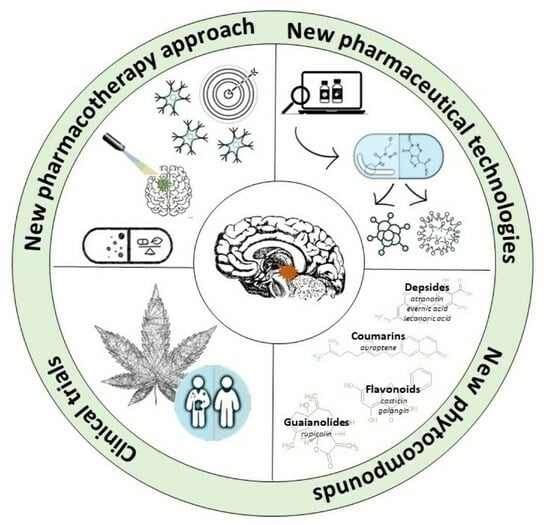
New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy
Abstract
1. Introduction
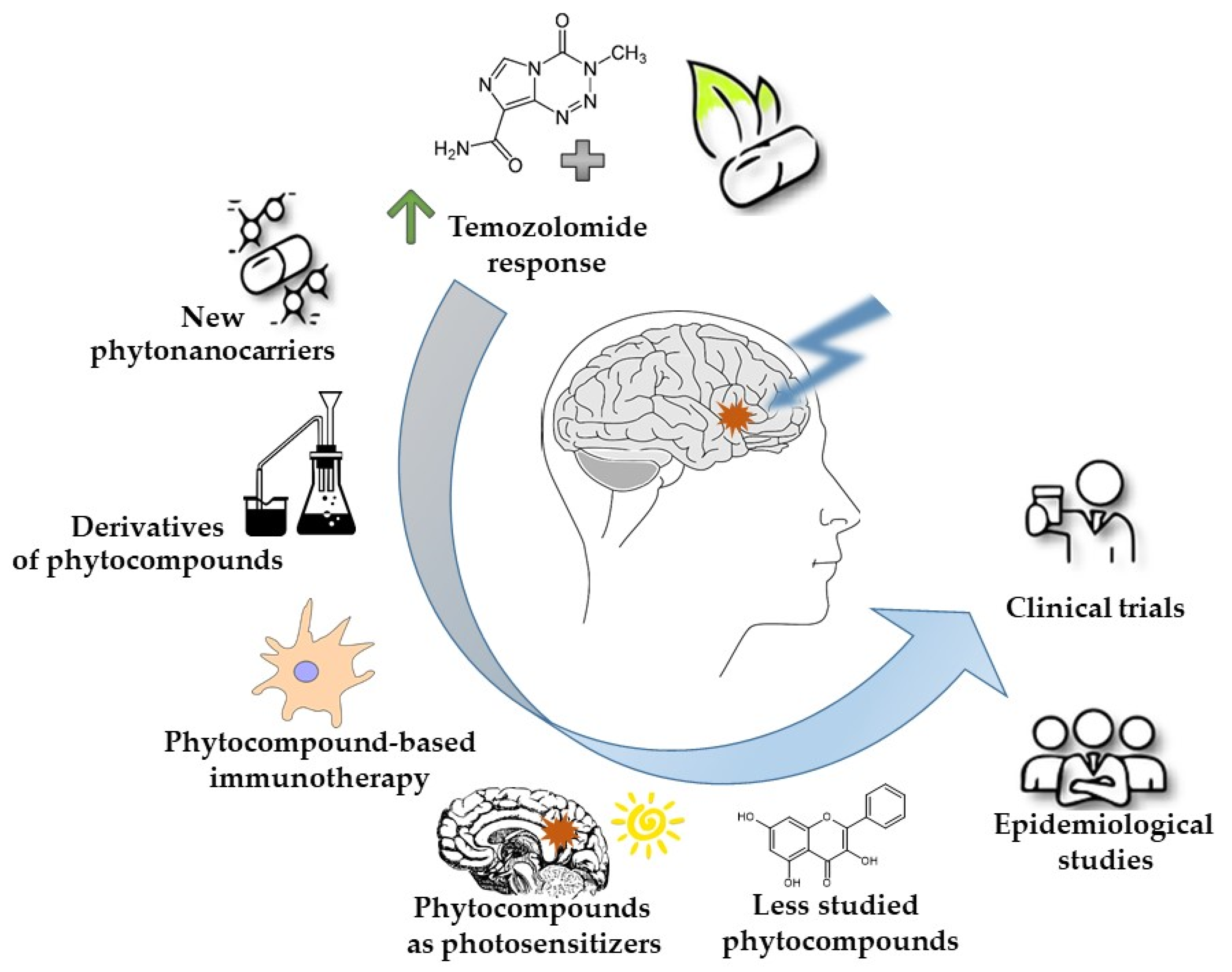
2. Enhancing Temozolomide Response with Phytochemicals
3. Strategies for the Improved Phytocompound Delivery to the Brain—The Advantages of Phyto-Nanocarriers
4. Chemical Modifications of Phytocompounds as a Way to Improve Their Solubility, Bioavailability, and Efficacy
5. Phytocompound-Based Immunotherapy
6. Phytocompounds Used as Photosensitizers in GBM Photodynamic Therapy (PDT)
7. Unraveling the Emerging Role of Less Studied Plant-Derived Substances with Anti-GBM Potential
8. Epidemiological Studies of Dietary Phytocompounds—Novel Trend in GBM Research?
9. Phytocompounds or Phytocompound-Based Products That Reached the Clinical Trials Phase in GBM Research
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silvani, A. New Perspectives: Glioma in Adult Patients. Tumori J. 2023, 109, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging Therapies for Glioblastoma: Current State and Future Directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Mowforth, O.D.; Brannigan, J.; El Khoury, M.; Sarathi, C.I.P.; Bestwick, H.; Bhatti, F.; Mair, R. Personalised Therapeutic Approaches to Glioblastoma: A Systematic Review. Front. Med. 2023, 10, 1166104. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Sidhu, A.; Miechowicz, I.; Nowak, W.; Barciszewska, A.-M. ABCB1 Is Frequently Methylated in Higher-Grade Gliomas and May Serve as a Diagnostic Biomarker of More Aggressive Tumors. J. Clin. Med. 2022, 11, 5655. [Google Scholar] [CrossRef] [PubMed]
- Radtke, L.; Majchrzak-Celińska, A.; Awortwe, C.; Vater, I.; Nagel, I.; Sebens, S.; Cascorbi, I.; Kaehler, M. CRISPR/Cas9-Induced Knockout Reveals the Role of ABCB1 in the Response to Temozolomide, Carmustine and Lomustine in Glioblastoma Multiforme. Pharmacol. Res. 2022, 185, 106510. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Misiorek, J.O.; Kruhlenia, N.; Przybyl, L.; Kleszcz, R.; Rolle, K.; Krajka-Kuźniak, V. COXIBs and 2,5-Dimethylcelecoxib Counteract the Hyperactivated Wnt/β-Catenin Pathway and COX-2/PGE2/EP4 Signaling in Glioblastoma Cells. BMC Cancer 2021, 21, 493. [Google Scholar] [CrossRef]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging Nanoformulation Strategies for Phytocompounds and Applications from Drug Delivery to Phototherapy to Imaging. Bioact. Mater. 2022, 14, 182–205. [Google Scholar] [CrossRef]
- Schaff, L.R.; Yan, D.; Thyparambil, S.; Tian, Y.; Cecchi, F.; Rosenblum, M.; Reiner, A.S.; Panageas, K.S.; Hembrough, T.; Lin, A.L. Characterization of MGMT and EGFR Protein Expression in Glioblastoma and Association with Survival. J. Neurooncol. 2020, 146, 163–170. [Google Scholar] [CrossRef]
- Tang, K.; Jin, Q.; Yan, W.; Zhang, W.; You, G.; Liu, Y.; Jiang, T. Clinical Correlation of MGMT Protein Expression and Promoter Methylation in Chinese Glioblastoma Patients. Med. Oncol. 2012, 29, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Limam, S.; Missaoui, N.; Abdessayed, N.; Mestiri, S.; Selmi, B.; Mokni, M.; Yacoubi, M.T. Prognostic Significance of MGMT Methylation and Expression of MGMT, P53, EGFR, MDM2 and PTEN in Glioblastoma Multiforme. Ann. Biol. Clin. 2019, 77, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of Temozolomide Resistance in Glioblastoma—A Comprehensive Review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The Role of Glioma Stem Cells in Chemotherapy Resistance and Glioblastoma Multiforme Recurrence. Expert Rev. Neurother. 2015, 15, 741–752. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Mehrpour, M.; Shojaei, S.; Harlos, C.; Pitz, M.; Hamai, A.; Siemianowicz, K.; Likus, W.; Wiechec, E.; Toyota, B.D.; et al. Glioblastoma and Chemoresistance to Alkylating Agents: Involvement of Apoptosis, Autophagy, and Unfolded Protein Response. Pharmacol. Ther. 2018, 184, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural Products: A Hope for Glioblastoma Patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in Cancer Cell Chemosensitization: Current Knowledge and Future Perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; et al. Natural Bioactive Molecules: An Alternative Approach to the Treatment and Control of Glioblastoma Multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef]
