Facilitated Unidirectional Electron Transmission by Ru Nano Particulars Distribution on MXene Mo2C@g-C3N4 Heterostructures for Enhanced Photocatalytic H2 Evolution
Abstract
1. Introduction
2. Results and Discussion
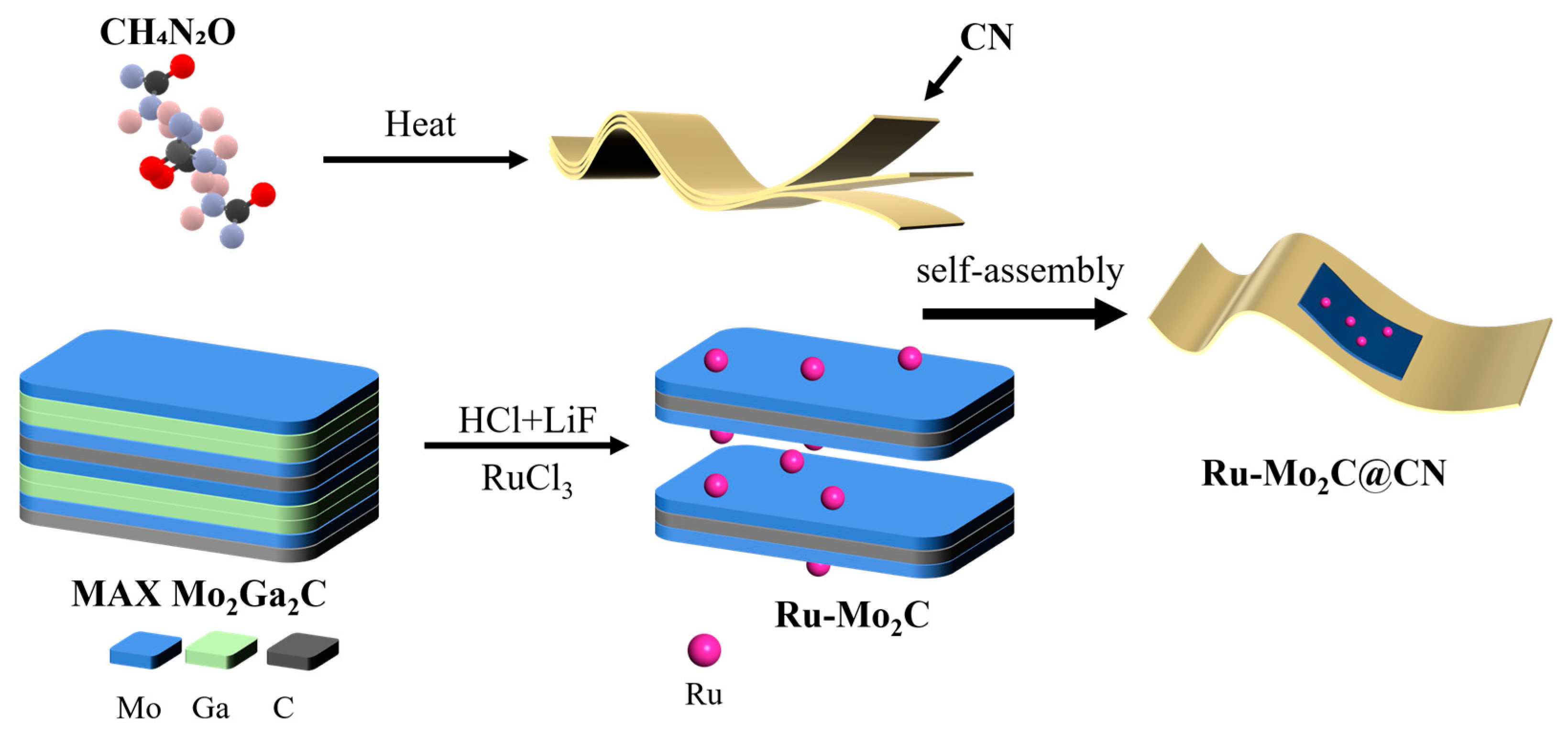
2.1. Construction Strategy of Ru-Mo2C@CN
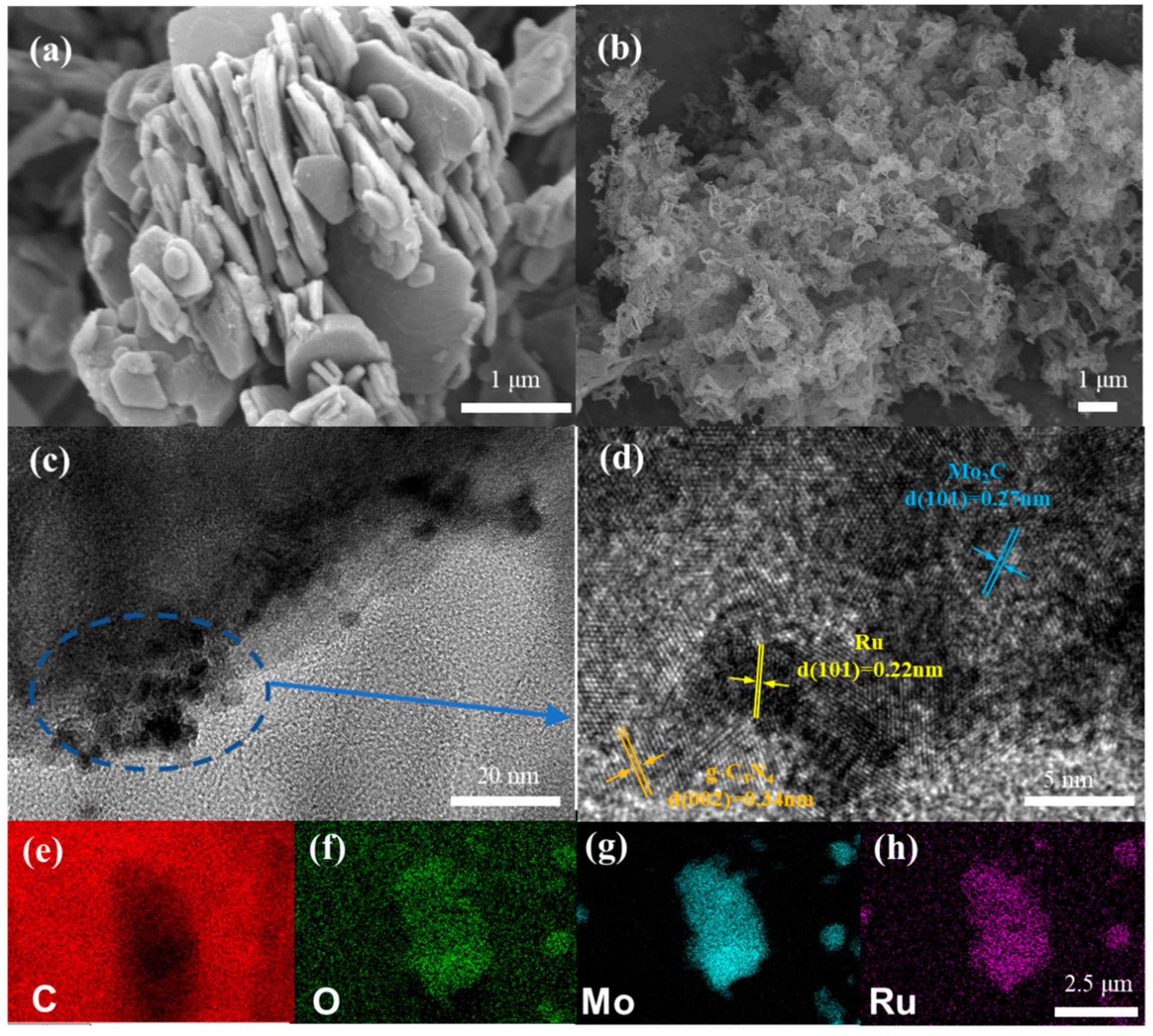
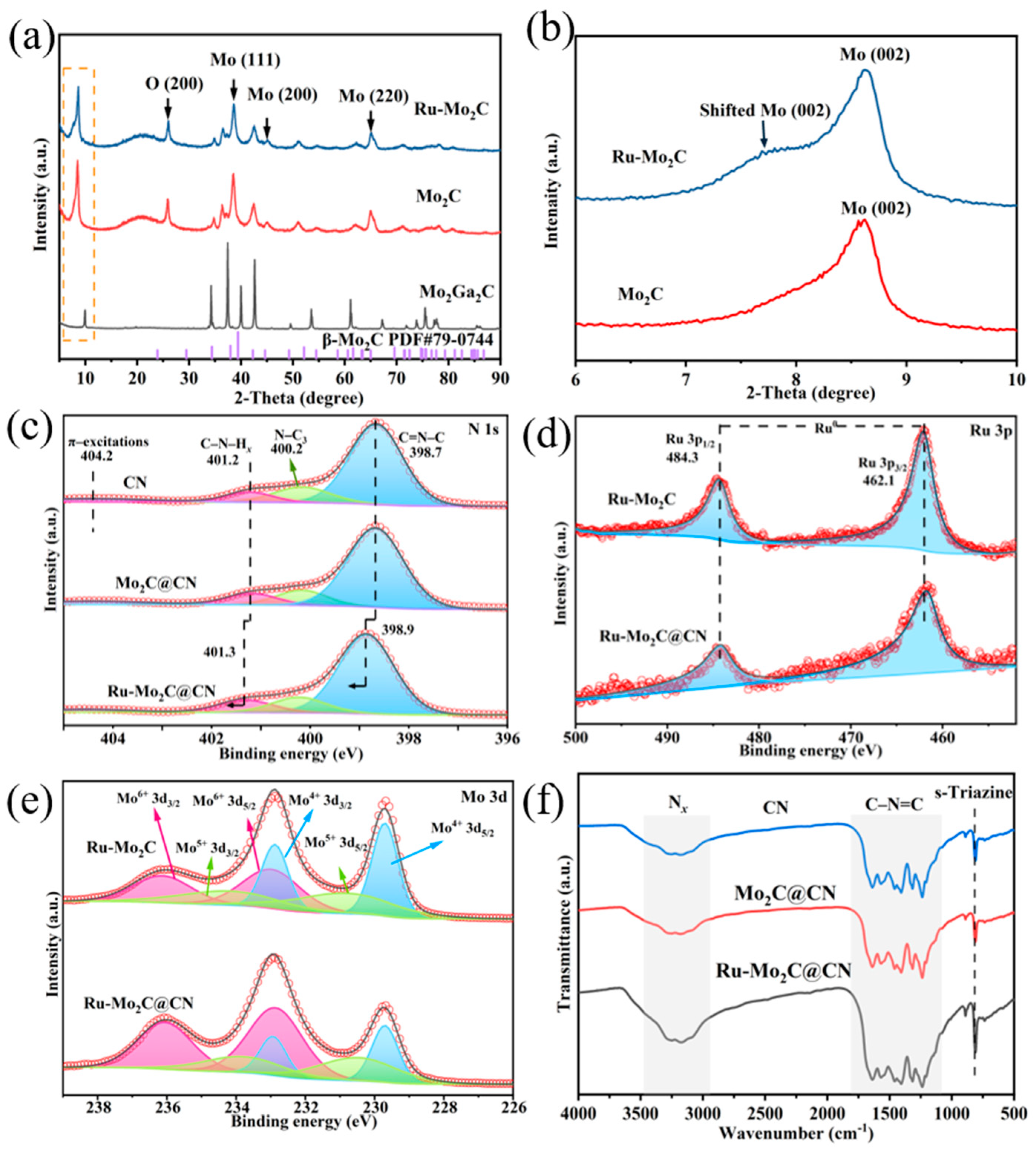
2.2. Synthesis and Structural Morphology of Ru-Mo2C@CN
2.3. Photocatalytic Performance
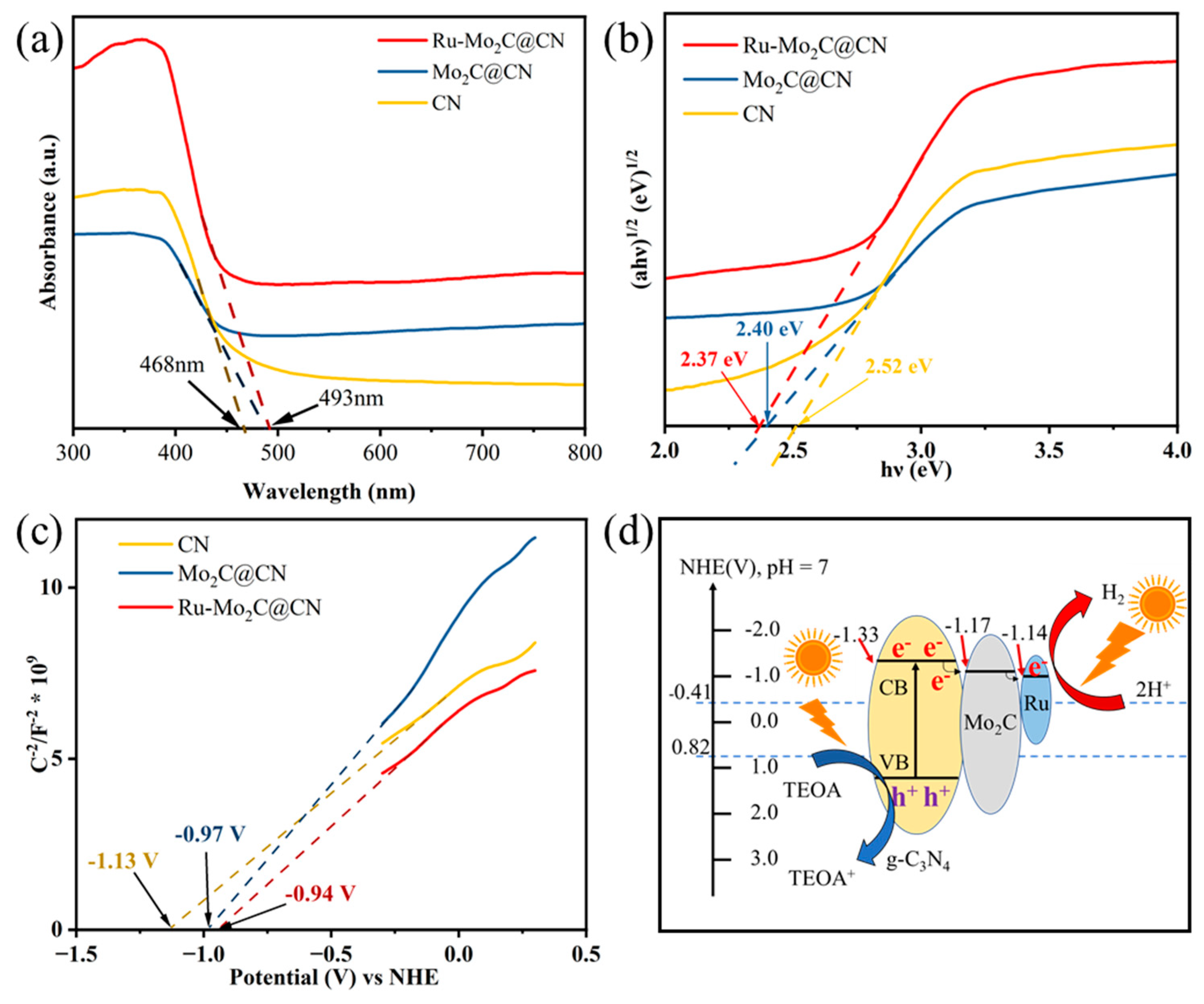
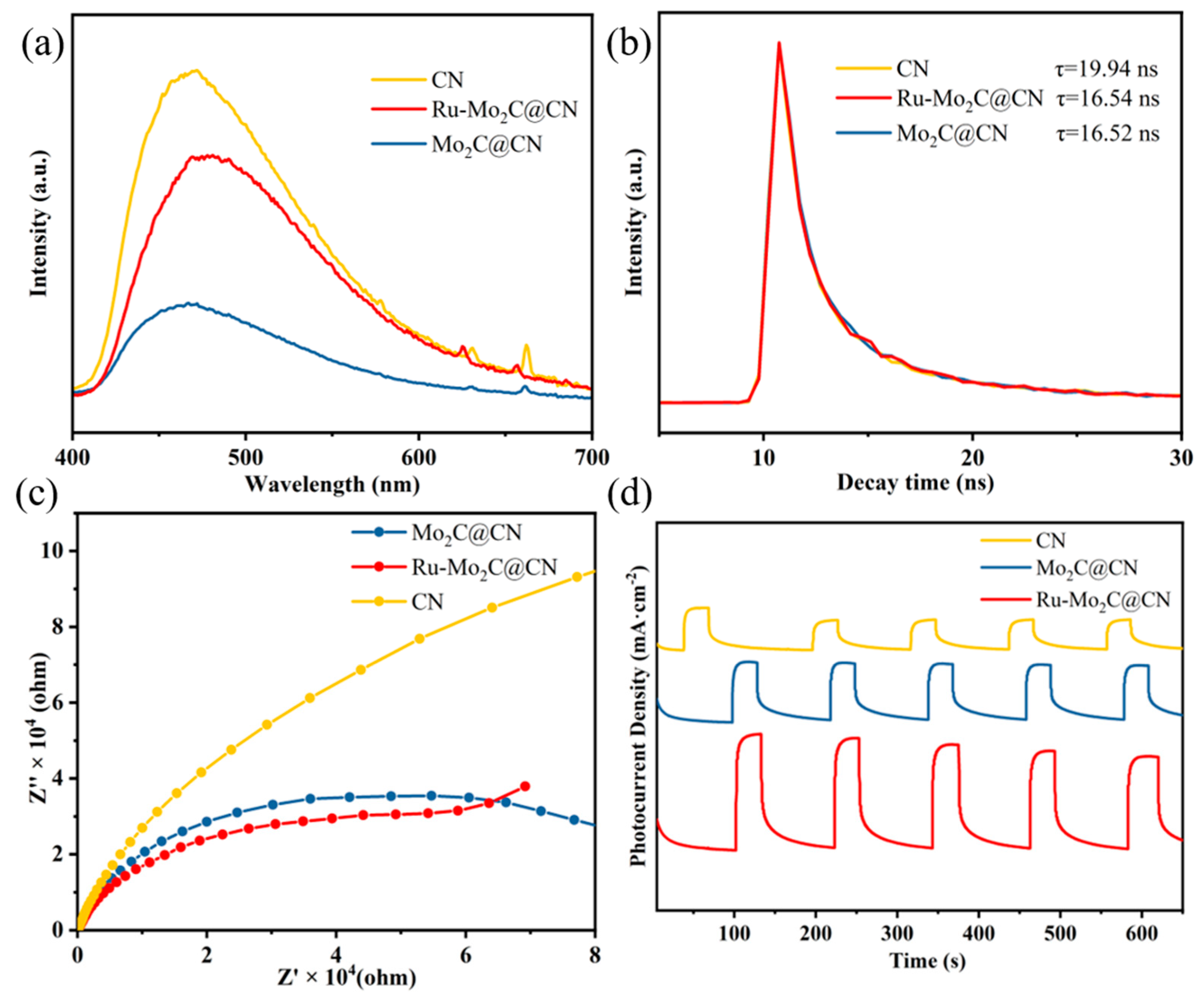
2.4. Photocatalytic Mechanism Analysis
3. Experimental Section
3.1. Materials
3.2. Preparation of Photocatalysts
3.2.1. Preparation of 2D g-C3N4 Nanosheets (CNs)
3.2.2. Preparation of MXene Mo2C (Mo2C) and Ru-Doped Mo2C (Ru-Mo2C)
3.2.3. Preparation of Bulk Mo2C
3.2.4. Preparation of MXene Mo2C@CN, Bulk Mo2C@CN, and Ru-Mo2C@CN Composites
3.3. Characterization and Experimentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hisatomi, T.; Domen, K. Reaction Systems for Solar Hydrogen Production Via Water Splitting with Particulate Semiconductor Photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent Advances in Metal Sulfides: From Controlled Fabrication to Electrocatalytic, Photocatalytic and Photoelectrochemical Water Splitting and Beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Zheng, X.-L.; Yang, Y.-J.; Liu, Y.-H.; Deng, P.-L.; Li, J.; Liu, W.-F.; Rao, P.; Jia, C.-M.; Huang, W.; Du, Y.-L.; et al. Fundamentals and Photocatalytic Hydrogen Evolution Applications of Quaternary Chalcogenide Semiconductor: Cu2ZnSnS4. Rare Met. 2022, 41, 2153–2168. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Z.; Wang, X. Crystalline Carbon Nitride Semiconductors for Photocatalytic Water Splitting. Angew. Chem. Int. Ed. Engl. 2019, 58, 6164–6175. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, B.; Xiong, L.; Bi, J.; Hao, H.; Yu, X.; Li, C.; Liu, J.; Yang, S. Boosting Photocatalytic Hydrogen Evolution of g-C3N4 Catalyst Via Lowering the Fermi Level of Co-Catalyst. Nano Res. 2021, 15, 1128–1134. [Google Scholar] [CrossRef]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors Vs True Water Splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef]
