Organophosphates as Versatile Substrates in Organic Synthesis
Abstract
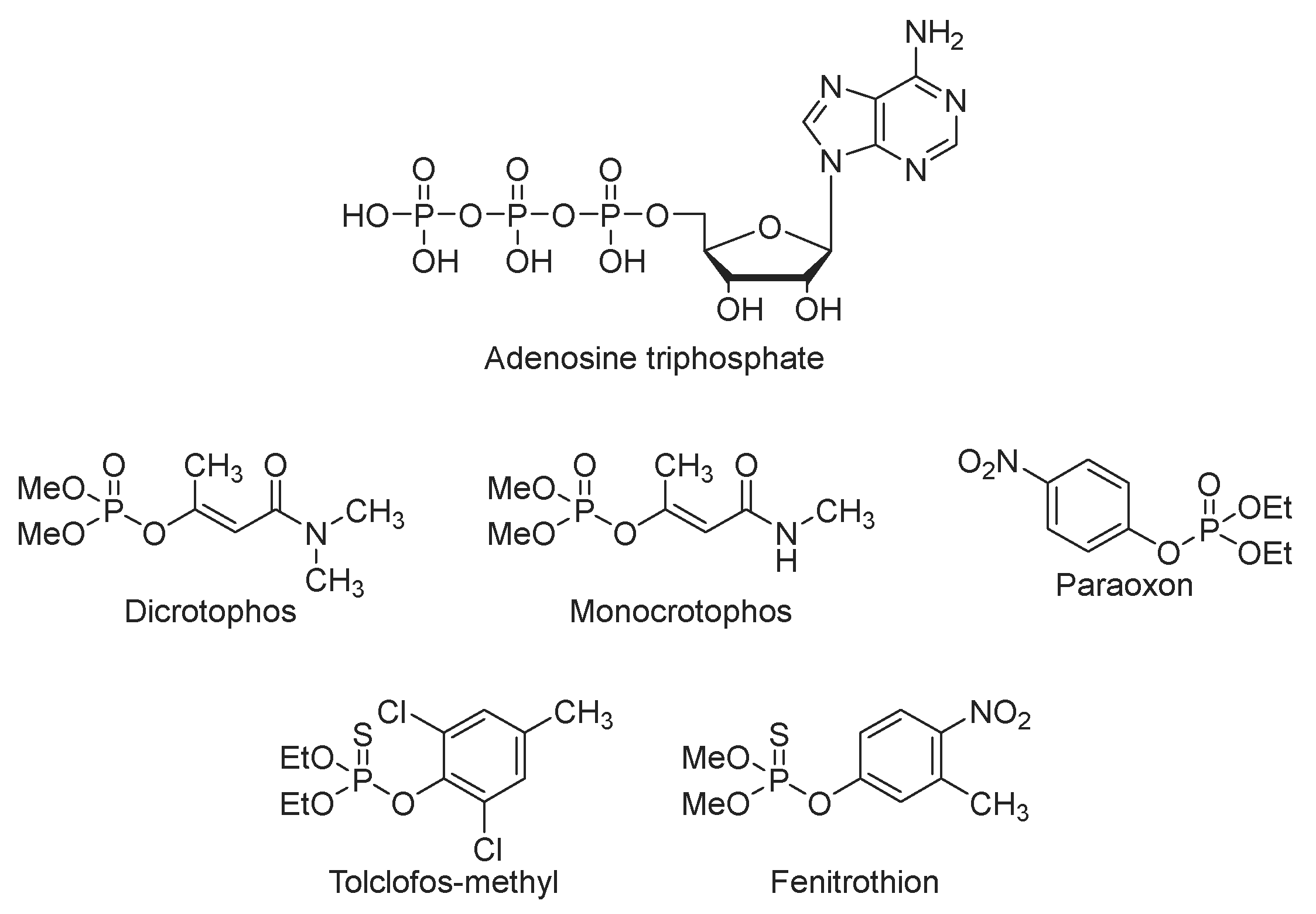
1. Introduction
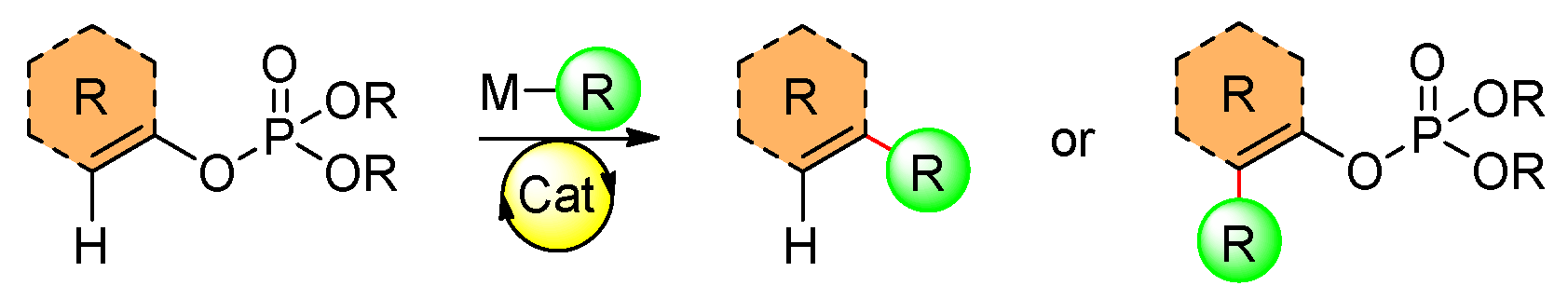
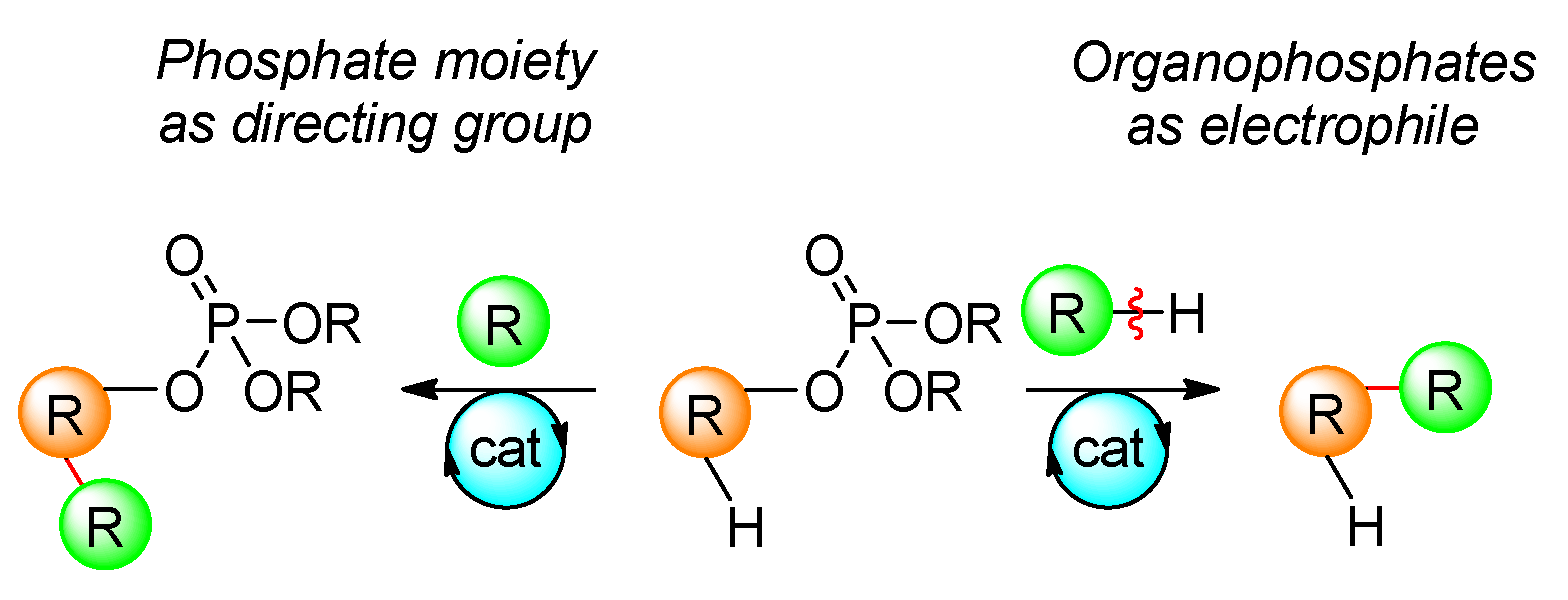
2. Organophosphates as Electrophiles in Transition-Metal-Catalyzed Reactions