- De Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; Da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of Tumor Cells to Chemotherapy by Natural Products: A Systematic Review of Preclinical Data and Molecular Mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef]
- Gautam, M.; Gabrani, R. Combinatorial Effect of Temozolomide and Naringenin in Human Glioblastoma Multiforme Cell Lines. Nutr. Cancer 2022, 74, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Netto, J.B.; Melo, E.S.A.; Oliveira, A.G.S.; Sousa, L.R.; Santiago, L.R.; Santos, D.M.; Chagas, R.C.R.; Gonçalves, A.S.; Thomé, R.G.; Santos, H.B.; et al. Matteucinol Combined with Temozolomide Inhibits Glioblastoma Proliferation, Invasion, and Progression: An in Vitro, in Silico, and in Vivo Study. Braz. J. Med. Biol. Res. 2022, 55, e12076. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Li, H.; Yi, D.; Sun, Y.; Bai, Y.; Zhong, S.; Song, Y.; Zhao, G.; Chen, Y. Cordycepin Augments the Chemosensitivity of Human Glioma Cells to Temozolomide by Activating AMPK and Inhibiting the AKT Signaling Pathway. Mol. Pharm. 2018, 15, 4912–4925. [Google Scholar] [CrossRef] [PubMed]
- Vibhavari, R.J.A.; Rao, V.; Cheruku, S.P.; Kumar, B.H.; Maity, S.; Nandakumar, K.; Kumar, L.; Mehta, C.H.; Nayak, U.; Chamallamudi, M.R.; et al. Enhancing Temozolomide Antiglioma Response by Inhibiting O6-Methylguanine-DNA Methyltransferase with Selected Phytochemicals: In Silico and in Vitro Approach. 3 Biotech 2023, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Meteoglu, I.; Erdemir, A. Genistein and Temozolomide-Loaded Polymeric Nanoparticles: A Synergistic Approach for Improved Anti-Tumor Efficacy Against Glioblastoma. Process Biochem. 2021, 110, 9–18. [Google Scholar] [CrossRef]
- Chang, K.-F.; Huang, X.-F.; Chang, J.T.; Huang, Y.-C.; Lo, W.-S.; Hsiao, C.-Y.; Tsai, N.-M. Cedrol, a Sesquiterpene Alcohol, Enhances the Anticancer Efficacy of Temozolomide in Attenuating Drug Resistance via Regulation of the DNA Damage Response and MGMT Expression. J. Nat. Prod. 2020, 83, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Çetin, A.; Biltekin, B.; Degirmencioglu, S. Ellagic Acid Enhances the Antitumor Efficacy of Bevacizumab in an In Vitro Glioblastoma Model. World Neurosurg. 2019, 132, e59–e65. [Google Scholar] [CrossRef] [PubMed]
- Altundağ, E.M.; Jannuzzi, A.T.; Özbilenler, C.; Ustürk, S.; Altınoğlu, G. Synergistic Role of Thymoquinone and 5-Fluorouracil in U-251MG Glioblastoma Cell Line. Turk. J. Biochem. 2023, 49, 82–89. [Google Scholar] [CrossRef]
- Cetin, A.; Biltekin, B.; Ozevren, H. Antitumor Activity of Irinotecan with Ellagic Acid in C6 Glioma Cells. Rev. Assoc. Méd. Bras. 2022, 68, 939–944. [Google Scholar] [CrossRef]
- Qu, H.; Song, X.; Song, Z.; Jiang, X.; Gao, X.; Bai, L.; Wu, J.; Na, L.; Yao, Z. Berberine Reduces Temozolomide Resistance by Inducing Autophagy via the ERK1/2 Signaling Pathway in Glioblastoma. Cancer Cell Int. 2020, 20, 592. [Google Scholar] [CrossRef]
- Kamani, M.; Ghanbari, A.; Taghadosi, M.; Mansouri, K.; Jalili, C. Harmine Augments the Cytotoxic and Anti-Invasive Potential of Temozolomide Against Glioblastoma Multiforme Cells. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e115464. [Google Scholar] [CrossRef]
- Jeong, S.; Jung, S.; Park, G.-S.; Shin, J.; Oh, J.-W. Piperine Synergistically Enhances the Effect of Temozolomide against Temozolomide-Resistant Human Glioma Cell Lines. Bioengineered 2020, 11, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol Inhibits Human Glioma by Induction of Lethal Mitophagy through Activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zając, A.; Bądziul, D.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. Coumarins Modulate the Anti-Glioma Properties of Temozolomide. Eur. J. Pharmacol. 2020, 881, 173207. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, D.; Li, L.; Wang, J.; Li, Q.; Duan, L.; Yin, H.; Wang, X.; Liu, Y.; Yuan, G.; et al. Biochanin A Sensitizes Glioblastoma to Temozolomide by Inhibiting Autophagy. Mol. Neurobiol. 2022, 59, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Z.; Dai, X.; Zhang, L.; Li, M. Apigenin and Temozolomide Synergistically Inhibit Glioma Growth Through the PI3K/AKT Pathway. Cancer Biother. Radiopharm. 2021, 39, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, Y.; Zhang, H.; Pan, J.; Yang, F.; Zhang, R.; Ahmad, N.; Yang, J.; Sun, M. Morusin Enhances Temozolomide Efficiency in GBM by Inducing Cytoplasmic Vacuolization and Endoplasmic Reticulum Stress. J. Clin. Med. 2022, 11, 3662. [Google Scholar] [CrossRef] [PubMed]