- Chun, W.-J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and Valence Band Positions of Ta2O5, TaON, and Ta3N5 by Ups and Electrochemical Methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Qiu, J.-Y.; Feng, H.-Z.; Chen, Z.-H.; Ruan, S.-H.; Chen, Y.-P.; Xu, T.-T.; Su, J.-Y.; Ha, E.-N.; Wang, L.-Y. Selective Introduction of Surface Defects in Anatase TiO2 Nanosheets for Highly Efficient Photocatalytic Hydrogen Generation. Rare Met. 2022, 41, 2074–2083. [Google Scholar] [CrossRef]
- Sun, L.-J.; Su, H.-W.; Xu, D.-F.; Wang, L.-L.; Tang, H.; Liu, Q.-Q. Carbon Hollow Spheres as Cocatalyst of Cu-Doped TiO2 Nanoparticles for Improved Photocatalytic H2 Generation. Rare Met. 2022, 41, 2063–2073. [Google Scholar] [CrossRef]
- Ma, Z.; Li, P.; Ye, L.; Wang, L.; Xie, H.; Zhou, Y. Selectivity Reversal of Photocatalytic CO2 Reduction by Pt Loading. Catal. Sci. Technol. 2018, 8, 5129–5132. [Google Scholar] [CrossRef]
- Yi, S.-S.; Zhang, X.-B.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Non-Noble Metals Applied to Solar Water Splitting. Energy Environ. Sci. 2018, 11, 3128–3156. [Google Scholar] [CrossRef]
- Wang, P.; Zong, L.; Guan, Z.; Li, Q.; Yang, J. Ptni Alloy Cocatalyst Modification of Eosin Y-Sensitized g-C3N4/Go Hybrid for Efficient Visible-Light Photocatalytic Hydrogen Evolution. Nanoscale Res. Lett. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiong, L.; Bi, J.; Zhang, X.; Yang, G.; Yang, S. Structural and Electronic Stabilization of Ptni Concave Octahedral Nanoparticles by P Doping for Oxygen Reduction Reaction in Alkaline Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 27009–27018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, Y.; Jian, G.; Tao, H.; Wang, X.; Fan, Y.; Lu, Y.; Hu, Z.; Chen, Y. Facile Construction of Pt-Co/CNx Nanotube Electrocatalysts and Their Application to the Oxygen Reduction Reaction. Adv. Mater. 2009, 21, 4953–4956. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yun, Q.; Tu, S.; Zhu, L.; Cao, W.; Lu, Q. Metallic Ruthenium-Based Nanomaterials for Electrocatalytic and Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 24691–24714. [Google Scholar] [CrossRef]
