Conofolidine: A Natural Plant Alkaloid That Causes Apoptosis and Senescence in Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Conofolidine Potently Inhibits Growth of Cancer Cells
2.2. Conofolidine Potently Inhibits Colony Formation
2.3. Conofolidine Caused Potent Perturbation of Cell Cycle Progression
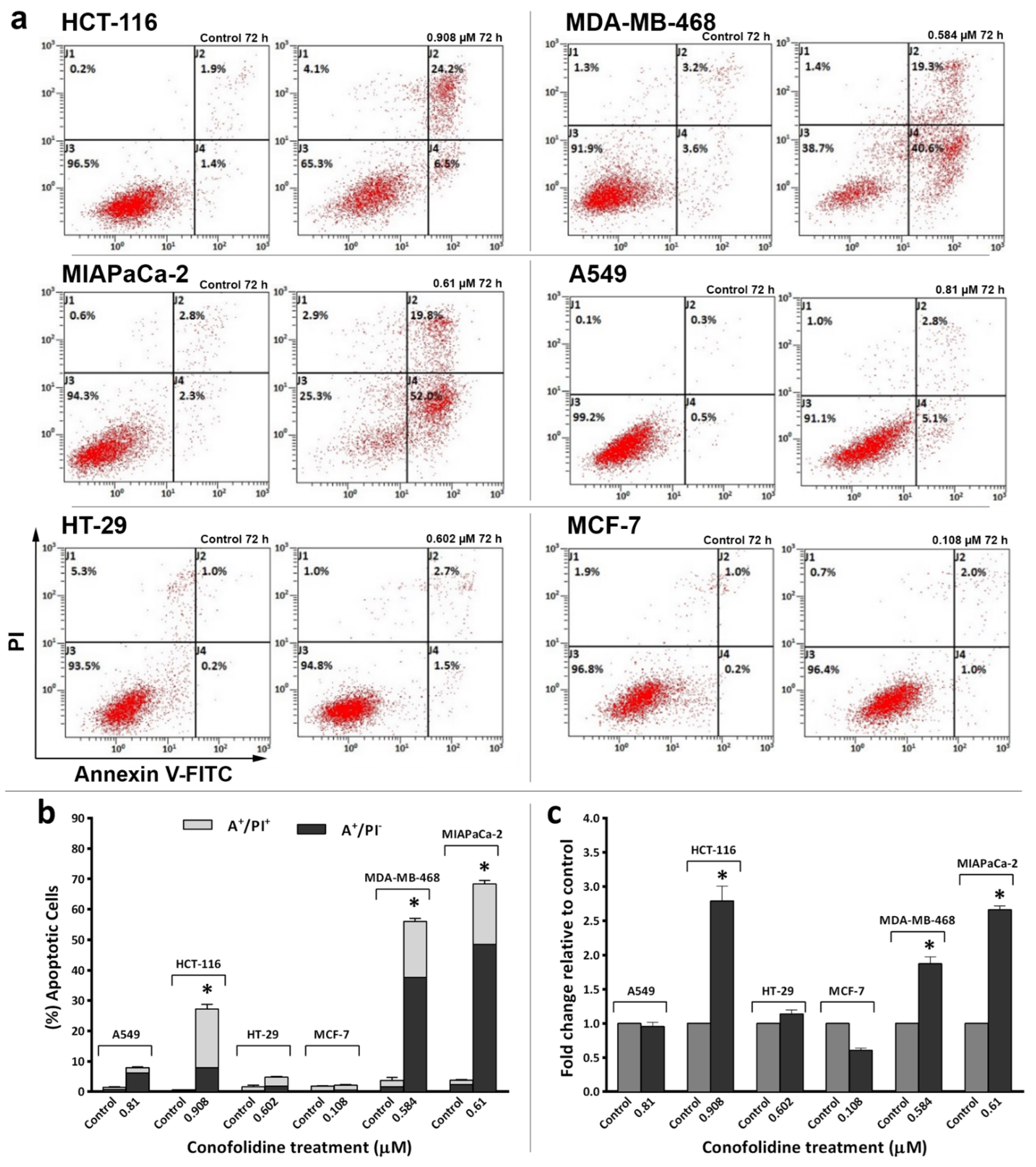
2.4. Conofolidine-Induced Apoptosis
2.5. Conofolidine-Induced Executioner Caspases and PARP Cleavage
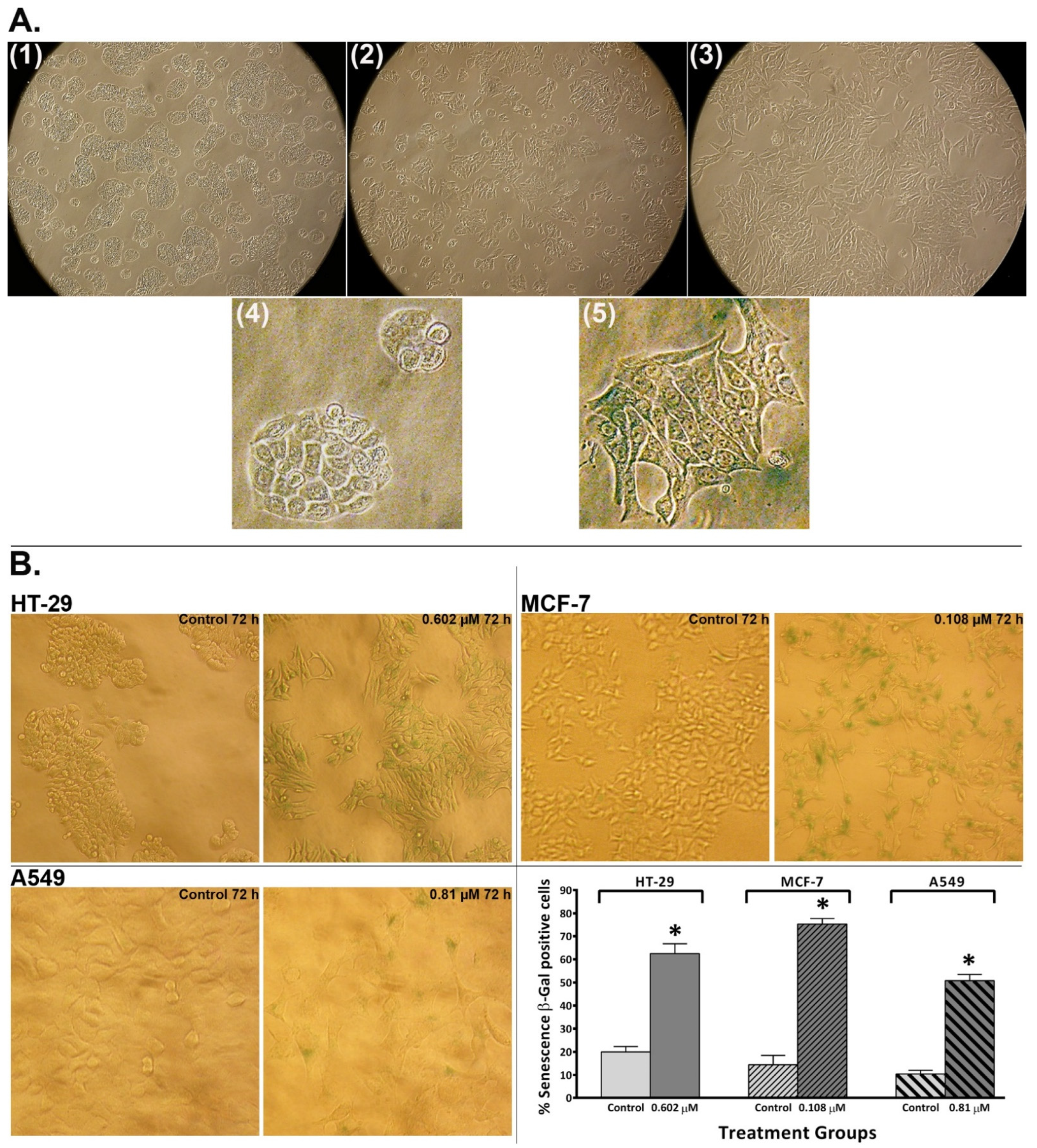
2.6. Conofolidine Promoted Morphological Changes Associated with Senescence Induction
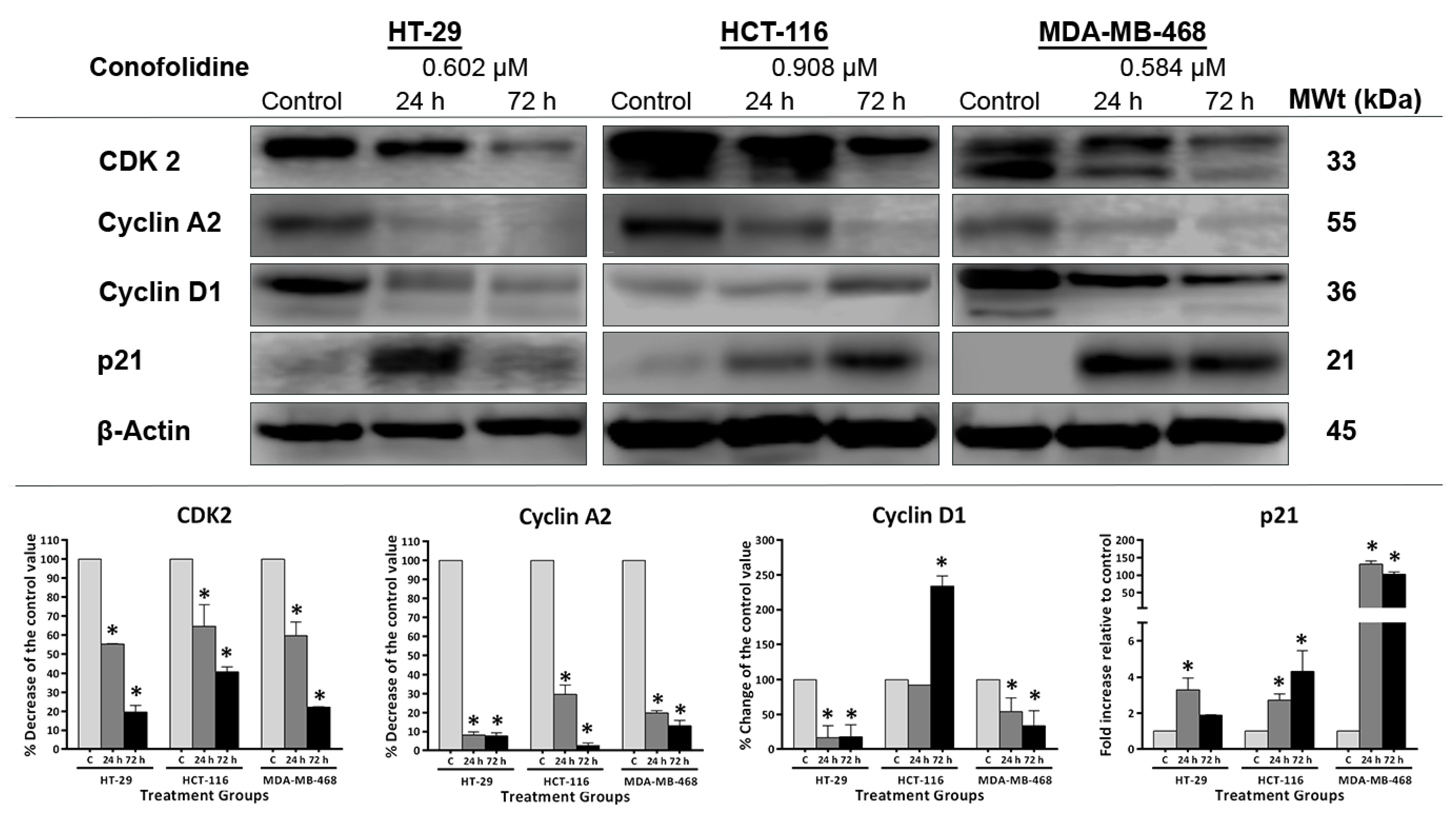
2.7. Conofolidine Downregulated CDK2, Cyclins (A2, D1), and Upregulated p21
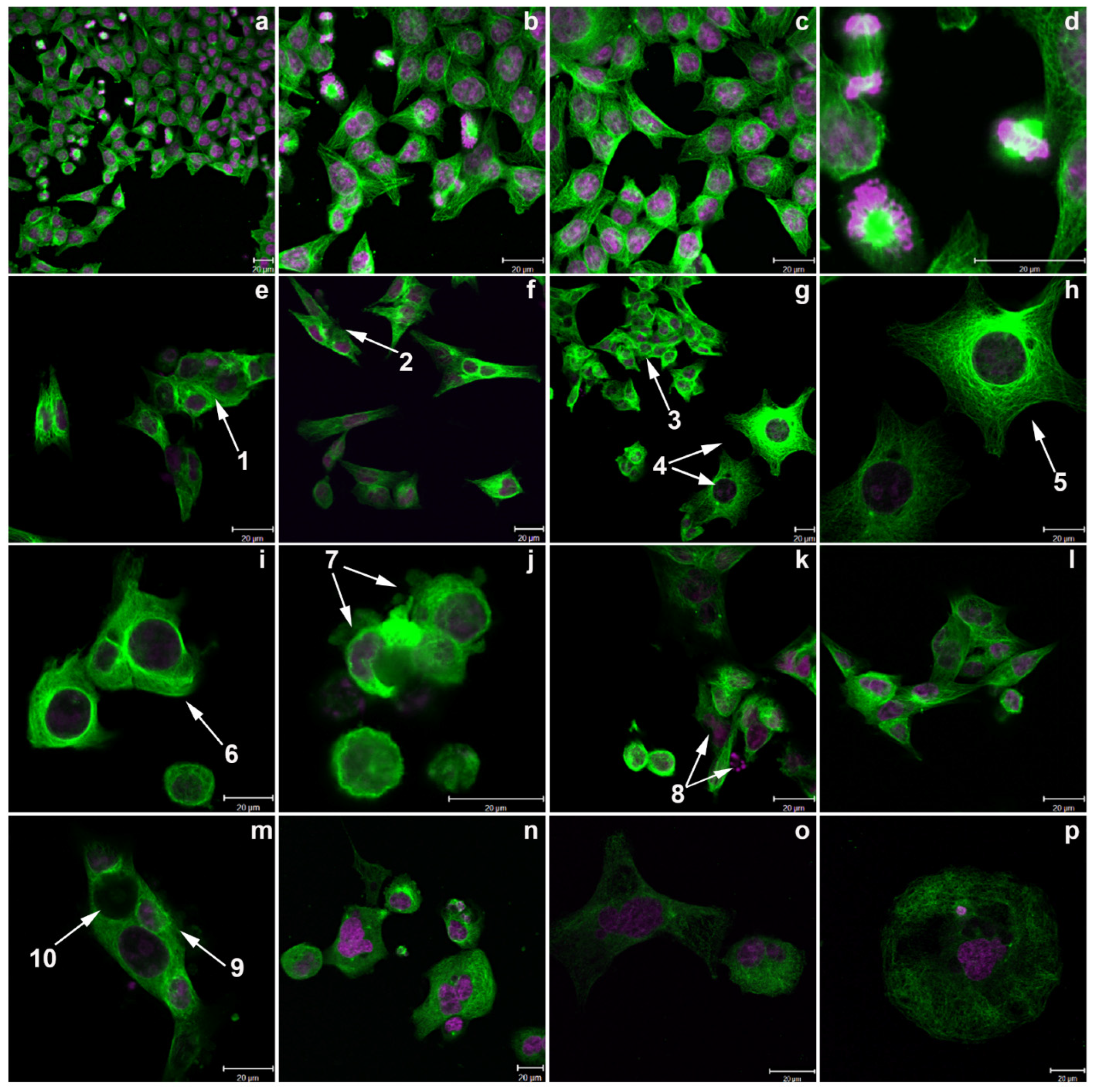
2.8. Confocal Images Confirmed the Induction of Aberrant Mitoses by Conofolidine
2.9. Conofolidine Increased the % of γ-H2AX (+) Events
2.10. Conofolidine-Induced γ-H2AX Foci Formation
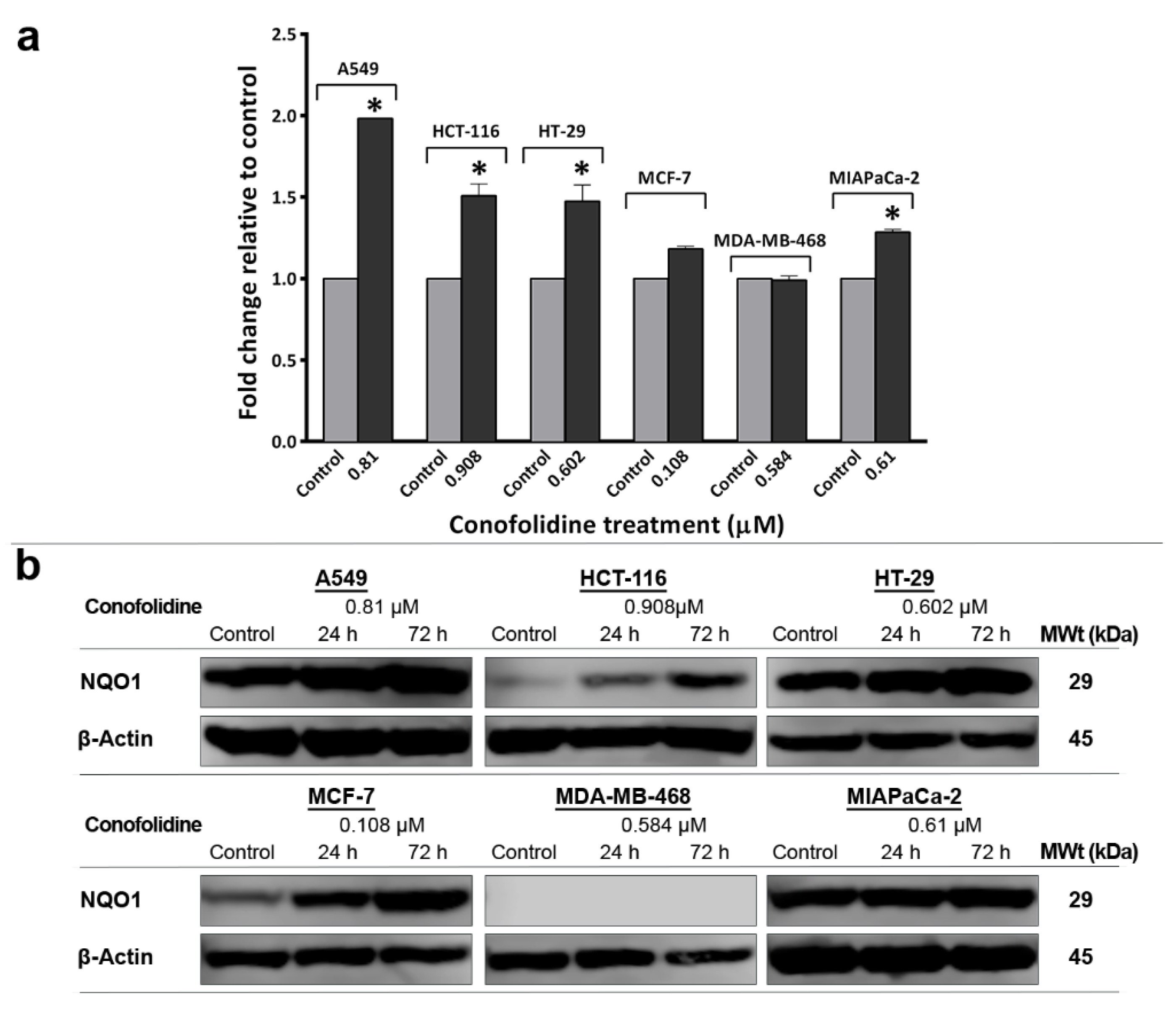
2.11. Conofolidine-Induced ROS Generation and NQO1 Expression
3. Discussion
4. Materials and Methods
4.1. Isolation and Characterization of Conofolidine
4.2. Cell Culture
4.3. MTT Assay
4.4. Clonogenic Assay
4.5. Cell Cycle Analysis
4.6. Annexin-V/PI Apoptosis Assay
4.7. Caspase 3/7 Assay
4.8. Senescence β-Galactosidase Cell Staining Assay
4.9. Western Blotting
4.10. Confocal Microscopy
4.11. Monitoring of γ-H2AX Phosphorylation Induction by Flow Cytometry
4.12. Reactive Oxygen Species Determination
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Rocha, A.B.; Lopes, R.M.; Schwartsmann, G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001, 1, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Wang, Z.; Yu, Q.; Yuan, L.; Liu, Y.; Sang, J.; Li, W.; Zhu, S.; Jiang, W.; et al. Sokotrasterol Sulfate Suppresses IFN-γ-Induced PD-L1 Expression by Inhibiting JAK