Citrus limon Wastes from Part of the Eastern Cape Province in South Africa: Medicinal, Sustainable Agricultural, and Bio-Resource Potential
Abstract
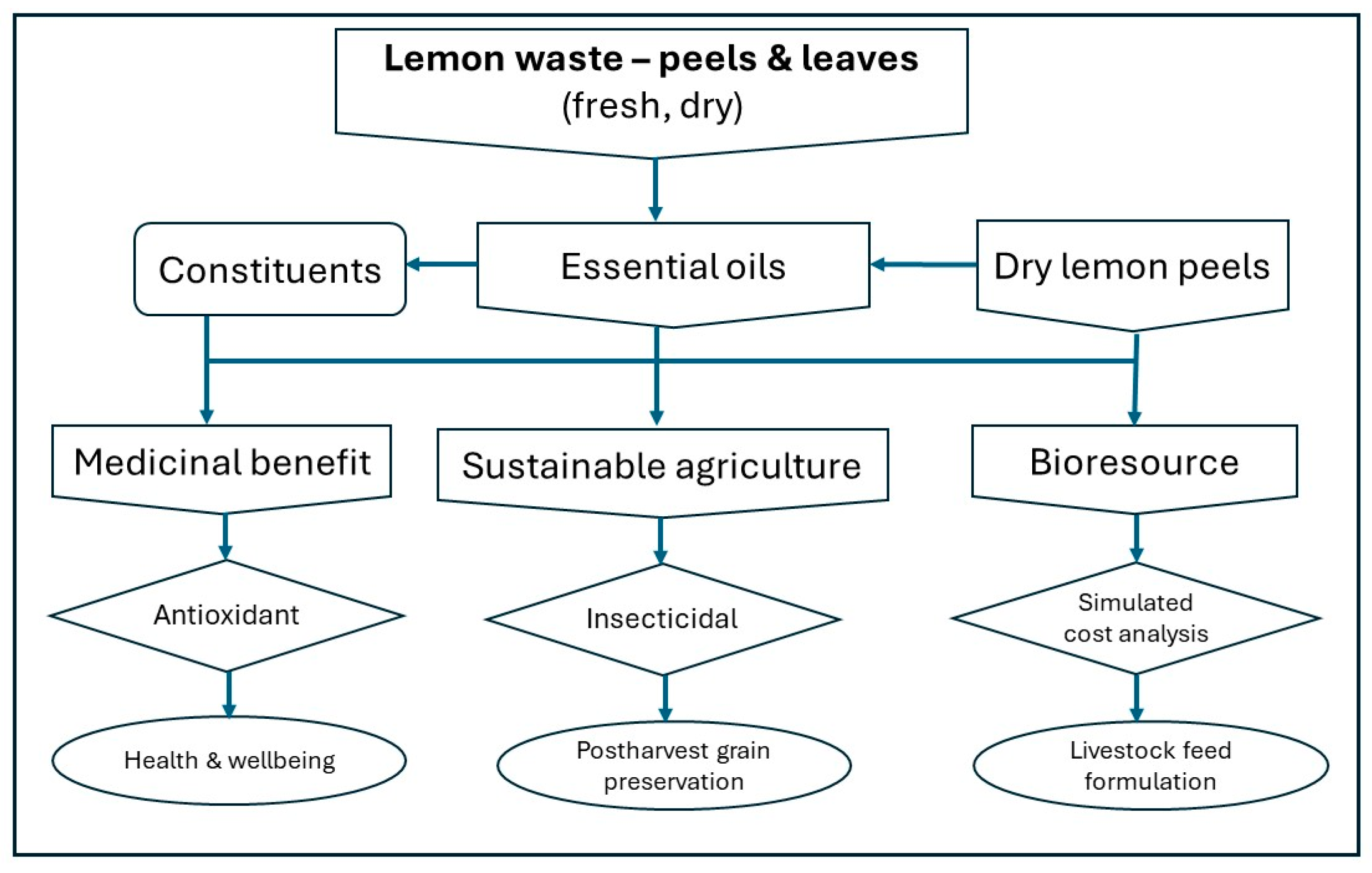
1. Introduction
2. Results
2.1. Physicochemical Properties of C. limon Oils
2.2. GC/MS Analysis of EOs of C. limon Leaves and Peel
2.3. Antioxidant Activity of C. limon Essential Oils
2.3.1. Ferric-Reducing Power
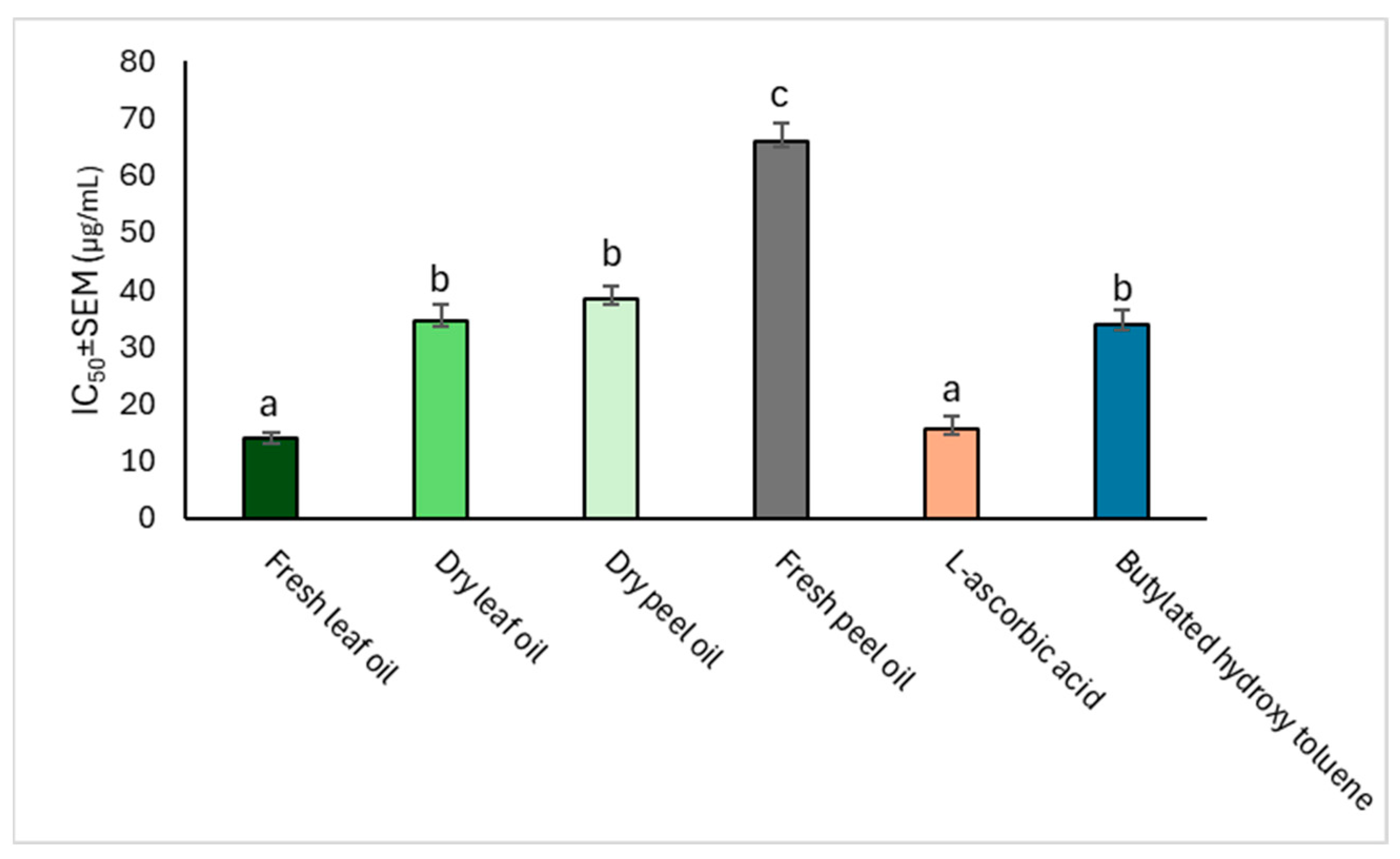
2.3.2. DPPH Radical-Scavenging Potential
2.4. Insecticidal Properties of C. limon Oils against Sitophilus zeamais
2.4.1. Repellent Activity
2.4.2. Fumigant Toxicity
2.4.3. Contact Toxicity
2.5. Agricultural and Socio-Economic Benefits of C. limon Peel
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of EOs from C. limon Leaves and Peel
4.3. GC and GC/MS Analysis of EOs
4.4. Antioxidant Analysis of C. limon EOs
4.4.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.4.2. DPPH Radical Scavenging Assay
4.5. Insecticidal Study on C. limon Leaf and Peel EOs
4.5.1. Test Insects
4.5.2. Repellent Assay
4.5.3. Fumigant Assay
4.5.4. Contact Toxicity Test
4.6. Statistical Analysis
4.7. Simulation of Sustainable Agricultural and Socio-Economic Benefits of Lemon Peel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Mehl, F.; Marti, G.; Boccard, J.; Debroux, B.; Raymo, V.; Velazco, M.I.; Sommer, H.; Wolfender, J.; Rudaz, S. Differentiation of lemon essential oil based on volatile and non-volatile fractions with various analytical techniques: A metabolomics approach. Food Chem. 2014, 143, 325–335. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The second life of citrus fruit waste: A valuable source of bioactive compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmental friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Kawaii, S.; Yasuhiko, T.; Eriko, K.; Kazunori, O.; Masamichi, Y.; Meisaku, K.; Chihiro, I.; Hiroshi, F. Quantitative study of flavonoids in leaves of Citrus plants. J. Agric. Food Chem. 2000, 48, 3865–3871. [Google Scholar] [CrossRef]