- Daisy Precilla, S.; Kuduvalli, S.S.; Angeline Praveena, E.; Thangavel, S.; Anitha, T.S. Integration of Synthetic and Natural Derivatives Revives the Therapeutic Potential of Temozolomide against Glioma—An in Vitro and in Vivo Perspective. Life Sci. 2022, 301, 120609. [Google Scholar] [CrossRef]
- Ho, K.-H.; Kuo, T.-C.; Lee, Y.-T.; Chen, P.-H.; Shih, C.-M.; Cheng, C.-H.; Liu, A.-J.; Lee, C.-C.; Chen, K.-C. Xanthohumol Regulates miR-4749-5p-Inhibited RFC2 Signaling in Enhancing Temozolomide Cytotoxicity to Glioblastoma. Life Sci. 2020, 254, 117807. [Google Scholar] [CrossRef]
- Chio, C.-C.; Chen, K.-Y.; Chang, C.-K.; Chuang, J.-Y.; Liu, C.-C.; Liu, S.-H.; Chen, R.-M. Improved Effects of Honokiol on Temozolomide-Induced Autophagy and Apoptosis of Drug-Sensitive and -Tolerant Glioma Cells. BMC Cancer 2018, 18, 379. [Google Scholar] [CrossRef]
- Chio, C.-C.; Tai, Y.-T.; Mohanraj, M.; Liu, S.-H.; Yang, S.-T.; Chen, R.-M. Honokiol Enhances Temozolomide-Induced Apoptotic Insults to Malignant Glioma Cells via an Intrinsic Mitochondrion-Dependent Pathway. Phytomed. Int. J. Phytother. Phytopharm. 2018, 49, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Das, S.; Nandi, S.; Dhara, D.; Mandal, M. Magnolol and Temozolomide Exhibit a Synergistic Anti-Glioma Activity through MGMT Inhibition. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2023, 1869, 166782. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Biltekin, B. Ellagic Acid Enhances Antitumor Efficacy of Temozolomide in an in Vitro Glioblastoma Model. Turk. Neurosurg. 2020, 30, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Bona, N.P.; Pedra, N.S.; Azambuja, J.H.; Soares, M.S.P.; Spohr, L.; Gelsleichter, N.E.; de Meine, B.M.; Sekine, F.G.; Mendonça, L.T.; de Oliveira, F.H.; et al. Tannic Acid Elicits Selective Antitumoral Activity in Vitro and Inhibits Cancer Cell Growth in a Preclinical Model of Glioblastoma Multiforme. Metab. Brain Dis. 2020, 35, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Çetin, A.; Biltekin, B. Combining Ellagic Acid with Temozolomide Mediates the Cadherin Switch and Angiogenesis in a Glioblastoma Model. World Neurosurg. 2019, 132, e178–e184. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-T.; Lee, I.-N.; Chen, C.-H.; Lu, F.-J.; Chung, C.-Y.; Lee, M.-H.; Cheng, Y.-C.; Chen, K.-T.; Peng, J.-Y.; Chen, C.-H. Gallic Acid Enhances the Anti-Cancer Effect of Temozolomide in Human Glioma Cell Line via Inhibition of Akt and P38-MAPK Pathway. Processes 2022, 10, 448. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Li, H.; Ji, Y.; Fang, F.; Tang, H.; Qiu, P. A Steroidal Saponin Form Paris Vietnamensis (Takht.) Reverses Temozolomide Resistance in Glioblastoma Cells via Inducing Apoptosis through ROS/PI3K/Akt Pathway. Biosci. Trends 2020, 14, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ercelik, M.; Tekin, C.; Tezcan, G.; Ak Aksoy, S.; Bekar, A.; Kocaeli, H.; Taskapilioglu, M.O.; Eser, P.; Tunca, B. Olea europaea Leaf Phenolics Oleuropein, Hydroxytyrosol, Tyrosol, and Rutin Induce Apoptosis and Additionally Affect Temozolomide against Glioblastoma: In Particular, Oleuropein Inhibits Spheroid Growth by Attenuating Stem-like Cell Phenotype. Life 2023, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic Effects of Resveratrol and Temozolomide Against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Li, A.; Liu, R.; Yang, H.; Xia, X. The Phytochemical Potential for Brain Disease Therapy and the Possible Nanodelivery Solutions for Brain Access. Front. Oncol. 2022, 12, 936054. [Google Scholar] [CrossRef]
- Gostyńska, A.; Czerniel, J.; Kuźmińska, J.; Brzozowski, J.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V.; Stawny, M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics 2023, 15, 448. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Mlynarczyk, D.T.; Krajka-Kuźniak, V.; Majchrzak-Celińska, A.; Budzianowska, A.; Tomczak, S.; Budzianowski, J.; Woźniak-Braszak, A.; Pietrzyk, R.; Baranowski, M.; et al. Natural Compounds in Liposomal Nanoformulations of Potential Clinical Application in Glioblastoma. Cancers 2022, 14, 6222. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Xu, H.; Li, X.; Yan, H.; Tang, H.; Wen, C.; Li, Y. The Construction of the Multifunctional Targeting Ursolic Acids Liposomes and Its Apoptosis Effects to C6 Glioma Stem Cells. Oncotarget 2017, 8, 64129–64142. [Google Scholar] [CrossRef]