Activity. J. Nat. Prod. 2024, 87, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Al-Hayali, M.; Garces, A.; Stocks, M.; Collins, H.; Bradshaw, T.D. Concurrent Reactive Oxygen Species Generation and Aneuploidy Induction Contribute to Thymoquinone Anticancer Activity. Molecules 2021, 26, 5136. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsalahat, I.; Daoud, S. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.M.; Emi, M.; Tanabe, K. The role of apoptosis in cancer cell survival and therapeutic outcome. Cancer Biol. Ther. 2006, 5, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Seevasant, I.; Mohamad, J.; Mukheem, A.; Huri, H.Z.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7, e2058. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, J.S. Exploiting tumor cell senescence in anticancer therapy. BMB Rep. 2014, 47, 51–59. [Google Scholar] [CrossRef]
- Milczarek, M. The Premature Senescence in Breast Cancer Treatment Strategy. Cancers 2020, 12, 1815. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.J.; Lim, K.-H.; Leong, C.-O.; Kam, T.-S.; Bradshaw, T.D. Novel antitumour indole alkaloid, Jerantinine A, evokes potent G2/M cell cycle arrest targeting microtubules. Investig. New Drugs 2014, 32, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Qazzaz, M.E.; Raja, V.J.; Lim, K.-H.; Kam, T.-S.; Lee, J.B.; Gershkovich, P.; Bradshaw, T.D. In vitro anticancer properties and biological evaluation of novel natural alkaloid jerantinine B. Cancer Lett. 2016, 370, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.-S.; Loh, K.-Y.; Lim, L.-H.; Loong, W.-L.; Chuah, C.-H.; Wei, C. New alkaloids from the leaves of Tabernaemontana divaricata. Tetrahedron Lett. 1992, 33, 969–972. [Google Scholar] [CrossRef]