- Mohanapriya, M. Health and medicinal properties of lemon (Citrus limonum). Int. J. Herb. Med. 2013, 3, 1095–1100. [Google Scholar]
- Rozza, A.L.; Moraes, T.M.; Kushima, H.; Tanimoto, A.; Marques, M.O.M.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus limon (Rutaceae) essential oil and its majority compounds limonene and α-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef]
- Mahalwal, V.S.; Ali, M. Volatile constituents of the fruits peels of C. limon (Linn) Burm. J. Essent. Oil Bear. Plants 2003, 6, 31–35. [Google Scholar] [CrossRef]
- Al-Jabri, N.N.; Hossain, M.A. Comparative chemical composition and antimicrobial activity study of essential oils from two imported lemon fruits samples against pathogenic bacteria. Aust. J. Basic Appl. Sci. 2014, 3, 247–253. [Google Scholar]
- Bialon, M.; Krzysko-Lupicka, T.; Koszalkowska, M.; Wieczorek, P.P. The Influence of chemical composition of commercial lemon essential oils on the growth of Candida strains. Mycopathologia 2014, 177, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior antibacterial activity of integral lemon pectin extracted via hydrodynamic cavitation. Chem. Open 2020, 9, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhai, Y.; Ou, M.; Bian, Y.; Tang, C.; Zhang, W.; Cheng, Y.; Li, G. Protective effect of lemon peel extract on oxidative stress in H9c2 rat heart cell injury. Drug Des. Dev. Ther. 2021, 15, 2047–2058. [Google Scholar] [CrossRef]
- Maruti, J.D.; Chidamber, B.J.; Jai, S.G.; Kailash, D.S. Study antimicrobial activity of lemon (Citrus limon L.) Peel extract. Br. J. Pharmacol. Toxicol. 2011, 2, 119–122. [Google Scholar]