- Xing, B.; Wang, T.; Zheng, Z.; Liu, S.; Mao, J.; Li, C.; Li, B. Synchronous Fabrication of Ru Single Atoms and RuO2 on Hierarchical TiO2 Spheres for Enhanced Photocatalytic Coproduction of H2 and Benzaldehyde. Chem. Eng. J. 2023, 461, 141871. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, N.N.; Zhang, C.; Sun, N.; Pan, Y.; Chen, C.; Li, J.; Tan, M.; Cui, R.; Shi, Z.; et al. Doping Ruthenium into Metal Matrix for Promoted Ph-Universal Hydrogen Evolution. Adv. Sci. 2022, 9, e2200010. [Google Scholar] [CrossRef]
- Boronat, M.; Leyva-Perez, A.; Corma, A. Theoretical and Experimental Insights into the Origin of the Catalytic Activity of Subnanometric Gold Clusters: Attempts to Predict Reactivity with Clusters and Nanoparticles of Gold. Acc. Chem. Res. 2014, 47, 834–844. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically Dispersed Supported Metal Catalysts. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M. Synergistic Effect of Ru Embedded 2D Ti3AlC2 Binary Cocatalyst with Porous g-C3N4 to Construct 2D/2D Ru-MAX/PCN Heterojunction for Enhanced Photocatalytic H2 Production. Mater. Res. Bull. 2021, 144, 111493. [Google Scholar] [CrossRef]
- Yi, W.-J.; Du, X.; Zhang, M.; Yi, S.-S.; Xia, R.-H.; Li, C.-Q.; Liu, Y.; Liu, Z.-Y.; Zhang, W.-L.; Yue, X.-Z. Rational Distribution of Ru Nanodots on 2D Ti3−XC2Ty/g-C3N4 Heterostructures for Boosted Photocatalytic H2 Evolution. Nano Res. 2023, 16, 6652–6660. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous Graphitic-C3N4@Carbon Metal-Free Electrocatalysts for Highly Efficient Oxygen Reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Jiang, L.; Zhang, J.; Yu, H.; Wang, H.; Zeng, G. Powerful Combination of 2D g-C3N4 and 2D Nanomaterials for Photocatalysis: Recent Advances. Chem. Eng. J. 2020, 390, 124475. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Xu, X.; Kang, Y.; Henzie, J.; Que, W.; Yamauchi, Y. Mxene Nanoarchitectonics: Defect-Engineered 2D Mxenes Towards Enhanced Electrochemical Water Splitting. Adv. Energy Mater. 2022, 12, 2103867. [Google Scholar] [CrossRef]