- Kam, T.-S.; Loh, K.-Y.; Wei, C. Conophylline and Conophyllidine: New Dimeric Alkaloids from Tabernaemontana divaricata. J. Nat. Prod. 1993, 56, 1865–1871. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Robinson, W.T.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T.-S. Leucophyllidine, a Cytotoxic Bisindole Alkaloid Constituted From the Union of an Eburnan and a New Vinylquinoline Alkaloid. Org. Lett. 2009, 11, 3962–3965. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.-S.; Tan, S.-J.; Ng, S.-W.; Komiyama, K. Bipleiophylline, an Unprecedented Cytotoxic Bisindole Alkaloid Constituted from the Bridging of Two Indole Moieties by an Aromatic Spacer Unit. Org. Lett. 2008, 10, 3749–3752. [Google Scholar] [CrossRef]
- Nge, C.E.; Sim, K.-S.; Lim, S.-H.; Thomas, N.F.; Low, Y.-Y.; Kam, T.-S. A Hexacyclic, Iboga-Derived Monoterpenoid Indole with a Contracted Tetrahydroazepine C-Ring and Incorporation of an Isoxazolidine Moiety, a Seco-Corynanthean, an Aspidosperma-Aspidosperma Bisindole with Anticancer Properties, and the Absolute Configuration of the Pyridopyrimidine Indole Alkaloid, Vernavosine. J. Nat. Prod. 2016, 79, 2709–2717. [Google Scholar]
- Jänicke, R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009, 117, 219–221. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.M.; Zhang, S.S.; Liu, X.Y.; Liu, D.X. Induction of cell cycle arrest at G1 and S phases and cAMP-dependent differentiation in C6 glioma by low concentration of cycloheximide. BMC Cancer 2010, 10, 684. [Google Scholar] [CrossRef]
- Maya-Mendoza, A.; Merchut-Maya, J.M.; Bartkova, J.; Bartek, J.; Streuli, C.H.; Jackson, D.A. Immortalised breast epithelia survive prolonged DNA replication stress and return to cycle from a senescent-like state. Cell Death Dis. 2014, 5, e1351. [Google Scholar] [CrossRef]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.S.; Pang, H.S.; Lim, T.M. Biologically active indole and