- Mathew, B.B.; Jatawa, S.K.; Tiwari, A. Phytochemical analysis of Citrus limonum pulp and peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 269–371. [Google Scholar]
- Chuku, L.C.; Chinak, N.C. Protein and mineral element levels of some fruit juices (Citrus spp.) in some Niger Delta areas of Nigeria. Int. J. Food Sci. Nutr. 2014, 3, 58–60. [Google Scholar]
- Otang, V.M.; Afolayan, A.J. Antimicrobial and antioxidant efficacy of Citrus limon L. peel extracts used for skin diseases by Xhosa tribe of Amathole District, Eastern Cape. S. Afr J. Bot. 2016, 102, 46–49. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Nassrallah, A.A.; Abdel-Raheem, M.A.; Elbehery, H.H. Lemon peel essential oil and its nano-formulation to control Agrotis ipsilon (Lepidoptera: Noctuidae). Sci. Rep. 2023, 13, 17922. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.L., Jr.; Ammerman, C.B.; Baker, F.S., Jr.; Hentges, J.F.; Hayes, B.W.; Cunha, T.J. Citrus Feeds for Beef Cattle, Series Paper; Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2000. [Google Scholar]
- Grigelmo-Miguel, N.; Martın-Belloso, O. Characterization of dietary fiber from orange juice extraction. Food Res. Int. 1998, 31, 355–361. [Google Scholar] [CrossRef]
- Khan, N.; le Roes-Hill, M.; Welz, P.J.; Grandin, K.A.; Kudanga, T.; Van Dyk, J.S.; Ohlhoff, C.; Van Zyl, W.H.E.; Pletschke, B.I. Fruit waste streams in South Africa and their potential role in developing a bio-economy. S. Afr. J. Sci. 2015, 111, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AgNet Media, Citrus Industry: Leading Citrus Producers of the World. 14 February 2023. Available online: https://citrusindustry.net/2023/02/14/leading-citrus-producers-of-the-world/ (accessed on 25 February 2024).