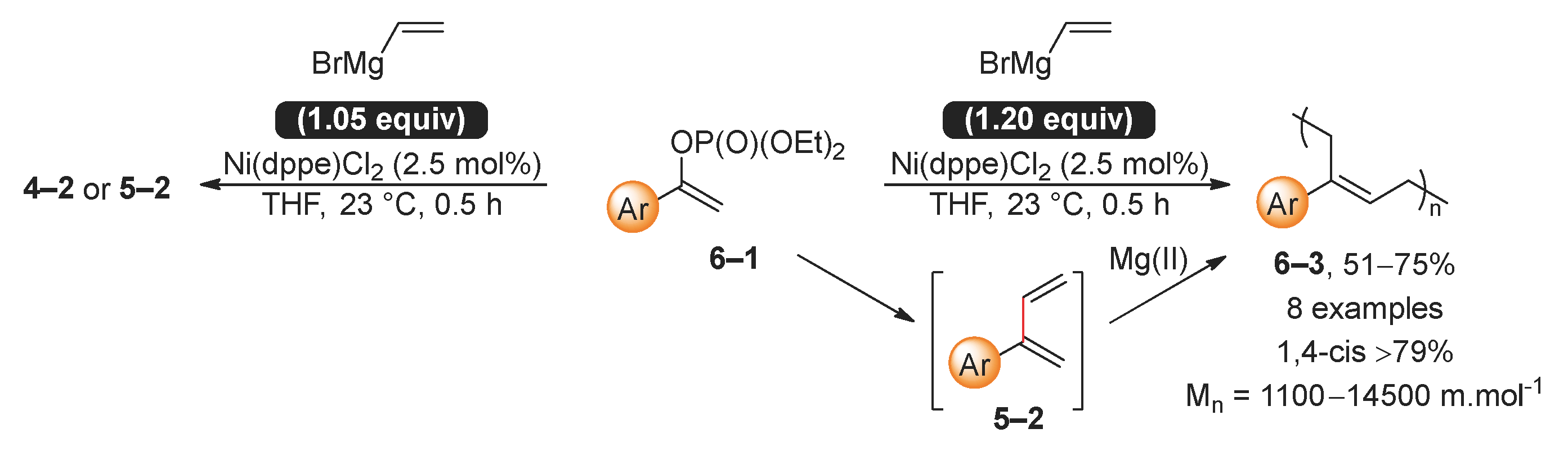
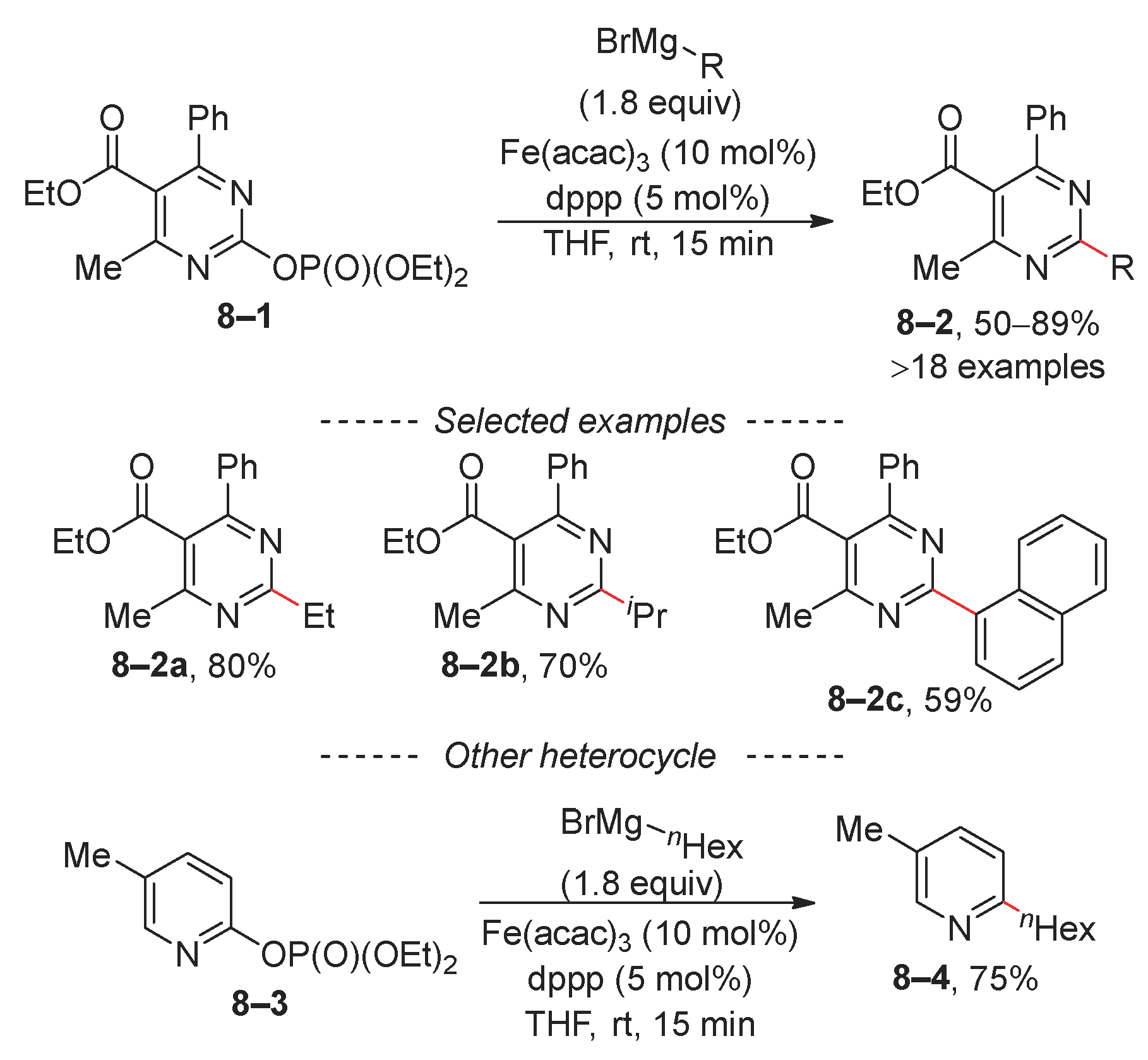
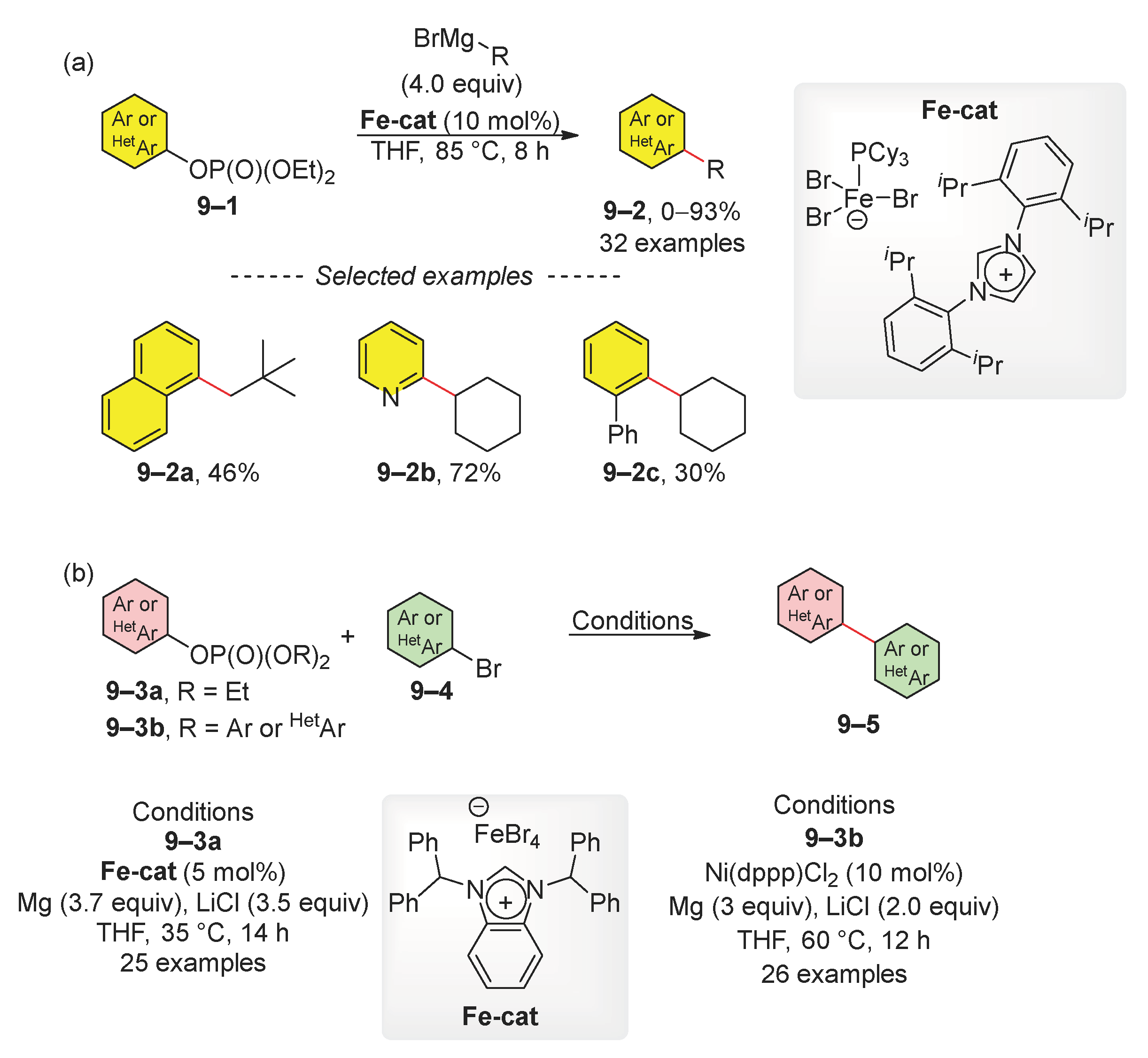
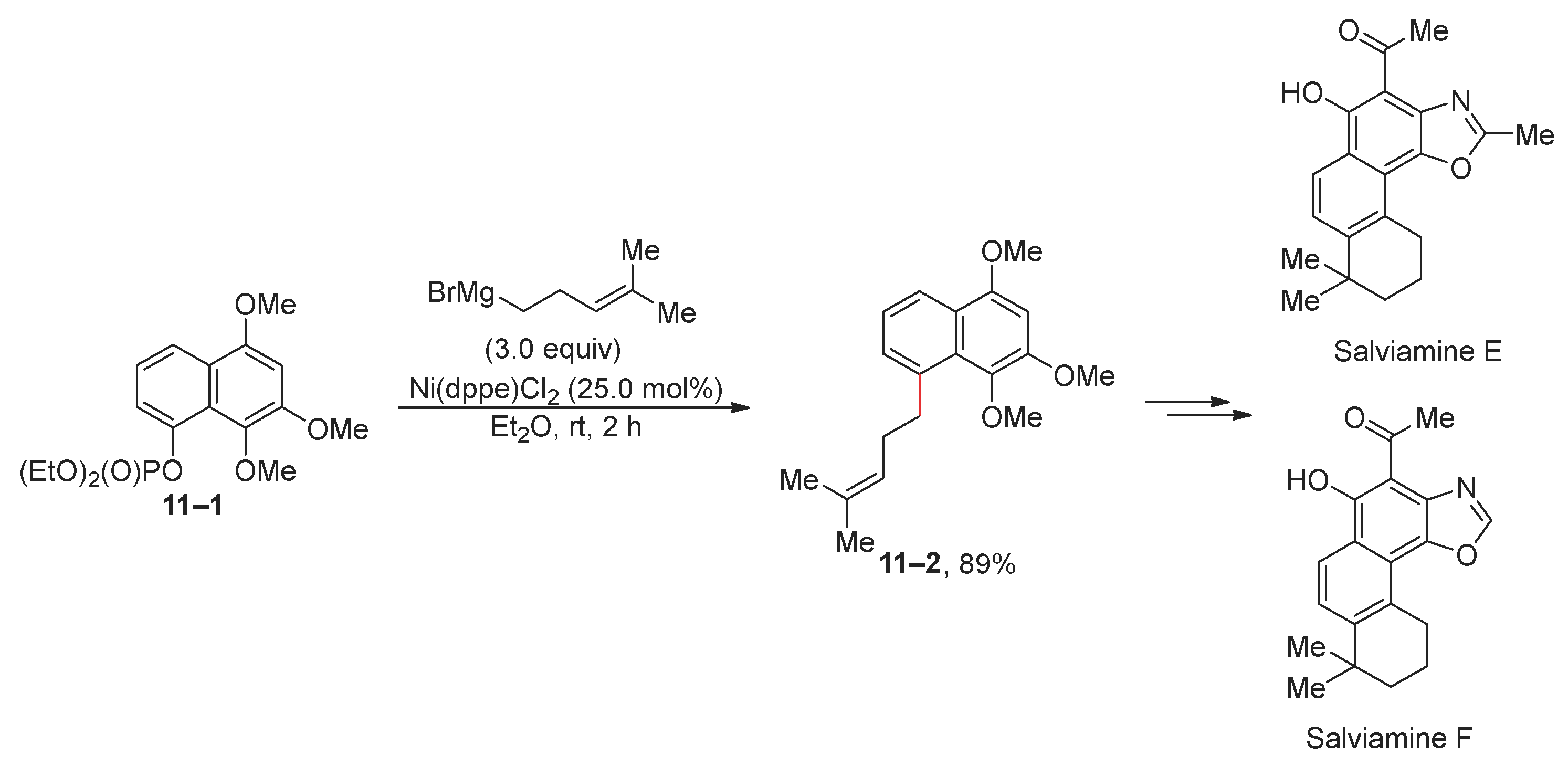
2.1. Kumada (Kumada–Tamao–Corriu) Reaction
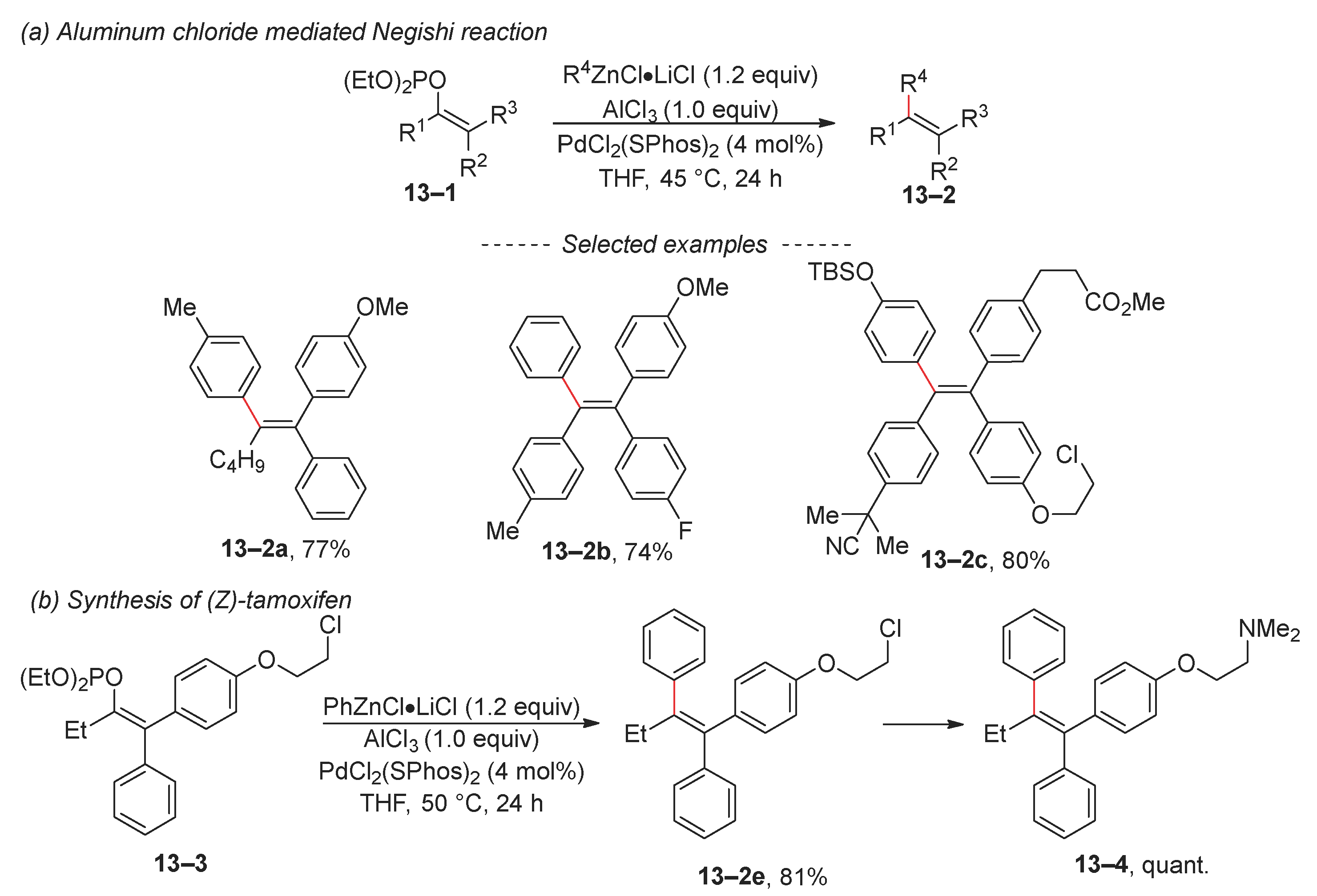