bisindole alkaloids from Tabernaemontana divaricata. Org. Biomol. Chem. 2003, 1, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Kubushiro, K.; Suzuki, K.; Tsukazaki, K.; Umezawa, K.; Nozawa, S. Inhibition of attachment and chemotactic invasion of uterine endometrial cancer cells by a new vinca alkaloid, conophylline. Anticancer Res. 1999, 19, 3061–3066. [Google Scholar]
- Bachur, N.R.; Yu, F.; Johnson, R.; Hickey, R.; Wu, Y.; Malkas, L. Helicase inhibition by anthracycline anticancer agents. Mol. Pharmacol. 1992, 41, 993–998. [Google Scholar]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Kawakami, M.; Hirayama, A.; Tsuchiya, K.; Ohgawara, H.; Nakamura, M.; Umezawa, K. Promotion of beta-cell differentiation by the alkaloid conophylline in porcine pancreatic endocrine cells. Biomed. Pharmacother. 2010, 64, 226–231. [Google Scholar] [CrossRef]
- Kojima, I.; Umezawa, K. Conophylline: A novel differentiation inducer for pancreatic beta cells. Int. J. Biochem. Cell Biol. 2006, 38, 923–930. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Bacso, Z.; Everson, R.B.; Eliason, J.F. The DNA of annexin V-binding apoptotic cells is highly fragmented. Cancer Res. 2000, 60, 4623–4628. [Google Scholar] [PubMed]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998, 391, 43. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Blagosklonny, M.V. Flavopiridol induces p53 via initial inhibition of Mdm2 and p21 and, independently of p53, sensitizes apoptosis-reluctant cells to tumor necrosis factor. Cancer Res. 2004, 64, 3653–3660. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Kalurupalle, S.; Hanko, C.; Lim, C.-U.; Broude, E.; Blagosklonny, M.V. Mechanism of G1-like arrest by low concentrations of paclitaxel: Next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene 2008, 27, 4402–4410. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 Is Required for DNA Fragmentation and Morphological Changes Associated with Apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef]
