Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance
Abstract
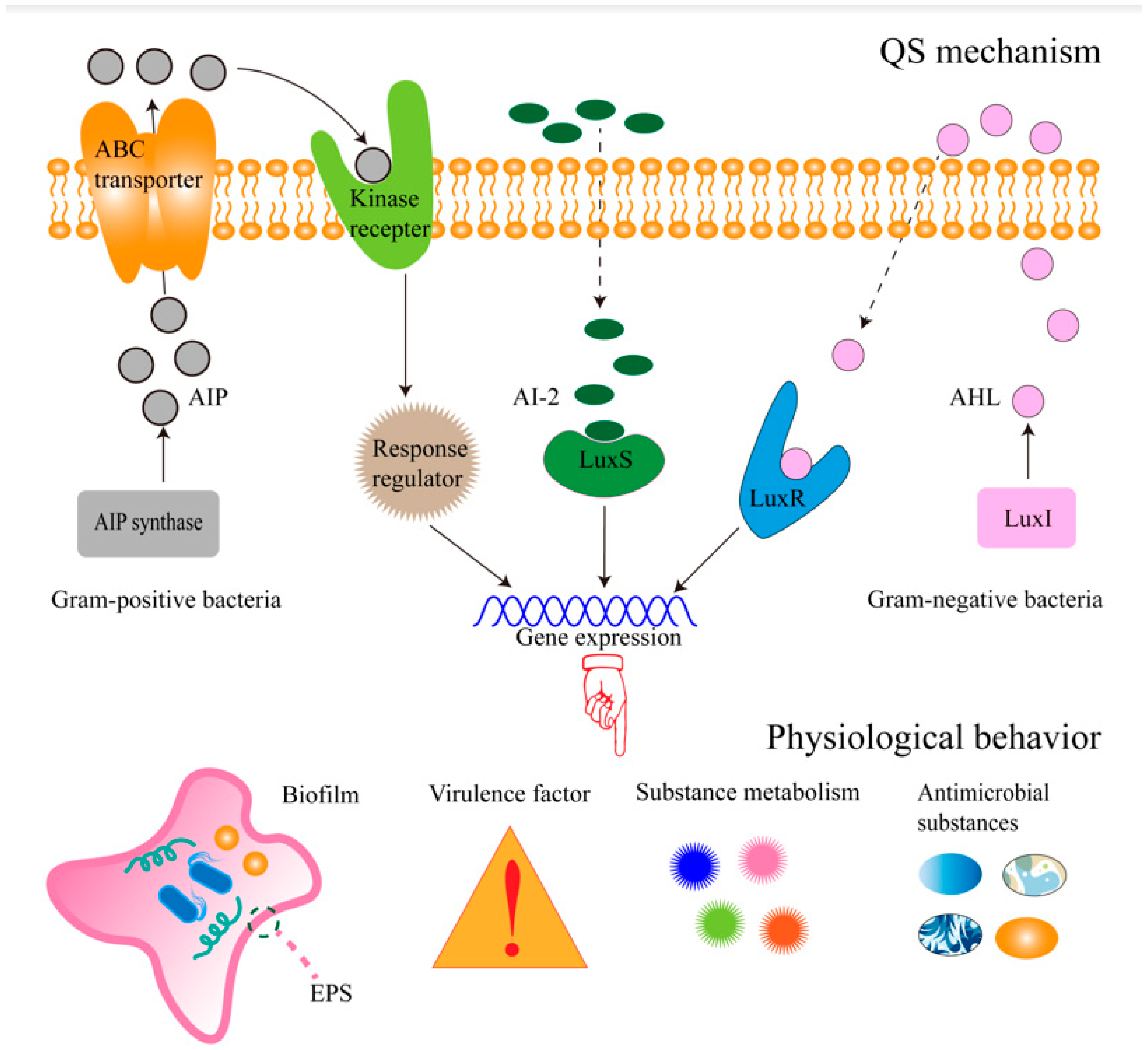
1. Introduction
2. Combination of Aminoglycoside Antibiotics and QSIs
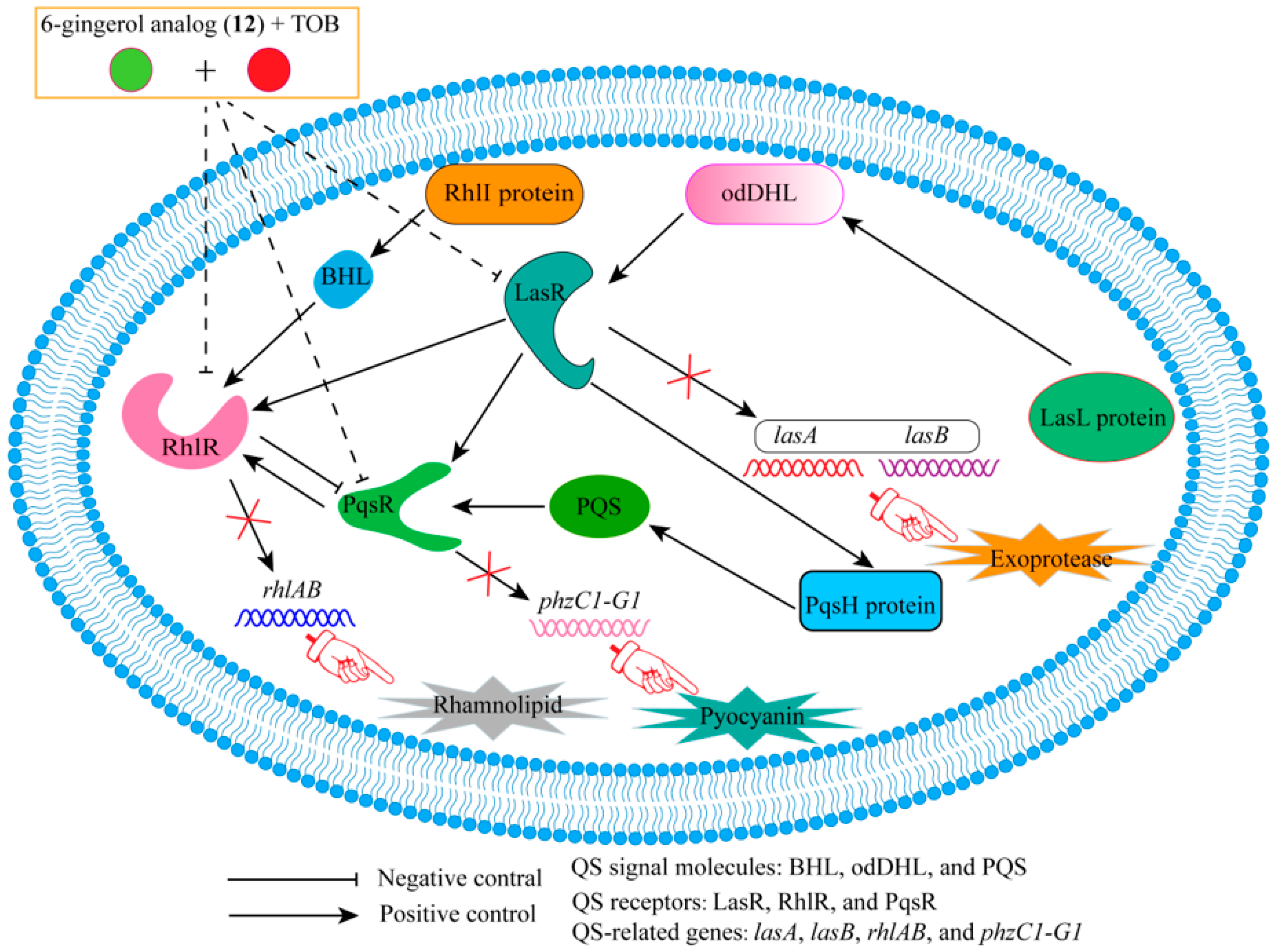
2.1. Combination of Tobramycin and QSIs
2.2. Combination of Gentamicin and QSIs
3. Combination of β-Lactam Antibiotics and QSIs
3.1. Combination of Ceftazidime and QSIs
3.2. Combination of Meropenem and QSIs
3.3. Combination of Penicillin G with QSIs
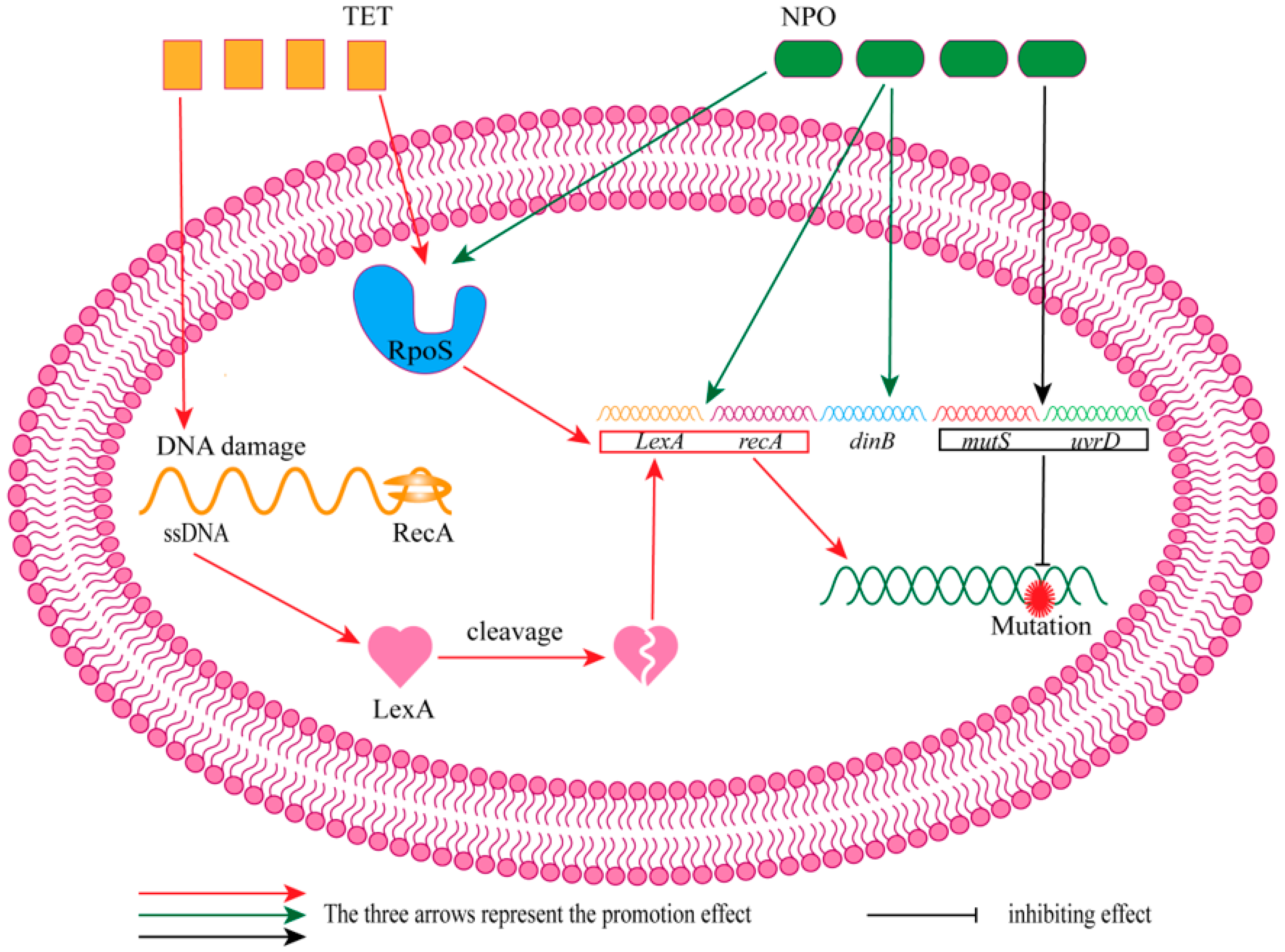
4. Combination of Tetracycline Antibiotics and QSIs
5. Combination of Macrolide Antibiotics and QSIs
6. Combination of Quinolone Antibiotics and QSIs
7. Combination of Polymyxin and QSIs
8. Combination of Other Antibiotics and QSIs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Zou, Y.; Zheng, J.; Qiu, S.; Liu, L.; Wei, C. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 273, 127414. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zou, H.; Li, J.; Song, T.; Lv, W.; Wang, W.; Wang, Z.; Tao, S. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: Current understanding and future perspectives. Gut Microbes 2022, 14, 2039048. [Google Scholar] [CrossRef] [PubMed]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Dong, X.; Grenier, D.; Wang, K.; Wang, Y. Research progress of bacterial quorum sensing receptors: Classification, structure, function and characteristics. Sci. Total Environ. 2021, 763, 143031. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; Zhu, H. A Cyclic Dipeptide from Marine Fungus Penicillium chrysogenum DXY-1 Exhibits Anti-quorum Sensing Activity. ACS Omega 2021, 6, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Jantaruk, P.; Pabuprapap, W.; Nakaew, A.; Kunthalert, D.; Suksamrarn, A. 4-methoxybenzalacetone, the cinnamic acid analog as a potential quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2021, 37, 153. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.X.; Liu, J.S.; Wang, Y.J.; Tang, S.; Wang, D.Y.; Deng, S.M.; Jia, A.Q. Actinomycin D: A novel Pseudomonas aeruginosa quorum sensing inhibitor from the endophyte Streptomyces cyaneochromogenes RC1. World J. Microbiol. Biotechnol. 2022, 38, 170. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Le, D.D.; Kim, W.G. Curvularin Isolated From Phoma macrostoma Is an Antagonist of RhlR Quorum Sensing in Pseudomonas aeruginosa. Front. Microbiol. 2022, 13, 913882. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, M.; Yi, G.; Liao, L.; Cheng, Q.; Zhu, J.; Zhang, B.; Wang, Y.; Chen, Y.; Zeng, M. Screening strategies for quorum sensing inhibitors in combating bacterial infections. J. Pharm. Anal. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Shi, M.; Wei, X.; Zhou, X.; Li, B.; Zhang, J. Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. Int. J. Mol. Sci. 2022, 23, 8809. [Google Scholar] [CrossRef]
- Rahmoun, L.A.; Azrad, M.; Peretz, A. Antibiotic Resistance and Biofilm Production Capacity in Clostridioides difficile. Front. Cell. Infect. Microbiol. 2021, 11, 683464. [Google Scholar] [CrossRef] [PubMed]
- Buzzo, J.R.; Devaraj, A.; Gloag, E.S.; Jurcisek, J.A.; Robledo-Avila, F.; Kesler, T.; Wilbanks, K.; Mashburn-Warren, L.; Balu, S.; Wickham, J.; et al. Z-form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell 2021, 184, 5740–5758.e17. [Google Scholar] [CrossRef] [PubMed]