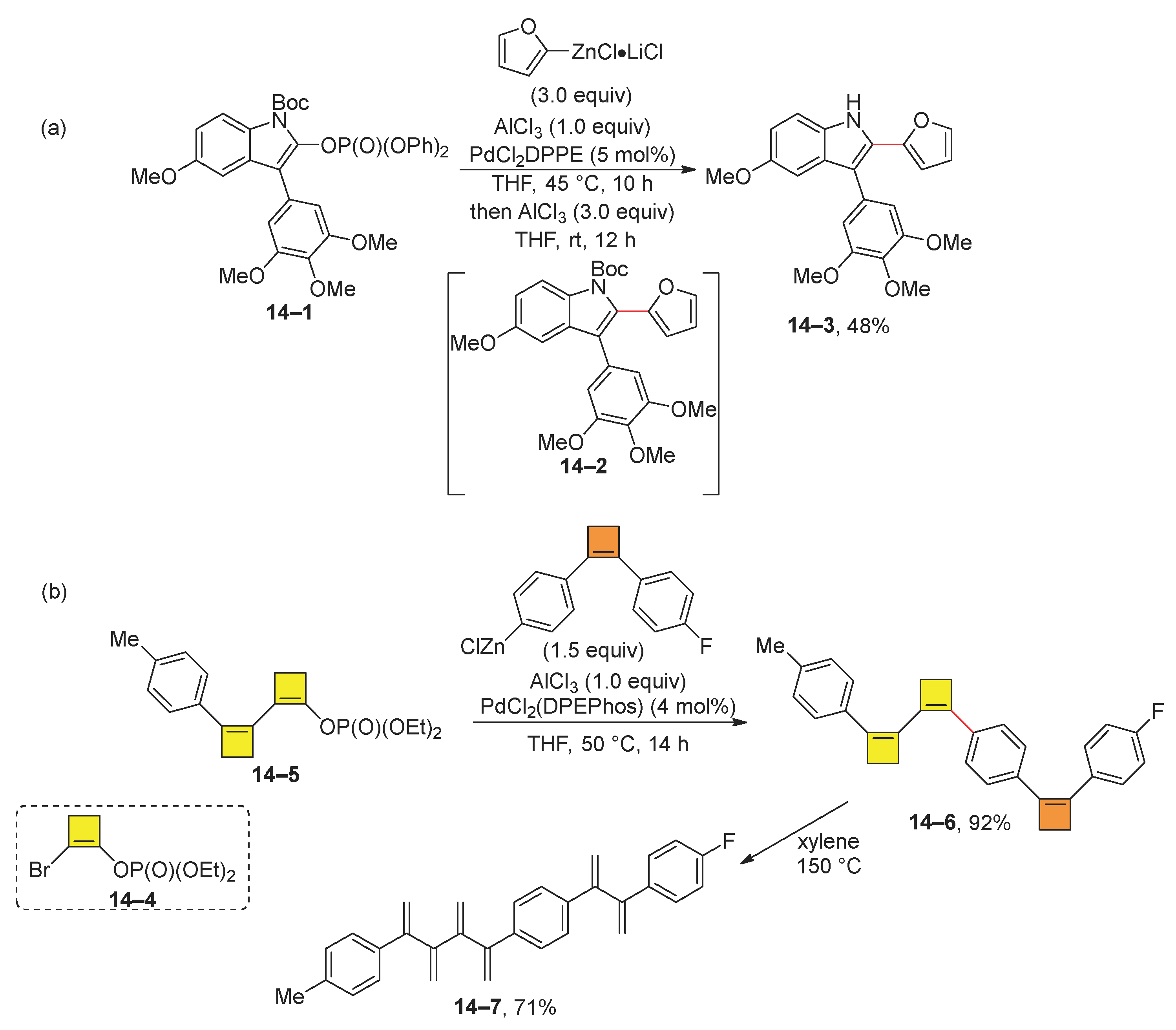
2.2. Negishi Reaction
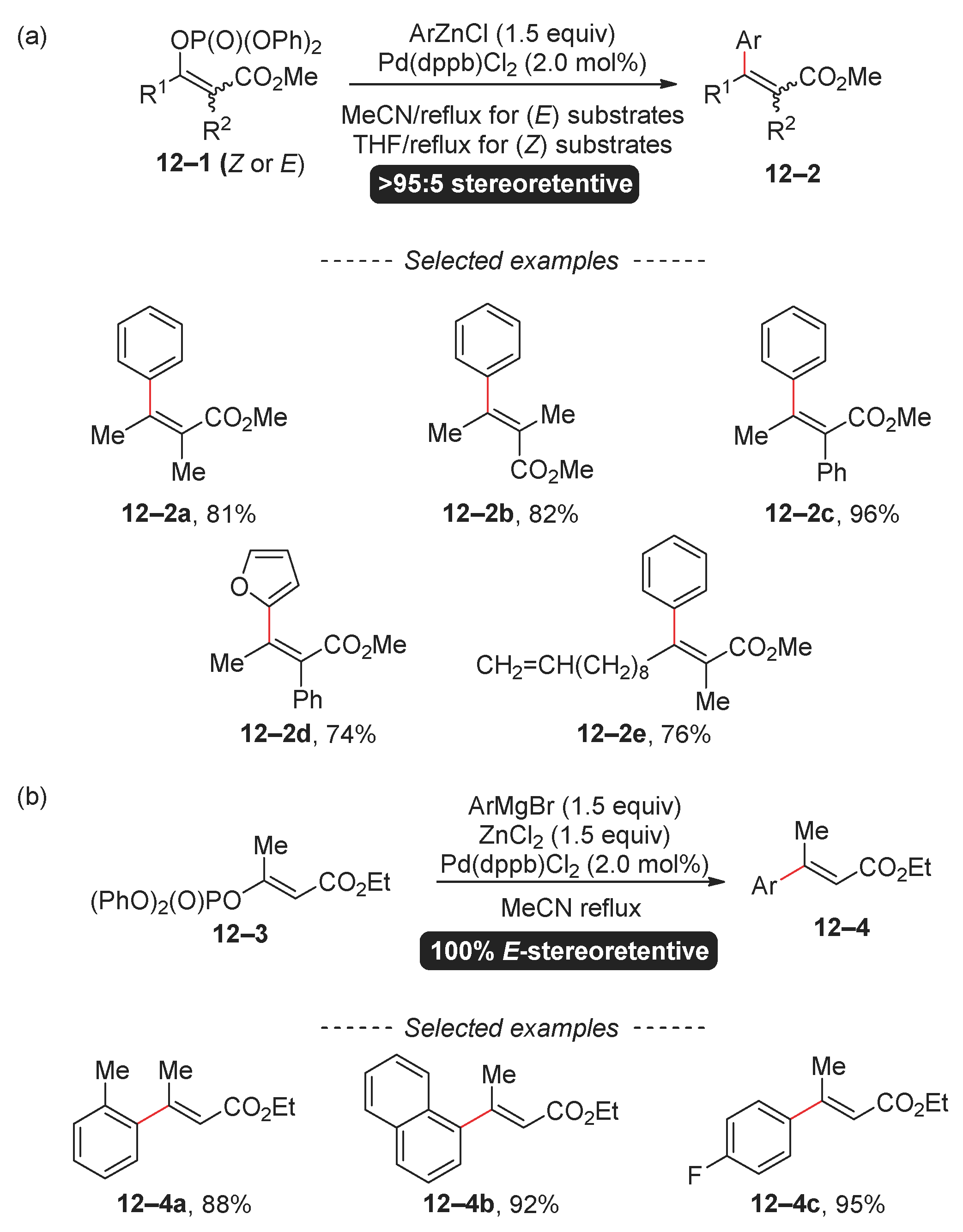
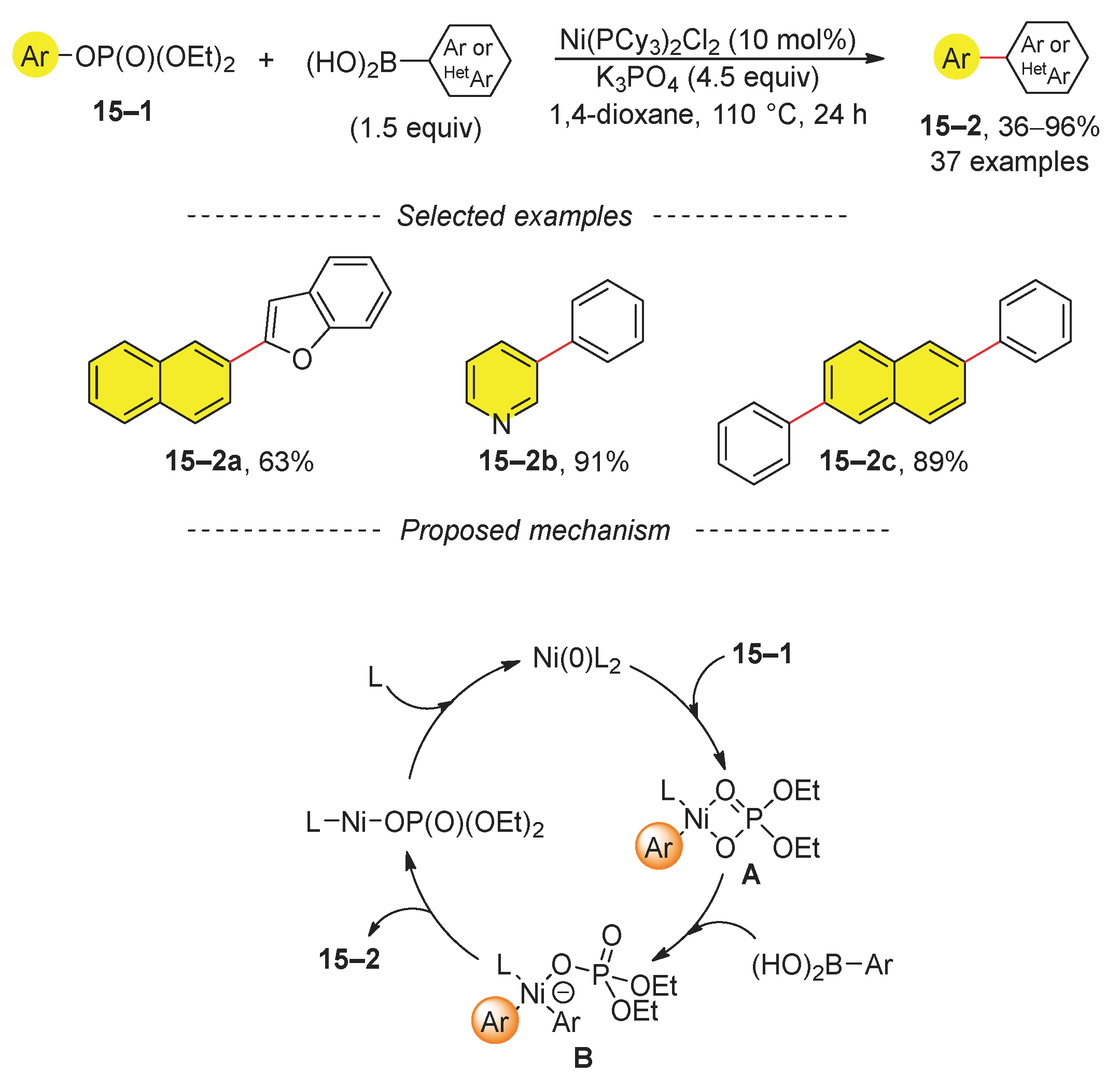
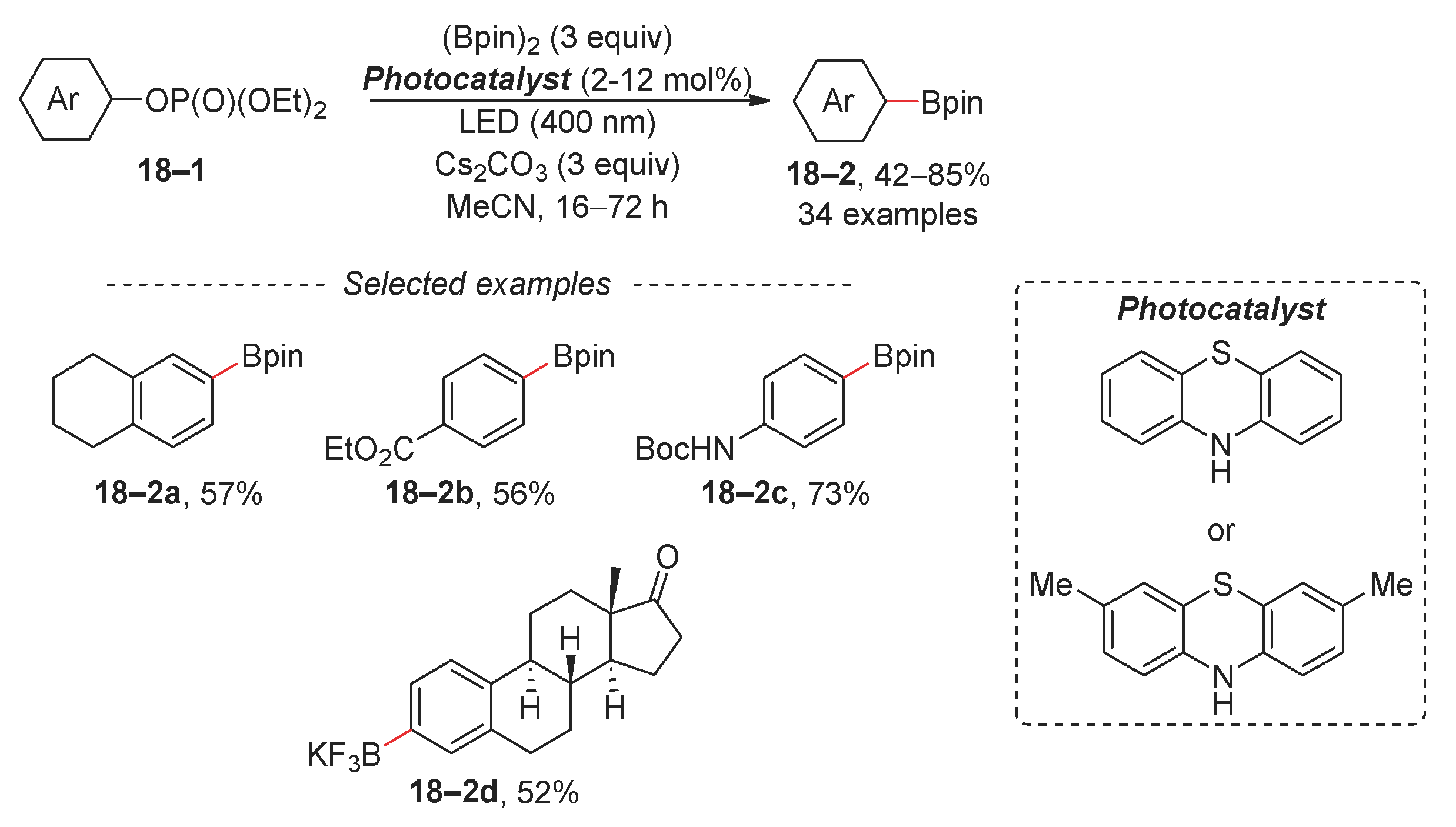
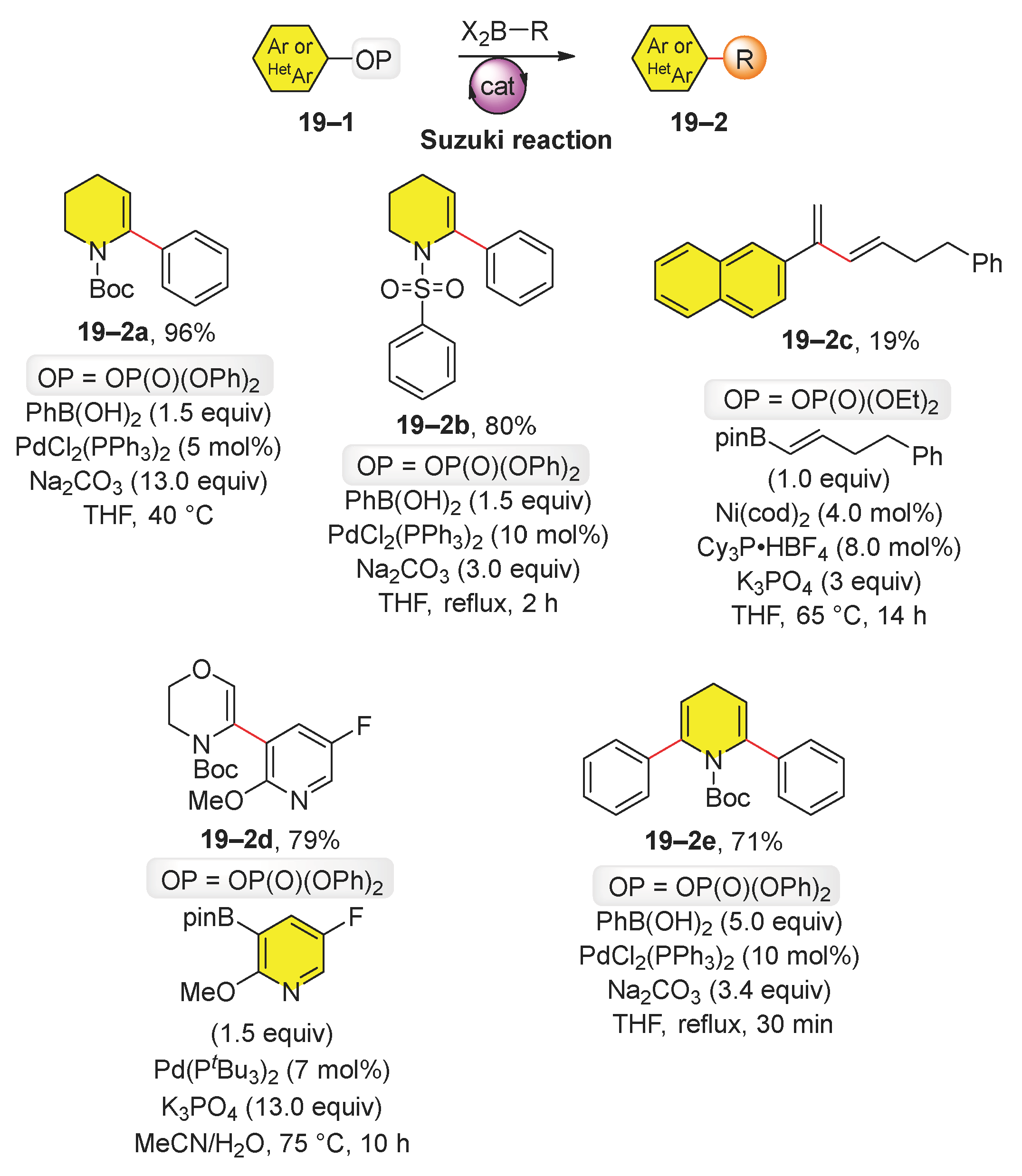
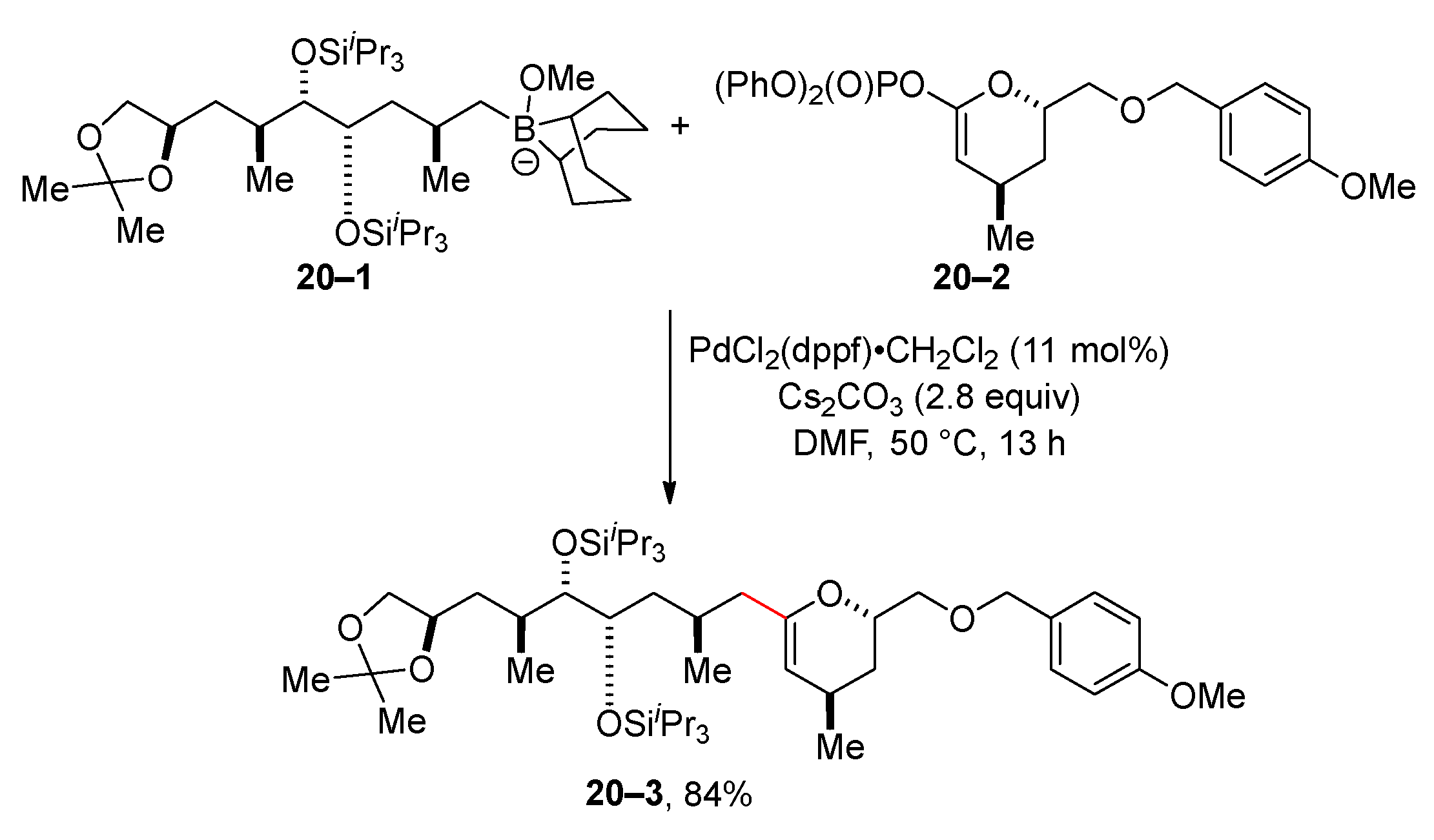
2.3. Suzuki Reaction