- Burton, D.G.; Faragher, R.G. Cellular senescence: From growth arrest to immunogenic conversion. Age 2015, 37, 27. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of p53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- DeGregori, J. The Rb network. J. Cell Sci. 2004, 117 Pt 16, 3411–3413. [Google Scholar] [CrossRef]
- Lauper, N.; Beck, A.R.; Cariou, S.; Richman, L.; Hofmann, K.; Reith, W.; Slingerland, J.M.; Amati, B. Cyclin E2: A novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene 1998, 17, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Woo, R.A.; Poon, R.Y. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2003, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, Y.; Suzuki, A.; Sugimura, K.; Okumura, K.; Zineldeen, D.H.; Shimada, M.; Niida, H.; Mizuno, T.; Hanaoka, F.; Nakanishi, M. Cyclin A–Cdk1 regulates the origin firing program in mammalian cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Earnshaw, W.C.; Lippincott-Schwartz, J.; Johnson, G.T. (Eds.) Chapter 43—G2 Phase, Responses to DNA Damage, and Control of Entry Into Mitosis, in Cell Biology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 743–754. [Google Scholar]
- Gong, D.; Pomerening, J.R.; Myers, J.W.; Gustavsson, C.; Jones, J.T.; Hahn, A.T.; Meyer, T.; Ferrell, J.E. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr. Biol. 2007, 17, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bendris, N.; Arsic, N.; Lemmers, B.; Blanchard, J.M. Cyclin A2, Rho GTPases and EMT. Small GTPases 2012, 3, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wei, H.; Yin, D.; Lu, Y.; Zhang, Y.; Jiang, D.; Jiang, Y.; Zhang, S. Cyclin D1b overexpression inhibits cell proliferation and induces cell apoptosis in cervical cancer cells in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 4016–4023. [Google Scholar] [PubMed]