- Global Agriculture Information Network Report (GAIN Report). South Africa Citrus Supply and Demand Report (USDA Foreign Agricultural Service). 2023. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Citrus%20Annual_Pretoria_South%20Africa%20-%20Republic%20of_SF2023-0047.pdf (accessed on 27 February 2024).
- Global Agriculture Information Network Report (GAIN Report). South Africa Citrus Supply and Demand Report (USDA Foreign Agricultural Service). 2016. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Citrus%20Annual_Pretoria_South%20Africa%20-%20Republic%20of_12-15-2016.pdf (accessed on 20 September 2017).
- Marriot, P.J.; Shellie, R.; Cornwell, C. Gas chromatographic technologies for the analysis of essential oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Denett, G.O.; Comelli, N.C.; Rodriguez, M.R.; Gómez, A.d.l.A.; Matías, M.d.H.S.; Sampietro, D.A. Chemical composition and insecticidal activity of essential oils from cultivated and native aromatic plants of Argentina against Carpophilus dimidiatus (Fabricius) (Nitidulidae) and Oryzaephilus mercator (L.) (Silvanidae). Nat. Prod. Res. 2023, 37, 4058–4062. [Google Scholar] [CrossRef] [PubMed]
- Bateki, C.A.; Dickhoefer, U. Evaluation of the modified livestock simulator for stall-fed dairy cattle in the tropics. Animals 2020, 10, 816. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lv, S.; Xu, J.G.; Zhang, L.F. Influence of drying methods on chemical compositions, antioxidant and antibacterial activity of essential oil from lemon peel. Nat. Prod. Res. 2018, 32, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Effect of drying on the yield and chemical composition of essential oils obtained from Mentha longifolia leaves. MOJ Food Process. Technol. 2020, 8, 67–69. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef]
- Ashafa, A.O.T.; Grierson, D.S.; Afolayan, A.J. Effects of drying methods on the chemical composition of essential oil from Felicia muricata leaves. Asian J. Plant Sci. 2008, 7, 603–606. [Google Scholar] [CrossRef]
- Sandra, B.G.; Milojevic, S.Z.; Dimitrijevic, S.I.; Orlovic, A.M.; Skala, D.U. Antimicrobial activity of the essential oil and different fractions of Juniperus communis L and a comparison with some commercial antibiotics. J. Serbian Chem. Soc. 2007, 72, 311–320. [Google Scholar]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene oxide, the active compound isolated from leaves of Hymenaea courbaril L. (Fabaceae) with antiproliferative and apoptotic effects on PC-3 androgen-independent prostate cancer cell line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Alam, W.; Ahmed, I.; Ali, M.; Khan, F.; Khan, H. Chapter 8—Neuroprotective effect of terpenoids. In Phytonutrients and Neurological Disorders; Khan, H., Aschner, M., Mirzaei, H., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 227–244. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Himed, L.; Merniz, S.; Monteagudo-Olivan, R.; Barkat, M.; Coronas, J. Antioxidant activity of the essential oil of Citrus limon before and after its encapsulation in amorphous SiO2. Sci. Afr. 2019, 6, e00181. [Google Scholar] [CrossRef]