- Sahab-Negah, S.; Ariakia, F.; Jalili-Nik, M.; Afshari, A.R.; Salehi, S.; Samini, F.; Rajabzadeh, G.; Gorji, A. Curcumin Loaded in Niosomal Nanoparticles Improved the Anti-Tumor Effects of Free Curcumin on Glioblastoma Stem-like Cells: An In Vitro Study. Mol. Neurobiol. 2020, 57, 3391–3411. [Google Scholar] [CrossRef]
- Ismail, M.; Yang, W.; Li, Y.; Chai, T.; Zhang, D.; Du, Q.; Muhammad, P.; Hanif, S.; Zheng, M.; Shi, B. Targeted Liposomes for Combined Delivery of Artesunate and Temozolomide to Resistant Glioblastoma. Biomaterials 2022, 287, 121608. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Kleszcz, R.; Stasiłowicz-Krzemień, A.; Cielecka-Piontek, J. Sodium Butyrate Enhances Curcuminoids Permeability through the Blood-Brain Barrier, Restores Wnt/β-Catenin Pathway Antagonists Gene Expression and Reduces the Viability of Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel Loaded Liposomes Decorated with a Multifunctional Tandem Peptide for Glioma Targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hong, W.; Yu, M.; Li, Y.; Zheng, Y.; Ying, X. Multifunctional Targeting Liposomes of Epirubicin Plus Resveratrol Improved Therapeutic Effect on Brain Gliomas. Int. J. Nanomed. 2022, 17, 1087–1110. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Zielińska-Przyjemska, M.; Wierzchowski, M.; Kleszcz, R.; Studzińska-Sroka, E.; Kaczmarek, M.; Paluszczak, J.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Methoxy-Stilbenes Downregulate the Transcription of Wnt/β-Catenin-Dependent Genes and Lead to Cell Cycle Arrest and Apoptosis in Human T98G Glioblastoma Cells. Adv. Med. Sci. 2021, 66, 6–20. [Google Scholar] [CrossRef]
- Rampogu, S.; Kim, S.M.; Shaik, B.; Lee, G.; Kim, J.H.; Kim, G.S.; Lee, K.W.; Kim, M.O. Novel Butein Derivatives Repress DDX3 Expression by Inhibiting PI3K/AKT Signaling Pathway in MCF-7 and MDA-MB-231 Cell Lines. Front. Oncol. 2021, 11, 712824. [Google Scholar] [CrossRef]
- Sun, M.; Song, L.; Zhou, T.; Gillespie, G.Y.; Jope, R.S. The Role of DDX3 in Regulating Snail. Biochim. Biophys. Acta 2011, 1813, 438–447. [Google Scholar] [CrossRef]
- He, Y.; Zhang, D.; Yang, Y.; Wang, X.; Zhao, X.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. A Double-Edged Function of DDX3, as an Oncogene or Tumor Suppressor, in Cancer Progression (Review). Oncol. Rep. 2018, 39, 883–892. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Yücer, R.; Dawood, M.; Hegazy, M.-E.F.; Drif, A.; Ooko, E.; Kadioglu, O.; Seo, E.-J.; Kamounah, F.S.; Titinchi, S.J.; et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. Int. J. Mol. Sci. 2022, 23, 3966. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Tarique, M.; Tabrez, S.; Zughaibi, T.A.; Rehan, M. Synergistic Inhibition of Glioblastoma Multiforme through an In-Silico Analysis of Luteolin and Ferulic Acid Derived from Angelica Sinensis and Cannabis Sativa: Advancements in Computational Therapeutics. PLoS ONE 2023, 18, e0293666. [Google Scholar] [CrossRef] [PubMed]
- Sucu, B.O.; Koc, E.B.; Savlug Ipek, O.; Mirat, A.; Almas, F.; Guzel, M.A.; Dogan, B.; Uludag, D.; Karakas, N.; Durdagi, S.; et al. Design and Synthesis of Novel Caffeic Acid Phenethyl Ester (CAPE) Derivatives and Their Biological Activity Studies in Glioblastoma Multiforme (GBM) Cancer Cell Lines. J. Mol. Graph. Model. 2022, 113, 108160. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kuo, H.-C.; Chu, C.-Y.; Wang, C.-J.; Lin, W.-C.; Tseng, T.-H. Involvement of Tumor Suppressor Protein P53 and P38 MAPK in Caffeic Acid Phenethyl Ester-Induced Apoptosis of C6 Glioma Cells. Biochem. Pharmacol. 2003, 66, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Łaba, A.E.; Ziółkowski, P. Trends in Glioblastoma Treatment Research: An Analysis of Clinical Trials and Literature. Neurol. Neurochir. Pol. 2021, 55, 269–280. [Google Scholar] [CrossRef]
- Huang, Q.; Pan, X.; Zhu, W.; Zhao, W.; Xu, H.; Hu, K. Natural Products for the Immunotherapy of Glioma. Nutrients 2023, 15, 2795. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Ye, J.; Gao, Y.; Liao, H.; Zhou, J.; Feng, Y.; Liu, D.; Meng, Y.; Chen, X.; et al. Construction of Chlorogenic Acid-Containing Liposomes with Prolonged Antitumor Immunity Based on T Cell Regulation. Sci. China Life Sci. 2021, 64, 1097–1115. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted Delivery of Chlorogenic Acid by Mannosylated Liposomes to Effectively Promote the Polarization of TAMs for the Treatment of Glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef]