- Poon, R.Y.; Jiang, W.; Toyoshima, H.; Hunter, T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J. Biol. Chem. 1996, 271, 13283–13291. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Elledge, S.J.; Keyomarsi, K.; Dynlacht, B.; Tsai, L.H.; Zhang, P.; Dobrowolski, S.; Bai, C.; Connell-Crowley, L.; Swindell, E.; et al. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 1995, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Waga, S.; Harry, J.B.; Espling, E.; Stillman, B.; Galloway, D.A. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997, 11, 2090–2100. [Google Scholar]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Coqueret, O.; Gascan, H. Functional interaction of STAT3 transcription factor with the cell cycle inhibitor p21WAF1/CIP1/SDI1. J. Biol. Chem. 2000, 275, 18794–18800. [Google Scholar] [CrossRef] [PubMed]
- Al Zaid Siddiquee, K.; Turkson, J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008, 18, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Masgras, I.; Carrera, S.; de Verdier, P.J.; Brennan, P.; Majid, A.; Makhtar, W.; Tulchinsky, E.; Jones, G.D.D.; Roninson, I.B.; Macip, S. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J. Biol. Chem. 2012, 287, 9845–9854. [Google Scholar] [CrossRef] [PubMed]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. GammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Billecke, C.A.; Ljungman, M.E.; McKay, B.C.; Rehemtulla, A.; Taneja, N.; Ethier, S.P. Lack of functional pRb results in attenuated recovery of mRNA synthesis and increased apoptosis following UV radiation in human breast cancer cells. Oncogene 2002, 21, 4481–4489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Müller-Tidow, C.; Ji, P.; Diederichs, S.; Potratz, J.; Bäumer, N.; Köhler, G.; Cauvet, T.; Choudary, C.; van der Meer, T.; Chan, W.