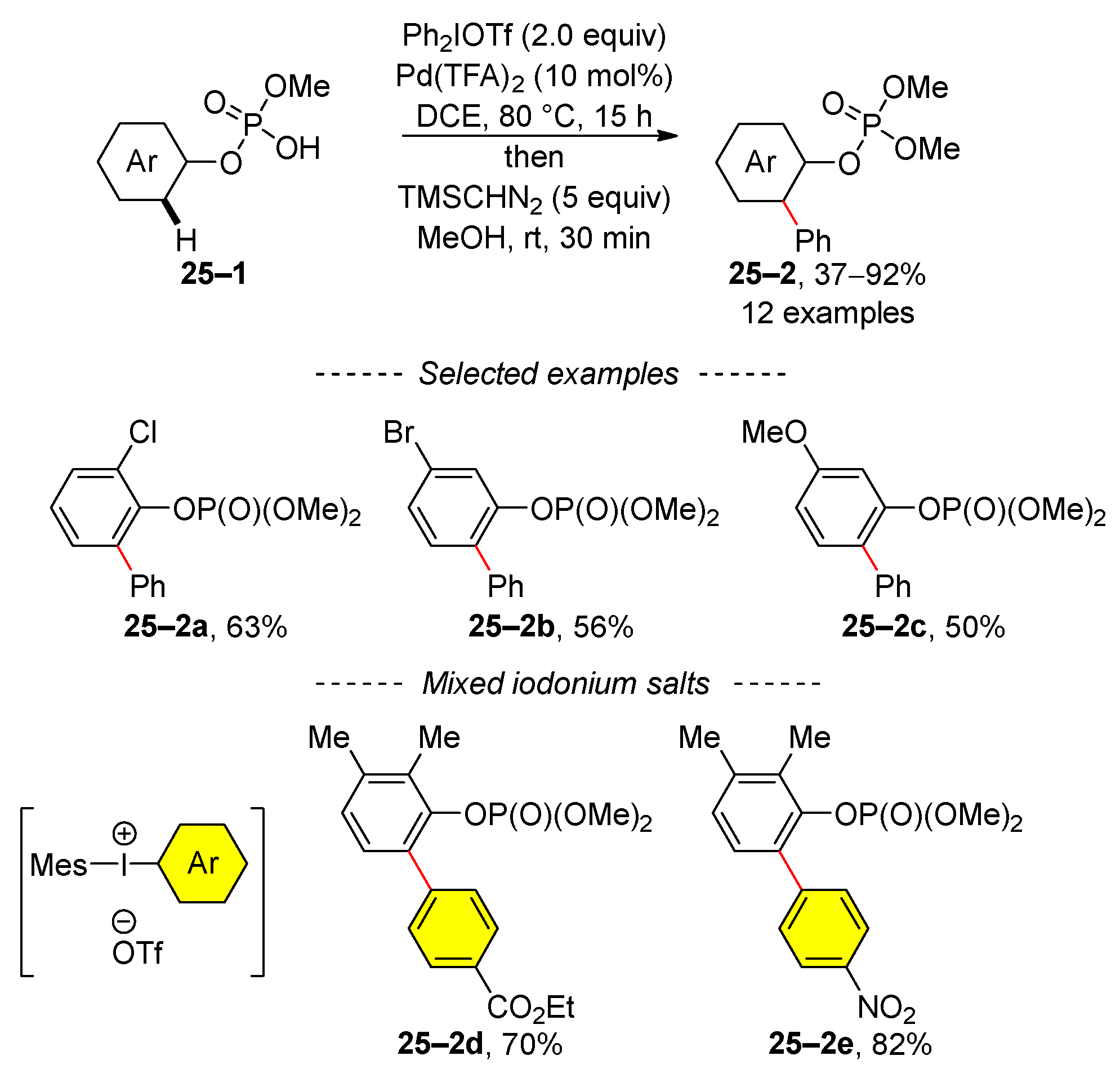
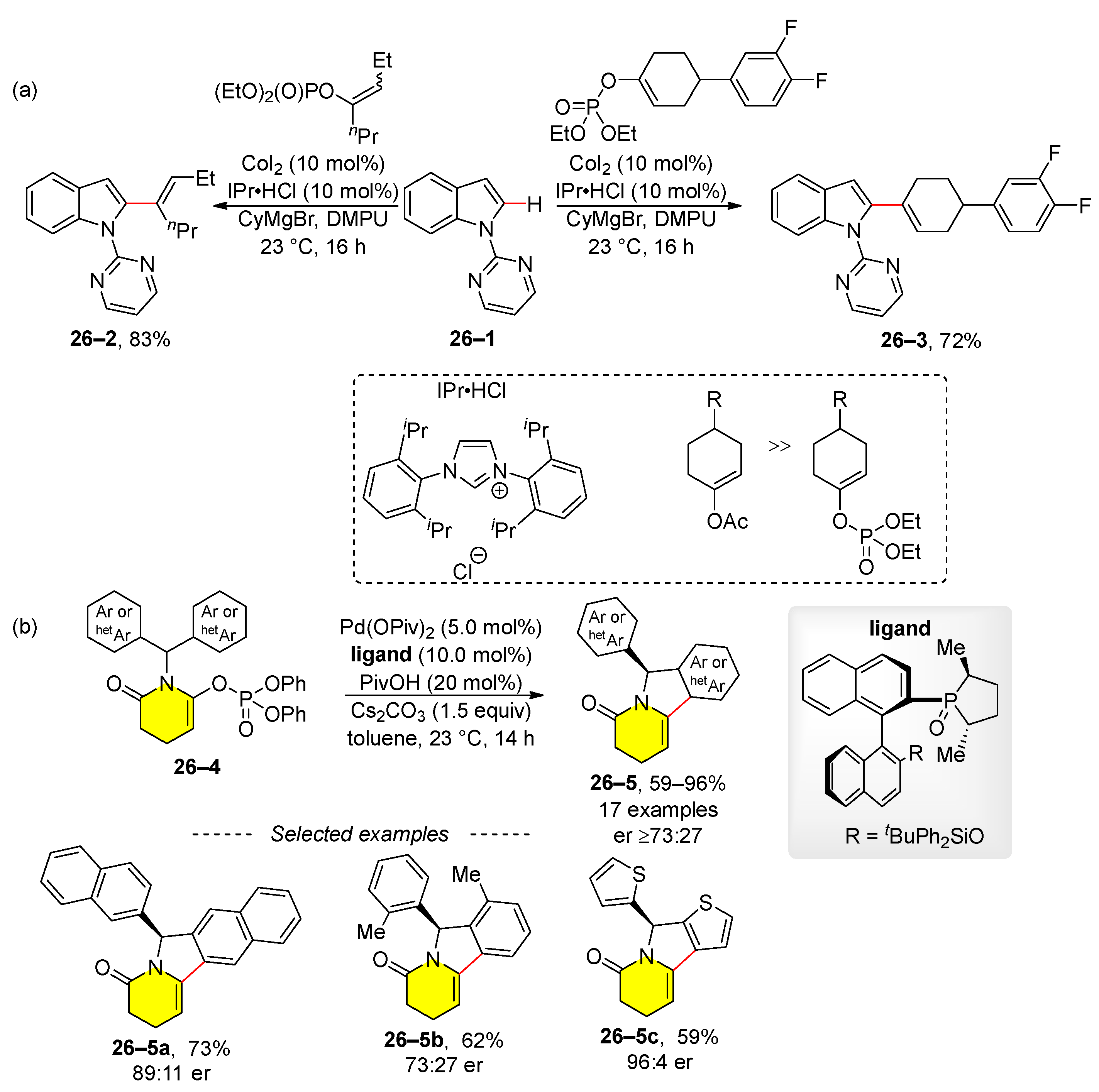
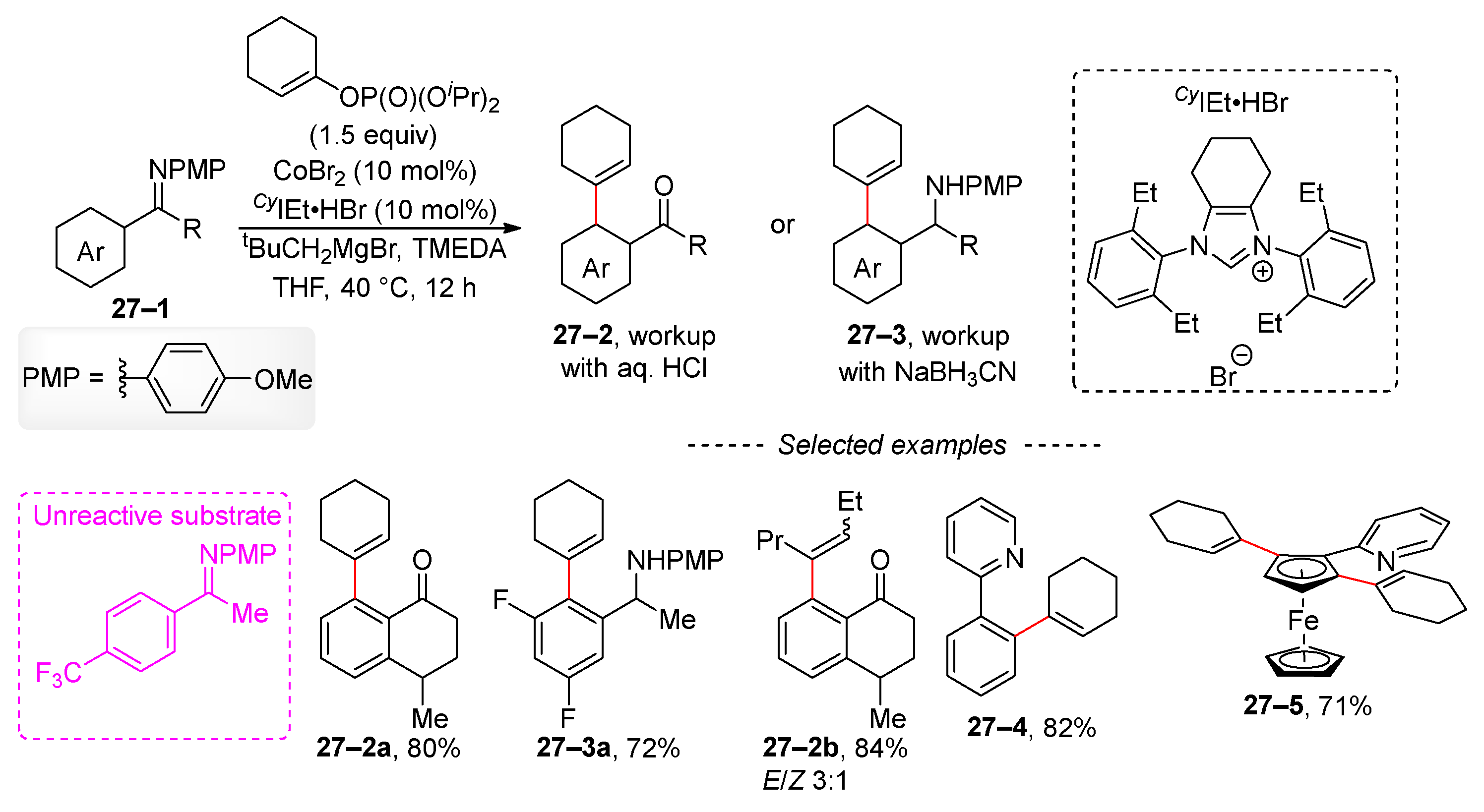
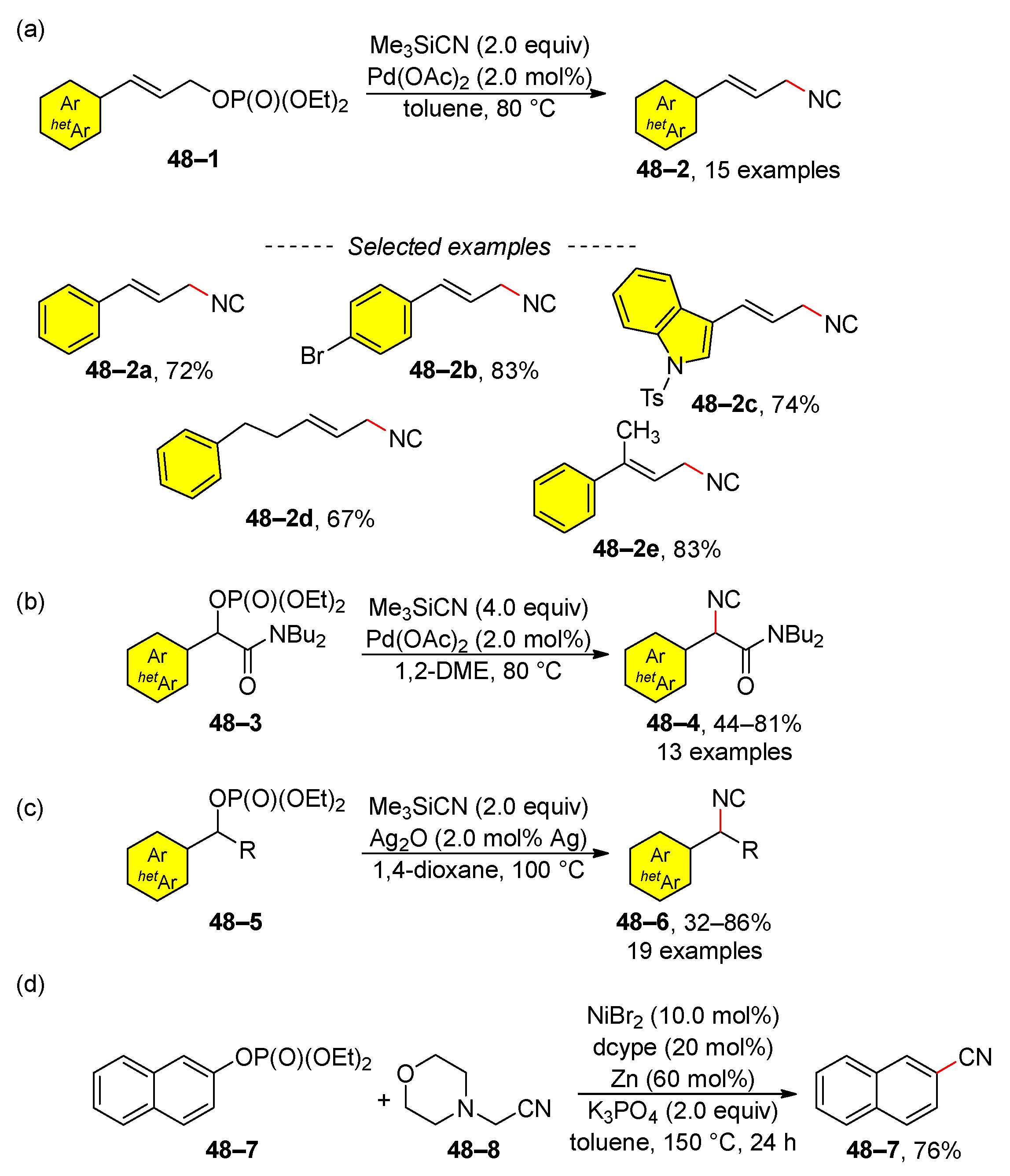
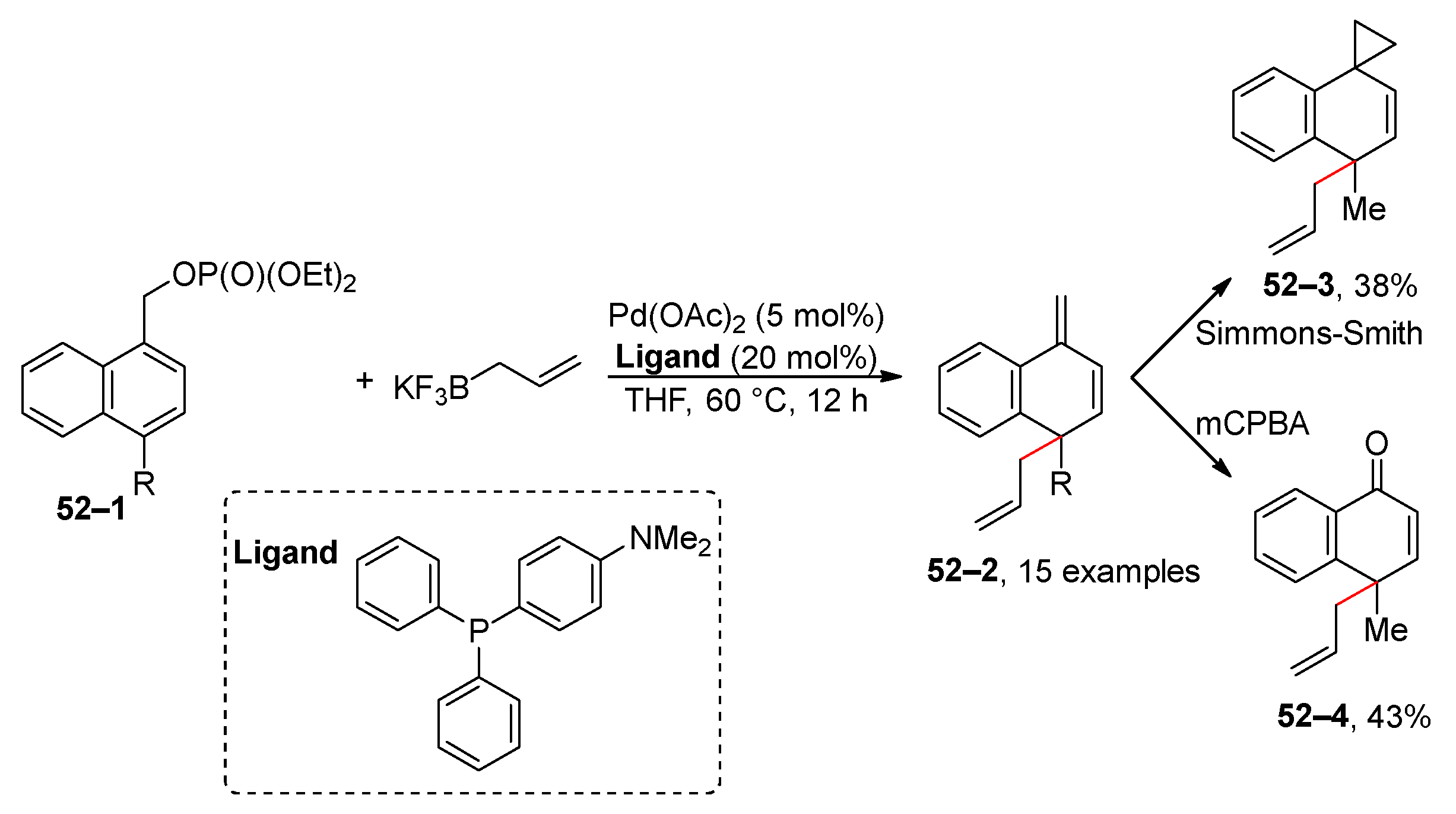
2.4. C–H Activation
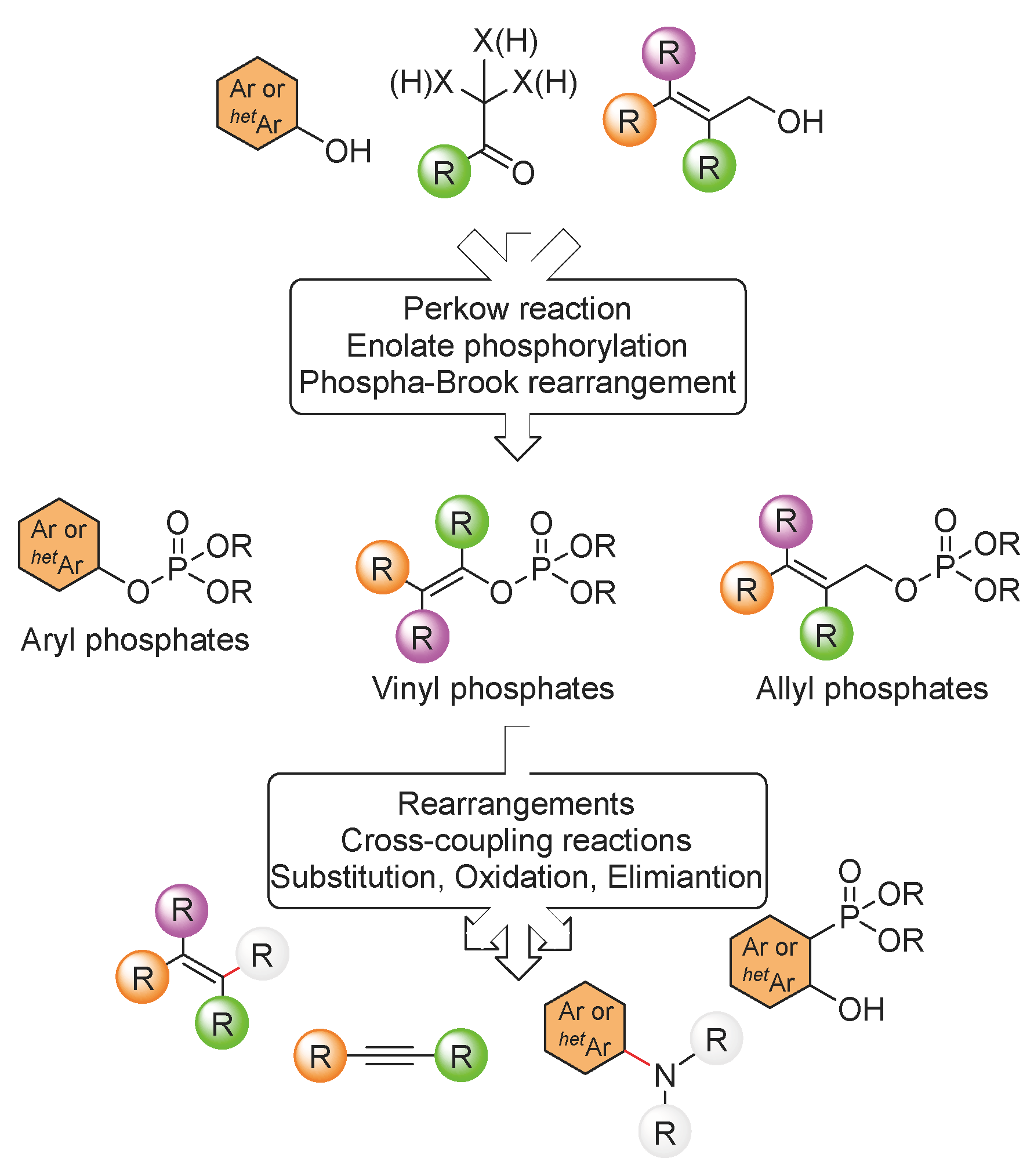