- Mukherjee, S.; Baidoo, J.N.E.; Sampat, S.; Mancuso, A.; David, L.; Cohen, L.S.; Zhou, S.; Banerjee, P. Liposomal TriCurin, A Synergistic Combination of Curcumin, Epicatechin Gallate and Resveratrol, Repolarizes Tumor-Associated Microglia/Macrophages, and Eliminates Glioblastoma (GBM) and GBM Stem Cells. Molecules 2018, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.L.C.; Amparo, J.A.O.; da Silva, A.B.; da Silva, K.C.; Braga-de-Souza, S.; Barbosa, P.R.; Lopes, G.P.d.F.; Costa, S.L. Apigenin from Croton betulaster Müll Restores the Immune Profile of Microglia against Glioma Cells. Phytother. Res. PTR 2019, 33, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Liu, H.; Sun, F.; Du, R.; Kong, J.; Wang, H.; Cheng, H.; Wang, G.; Gao, F.; Liang, P. Intratumor Injection of Thermosensitive Polypeptide with Resveratrol Inhibits Glioblastoma Growth. Tissue Eng. Part C Methods 2023, 29, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Stuppner, H. The Photoactivity of Natural Products—An Overlooked Potential of Phytomedicines? Phytomed. Int. J. Phytother. Phytopharm. 2019, 60, 152985. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. In Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef]
- Eom, K.S.; Kim, H.-J.; So, H.-S.; Park, R.; Kim, T.Y. Berberine-Induced Apoptosis in Human Glioblastoma T98G Cells Is Mediated by Endoplasmic Reticulum Stress Accompanying Reactive Oxygen Species and Mitochondrial Dysfunction. Biol. Pharm. Bull. 2010, 33, 1644–1649. [Google Scholar] [CrossRef]
- Werner, M.; Lyu, C.; Stadlbauer, B.; Schrader, I.; Buchner, A.; Stepp, H.; Sroka, R.; Pohla, H. The Role of Shikonin in Improving 5-Aminolevulinic Acid-Based Photodynamic Therapy and Chemotherapy on Glioblastoma Stem Cells. Photodiagn. Photodyn. Ther. 2022, 39, 102987. [Google Scholar] [CrossRef]
- Bassler, M.; Hiller, J.; Wackenhut, F.; Zur Oven-Krockhaus, S.; Frech, P.; Schmidt, F.; Kertzscher, C.; Rammler, T.; Ritz, R.; Braun, K.; et al. Fluorescence Lifetime Imaging Unravels the Pathway of Glioma Cell Death upon Hypericin-Induced Photodynamic Therapy. Chemistry 2023, preprint. [Google Scholar]
- Pevna, V.; Wagnières, G.; Huntosova, V. Autophagy and Apoptosis Induced in U87 MG Glioblastoma Cells by Hypericin-Mediated Photodynamic Therapy Can Be Photobiomodulated with 808 Nm Light. Biomedicines 2021, 9, 1703. [Google Scholar] [CrossRef]
- Bassler, M.C.; Rammler, T.; Wackenhut, F.; zur Oven-Krockhaus, S.; Secic, I.; Ritz, R.; Meixner, A.J.; Brecht, M. Accumulation and Penetration Behavior of Hypericin in Glioma Tumor Spheroids Studied by Fluorescence Microscopy and Confocal Fluorescence Lifetime Imaging Microscopy. Anal. Bioanal. Chem. 2022, 414, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Aebisher, D. Photosensitizers for Photodynamic Therapy of Brain Cancers—A Review. Brain Sci. 2023, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.R.; Karimi Roshan, M.; Soukhtanloo, M.; Ghorbani, A.; Rahmani, F.; Jalili-Nik, M.; Vahedi, M.M.; Hoseini, A.; Sadeghnia, H.R.; Mollazadeh, H.; et al. Cytotoxic Effects of Auraptene against a Human Malignant Glioblastoma Cell Line. Avicenna J. Phytomed. 2019, 9, 334–346. [Google Scholar]
- Izadi, A.; Soukhtanloo, M.; Mirzavi, F.; Jalili-Nik, M.; Sadeghi, A. Alpha-Lipoic Acid, Auraptene, and Particularly Their Combination Prevent the Metastasis of U87 Human Glioblastoma Cells. Evid. Based Complement. Alternat. Med. 2023, 2023, e8618575. [Google Scholar] [CrossRef]
- Tsiftsoglou, O.S.; Krigas, N.; Gounaris, C.; Papitsa, C.; Nanouli, M.; Vartholomatos, E.; Markopoulos, G.S.; Isyhou, R.; Alexiou, G.; Lazari, D. Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model. Pharmaceutics 2023, 15, 1383. [Google Scholar] [CrossRef]
- Chen, X.-M.; Lu, W.; Zhang, Z.-H.; Zhang, J.-Y.; Tuong, T.M.L.; Liu, L.-L.; Kim, Y.H.; Li, C.-H.; Gao, J.-M. Cassane Diterpenoids from the Aerial Parts of Caesalpinia pulcherrima and Their Antibacterial and Anti-Glioblastoma Activity. Phytochemistry 2022, 196, 113082. [Google Scholar] [CrossRef]