-Y.I.; et al. The Cyclin A1-CDK2 Complex Regulates DNA Double-Strand Break Repair. Mol. Cell. Biol. 2004, 24, 8917–8928. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Liu, K.; Jin, B.; Wu, C.; Yang, J.; Zhan, X.; Wang, L.; Shen, X.; Chen, J.; Chen, H.; Mao, Z. NQO1 Stabilizes p53 in Response to Oncogene-Induced Senescence. Int. J. Biol. Sci. 2015, 11, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Lotem, J.; Kama, R.; Sachs, L.; Shaul, Y. NQO1 stabilizes p53 through a distinct pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 3099–3104. [Google Scholar] [CrossRef]
- Chiu, W.H.; Luo, S.-J.; Chen, C.-L.; Cheng, J.-H.; Hsieh, C.-Y.; Wang, C.-Y.; Huang, W.-C.; Su, W.-C.; Lin, C.-F. Vinca alkaloids cause aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA damage, mitochondrial dysfunction, and apoptosis in lung adenocarcinoma cells. Biochem. Pharmacol. 2012, 83, 1159–1171. [Google Scholar] [CrossRef]
- Sutton, K.M.; Doucette, C.D.; Hoskin, D.W. NADPH quinone oxidoreductase 1 mediates breast cancer cell resistance to thymoquinone-induced apoptosis. Biochem. Biophys. Res. Commun. 2012, 426, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.J.; Planchon, S.M.; Tagliarino, C.; Varnes, M.E.; Siegel, D.; Boothman, D.A. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J. Biol. Chem. 2000, 275, 5416–5424. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abuzaid, H.; Abdelrazig, S.; Ferreira, L.; Collins, H.M.; Kim, D.-H.; Lim, K.-H.; Kam, T.-S.; Turyanska, L.; Bradshaw, T.D. Apoferritin-Encapsulated Jerantinine A for Transferrin Receptor Targeting and Enhanced Selectivity in Breast Cancer Therapy. ACS Omega 2022, 7, 21473–21482. [Google Scholar] [CrossRef]