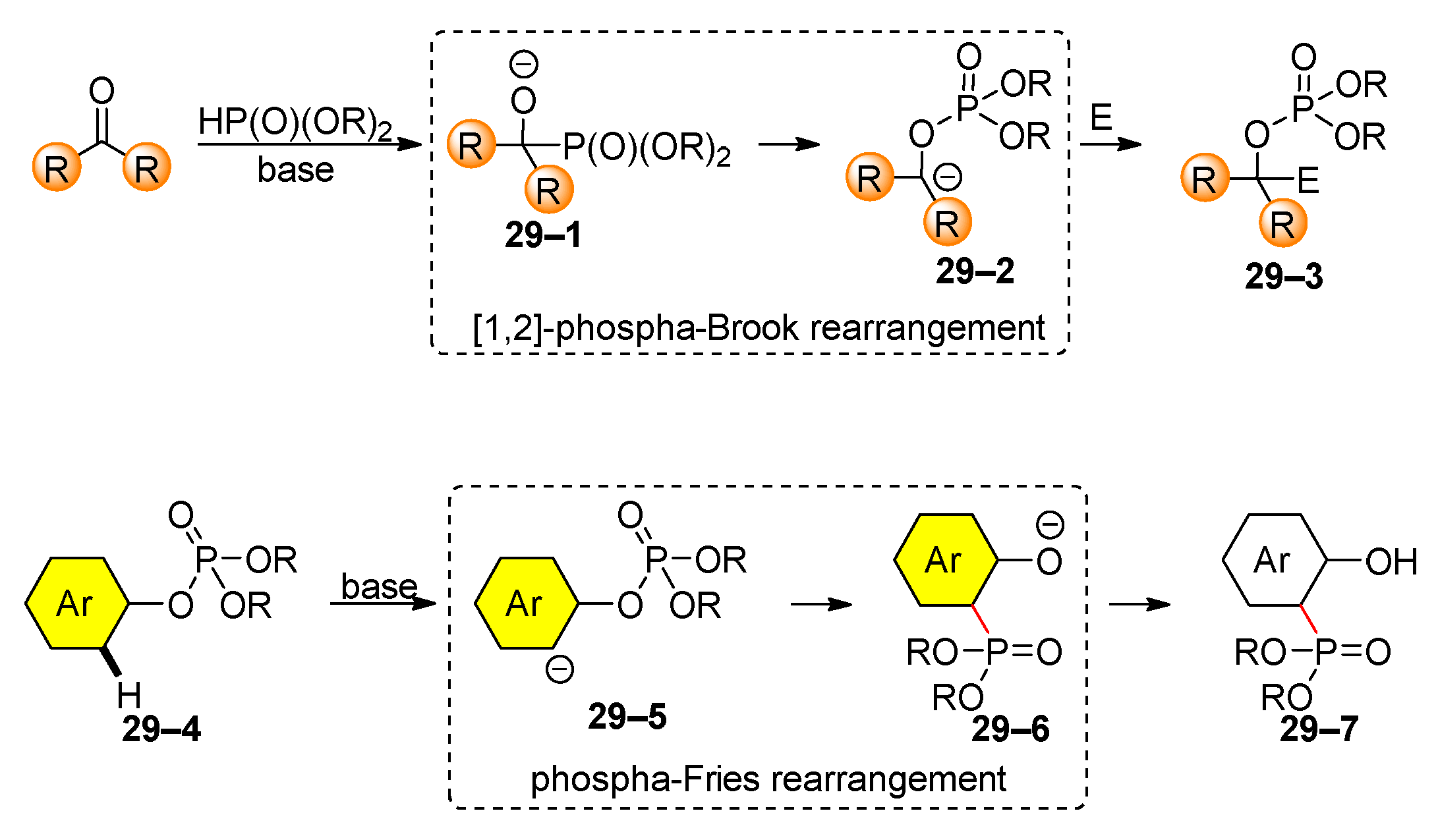
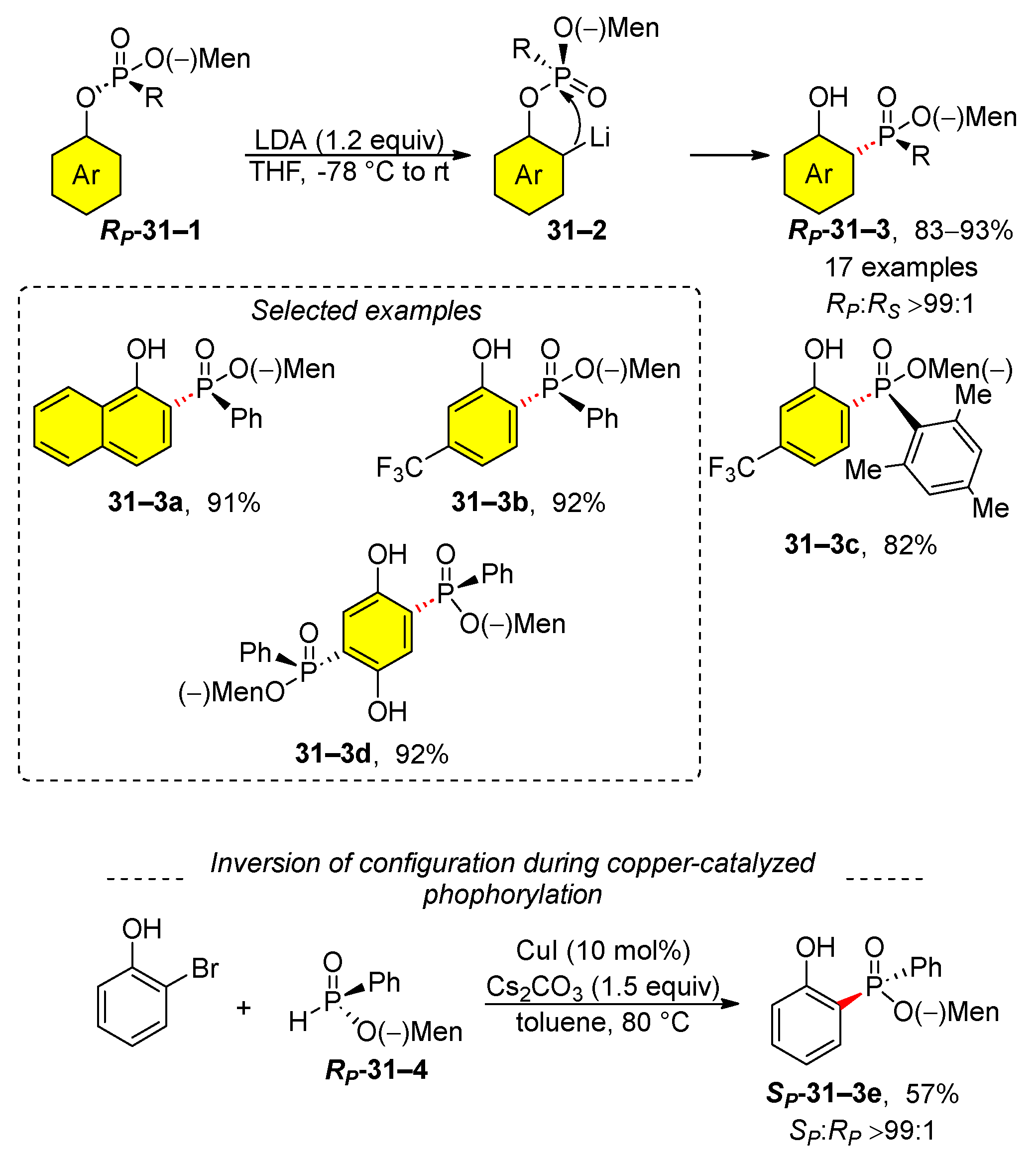
3. Organophosphate Rearrangements
4. Transition-Metal-Free Substitution Reactions of Organophosphates
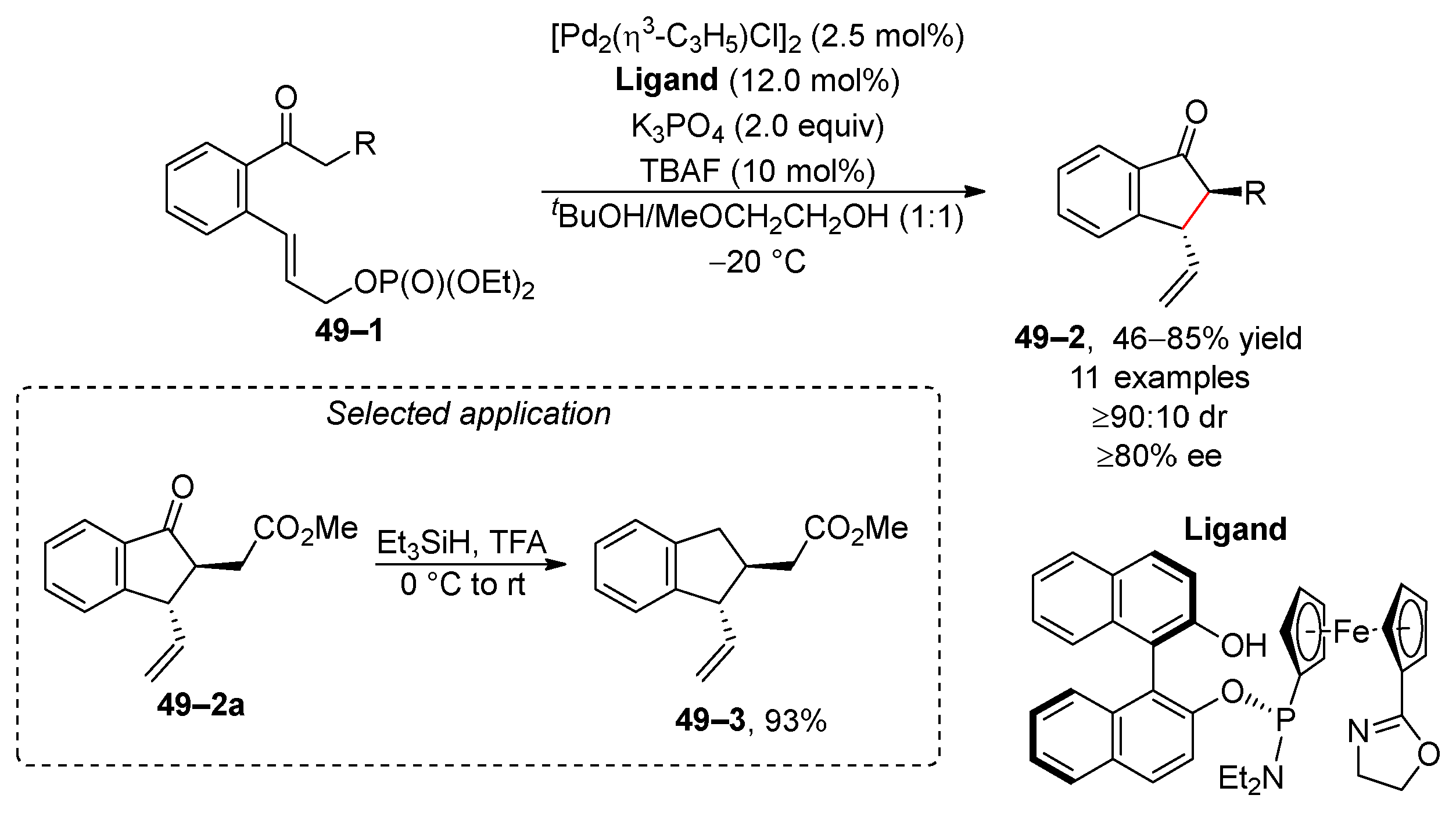
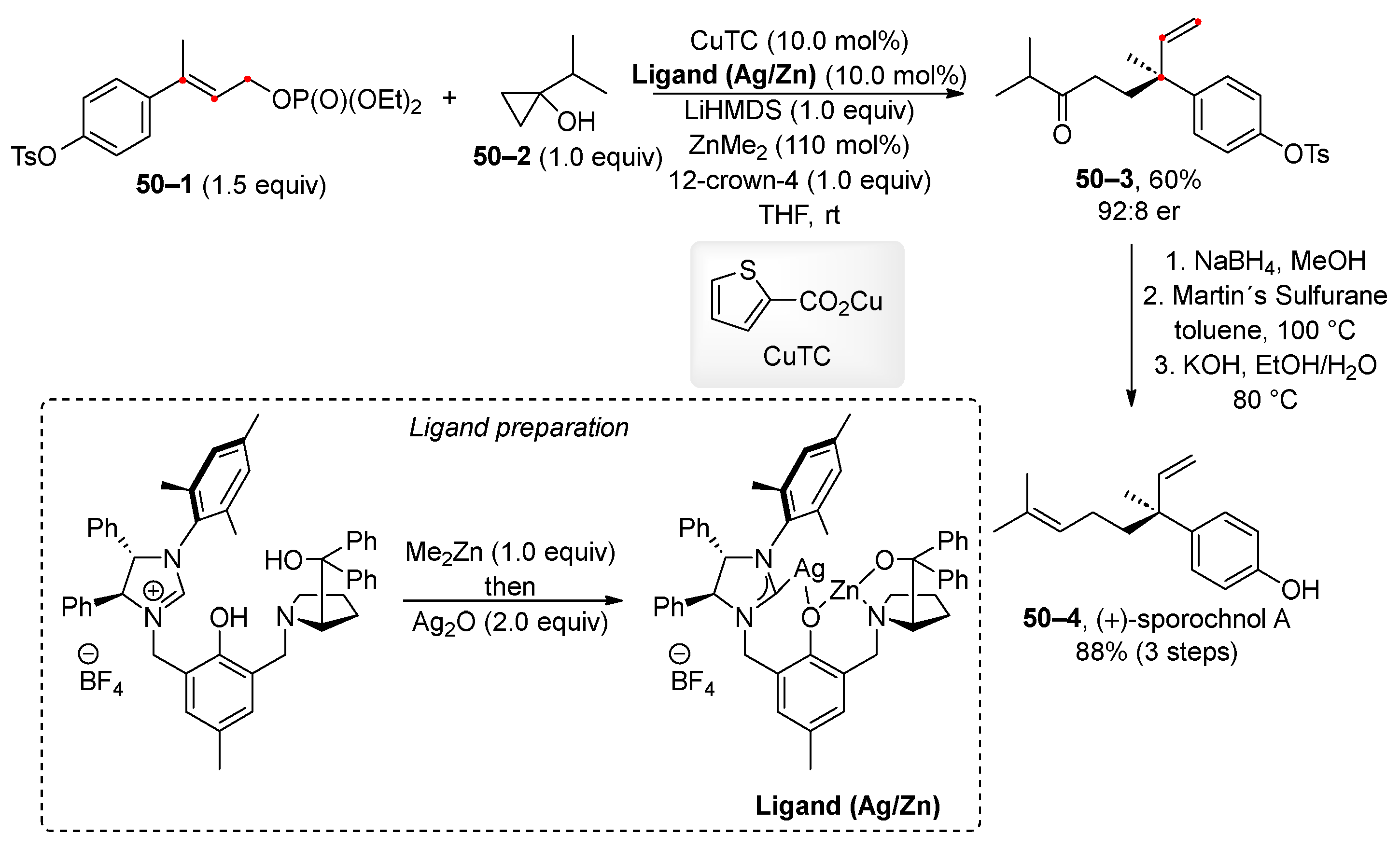