- Ou, M.; Tu, W.; Yin, S.; Xing, W.; Wu, S.; Wang, H.; Wan, S.; Zhong, Q.; Xu, R. Amino-Assisted Anchoring of CsPbBr3 Perovskite Quantum Dots on Porous g-C3N4 for Enhanced Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. Engl. 2018, 57, 13570–13574. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, D.; Wang, R.; Zhang, Z.; Qiu, S. 2D/2D Interface Engineering Promotes Charge Separation of Mo2C/g-C3N4 Nanojunction Photocatalysts for Efficient Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2022, 14, 31782–31791. [Google Scholar] [CrossRef]
- Gao, L.; Liu, J.; Long, H.; Wang, P.; Yu, H. One-Step Calcination Synthesis of WC–Mo2C Heterojunction Nanoparticles as Novel H2-Production Cocatalysts for Enhanced Photocatalytic Activity of TiO2. Catal. Sci. Technol. 2021, 11, 7307–7315. [Google Scholar] [CrossRef]
- Kim, S.; Choi, C.; Hwang, J.; Park, J.; Jeong, J.; Jun, H.; Lee, S.; Kim, S.K.; Jang, J.H.; Jung, Y.; et al. Interaction Mediator Assisted Synthesis of Mesoporous Molybdenum Carbide: Mo-Valence State Adjustment for Optimizing Hydrogen Evolution. ACS Nano 2020, 14, 4988–4999. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Fan, J.; Yu, H.; Yu, J. Hetero-Phase MoC-Mo2C Nanoparticles for Enhanced Photocatalytic H2-Production Activity of TiO2. Nano Res. 2020, 14, 1095–1102. [Google Scholar] [CrossRef]
- Pan, M.; Wang, P.; Wang, X.; Chen, F.; Yu, H. Weakening Mo–H Bond of Mo2C Mxene Cocatalyst by Increased Antibonding-Orbital Occupancy State for Superior Photocatalytic Hydrogen Production. ACS Sustain. Chem. Eng. 2023, 11, 13222–13231. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Bo, T.; Chai, Z.; Gibson, J.K.; Shi, W. Boosting Hydrogen Evolution in Neutral Medium by Accelerating Water Dissociation with Ru Clusters Loaded on Mo2CTx Mxene. Adv. Funct. Mater. 2023, 33, 2214375. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface Charge Modification Via Protonation of Graphitic Carbon Nitride (g-C3N4) for Electrostatic Self-Assembly Construction of 2D/2D Reduced Graphene Oxide (rGO)/g-C3N4 Nanostructures toward Enhanced Photocatalytic Reduction of Carbon Dioxide to Methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Lei, C.; Zhou, W.; Shen, L.; Zheng, X.; Feng, Q.; Liu, Y.; Lei, Y.; Liang, S.; Zhang, D.; Jiang, L.; et al. Enhanced Selective H2S Oxidation Performance on Mo2C-Modified g-C3N4. ACS Sustain. Chem. Eng. 2019, 7, 16257–16263. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Li, H.; Cai, P.; Li, Y.; Wen, Z. Ru-RuO2/CNT Hybrids as High-Activity PH-Universal Electrocatalysts for Water Splitting within 0.73 V in an Asymmetric-Electrolyte Electrolyzer. Nano Energy 2019, 61, 576–583. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, T.; Zheng, Z.; Xing, B.; Li, C.; Li, B. Constructing Interfacial Active Sites in Ru/g-C3N4−X Photocatalyst for Boosting H2 Evolution Coupled with Selective Benzyl-Alcohol Oxidation. Appl. Catal. B Environ. 2022, 315, 121575. [Google Scholar] [CrossRef]
- Zhao, Y.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. In Situ CO2-Emission Assisted Synthesis of Molybdenum Carbonitride Nanomaterial as Hydrogen Evolution Electrocatalyst. J. Am. Chem. Soc. 2015, 137, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine Molybdenum Carbide Nanoparticles Composited with Carbon as a Highly Active Hydrogen-Evolution Electrocatalyst. Angew. Chem. Int. Ed. Engl. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Alex, C.; Jana, R.; Ramakrishnan, V.; Naduvil Kovilakath, M.S.; Datta, A.; John, N.S.; Tayal, A. Probing the Evolution of Active Sites in MoO2 for Hydrogen Generation in Acidic Medium. ACS Appl. Energy Mater. 2023, 6, 5342–5351. [Google Scholar] [CrossRef]