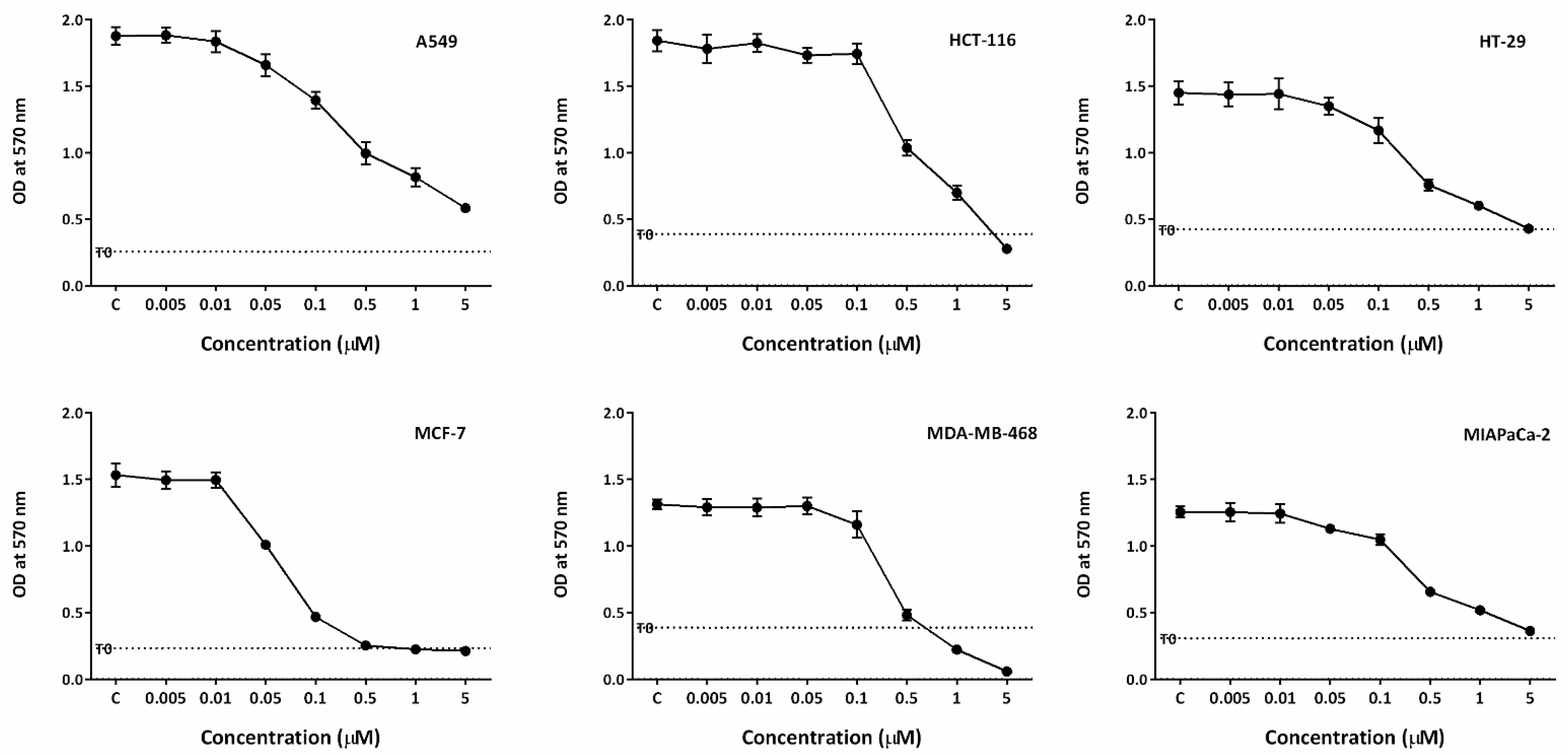





| Human Cell Line | 72 h Exposure MTT GI50 (μM) | ||||
|---|---|---|---|---|---|
| Origin | Designation | Conofolidine | Conophylline | Leucophyllidine | Bipleiophylline |
| Breast carcinoma | MCF-7 | 0.054 ± 0.08 | 0.066 ± 0.03 | 2.79 ± 0.98 | 3.49 ± 0.47 |
| MDA-MB-468 | 0.292 ± 0.03 | 0.557 ± 0.08 | 1.99 ± 0.03 | 5.81 ± 1.11 | |
| Colon carcinoma | HCT-116 | 0.454 ± 0.07 | 0.737 ± 0.27 | 2.43 ± 0.08 | 3.86 ± 1.04 |
| HT-29 | 0.301 ± 0.03 | 0.350 ± 0.52 | 2.48 ± 0.17 | 7.72 ± 4.08 | |
| Pancreatic carcinoma | MIAPaCa-2 | 0.305 ± 0.03 | 0.663 ± 0.03 | 3.29 ± 0.98 | 12.37 ± 3.12 |
| Lung carcinoma | A549 | 0.405 ± 0.07 | 1.743 ± 0.34 | 4.45 ± 0.27 | 13.63 ± 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hayali, M.Z.; Nge, C.-E.; Lim, K.H.; Collins, H.M.; Kam, T.-S.; Bradshaw, T.D. Conofolidine: A Natural Plant Alkaloid That Causes Apoptosis and Senescence in Cancer Cells. Molecules 2024, 29, 2654. https://doi.org/10.3390/molecules29112654
Al-Hayali MZ, Nge C-E, Lim KH, Collins HM, Kam T-S, Bradshaw TD. Conofolidine: A Natural Plant Alkaloid That Causes Apoptosis and Senescence in Cancer Cells. Molecules. 2024; 29(11):2654. https://doi.org/10.3390/molecules29112654
Chicago/Turabian StyleAl-Hayali, Mohammed Zuhair, Choy-Eng Nge, Kuan Hon Lim, Hilary M. Collins, Toh-Seok Kam, and Tracey D. Bradshaw. 2024. "Conofolidine: A Natural Plant Alkaloid That Causes Apoptosis and Senescence in Cancer Cells" Molecules 29, no. 11: 2654. https://doi.org/10.3390/molecules29112654
APA StyleAl-Hayali, M. Z., Nge, C.-E., Lim, K. H., Collins, H. M., Kam, T.-S., & Bradshaw, T. D. (2024). Conofolidine: A Natural Plant Alkaloid That Causes Apoptosis and Senescence in Cancer Cells. Molecules, 29(11), 2654. https://doi.org/10.3390/molecules29112654