- Dillon, N.; Holland, M.; Tsunemoto, H.; Hancock, B.; Cornax, I.; Pogliano, J.; Sakoulas, G.; Nizet, V. Surprising synergy of dual translation inhibition vs. Acinetobacter baumannii and other multidrug-resistant bacterial pathogens. EBioMedicine 2019, 46, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fatsis-Kavalopoulos, N.; Roelofs, L.; Andersson, D.I. Potential risks of treating bacterial infections with a combination of β-lactam and aminoglycoside antibiotics: A systematic quantification of antibiotic interactions in E. coli blood stream infection isolates. EBioMedicine 2022, 78, 103979. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin - an anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, H.; Yan, Z.; Zhao, C.; Ren, J.; Qu, X. Combating Biofilm Associated Infection In Vivo: Integration of Quorum Sensing Inhibition and Photodynamic Treatment based on Multidrug Delivered Hollow Carbon Nitride Sphere. Adv. Funct. Mater. 2019, 29, 1808222. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Jospe-Kaufman, M.; Siomin, L.; Fridman, M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorg. Med. Chem. Lett. 2020, 30, 127218. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, T.; Yau, Y.C.W.; Stapleton, P.J.; Gong, Y.; Wang, P.W.; Guttman, D.S.; Waters, V. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbomes 2017, 3, 25. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Tajani, A.S.; Amiri Tehranizadeh, Z.; Pourmohammad, A.; Pourmohammad, A.; Iranshahi, M.; Farhadi, F.; Soheili, V.; Fazly Bazzaz, B.S. Anti-quorum sensing and antibiofilm activity of coumarin derivatives against Pseudomonas aeruginosa PAO1: Insights from in vitro and in silico studies. Iran. J. Basic. Med. Sci. 2023, 26, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, S.Y.; Hu, J.Y.; Chen, Q.X.; Jiao, S.M.; Xiao, H.C.; Zhang, Q.; Xu, J.; Zhao, J.F.; Zhou, H.B.; et al. Novel Coumarin Derivatives Inhibit the Quorum Sensing System and Iron Homeostasis as Antibacterial Synergists against Pseudomonas aeruginosa. J. Med. Chem. 2023, 66, 14735–14754. [Google Scholar] [CrossRef]
- Ho, D.K.; Murgia, X.; De Rossi, C.; Christmann, R.; Hufner de Mello Martins, A.G.; Koch, M.; Andreas, A.; Herrmann, J.; Muller, R.; Empting, M.; et al. Squalenyl Hydrogen Sulfate Nanoparticles for Simultaneous Delivery of Tobramycin and an Alkylquinolone Quorum Sensing Inhibitor Enable the Eradication of P. aeruginosa Biofilm Infections. Angew. Chem. Int. Ed. Engl. 2020, 59, 10292–10296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Huang, Y.; Jin, Q.; Ji, J. Inhibiting Quorum Sensing by Active Targeted pH-Sensitive Nanoparticles for Enhanced Antibiotic Therapy of Biofilm-Associated Bacterial Infections. ACS Nano 2023, 17, 10019–10032. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.Y.; Zhang, X.Y.; Yang, M.H.; Huang, Y.J.; Lin, J.; Chen, W.M. 3-Hydroxypyridin-4(1H)-one Derivatives as pqs Quorum Sensing Inhibitors Attenuate Virulence and Reduce Antibiotic Resistance in Pseudomonas aeruginosa. J. Med. Chem. 2023, 66, 15823–15846. [Google Scholar] [CrossRef] [PubMed]
- Tajani, A.S.; Jangi, E.; Davodi, M.; Golmakaniyoon, S.; Ghodsi, R.; Soheili, V.; Fazly Bazzaz, B.S. Anti-quorum sensing potential of ketoprofen and its derivatives against Pseudomonas aeruginosa: Insights to in silico and in vitro studies. Arch. Microbiol. 2021, 203, 5123–5132. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Cui, D.; Fan, Y.; Li, G.; Zhong, Z.; Wang, Y. Recent advances in bioaffinity strategies for preclinical and clinical drug discovery: Screening natural products, small molecules and antibodies. Drug Discov. Today 2024, 29, 103885. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.M.; Abdelsamie, A.S.; Rox, K.; Schutz, C.; Kany, A.M.; Rohrig, T.; Schmelz, S.; Blankenfeldt, W.; Arce-Rodriguez, A.; Borrero-de Acuna, J.M.; et al. Towards Translation of PqsR Inverse Agonists: From In Vitro Efficacy Optimization to In Vivo Proof-of-Principle. Adv. Sci. 2023, 10, e2204443. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.-Y.; Kim, H.-S.; Jo, M.J.; Lee, J.-H.; Byun, Y.; Ko, G.-J.; Park, H.-D. Combined Treatment of 6-Gingerol Analog and Tobramycin for Inhibiting Pseudomonas aeruginosa Infections. Microbiol. Spectr. 2021, 9, e00192-21. [Google Scholar] [CrossRef]
- Tahrioui, A.; Duchesne, R.; Bouffartigues, E.; Rodrigues, S.; Maillot, O.; Tortuel, D.; Hardouin, J.; Taupin, L.; Groleau, M.C.; Dufour, A.; et al. Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. NPJ Biofilms Microbomes 2019, 5, 15. [Google Scholar] [CrossRef]