- Borgschulte, A.; Sambalova, O.; Delmelle, R.; Jenatsch, S.; Hany, R.; Nuesch, F. Hydrogen Reduction of Molybdenum Oxide at Room Temperature. Sci. Rep. 2017, 7, 40761. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ma, D.; Sun, D.; Mao, S.; He, C.; Wang, Z.; Ji, X.; Shi, J.-W. Carbon Nanosheet Facilitated Charge Separation and Transfer between Molybdenum Carbide and Graphitic Carbon Nitride toward Efficient Photocatalytic H2 Production. Appl. Surf. Sci. 2019, 473, 91–101. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, Y.; Luo, Z.; Li, H.; Chen, L.; Cao, X.; Wei, S.; Zhou, B.; Zhang, Z.; Chen, S.; et al. Water Induced Ultrathin Mo2C Nanosheets with High-Density Grain Boundaries for Enhanced Hydrogen Evolution. Nat. Commun. 2022, 13, 7225. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Song, H.; Han, F.; Wei, L. Photocatalytic Oxidative Desulfurization and Denitrogenation for Fuels in Ambient Air over Ti3C2/g-C3N4 Composites under Visible Light Irradiation. Appl. Catal. B Environ. 2020, 269, 118845. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. 2D/2D Heterojunction of Ti3C2/g-C3N4 Nanosheets for Enhanced Photocatalytic Hydrogen Evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zong, S.; Shi, J.; Xue, F.; Zhang, Y.; Guan, X.; Zheng, B.; Deng, J.; Guo, L. Facile Preparation of Nanosized Mop as Cocatalyst Coupled with g-C3N4 by Surface Bonding State for Enhanced Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2020, 265, 118620. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, W.; Du, L.; Zhu, J.; Phillips, D.L.; Xu, J. Interpreting the Enhanced Photoactivities of 0d/1d Heterojunctions of CdS Quantum Dots/TiO2 Nanotube Arrays Using Femtosecond Transient Absorption Spectroscopy. Appl. Catal. B Environ. 2020, 275, 119151. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Liu, E.; Fan, J.; Hu, X. Facile Strategy to Construction Z-Scheme ZnCo2O4/g-C3N4 Photocatalyst with Efficient H2 Evolution Activity. Appl. Surf. Sci. 2020, 515, 146039. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Zhou, Y.; Yu, Y.; Li, L.; Hao, S.; Liu, B. ZIF-8 Derived Carbon (C-ZIF) as a Bifunctional Electron Acceptor and Her Cocatalyst for g-C3N4: Construction of a Metal-Free, All Carbon-Based Photocatalytic System for Efficient Hydrogen Evolution. J. Mater. Chem. A 2016, 4, 3822–3827. [Google Scholar] [CrossRef]