- Hua, D.; Zhao, Q.; Yu, Y.; Yu, H.; Yu, L.; Zhou, X.; Wang, Q.; Sun, C.; Shi, C.; Luo, W.; et al. Eucalyptal A Inhibits Glioma by Rectifying Oncogenic Splicing of MYO1B mRNA via Suppressing SRSF1 Expression. Eur. J. Pharmacol. 2021, 890, 173669. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, D.; Xu, X.; Qiu, S.; Luo, S.; Qiu, E.; Rong, Z.; Zhang, J.; Zheng, D. Galangin Inhibits Epithelial-Mesenchymal Transition and Angiogenesis by Downregulating CD44 in Glioma. J. Cancer 2019, 10, 4499–4508. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y.; Zhang, X.; Yang, N.; Wang, J.; et al. The Natural Flavonoid Galangin Elicits Apoptosis, Pyroptosis, and Autophagy in Glioblastoma. Front. Oncol. 2019, 9, 942. [Google Scholar] [CrossRef]
- Xiong, Y.; Lai, X.; Xiang, W.; Zhou, J.; Han, J.; Li, H.; Deng, H.; Liu, L.; Peng, J.; Chen, L. Galangin (GLN) Suppresses Proliferation, Migration, and Invasion of Human Glioblastoma Cells by Targeting Skp2-Induced Epithelial–Mesenchymal Transition (EMT). OncoTargets Ther. 2020, 13, 9235–9244. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Z.; Xu, X.; He, M.; Xiong, H.; Liu, L. Casticin Induces Apoptosis and Cytoprotective Autophagy While Inhibiting Stemness Involving Akt/mTOR and JAK2/STAT3 Pathways in Glioblastoma. Phytother. Res. 2024, 38, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef]
- Mutai, C.; Abatis, D.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity. Molecules 2007, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, W.; Lou, H. Antifungal Constituents from the Chinese Moss Homalia Trichomanoides. Chem. Biodivers. 2005, 2, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Kleszcz, R.; Studzińska-Sroka, E.; Łukaszyk, A.; Szoszkiewicz, A.; Stelcer, E.; Jopek, K.; Rucinski, M.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Lichen Secondary Metabolites Inhibit the Wnt/β-Catenin Pathway in Glioblastoma Cells and Improve the Anticancer Effects of Temozolomide. Cells 2022, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia Physodes Extract Component-Physodic Acid through the Blood-Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia Glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef]
- Murugesan, M.; Kandhavelu, M.; Thiyagarajan, R.; Natesan, S.; Rajendran, P.; Murugesan, A. Marine Halophyte Derived Polyphenols Inhibit Glioma Cell Growth through Mitogen-Activated Protein Kinase Signaling Pathway. Biomed. Pharmacother. 2023, 159, 114288. [Google Scholar] [CrossRef]
- Kakouri, E.; Hatziagapiou, K.; Kanakis, C.; Nikola, O.; Lambrou, G.I.; Trigas, P.; Kanaka-Gantenbein, C.; Tarantilis, P.A. Cytotoxic and Antioxidant Activity of a Chemically Characterized Extract of Smilax Aspera Leaves and Stems. Appl. Sci. 2023, 13, 4784. [Google Scholar] [CrossRef]
- Kamarudin, N.A.; Nik Salleh, N.N.H.; Tan, S.C. Gallotannin-Enriched Fraction from Quercus infectoria Galls as an Antioxidant and Inhibitory Agent against Human Glioblastoma Multiforme. Plants 2021, 10, 2581. [Google Scholar] [CrossRef]
- Kleszcz, R.; Majchrzak-Celińska, A.; Baer-Dubowska, W. Tannins in Cancer Prevention and Therapy. Br. J. Pharmacol. 2023. early access. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Chen, F.; He, Y.; Yin, S.; Peng, Y.; Li, W. Phytochemicals and Glioma: Results from Dietary Mixed Exposure. Brain Sci. 2023, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Ghorbani, A. Cancer Therapy with Phytochemicals: Evidence from Clinical Studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar] [PubMed]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A Pilot Clinical Study of Δ9-Tetrahydrocannabinol in Patients with Recurrent Glioblastoma Multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Lah, T.T.; Majc, B.; Novak, M.; Sušnik, A.; Breznik, B.; Porčnik, A.; Bošnjak, R.; Sadikov, A.; Malavolta, M.; Halilčević, S.; et al. The Cytotoxic Effects of Cannabidiol and Cannabigerol on Glioblastoma Stem Cells May Mostly Involve GPR55 and TRPV1 Signalling. Cancers 2022, 14, 5918. [Google Scholar] [CrossRef]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants 2023, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Alptekin, A.; Mehrabian, D.; Rutkowski, M.; Arbab, A.S.; Yeudall, W.A.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; et al. Inhalant Cannabidiol Inhibits Glioblastoma Progression Through Regulation of Tumor Microenvironment. Cannabis Cannabinoid Res. 2023, 8, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Singer, E.; Dighe, P.; Sidorov, M.; Limbad, C.; Rodriquez-Brotons, A.; Rix, P.; Woo, R.W.L.; Dickinson, L.; Desprez, P.-Y.; et al. Cannabidiol Inhibits RAD51 and Sensitizes Glioblastoma to Temozolomide in Multiple Orthotopic Tumor Models. Neuro-Oncol. Adv. 2022, 4, vdac019. [Google Scholar] [CrossRef]