- Hollingsworth, R.G. Limonene: A citrus extract, for control of mealybugs and scale insects. J. Econ. Entomol. 2005, 98, 772–779. [Google Scholar] [CrossRef]
- Swamy, C.T.; Sivadurga, K.; Reddy, M.P.; Marimuthu, G.; Prashantkumar, C.S.; Premkumar, C.; Purewal, S.S. Citrus Waste: A Treasure of Promised Phytochemicals and Its Nutritional-Nutraceutical Value in Health Promotion and Industrial Applications; Singh, P.S., Punia, B.S., Kaur, P., Eds.; Recent Advances in Citrus Fruits; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Miya, G.M.; Oyemitan, I.A.; Oyedeji, O.O.; Oluwafemi, S.O.; Nkeh-Chungag, B.N.; Songca, S.P.; Oyedeji, A.O. Phytochemical screening, anti-inflammatory and analgesic properties of Pentanisia prunelloides from the Eastern Cape Province, South Africa. Afr. J. Tradi. Complement. Altern. Med. 2016, 13, 179–185. [Google Scholar]
- Okeleye, B.I.; Nongogo, V.; Mkwetshana, N.T.; Ndip, R.N. Polyphenolic content and in vitro antioxidant evaluation of the stem bark extract of Peltophorum africanum Sond (Fabaceae). Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of selenium-contained protein from the Se-enriched Bifidobacterium animals. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef]
- Yang, Y.; Isman, M.B.; Tak, J.-H. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Adler, C.; Bouda, H.; Fontem, D.A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Peebles, J.; Gwebu, E.; Oyedeji, O.; Nanyonga, S.; Kunene, N.; Jackson, D.; Setzer, W.; Oyedeji, A. Composition and biological potential of essential oil from Thelechitonia trilobata growing in South Africa. Nat. Prod. Commun. 2011, 12, 1945–1948. [Google Scholar] [CrossRef]
| Citrus limon EO | EO Smell | EO Color | Starting Material (g) | Mass of Oil (g) | % (w/w) |
|---|---|---|---|---|---|
| Fresh leaf | Sharp pungent citric smell | Colorless | 600.00 | 2.59 | 0.39 |
| Dried leaf | Herbaceous smell | Yellowish | 600.00 | 4.14 | 0.69 |
| Fresh peel | Sweet lemonade smell | Colorless | 600.00 | 4.98 | 0.83 |
| Dried peel | Sharp lemon smell | Light yellowish | 600.00 | 9.72 | 1.62 |
| Compound | KI Value | Percentage Composition (%) | |||
|---|---|---|---|---|---|
| C. limon (Peel) | C. limon (Leaf) | ||||
| Fresh | Dry | Fresh | Dry | ||
| Thujene | 932 | 0.3 | - | - | - |
| α-Pinene | 934 | 1.5 | 0.2 | 1.1 | - |
| Sabinene | 957 | 0.3 | 0.3 | - | - |
| δ-3-Carene | 974 | - | - | 1.0 | - |
| β-Pinene | 980 | 10.6 | 2.2 | 13.3 | - |
| Myrcene | 991 | 1.4 | - | 1.4 | - |