- Che, H.; Liu, C.; Che, G.; Liao, G.; Dong, H.; Li, C.; Song, N.; Li, C. Facile Construction of Porous Intramolecular g-C3N4-Based Donor-Acceptor Conjugated Copolymers as Highly Efficient Photocatalysts for Superior H2 Evolution. Nano Energy 2020, 67, 104273. [Google Scholar] [CrossRef]
- Boppella, R.; Yang, W.; Tan, J.; Kwon, H.-C.; Park, J.; Moon, J. Black Phosphorus Supported Ni2P Co-Catalyst on Graphitic Carbon Nitride Enabling Simultaneous Boosting Charge Separation and Surface Reaction. Appl. Catal. B Environ. 2019, 242, 422–430. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Y.; Luo, J.; Jin, B.; Wu, Z.; Ning, X.; Zhan, L.; Fan, X.; Zhou, T.; Zhang, S.; et al. MoC Quantum Dots@N-Doped-Carbon for Low-Cost and Efficient Hydrogen Evolution Reaction: From Electrocatalysis to Photocatalysis. Adv. Funct. Mater. 2022, 32, 2201518. [Google Scholar] [CrossRef]
- Huang, Z.; Long, X.; Liu, M.; Li, X.; Du, Y.; Liu, Q.; Chen, Y.; Guo, S.; Chen, R. Constructing CoP-C/g-C3N4 Nanocomposites with P-C Bond Bridged Interface and Van Der Waals Heterojunctions for Enhanced Photocatalytic H2 Evolution. J. Colloid Interface Sci. 2024, 653, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Shi, Y.; Huang, C.; Wu, Q.; Zeng, T.; Yao, W. A New and stable Mo-Mo2C modified g-C3N4 photocatalyst for efficient visible light photocatalytic H2 production. Appl. Catal. B Environ. 2019, 243, 27–35. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; He, B.; Wang, R.; Wang, H.; Gong, Y. Facile synthesis of rod-like g-C3N4 by decorating Mo2C co-catalyst for enhanced visible-light photocatalytic activity. Applied Surface Science 2019, 470, 565–572. [Google Scholar] [CrossRef]
- Liu, R.-Y.; Ding, L.; Yang, G.-D.; Zhang, J.-Y.; Jiao, R.; Sun, H.-Z. Hollow Mo2C nanospheres modified B-doped g-C3N4 for high efficient photocatalysts. J. Phys. D Appl. Phys. 2022, 55, 454001. [Google Scholar] [CrossRef]
- Du, J.; Shen, Y.; Yang, F.; Zhang, B.; Jiang, X.; An, C.; Ye, J. In situconstruction of an α-Mo2C/g-C3N4 Mott–Schottky heterojunction with high-speed electron transfer channel for efficient photocatalytic H2 evolution. Inorg. Chem. Front. 2023, 10, 832–840. [Google Scholar] [CrossRef]