- Kuźmińska, J.; Sobczak, A.; Majchrzak-Celińska, A.; Żółnowska, I.; Gostyńska, A.; Jadach, B.; Krajka-Kuźniak, V.; Jelińska, A.; Stawny, M. Etoricoxib-Cannabidiol Combo: Potential Role in Glioblastoma Treatment and Development of PLGA-Based Nanoparticles. Pharmaceutics 2023, 15, 2104. [Google Scholar] [CrossRef]
- Volmar, M.N.M.; Cheng, J.; Alenezi, H.; Richter, S.; Haug, A.; Hassan, Z.; Goldberg, M.; Li, Y.; Hou, M.; Herold-Mende, C.; et al. Cannabidiol Converts NF-κB into a Tumor Suppressor in Glioblastoma with Defined Antioxidative Properties. Neuro-Oncolology 2021, 23, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Likar, R.; Koestenberger, M.; Stutschnig, M.; Nahler, G. Cannabidiol Μay Prolong Survival in Patients with Glioblastoma Multiforme. Cancer Diagn. Progn. 2021, 1, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. The Application of Cannabidiol in the Treatment of Glioblastoma. Acta Pol. Pharm. Drug Res. 2023, 80, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Mathur, A.; Pandey, V.K.; Kakkar, P. Endoplasmic Reticulum Stress-Dependent Activation of TRB3-FoxO1 Signaling Pathway Exacerbates Hyperglycemic Nephrotoxicity: Protection Accorded by Naringenin. Eur. J. Pharmacol. 2022, 917, 174745. [Google Scholar] [CrossRef]
- Twelves, C.; Sabel, M.; Checketts, D.; Miller, S.; Tayo, B.; Jove, M.; Brazil, L.; Short, S.C.; GWCA1208 study group. A Phase 1b Randomised, Placebo-Controlled Trial of Nabiximols Cannabinoid Oromucosal Spray with Temozolomide in Patients with Recurrent Glioblastoma. Br. J. Cancer 2021, 124, 1379–1387. [Google Scholar] [CrossRef]
| Compound | Group | Cell Line | Model | Results | Reference |
|---|---|---|---|---|---|
| Bevacizumab + elagic acid | phenolic acid | C6 | in vitro | Antiproliferative efficacy. Inhibition of MGMT expression and time-dependent inhibition of MDR1. | [27] |
| 5-Fluorouracil + thymoquinone | quinones | U-251MG | in vitro | Reduced cell viability and proliferation in GBM cells. Strong synergistic anticancer effect. | [28] |
| Irinotekan + elagic acid | phenolic acid | C6 | in vitro | Synergistic effect. Reduced cell proliferation by inhibiting the cadherin switch and promoting the antiangiogenic processes. | [29] |
| Temozolomide + berberine | alkalod | U87 U251 | in vitro/ in vivo mice | Enhanced autophagy and apoptosis in TMZ-resistant cells linked with ERK1/2 signaling. In vivo increased GBM sensitivity to TMZ through the ERK1/2 signaling pathway. | [30] |
| Temozolomide + harmine | alkaloid | T98G | in vitro | Decreased cancer cells’ migration, invasion, and adhesion potentials, as well as the expression of metalloproteinases 2 and 9. | [31] |
| Temozlomide + piperine | alkaloid | U251MG T98G | in vitro | Apoptosis induction by activation of caspase-8/-9/-3, MMP loss, and inhibition of cell motility. | [32] |
| Temozlomide + cannabidiol | canabinoid | U87MG | in vivo mice | Controlling tumor size and improving survival. | [33] |
| Temozolomide + osthol | coumarin | T98G | in vitro | Apoptosis, correlated with Bcl-2/Beclin 1 complex formation. | [34] |
| Temozolomide + biohanin A | isoflavone | U251 U87 C6 | in vitro/ in vivo rats/ in silico | Enhanced cells sensitivity to TMZ in vitro and in vivo. Inhibited TMZ-induced autophagy in GBM cells by activating the AMPK/ULK1 pathway in silico. | [35] |
| Temozolomide + apigenine | flavonoid | glioma cells | in vitro/ in vivo mice | Synergistic inhibition of glioma growth through the PI3K/AKT pathway. | [36] |
| Temozolomide + morusin | flavonoid | U87 U251 | in vitro/ in vivo mice | Enhanced endoplasmic reticulum stress, synergistic effect in GBM cells, suppressed tumor progression in an orthotopic xenograft model. | [37] |
| Temozolomide + naringenin | flavonoid | C6 U87MG LN229 HEK-293 T | in vitro/ in vivo rats | Synergistically increased efficacy of TMZ on glioma in vitro and in vivo. | [38] |
| Temozolomide + xantohumol | flavonoid | U87 MG A172 | in vitro | miR-4749-5p targeting RFC2 signaling participates in XN-enhanced TMZ cytotoxicity. | [39] |
| Temozolomide + honokiol | lignan | U87MG GL261 U87MG-R9 | in vitro | Significantly enhanced TMZ-induced insults. Induced greater caspase-3 activation, DNA fragmentation, cell apoptosis, and cell-cycle arrest at the G1 phase. Autophagy and consequent apoptosis in U87-MG-R9. | [40] |
