich-type electrochemical immunosensor using HP-AgPt/NGR as a dual signal amplification label and PPy-PEDOT-Au as the substrate | I-T | 50 fg/mL to 100 ng/mL | 18.5 fg/mL | Serum | [105] |
| Ratiometric electrochemical immunosensor based on Cu-MOF-Au as the electrode sensing surface and Fc-L-Cys as the label of Ab2 | DPV | 1 pg/mL to 1 μg/mL | 0.011 pg/mL | Serum | [106] |
| An electrochemical NSE immunosensor using a AuNPs@MoS2/rGO platform and a CoFe2O4@Ag label for signal amplification | DPV | 0.01 to 1.00 pg/mL | 3.00 fg/mL | Serum | [107] |
| PEC immunosensing using ZnO/CdSe and an antifouling interface | DPV | 0.10 pg/mL–100 ng/mL | 34 fg/mL | Serum | [108] |
| Sensing Strategy | Technology | Linear Detection Range | Limit of Detection | Real Sample | Ref. |
|---|---|---|---|---|---|
| Electrochemical immunosensor based on a AuNP–dextran–rGO nanocomposite | DPV | 0.01–20 ng/mL | 0.05 pg/mL | Serum | [110] |
| Electrochemical immunosensing using an anti-alpha fetoprotein antibody labeled with horseradish peroxidase immobilized on poly-L-lysine-functionalized SWCNT/PB composite film | DPV | 0.05–10.0 ng/mL 10.0–50.0 ng/mL | 0.011 ng/mL | Serum | [111] |
| Chemiluminescent immunoassay based on dual signal amplification using HRP and an HRP-labeled antibody co-immobilized on mesoporous silica nanoparticles | ECL | 0.01 to 0.5 ng/mL 0.5 to 100 ng/mL | 0.005 ng/mL | Serum | [112] |
| Sandwich-type electrochemical immunosensor using a signal amplification strategy | DPV | 0.02–10,000 pg/mL 10,000–100,000 pg/mL | 0.01 pg/mL | Serum | [114] |
| Sandwich-type electrochemical immunosensor using rGO-TEPA-Thi-Au as a sensitive platform and CMK-3@AuPtNPs as a signal probe | I-T | 0.005 to 100 ng/mL | 0.0022 ng/mL | Serum | [115] |
| Monitoring the electrochemical response current of AuPt-vertical graphene/GCE for the oxidation of the methyl orange redox probe | DPV | 1 fg/mL to 100 ng/mL | 0.7 fg/mL | Serum | [116] |
| Ordered mesoporous carbon (OMC) doped with AuNPs as a substrate to immobilize AFP antibodies, along with AuPt-MB nanorods as signal probes to bind secondary AFP antibodies and amplify detection | DPV | 10 fg/mL to 100 ng/mL | 3.33 fg/mL | Serum | [117] |
| Electrochemical immunosensor based on Fe3O4NPs@COF-decorated gold nanoparticles and magnetic nanoparticles including SiO2@TiO2 | DPV | 0.01 pg/mL to 1 pg/mL | 3.30 fg/mL | Serum | [118] |
| Label-free electrochemical aptasensing using rGO–chitosan–Fc nanocomposites and Au-Pt NPs | DPV | 0.001 to 10.0 mg/mL | 0.3013 ng/mL | Serum | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Wu, A.; Li, J.; Tang, Z.; Zhang, J.; Zhang, M.; Wei, Z. Progress and Outlook on Electrochemical Sensing of Lung Cancer Biomarkers. Molecules 2024, 29, 3156. https://doi.org/10.3390/molecules29133156
Zheng R, Wu A, Li J, Tang Z, Zhang J, Zhang M, Wei Z. Progress and Outlook on Electrochemical Sensing of Lung Cancer Biomarkers. Molecules. 2024; 29(13):3156. https://doi.org/10.3390/molecules29133156
Chicago/Turabian StyleZheng, Rui, Aochun Wu, Jiyue Li, Zhengfang Tang, Junping Zhang, Mingli Zhang, and Zheng Wei. 2024. "Progress and Outlook on Electrochemical Sensing of Lung Cancer Biomarkers" Molecules 29, no. 13: 3156. https://doi.org/10.3390/molecules29133156
APA StyleZheng, R., Wu, A., Li, J., Tang, Z., Zhang, J., Zhang, M., & Wei, Z. (2024). Progress and Outlook on Electrochemical Sensing of Lung Cancer Biomarkers. Molecules, 29(13), 3156. https://doi.org/10.3390/molecules29133156