- Song, Y.; Xia, K.; Gong, Y.; Chen, H.; Li, L.; Yi, J.; She, X.; Chen, Z.; Wu, J.; Li, H.; et al. Controllable synthesized heterostructure photocatalyst Mo2C@C/2D g-C3N4: Enhanced catalytic performance for hydrogen production. Dalton Trans. 2018, 47, 14706–14712. [Google Scholar] [CrossRef]
- Tan, X.Q.; Zhang, P.; Chen, B.; Mohamed, A.R.; Ong, W.J. Synergistic effect of dual phase cocatalysts: MoC-Mo2C quantum dots anchored on g-C3N4 for high-stability photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2024, 662, 870–882. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Huang, Z.; Liu, M.; Li, X.; Du, Y.; Chen, X.; Ding, D.; Yang, S.; Chen, Y.; Chen, R. Facilitated Unidirectional Electron Transmission by Ru Nano Particulars Distribution on MXene Mo2C@g-C3N4 Heterostructures for Enhanced Photocatalytic H2 Evolution. Molecules 2024, 29, 1684. https://doi.org/10.3390/molecules29071684
Chen Q, Huang Z, Liu M, Li X, Du Y, Chen X, Ding D, Yang S, Chen Y, Chen R. Facilitated Unidirectional Electron Transmission by Ru Nano Particulars Distribution on MXene Mo2C@g-C3N4 Heterostructures for Enhanced Photocatalytic H2 Evolution. Molecules. 2024; 29(7):1684. https://doi.org/10.3390/molecules29071684
Chicago/Turabian StyleChen, Qiuyu, Zonghan Huang, Meng Liu, Xiaoping Li, Yuxuan Du, Xiaobao Chen, Dahu Ding, Shengjiong Yang, Yang Chen, and Rongzhi Chen. 2024. "Facilitated Unidirectional Electron Transmission by Ru Nano Particulars Distribution on MXene Mo2C@g-C3N4 Heterostructures for Enhanced Photocatalytic H2 Evolution" Molecules 29, no. 7: 1684. https://doi.org/10.3390/molecules29071684
APA StyleChen, Q., Huang, Z., Liu, M., Li, X., Du, Y., Chen, X., Ding, D., Yang, S., Chen, Y., & Chen, R. (2024). Facilitated Unidirectional Electron Transmission by Ru Nano Particulars Distribution on MXene Mo2C@g-C3N4 Heterostructures for Enhanced Photocatalytic H2 Evolution. Molecules, 29(7), 1684. https://doi.org/10.3390/molecules29071684