| 6-Methyl-5-hepten-2-one | 989 | - | 0.3 | 2.7 | 1.8 |
| Octanal | 1019 | 0.1 | 0.5 | - | - |
| 1,8-Cineole | 1022 | - | 0.2 | - | - |
| Limonene | 1034 | 52.5 | 36.0 | 31.0 | 12.0 |
| Limonene glycol | 1035 | - | - | - | 5.2 |
| 1,8 Cineole | 1036 | - | - | 1.2 | 2.1 |
| Trans-β-ocimene | 1047 | 0.1 | 0.3 | 2.3 | - |
| Linalool oxide | 1056 | - | - | - | 3.8 |
| γ-Terpinene | 1066 | 8.8 | - | 0.5 | 8.6 |
| Cyclo-octane | 1071 | - | 0.1 | - | - |
| Epoxylinalool | 1079 | - | 0.7 | - | - |
| α-Terpinolene | 1084 | 0.5 | - | 0.3 | - |
| Trans-linalool oxide | 1088 | - | 0.7 | 0.2 | 4.2 |
| p-Cymene | 1092 | - | 0.2 | - | - |
| Linalool | 1099 | 1.2 | 1.3 | 2.3 | |
| Nonanal | 1104 | 0.2 | 0.2 | - | - |
| Fenchol | 1117 | - | 0.1 | - | - |
| Trans-p-2,8-menthadien-1-ol | 1122 | - | 1.1 | - | - |
| Cis-limonene oxide | 1135 | - | 1.7 | 0.1 | - |
| Trans-pinocarveol | 1152 | - | 0.8 | - | - |
| Citronellal | 1155 | - | 0.2 | 0.3 | - |
| Camphor | 1159 | - | 0.2 | - | - |
| p-Menth-1-en-9-al | 1162 | - | 0.2 | - | - |
| Pinocarvone | 1163 | - | 0.8 | - | - |
| Borneol | 1165 | - | 0.3 | - | - |
| 1-Tert-butyl-3,3-dimethylcyclopropene | 1181 | - | 1.3 | - | - |
| Terpinen-4-ol | 1182 | 1.1 | 1.1 | 1.0 | 1.5 |
| α-Terpineol | 1192 | 2.7 | - | 1.5 | - |
| Epoxy-linalol | 1198 | - | - | - | 1.1 |
| Alloocimene | 1207 | - | 5.6 | - | - |
| 2-Butenal, 3-methyl | 1209 | - | 0.5 | - | - |
| Citronellol | 1211 | - | 0.7 | - | 1.8 |
| Nerol | 1228 | 1.7 | - | 0.7 | 2.3 |
| Carveol | 1233 | 0.3 | - | - | 1.0 |
| Trans-(+)-carveol | 1235 | - | 3.5 | - | - |
| Exo-2-hydroxycineole | 1238 | - | 0.5 | - | - |
| Z-Citral | 1240 | 4.3 | 0.7 | 10.3 | - |
| Cis-carveol | 1242 | 1.0 | - | - | - |
| Geraniol | 1245 | 1.3 | 4.1 | 1.3 | 1.3 |
| S-Carvone | 1249 | 0.3 | - | 0.2 | 1.3 |
| Piperitone | 1251 | - | 0.2 | - | - |
| Cis-salvene | 1254 | - | 0.1 | - | - |
| Geranial | 1258 | 5.7 | - | 13.2 | 4.1 |
| Perillaldehyde | 1270 | - | 0.3 | - | - |
| Methyl geraniate | 1323 | - | - | 0.2 | - |
| Citronellyl acetate | 1338 | - | - | 0.3 | - |
| Farnesol | 1345 | - | 0.3 | - | - |
| α-Humulene | 1443 | - | - | 0.1 | - |
| Neryl acetate | 1470 | 1.3 | 2.7 | 4.6 | 2.6 |
| Spiro(5.6)dodecane | 1496 | - | - | - | 1.8 |
| D-Nerolidol | 1539 | - | 0.2 | - | - |
| 1,10-Decanediol | 1548 | - | - | 0.2 | - |
| Geranyl acetate | 1560 | 0.5 | - | 4.4 | - |
| (+) Spathulenol | 1579 | - | - | - | 3.9 |
| β-Caryophyllene | 1594 | 0.3 | - | 0.5 | - |
| (-)-Humulene epoxide II | 1603 | - | - | - | 1.3 |
| Zingiberene | 1611 | 0.6 | 0.2 | - | - |
| Trans-sobrerol | 1623 | - | 0.1 | - | - |
| 4-Methyl-5-vinyl thiazole | 1673 | - | 0.2 | - | - |
| 1-(Dimethylamino)-3-borolene | 1681 | - | 0.2 | - | - |
| Geranylacetate,2,3-epoxy | 1699 | - | 0.2 | - | - |
| 5-Methyl-tetradecane | 1710 | - | 1.2 | - | - |