- Li, W.R.; Ma, Y.K.; Shi, Q.S.; Xie, X.B.; Sun, T.L.; Peng, H.; Huang, X.M. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 2018, 102, 7555–7564. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.D.; van Gennip, M.; Jakobsen, T.H.; Alhede, M.; Hougen, H.P.; Hoiby, N.; Bjarnsholt, T.; Givskov, M. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J. Antimicrob. Chemother. 2012, 67, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Furiga, A.; Lajoie, B.; El Hage, S.; Baziard, G.; Roques, C. Impairment of Pseudomonas aeruginosa Biofilm Resistance to Antibiotics by Combining the Drugs with a New Quorum-Sensing Inhibitor. Antimicrob. Agents Chemother. 2015, 60, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Piras, A.M.; Motta, V.; Braccini, S.; Mazzantini, D.; Chiellini, F.; Zambito, Y.; Esin, S.; Batoni, G. Antivirulence Properties of a Low-Molecular-Weight Quaternized Chitosan Derivative against Pseudomonas aeruginosa. Microorganisms 2021, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Vandeputte, O.M.; Pottier, L.; Huet, J.; Rabemanantsoa, C.; Kiendrebeogo, M.; Andriantsimahavandy, A.; Rasamindrakotroka, A.; Stevigny, C.; Duez, P.; et al. Pseudomonas aeruginosa Biofilm Formation and Persistence, along with the Production of Quorum Sensing-Dependent Virulence Factors, Are Disrupted by a Triterpenoid Coumarate Ester Isolated from Dalbergia trichocarpa, a Tropical Legume. PLoS ONE 2015, 10, e0132791. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Mortensen, K.T.; Norskov, A.; Qvortrup, K.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Itaconimides as Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2018, 8, 443. [Google Scholar] [CrossRef]
- Hallan, S.S.; Marchetti, P.; Bortolotti, D.; Sguizzato, M.; Esposito, E.; Mariani, P.; Trapella, C.; Rizzo, R.; Cortesi, R. Design of Nanosystems for the Delivery of Quorum Sensing Inhibitors: A Preliminary Study. Molecules 2020, 25, 5655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.J.; Zhou, J.W.; Zhang, P.P.; Luo, H.Z.; Tang, S.; Li, J.J.; Deng, S.M.; Jia, A.Q. Quorum sensing inhibition and tobramycin acceleration in Chromobacterium violaceum by two natural cinnamic acid derivatives. Appl. Microbiol. Biotechnol. 2020, 104, 5025–5037. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, S. In silico identification of albendazole as a quorum sensing inhibitor and its in vitro verification using CviR and LasB receptors based assay systems. Bioimpacts 2018, 8, 201–209. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, A.; Sandhu, P.; Daware, A.; Das, M.C.; Akhter, Y.; Bhattacharjee, S. Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: A study with plumbagin and gentamicin. J. Appl. Microbiol. 2017, 123, 246–261. [Google Scholar] [CrossRef]
- Zore, M.; Gilbert-Girard, S.; San-Martin-Galindo, P.; Reigada, I.; Hanski, L.; Savijoki, K.; Fallarero, A.; Yli-Kauhaluoma, J.; Patel, J.Z. Repurposing the Sphingosine-1-Phosphate Receptor Modulator Etrasimod as an Antibacterial Agent Against Gram-Positive Bacteria. Front. Microbiol. 2022, 13, 926170. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, C.; Jha, N.K.; Ghosh, R.; Shetty, P.H. Anti quorum sensing and anti virulence activity of tannic acid and it’s potential to breach resistance in Salmonella enterica Typhi/Paratyphi A clinical isolates. Microb. Pathog. 2020, 138, 103813. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Ahn, J. Characterization of β-lactamase- and efflux pump-mediated multiple antibiotic resistance in Salmonella Typhimurium. Food Sci. Biotechnol. 2018, 27, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ochomogo, M.; Lohans, C.T. β-Lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates. RSC Med. Chem. 2021, 12, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum sensing inhibitors as Therapeutics: Bacterial biofilm inhibition. Bioorg. Chem. 2023, 136, 106551. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Han, X.; Du, W.; Kou, Z.; Jiang, F. Trp-Containing Antibacterial Peptides Impair Quorum Sensing and Biofilm Development in Multidrug-Resistant Pseudomonas aeruginosa and Exhibit Synergistic Effects With Antibiotics. Front. Microbiol. 2021, 12, 611009. [Google Scholar] [CrossRef] [PubMed]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef] [PubMed]