| Temozolomide + honokiol | lignan | U373MG GL261 U87MG | in vitro | Improved TMZ-induced insults to human malignant glioma cells. Enhanced TMZ-induced apoptosis and suppression of proliferation in human glioma cells. | [41] |
| Temozolomide + magnolol | lignan | LN18 U87MG LN229 T98G HEK293 C6 | in vitro/ in vivo | Potentiation of TMZ-induced apoptosis in glioma by inhibiting NF-κB pathway-mediated MGMT activation. | [42] |
| Temozoomide + elagic acid | phenolic acid | C6 | in vitro | Antiproliferative efficacy by inhibiting MGMT expression and activating apoptotic protein, p53, and caspase-3 expression. | [43] |
| Temozolomide + tannic acid | phenolic acid | C6 | in vitro/ in vivo rats | Not cytotoxic to astrocytes. Induced anti-glioma activity, apoptosis, and cell-cycle arrest. Reduced the formation and size of colonies, and cell migration/adhesion. In vivo: decreased tumor volume and increased the area of intratumoral necrosis and infiltration of lymphocytes. | [44] |
| Temozolomide + elagic acid | phenolic acid | C6 | in vitro | Inhibited the cadherin switch and angiogenesis. | [45] |
| Temozolomide + gallic acid | phenolic acid | U87MG | in vitro | Potential augmentation of the anticancer effect of TMZ via the repression of Bcl-2 expression and Akt activation and the enhancement of the p38 MAPK pathway. | [46] |
| Temozolomide + steroidal saponin (N45) | saponin | U87R | in vitro | Induced mitochondrial apoptosis, and decreased drug resistance by downregulation of NF-κB p65. | [47] |
| Temozolomide + oleuropein | secoiridoid | T98G A172 | in vitro | Demonstrated additive effects that can augment the effect of TMZ. | [48] |
| Temozolomide + resveratrol | stilbenoid | RG-2 LN-18 LN-428 | in vitro | Downregulated MGMT overexpression. Inhibition of the STAT3/Bcl-2/survivin signaling pathway. | [49] |
| Compound/Product | ClinicalTrials.gov ID | Description | Study Type/Phase | Status |
|---|---|---|---|---|
| Cannabidiol | NCT05753007 | A Clinical Trial of a Hemp-Derived, High-Cannabidiol Product for Anxiety in Glioblastoma Patients | Phase 2 | Not yet recruiting |
| NCT03607643 | A Study of the Efficacy of Cannabidiol in Patients with Multiple Myeloma, Glioblastoma Multiforme, and GI Malignancies | Phases 1 and 2 | Unknown status | |
| TN-TC11G (Δ9-tetrahydrocannabinol + cannabidiol) | NCT03529448 | TN-TC11G (THC+CBD) Combination with Temozolomide and Radiotherapy in Patients with Newly Diagnosed Glioblastoma | Phases 1 and 2 | Recruiting |
| Cannabis (for smoking) | NCT03246113 | Tolerability of Cannabis in Patients Receiving Concurrent Chemoradiation for Glioblastoma | Phase 1 | Terminated |
| Sativex® (Nabiximols oromucosal spray) | NCT01812603 | A Safety Study of Sativex in Combination with Dose-Intense Temozolomide in Patients with Recurrent Glioblastoma | Phases 1 and 2 | Completed |
| NCT01812616 | A Safety Study of Sativex Compared with Placebo (Both with Dose-Intense Temozolomide) in Recurrent Glioblastoma Patients | Phases 1 and 2 | Completed | |
| NCT05629702 | ARISTOCRAT: Blinded Trial of Temozolomide +/− Cannabinoids | Phase 2 | Recruiting | |
| Chlorogenic Acid | NCT02728349 | Tolerance and Pharmacokinetic Study of Chlorogenic Acid to Advanced Glioblastoma | Phase 1 | Completed |
| Curcumin | NCT01712542 | Curcumin Bioavailability in Glioblastoma Patients | Observational | Completed |
| NCT05768919 | Study of Liposomal Curcumin in Combination with RT and TMZ in Patients with Newly Diagnosed High-Grade Gliomas | Phases 1 and 2 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majchrzak-Celińska, A.; Studzińska-Sroka, E. New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy. Molecules 2024, 29, 1682. https://doi.org/10.3390/molecules29071682
Majchrzak-Celińska A, Studzińska-Sroka E. New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy. Molecules. 2024; 29(7):1682. https://doi.org/10.3390/molecules29071682
Chicago/Turabian StyleMajchrzak-Celińska, Aleksandra, and Elżbieta Studzińska-Sroka. 2024. "New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy" Molecules 29, no. 7: 1682. https://doi.org/10.3390/molecules29071682
APA StyleMajchrzak-Celińska, A., & Studzińska-Sroka, E. (2024). New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy. Molecules, 29(7), 1682. https://doi.org/10.3390/molecules29071682