| Valencene | 1726 | - | 0.3 | - | - |
| Dimethyl-2,6-octadienoic acid | 1730 | - | - | - | 2.8 |
| β-Bisabolene | 1788 | 1.0 | 0.4 | - | - |
| Caryophyllene oxide | 1962 | - | 0.5 | 0.2 | 17.7 |
| Isonicotinic acid | 2088 | - | 0.1 | - | - |
| 3,5-Dimethyladamantan-1-ol | 2102 | - | 0.3 | - | - |
| (+,-)-(Z)-Dihydrofarnesal | 2166 | - | 0.1 | - | - |
| Trans-chrysanthenol | 2174 | - | 0.2 | - | - |
| Total % of compounds | 81.9 | 86.3 | 96.4 | 82.4 | |
| Concentration (μg mL−1) | Mean Percentage Ferric-Reducing Power (%) | |||||
|---|---|---|---|---|---|---|
| Fresh Leaf | Dry Leaf | Dry Peel | Fresh Peel | ASC | BHT | |
| 160 | 65.91 ± 1.21 b | 65.43 ± 1.19 b | 64.30 ± 1.07 b | 63.65 ± 0.29 b | 63.81 ± 0.33 b | 58.48 ± 0.97 a |
| 80 | 64.94 ± 0.43 c | 61.71 ± 1.26 b | 63.81 ± 0.54 bc | 63.33 ± 0.20 b | 63.33 ± 0.24 b | 56.06 ± 1.03 a |
| 40 | 62.19 ± 0.25 c | 53.63 ± 2.18 a | 58.64 ± 1.69 b | 57.51 ± 1.55 b | 57.51 ± 0.94 b | 52.99 ± 1.36 a |
| 20 | 49.11 ± 1.14 c | 45.23 ± 2.05 b | 43.78 ± 3.15 b | 29.24 ± 2.79 a | 48.79 ± 1.18 c | 47.17 ± 1.22 bc |
| Mean Percentage Inhibition of the DPPH Radical (%) | IC50 ± SEM (µg mL−1) | ||||
|---|---|---|---|---|---|
| C. limon Oil | 20 µg mL−1 | 40 µg mL−1 | 80 µg mL−1 | 160 µg mL−1 | |
| Fresh leaf | 43.44 ± 1.74 b | 54.89 ± 0.43 a | 62.93 ± 0.28 b | 73.75 ± 0.15 a | 34.54 ± 3.01 e |
| Dry leaf | 50.78 ± 0.69 c | 56.95 ± 0.87 b | 66.88 ± 0.56 d | 84.01 ± 0.45 b | 12.59 ± 1.48 c |
| Fresh peel | 45.32 ± 2.56 b | 55.92 ± 0.80 ab | 64.27 ± 0.21 c | 75.82 ± 0.61 a | 27.15 ± 2.72 d |
| Dry peel | 52.41 ± 0.26 d | 58.70 ± 0.62 c | 67.00 ± 0.02 e | 87.68 ± 0.01 c | 8.79 ± 1.30 b |
| ASC | 54.25 ± 3.55 cd | 61.09 ± 1.17 d | 70.08 ± 0.17 f | 92.81 ± 0.95 d | 3.01 ± 0.23 a |
| BHT | 36.59 ± 2.85 a | 55.82 ± 5.51 abcd | 60.67 ± 0.32 a | 73.53 ± 4.79 a | 27.58 ± 3.16 d |
| C. limon Oil | Repellent Activity (%) | Statistical Analysis | ||||
|---|---|---|---|---|---|---|
| 10 µL | 20 µL | 30 µL | 40 µL | F3,56 | p-Value | |
| Dry peel | 52.00 ± 5.09 | 69.33 ± 5.47 | 57.33 ± 5.81 | 82.67 ± 4.73 | 6.6455 | <0.001 |
| Dry leaf | 73.33 ± 6.95 | 72.00 ± 5.45 | 82.67 ± 4.73 | 78.33 ± 2.82 | 1.4180 | >0.05 |
| C. limon Essential Oil | Exposure Time (h) | No. of Insects Tested | LC50 (95% CI) (µL mL−1 air) | Slope ± SEM | χ2 (df) | p-Value |
|---|---|---|---|---|---|---|
| Dry peel | 48 | 150 | 0.34 b | 1.35 ± 0.55 | 17.92 (10) | >0.05 |
| 72 | 150 | 0.28 (0.13–49.30) | 1.20 ± 0.50 | 13.62 (10) | >0.05 | |
| 96 | 150 | 0.08 (0.06–0.16) | 2.15 ± 0.49 | 4.21 (10) | >0.05 | |
| Dry leaf | 48 | 150 | 0.17 (0.12–0.48) | 3.08 ± 0.96 | 5.45 (10) | >0.05 |
| 72 | 150 | 0.09 (0.07–0.11) | 3.11 ± 0.61 | 7.87 (10) | >0.05 | |
| 96 | 150 | 0.06 (0.05–0.07) | 2.54 ± 0.49 | 5.58 (10) | >0.05 |