- Muslim, S.N.; Kadmy, I.; Ali, A.N.M.; Salman, B.K.; Ahmad, M.; Khazaal, S.S.; Hussein, N.H.; Muslim, S.N. Chitosan extracted from Aspergillus flavus shows synergistic effect, eases quorum sensing mediated virulence factors and biofilm against nosocomial pathogen Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2018, 107, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Glišić, B.Đ.; Aleksic, I.; Comba, P.; Wadepohl, H.; Ilic-Tomic, T.; Nikodinovic-Runic, J.; Djuran, M.I. Copper(ii) complexes with aromatic nitrogen-containing heterocycles as effective inhibitors of quorum sensing activity in Pseudomonas aeruginosa. RSC Adv. 2016, 6, 86695–86709. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, Y.; Yuan, B.; Yue, Y.; Zhao, M.; Luo, R.; Wu, H.; Wang, L.; Zhang, Y.; Xiao, J.; et al. Effect of Autoinducer-2 Quorum Sensing Inhibitor on Interspecies Quorum Sensing. Front. Microbiol. 2022, 13, 791802. [Google Scholar] [CrossRef]
- Jiang, K.; Yan, X.; Yu, J.; Xiao, Z.; Wu, H.; Zhao, M.; Yue, Y.; Zhou, X.; Xiao, J.; Lin, F. Design, synthesis, and biological evaluation of 3-amino-2-oxazolidinone derivatives as potent quorum-sensing inhibitors of Pseudomonas aeruginosa PAO1. Eur. J. Med. Chem. 2020, 194, 112252. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.K.; Alvarado, C.L.; Sutton, M.D. Role of the SOS Response in the Generation of Antibiotic Resistance In Vivo. Antimicrob. Agents Chemother. 2021, 65, e0001321. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, A.; Bakhlanova, I.; Baitin, D. Targeting evolution of antibiotic resistance by SOS response inhibition. Comp. Struct. Biotechnol. J. 2021, 19, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Wang, D.; You, J. Joint effects of antibiotics and quorum sensing inhibitors on resistance development in bacteria. Environ. Sci.-Process Impacts 2021, 23, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Chawla, M.; Ahrodia, T.; Narendrakumar, L.; Das, B. Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens. Antibiotics 2023, 12, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Li, L.; Cao, R.; Zheng, Q.; Xu, Z.; Wu, C.J.; Zhu, H. Glycosylation increases the anti-QS as well as anti-biofilm and anti-adhesion ability of the cyclo (L-Trp-L-Ser) against Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 238, 114457. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Li, L.; Liu, L. Synergistic Activity of Berberine with Azithromycin against Pseudomonas Aeruginosa Isolated from Patients with Cystic Fibrosis of Lung In Vitro and In Vivo. Cell Physiol. Biochem. 2017, 42, 1657–1669. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Chhibber, S.; Harjai, K. Efficacy of purified lactonase and ciprofloxacin in preventing systemic spread of Pseudomonas aeruginosa in murine burn wound model. Burns 2015, 41, 153–162. [Google Scholar] [CrossRef]
- Vadekeetil, A.; Alexandar, V.; Chhibber, S.; Harjai, K. Adjuvant effect of cranberry proanthocyanidin active fraction on antivirulent property of ciprofloxacin against Pseudomonas aeruginosa. Microb. Pathog. 2016, 90, 98–103. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, W.; Ma, Y.C.; Bai, B.; Sun, G.; Zhang, S.; Chang, X.; Wang, Y.; Jiang, N.; Zhang, X.; et al. Design and Synthesis of 4-Fluorophenyl-5-methylene-2(5H)-furanone Derivatives as Potent Quorum Sensing Inhibitors. J. Med. Chem. 2023, 66, 8441–8463. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-Z.; Tan, X.-J.; Chang, Z.-Y.; Li, J.-J.; Jia, A.-Q. 2-tert-Butyl-1,4-benzoquinone, a food additive oxidant, reduces virulence factors of Chromobacterium violaceum. LWT-Food Sci. Technol. 2022, 163, 113569. [Google Scholar] [CrossRef]
- Liu, J.; Meng, Y.; Yang, M.H.; Zhang, X.Y.; Zhao, J.F.; Sun, P.H.; Chen, W.M. Design, synthesis and biological evaluation of novel 3-hydroxypyridin-4(1H)-ones based hybrids as Pseudomonas aeruginosa biofilm inhibitors. Eur. J. Med. Chem. 2023, 259, 115665. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Trapella, C.; Bragonzi, A.; Marchetti, P.; Zanirato, V.; Alogna, A.; Gentili, V.; Cervellati, C.; Valacchi, G.; Sicolo, M.; et al. Conjugation of LasR Quorum-Sensing Inhibitors with Ciprofloxacin Decreases the Antibiotic Tolerance of P. aeruginosa Clinical Strains. J. Chem. 2019, 2019, 8143739. [Google Scholar] [CrossRef]
- Bose, S.K.; Chauhan, M.; Dhingra, N.; Chhibber, S.; Harjai, K. Terpinen-4-ol attenuates quorum sensing regulated virulence factors and biofflm formation in Pseudomonas aeruginosa. Future Microbiol. 2020, 15, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, P.; Guo, T.; Gu, X.; Bai, B.; Zhang, S.; Chang, X.; Wang, Y.; Ma, S. Design, synthesis and evaluation of oxazolopyridinone derivatives as quorum sensing inhibitors. Bioorganic Chem. 2023, 130, 106266. [Google Scholar] [CrossRef] [PubMed]
- Thomann, A.; de Mello Martins, A.G.; Brengel, C.; Empting, M.; Hartmann, R.W. Application of Dual Inhibition Concept within Looped Autoregulatory Systems toward Antivirulence Agents against Pseudomonas aeruginosa Infections. ACS Chem. Biol. 2016, 11, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.W.; Ruan, L.Y.; Chen, H.J.; Luo, H.Z.; Jiang, H.; Wang, J.S.; Jia, A.Q. Inhibition of Quorum Sensing and Virulence in Serratia marcescens by Hordenine. J. Agric. Food Chem. 2019, 67, 784–795. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Jiang, K.; Wang, H.; Lin, F. Inhibitory effects of novel 1,4-disubstituted 1,2,3-triazole compounds on quorum-sensing of P. aeruginosa PAO1. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 40, 373–379. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Ye, F.; Zhang, H.; Zhou, Y.; Li, J.; Wu, Q.; Xu, X.; Wu, Q.; Wei, B.; et al. A Small-Molecule Inhibitor of the Anthranilyl-CoA Synthetase PqsA for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa. Microbiol. Spectr. 2022, 10, e02764-21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Borges, A.; Ruiz, R.M.; Negrin, Z.R.; Distinto, S.; Borges, F.; Simoes, M. 2-(2-Methyl-2-nitrovinyl)furan but Not Furvina Interfere with Staphylococcus aureus Agr Quorum-Sensing System and Potentiate the Action of Fusidic Acid against Biofilms. Int. J. Mol. Sci. 2021, 22, 613. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, G.; Dal Pra, M.; Ronca, V.; Pauletto, A.; Marzaro, G.; Saluzzo, F.; Stefani, A.; Artusi, I.; De Filippis, V.; Ferlin, M.G.; et al. A Novel Aza-Derivative Inhibits agr Quorum Sensing Signaling and Synergizes Methicillin-Resistant Staphylococcus aureus to Clindamycin. Front. Microbiol. 2021, 12, 610859. [Google Scholar] [CrossRef] [PubMed]
- Iobbi, V.; Brun, P.; Bernabe, G.; Dougue Kentsop, R.A.; Donadio, G.; Ruffoni, B.; Fossa, P.; Bisio, A.; De Tommasi, N. Labdane Diterpenoids from Salvia tingitana Etl. Synergize with Clindamycin against Methicillin-Resistant Staphylococcus aureus. Molecules 2021, 26, 6681. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Lee, S.S.; Blumberg, L.; Levison, M.E. Antimicrobial resistance-A global problem in need of global solutions. Int. J. Infect. Dis. 2023, 137, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Cirz, R.T.; Romesberg, F.E. Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob. Agents Chemother. 2006, 50, 220–225. [Google Scholar] [CrossRef]






| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