| C. limon Essential Oil | Exposure Period (h) | Number of Weevils Tested | LC50 (95% CI) (µL g−1) | Slope ± SE | χ2 (df) | p-Value |
|---|---|---|---|---|---|---|
| Dry peel | 24 | 300 | 2.02 (1.76–2.27) | 4.07 ± 0.46 | 8.29 (10) | >0.05 |
| 48 | 300 | 1.66 (1.41–1.89) | 3.82 ± 0.49 | 10.80 (10) | >0.05 | |
| 72 | 300 | 1.39 (0.95–1.74) | 3.34 ± 0.47 | 15.33 (10) | >0.05 | |
| Dry leaf | 24 | 300 | 4.09 (3.01–5.61) | 3.94 ± 0.44 | 42.02 (10) | <0.001 |
| 48 | 300 | 3.19 (2.44–4.11) | 2.91 ± 0.34 | 21.84 (10) | <0.05 | |
| 72 | 300 | 2.39(1.76–3.02) | 2.92 ± 0.35 | 20.41(10) | <0.05 |
| Simulated Agricultural and Socio-Economic Benefits of C. limon (Lemon) Peel Waste | Values |
|---|---|
| Total amount of lemons produced (2023) | 653,000 MT |
| Total amount of lemons imported | 3000 MT |
| Total amount of lemons exported (2023) | 573,000 MT |
| Total amount of lemons consumed/wasted within South Africa (2023) | 83,000 MT |
| Average weight (wet) of fruit peel per lemon | 39.70 g |
| Average dehydrated (dry) of fruit peel per lemon | 9.19 g |
| Total available dehydrated lemon peel within South Africa (2023) | 0.762 MT |
| Total maize used for animal feed (2023) in South Africa | 6.650 MT |
| Cost of lemon peel as an alternative energy-producing ingredient in animal feed (at the current maize market price of ZAR 3754/ton) | ZAR 2,869,548,000:00 |
| Cost of maize used in animal feed (2024) in South Africa (January 2024 market price/ton) | ZAR 24,964,100,000:00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nodola, P.; Miya, G.M.; Mazwi, V.; Oriola, A.O.; Oyedeji, O.O.; Hosu, Y.S.; Kuria, S.K.; Oyedeji, A.O. Citrus limon Wastes from Part of the Eastern Cape Province in South Africa: Medicinal, Sustainable Agricultural, and Bio-Resource Potential. Molecules 2024, 29, 1675. https://doi.org/10.3390/molecules29071675
Nodola P, Miya GM, Mazwi V, Oriola AO, Oyedeji OO, Hosu YS, Kuria SK, Oyedeji AO. Citrus limon Wastes from Part of the Eastern Cape Province in South Africa: Medicinal, Sustainable Agricultural, and Bio-Resource Potential. Molecules. 2024; 29(7):1675. https://doi.org/10.3390/molecules29071675
Chicago/Turabian StyleNodola, Phumelele, Gugulethu M. Miya, Vuyokazi Mazwi, Ayodeji O. Oriola, Opeoluwa O. Oyedeji, Yiseyon S. Hosu, Simon K. Kuria, and Adebola O. Oyedeji. 2024. "Citrus limon Wastes from Part of the Eastern Cape Province in South Africa: Medicinal, Sustainable Agricultural, and Bio-Resource Potential" Molecules 29, no. 7: 1675. https://doi.org/10.3390/molecules29071675
APA StyleNodola, P., Miya, G. M., Mazwi, V., Oriola, A. O., Oyedeji, O. O., Hosu, Y. S., Kuria, S. K., & Oyedeji, A. O. (2024). Citrus limon Wastes from Part of the Eastern Cape Province in South Africa: Medicinal, Sustainable Agricultural, and Bio-Resource Potential. Molecules, 29(7), 1675. https://doi.org/10.3390/molecules29071675