| 1 | farnesifrol A | PqsR | TOB | Compared with TOB treatment alone, the sterilization efficiency of TOB combined with 1 was increased by 59%. | [21] |
| 2 | farnesifrol B | PqsR | TOB | Compared with TOB treatment alone, the sterilization efficiency of TOB combined with 2 was increased by 59%. | |
| 3 | farnesifrol C | PqsR | TOB | Compared with TOB treatment alone, the sterilization efficiency of TOB combined with 3 was increased by 51.4%. | |
| 4 | gummosin | PqsR | TOB | Compared with TOB treatment alone, the sterilization efficiency of TOB combined with 4 was increased by 19.4%. | |
| 5 | 4-farnesyloxycoumarin | PqsR | TOB | Compared with TOB treatment alone, the sterilization efficiency of TOB combined with 5 was increased by 17%. | |
| 6 | N-Hydroxy-7-((6-methyl-2-oxo-2H-chromen-4-yl) oxy)- heptanamide | LasR PqsR FpvA | TOB | Compound 6 increased TOB activity by 200-fold by inhibiting biofilm formation and efflux pump gene expression. | [22] |
| 7 | 3-Hydroxy-1,6-dimethyl-2-((((1-(4-phenoxyphenyl)-1H-1,2,3-triazol-4-yl) methyl) amino) methyl) pyridin-4(1H)-one | PqsR | TOB | Compared with TOB treatment alone, the combination of 7 (10 μM and 50 μM) with TOB inhibited the activity of P. aeruginosa, and increased the survival rate of infected C. elegans by 28.6% and 64.2%, respectively. | [25] |
| 8 | - | PqsR | TOB | Compared with TOB alone, the antibacterial activity increased by about 62.5%. | [26] |
| 9 | - | PqsR | TOB | Compared with TOB alone, the antibacterial activity increased by about 50%. | [26] |
| 10 | - | PqsR | TOB | Compared with TOB alone, the antibacterial activity increased by about 37.5%. | |
| 11 | - | PqsR | TOB | Compared with TOB treatment alone, the combined treatment resulted in a more than 3-fold reduction in the total number of CFU. | [28] |
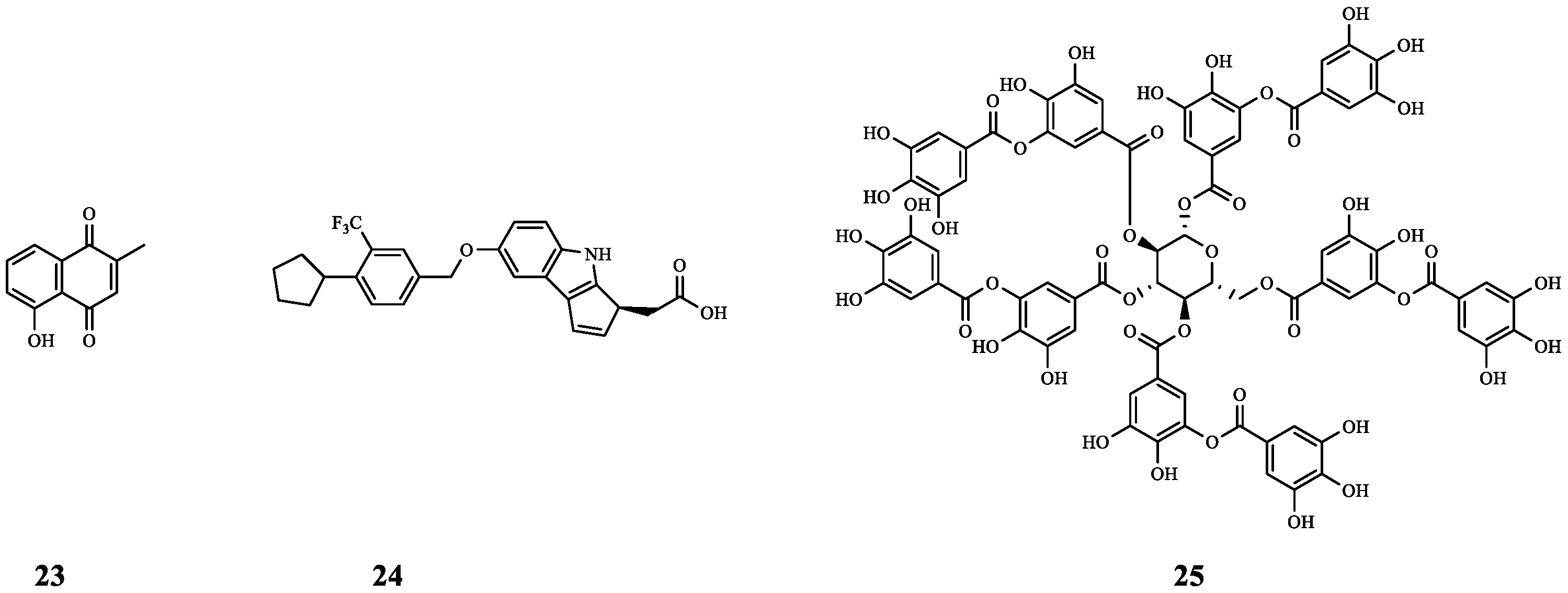
| 12 | - | RhlR | TOB | Synergistic effect (FICI = 0.39). | [29] |
| 13 | Furanone C-30 | P. aeruginosa | TOB | Compared with 13 or TOB treatment alone, the combination reduced the CFU of P. aeruginosa in each implant from above 105 to around 105. | [30,31,32] |
| 14 | Ajoene | P. aeruginosa | TOB | In the early treatment, the therapeutic effect of the combined administration group was ~100–150-fold that of the control group, but in the late treatment, the therapeutic effect of the combined administration group was weak, and the effect was only 1.3–4-fold that of the control group. | |
| 15 | N-(2-pyrimidinyl) butanamide | P. aeruginosa | TOB | Synergistic anti-biofilm effect. | [33] |
| 16 | QAL | P. aeruginosa | TOB | Synergistic anti-biofilm effect. | [34] |
| 17 | Oleanolic aldehyde coumarate | P. aeruginosa | TOB | Synergistic anti-biofilm effect. | [35] |
| 18 | - | P. aeruginosa | TOB | Synergistic anti-biofilm effect. | [36] |
| 19 | - | P. aeruginosa | TOB | No synergistic anti-biofilm activity. | |
| 20 | 4-dimethylaminocinnamic acid | C. violaceuma | TOB | 20 and 21 decreased ethanolamine (biofilm component) and D-proline (osmotic pressure regulator), which promoted TOB to enter bacteria more easily and exert antibacterial activity. | [38] |
| 21 | 4-methoxycinnamic acid | C. violaceuma | TOB | ||
| 22 | Albendazole | CviR LasR | TOB | Synergistic anti-biofilm effect. | [39] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
| 23 | Plumbagin | P. aeruginosa MTCC 424 | GM | Synergistic effect (FICI = 0.192). | [40] |
| P. aeruginosa MTCC 2488 | Synergistic effect (FICI = 0.485). | ||||
| 24 | Etrasimod | S. aureus | GM | Synergistic effect (FICI = 0.5). | [41] |
| 25 | Tannic acid | S. Paratyphi 3336 S. Typhi 950 | GM | By reducing the formation of bacterial EPS (biofilm component), 25 improved the inhibitory effect of GM on S. Paratyphi 3336 (inhibition zone from 18.7 mm to 27.0 mm) and S. Typhi 950 (inhibition zone from 20.3 mm to 25.0 mm). | [42] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
| 26 | Curcumin | P. aeruginosa | CAZ | Synergistic effect (FICI = 0.26). | [47] |
| CIP | Additive effect (FICI = 1.0). | ||||
| 27 | Chitosan | P. aeruginosa | CAZ | Compared to CAZ alone, the MIC was 8-fold smaller. | [48] |
| 28 | - | P. aeruginosa | CAZ | The MIC values of CAZ decreased by 2- fold and 4-fold after CAZ was combined with CAZ at 500 μg mL−1/1000 μg mL−1 of 28, respectively. | [49] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
| 29 | STR7410 | LuxP | MEPM | The combination of the two had a significant inhibitory effect on the biofilm of mixed P. aeruginosa PAO1 and S. aureus ATCC 25923 cells, and increased the survival rate of infected C. elegans (12.67% higher than that of 29 and 10.67% higher than that of MEPM). | [50] |
| 30 | YXL-13 | CviR | MEPM | Synergistic effect (FICI < 0.5). | [51] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
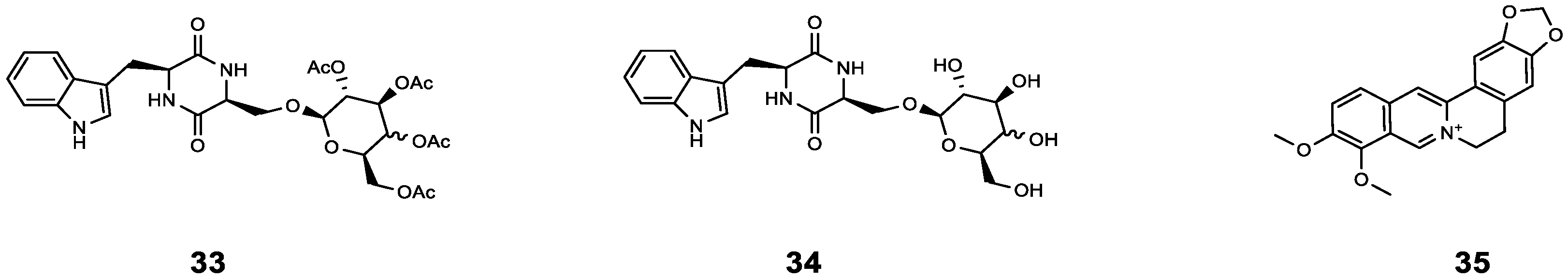
| 33 | cyclo(L-Trp-L-Ser) [c(WS)-Gal] | P. aeruginosa | AZM | Compared with AZM treatment alone, the sterilization rate of AZM combined with 33 increased by 20%, and the bacterial density further decreased (from 99% to 81%). | [56] |
| 34 | cyclo(L-Trp-L-Ser) [c(WS)-Glc] | P. aeruginosa | AZM | Compared with AZM treatment alone, the sterilization rate of AZM combined with 34 increased by 22%, and the bacterial density further decreased (from 99% to 73%). | [56] |
| 35 | Berberine | P. aeruginosa | AZM | Synergistic effect (0.13 < FICI < 0.5). | [57] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
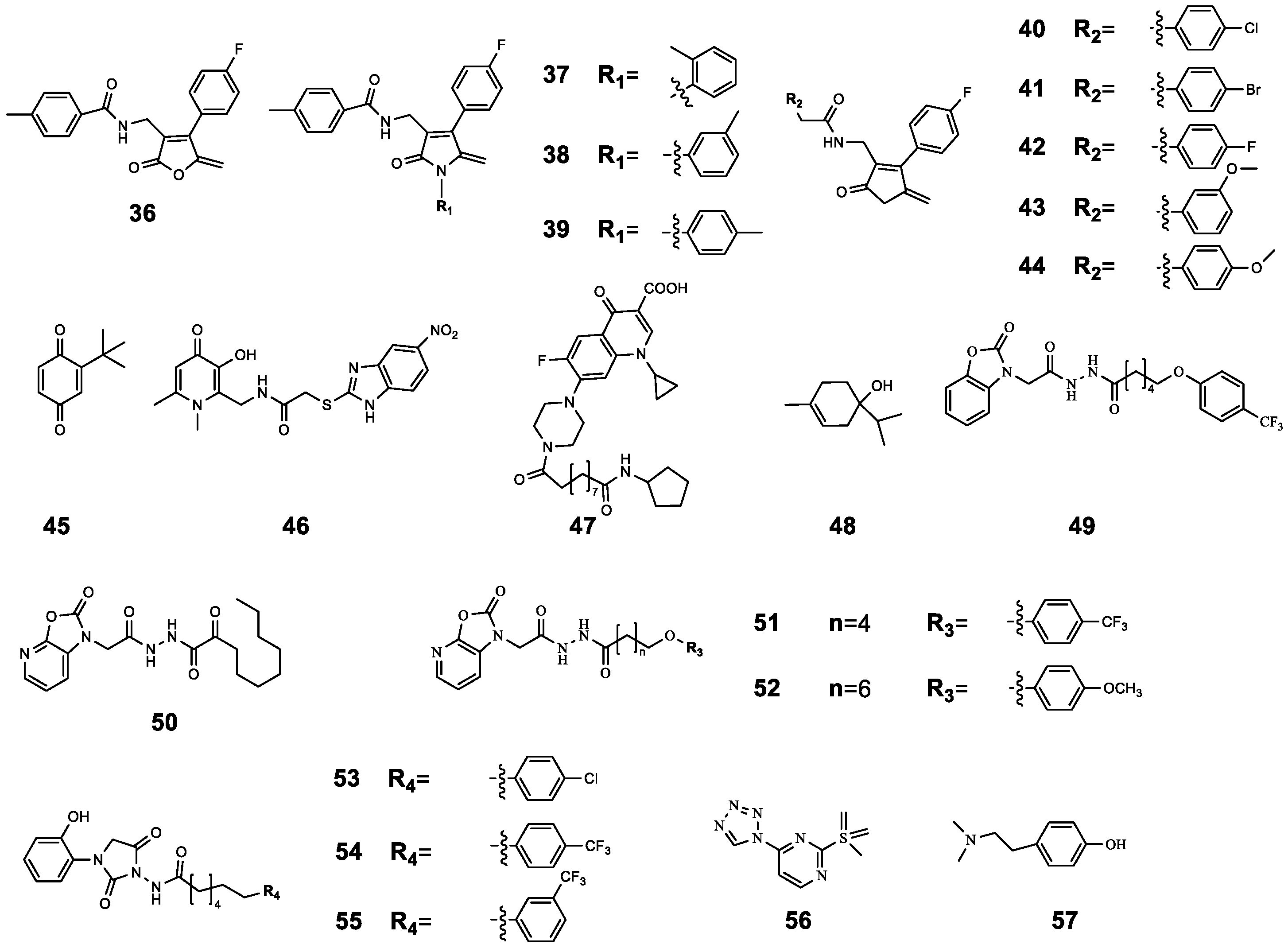
| 36 | 4-Fluoro-N-((4-(4-fluorophenyl)-5-methylene-2-oxo-2,5-dihydrofuran-3-yl) methyl) benzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | Compared with CIP treatment alone, CIP-binding compound 36–44 had a better antibacterial effect, and this effect was enhanced with the increase in the concentration of 36–44 (from 1 μg mL−1 to 256 μg mL−1). | [61] |
| 37 | N-((4-(4-Fluorophenyl)-5-methylene-2-oxo-1-(o-tolyl)-2,5-dihydro-1H-pyrrol-3-yl) methyl)-4-methylbenzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 38 | N-((4-(4-Fluorophenyl)-5-methylene-2-oxo-1-(m-tolyl)-2,5-dihydro-1H-pyrrol-3-yl) methyl)-4-methylbenzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 39 | N-((4-(4-Fluorophenyl)-5-methylene-2-oxo-1-(p-tolyl)-2,5-dihydro-1H-pyrrol-3-yl) methyl)-4-methylbenzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 40 | 4-Chloro-N-((4-(4-fluorophenyl)-5-methylene-2-oxo-2,5-dihydrofuran-3-yl) methyl) benzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 41 | 4-Bromo-N-((4-(4-fluorophenyl)-5-methylene-2-oxo-2,5-dihydrofuran-3-yl) methyl) benzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 42 | 4-Fluoro-N-((4-(4-fluorophenyl)-5-methylene-2-oxo-2,5-dihydrofuran-3-yl) methyl) benzamide | LasR RhlR PqsR | CIP | ||
| 43 | N-((4-(4-Fluorophenyl)-5-methylene-2-oxo-2,5-dihydro-furan-3-yl) methyl)-3-methoxybenzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 44 | N-((4-(4-Fluorophenyl)-5-methylene-2-oxo-2,5-dihydro-furan-3-yl) methyl)-4-methoxybenzamide | P. aeruginosa 27853 P. aeruginosa PAO1 | CIP | ||
| 45 | 2-tert-butyl-1,4-benzoquinone | C. violaceum ATCC12472 | CIP | Compared with CIP treatment alone, the combined treatment significantly inhibited biofilm formation; in particular, 45 (50 μg mL−1) combined with CIP (0.2 μg mL−1) inhibited biofilm formation by 73.27% and reduced the survival rate of biofilm cells to 26.73%. | [62] |
| 46 | 1,6-Dimethyl-2-((5-nitro-2-benzimidazolyl)-thioacetaminomethyl)-3-hydroxy-4-pyridone | LasR PqsR | CIP | The MIC value of CIP was reduced by 50% after combination. | [63] |
| 47 | ET-37 | P. aeruginosa | CIP | 47 assists CIP antibacterial by destroying biofilm and promoting the oxidative stress response. | [64] |
| 48 | Terpinen-4-ol | LasR RhlR PqsR | CIP | Synergistic effect (FICI = 0.36) | [65] |
| 49 | N’-(2-(2-Oxobenzo[d]oxazol-3(2H)-yl) acetyl)-6-(3-(trifluoromethyl)phenoxy) hexanehydrazide | P. aeruginosa PAO1 | CIP | Compared with CIP treatment alone (16.99%), the combined effect resulted in higher bacterial mortality (40% to 50%). | [66] |
| 50 | N’-(2-(2-Oxooxazolo[5,4-b] pyridin-1(2H)-yl) acetyl) nonanehydrazide | P. aeruginosa PAO1 | CIP | ||
| 51 | N’-(2-(2-Oxooxazolo[5,4-b] pyridin-1(2H)-yl) acetyl)-6(4(trifluoromethyl)phenoxy) hexanehydrazide | P. aeruginosa PAO1 | CIP | ||
| 52 | 8-(4-Methoxyphenoxy)-N’-(2-(2-oxooxazolo[5,4-b] pyridin-1(2H)-yl) acetyl) octanehydrazide | LasR | CIP | Compared with CIP treatment alone, the combination resulted in higher bacterial mortality (from 16.99% to 48.27%). | [66] |
| 53 | 6-(4-Chlorophenoxy)-N-(3-(2-hydroxyphenyl)-2,5-dioxoimidazolidin-1-yl) hexanamide | P. aeruginosa PAO1 | CIP | Compared with CIP treatment alone (16.99%), the combined effect resulted in higher bacterial mortality (40% to 50%). | |
| 54 | N-(3-(2-Hydroxyphenyl)-2,5-dioxoimidazolidin-1-yl)-6-(4-(trifluoromethyl)phenoxy) hexanamide | P. aeruginosa PAO1 | CIP | Compared with CIP treatment alone (16.99%), the combined effect resulted in higher bacterial mortality (about 30%). | |
| 55 | N-(3-(2-Hydroxyphenyl)-2,5-dioxoimidazolidin-1-yl)-6-(3-(trifluoromethyl)phenoxy) hexanamide | P. aeruginosa PAO1 | CIP | Compared with CIP treatment alone (16.99%), the combined effect resulted in higher bacterial mortality (nearly 40%). | |
| 56 | - | PqsR PqsD | CIP | 56 increased the antibacterial activity of CIP by inhibiting the release of eDNA and reducing the content of polysaccharide (PS) and protein BF. | [67] |
| 57 | Hordenine | Serratia marcescens NJ01 | CIP | 57 significantly increased the susceptibility of pre-formed biofilms to CIP by reducing the synthesis of EPS and destroying the structural integrity of biofilms. | [68] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
| 58 | - | P. aeruginosa | polymyxin E | Synergistic anti-biofilm effect. | [70] |
| 59 | - | P. aeruginosa | polymyxin E | Synergistic anti-biofilm effect. | |
| 60 | - | P. aeruginosa | polymyxin E | Synergistic anti-biofilm effect. | |
| 61 | Norharmane | PqsA | PB | Synergistic effect (FICI = 0.266). | [71] |
| Compound | Name | Target/Targeted Bacteria | Antibiotic | Combined Results | Ref. |
|---|---|---|---|---|---|
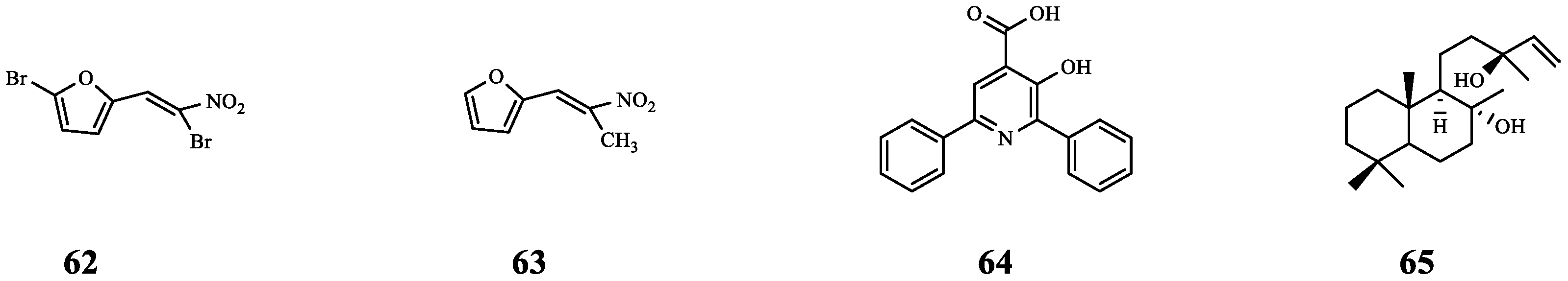
| 62 | Furvina | S. aureus ALC1742 | FA | Compared with FA alone, the ALC1742 biofilm was further reduced (from 20% to 38%) after combination. | [72] |
| S. aureus ALC1743 | Compared with FA alone, the ALC1743 biofilm was further reduced (from 23% to 29%) after combination. | ||||
| 63 | - | S. aureus ALC1742 | FA | Compared with FA alone, the ALC1742 biofilm was further reduced (from 20% to 34%) after combination. | |
| S. aureus ALC1743 | There is no effect on biofilm. | ||||
| 64 | Azan-7 | MRSA | CD | Synergistic effect (FICI = 0.45). | [73] |
| 65 | Sclareol | MRSA | CD | Synergistic effect (FICI = 0.45). | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lu, X.; Wang, C.; Yue, Y.; Wei, B.; Zhang, H.; Wang, H.; Chen, J. Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance. Molecules 2024, 29, 1674. https://doi.org/10.3390/molecules29071674
Wang J, Lu X, Wang C, Yue Y, Wei B, Zhang H, Wang H, Chen J. Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance. Molecules. 2024; 29(7):1674. https://doi.org/10.3390/molecules29071674
Chicago/Turabian StyleWang, Jiahao, Xingyue Lu, Chenjie Wang, Yujie Yue, Bin Wei, Huawei Zhang, Hong Wang, and Jianwei Chen. 2024. "Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance" Molecules 29, no. 7: 1674. https://doi.org/10.3390/molecules29071674
APA StyleWang, J., Lu, X., Wang, C., Yue, Y., Wei, B., Zhang, H., Wang, H., & Chen, J. (2024). Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance. Molecules, 29(7), 1674. https://doi.org/10.3390/molecules29071674