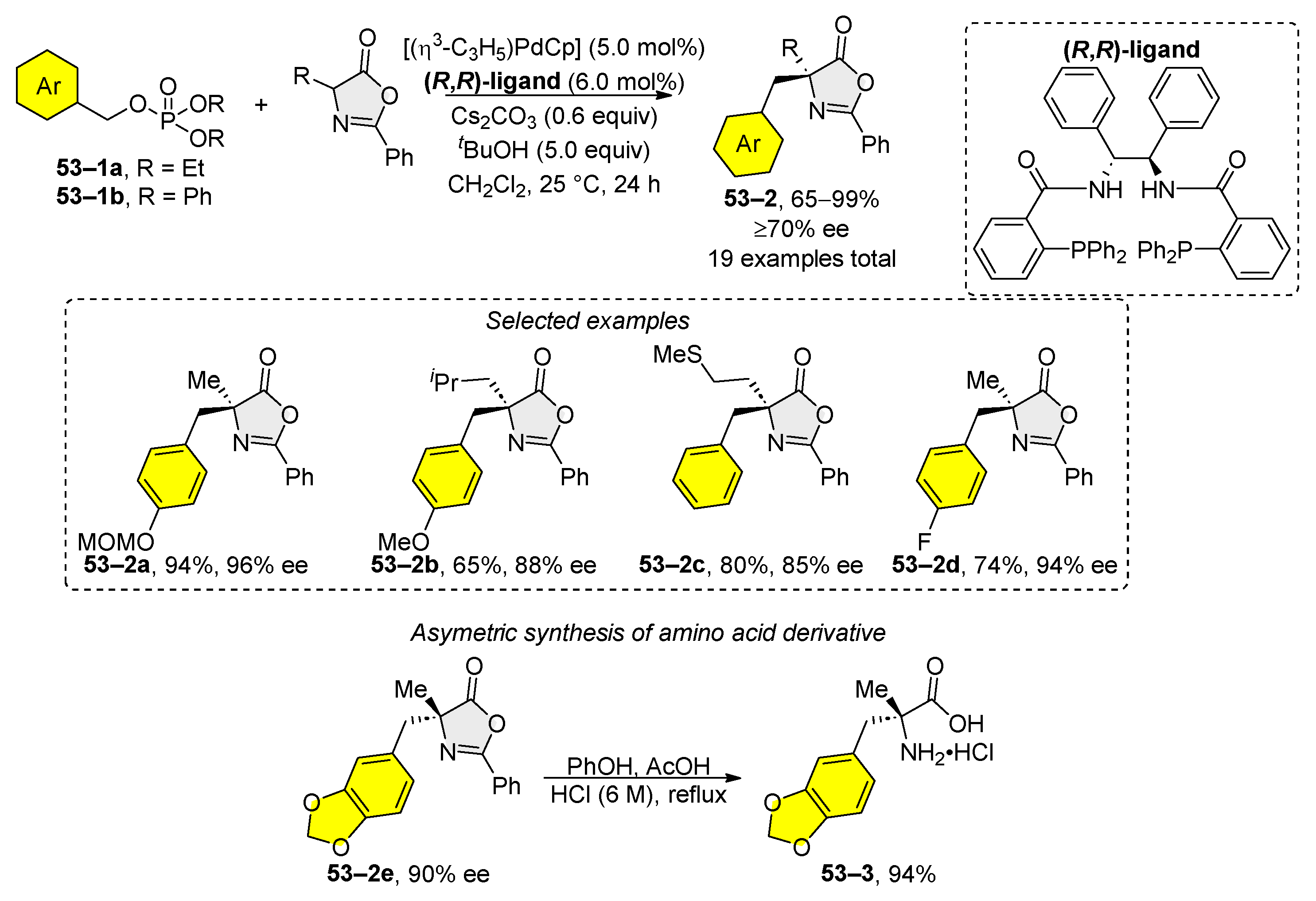
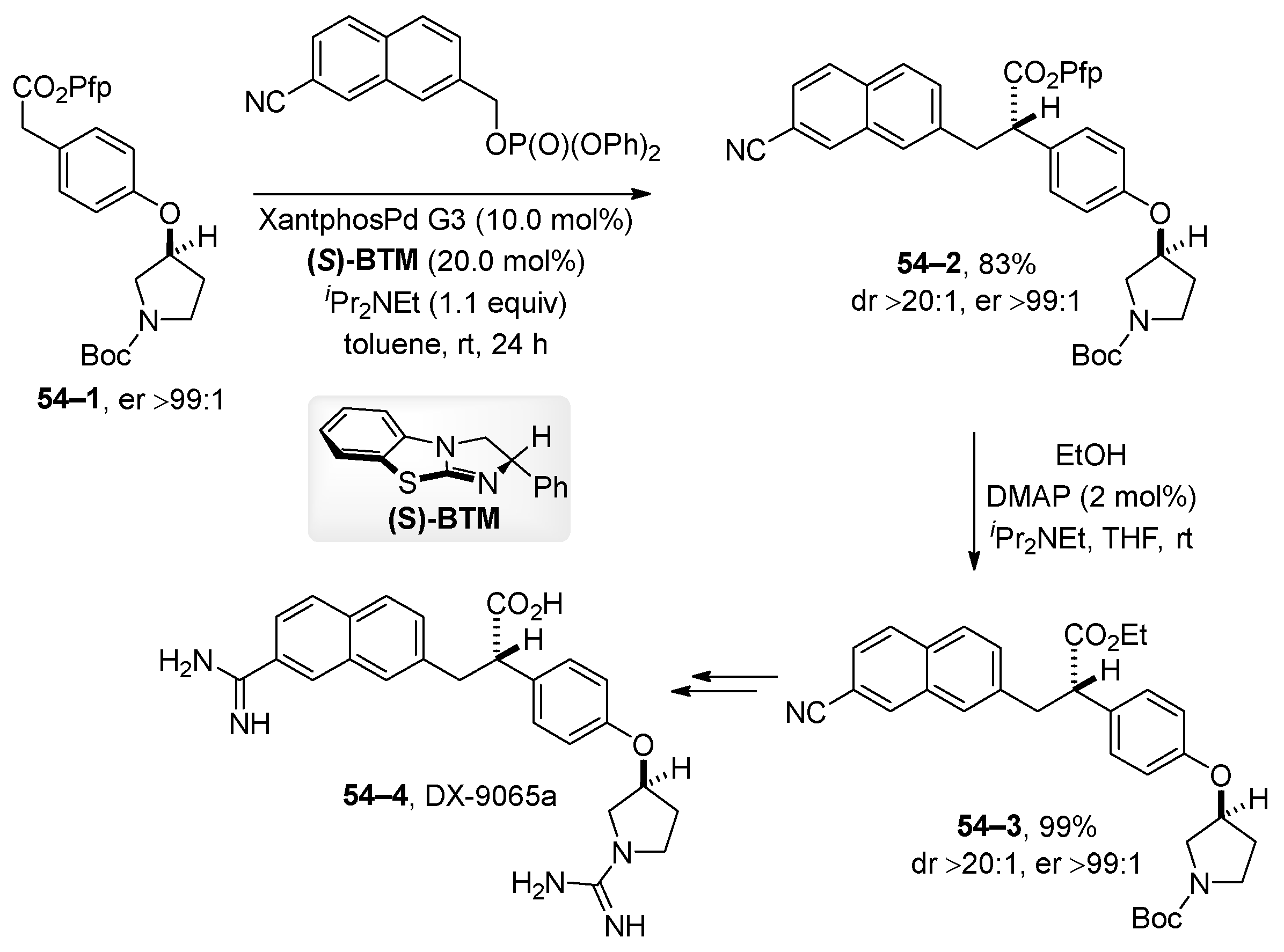
5. Transition-Metal-Catalyzed Allylic and Benzylic Substitution of Organophosphates
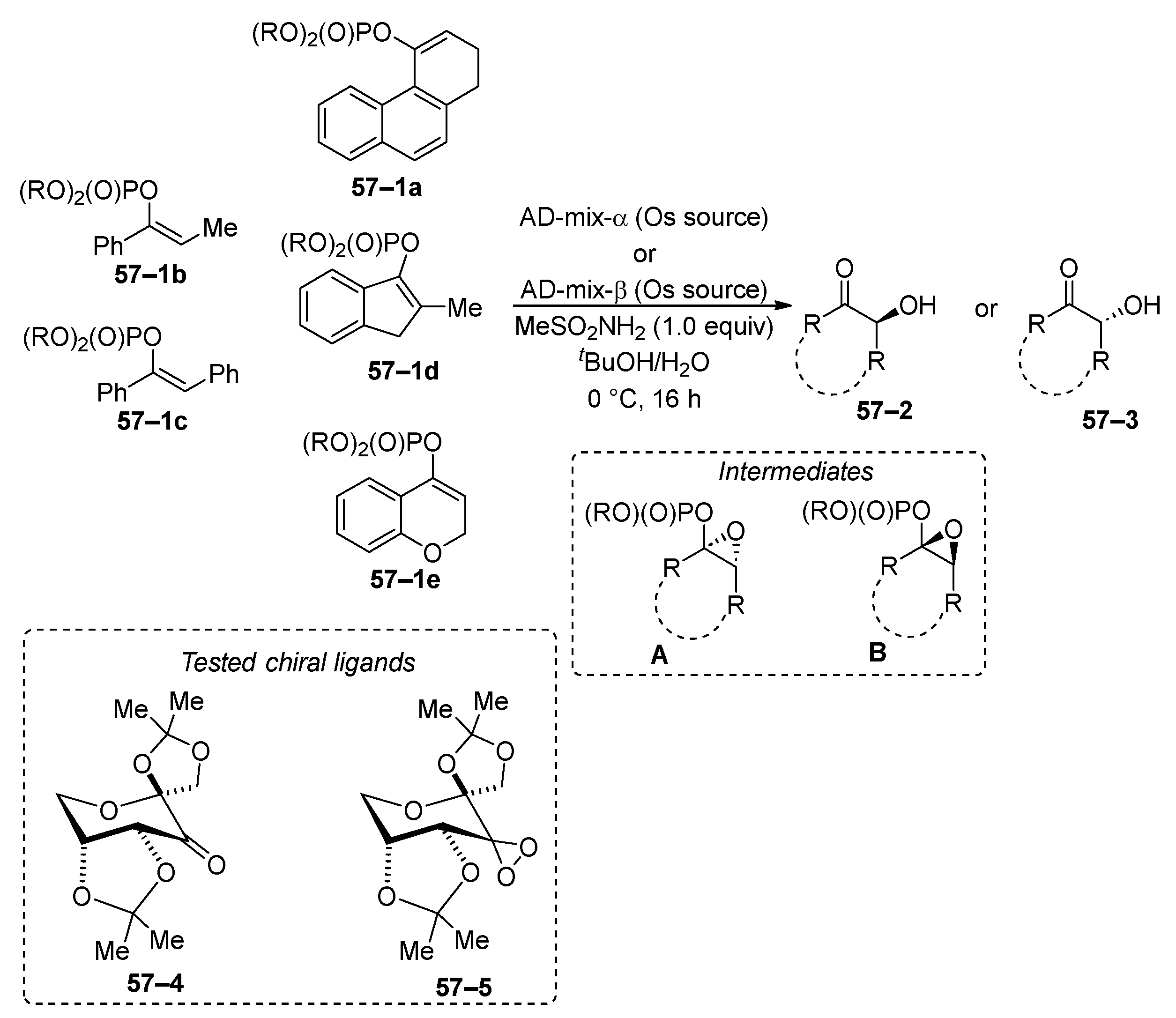
6. Oxidation and Reduction Reactions of Organophosphates
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lorke, D.E.; Petroianu, G.A. Reversible cholinesterase inhibitors as pretreatment for exposure to organophosphates. A review. J. Appl. Toxicol. 2019, 39, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Beynon, K.I.; Hutson, D.H.; Wright, A.N. The Metabolism and Degradation of Vinyl Phosphate Insecticides; Springer: New York, NY, USA, 1973; pp. 55–142. [Google Scholar]
- Lorke, D.E.; Petroianu, G.A. Minireview: Does in-vitro testing of oximes help predict their in-vivo action after paraoxon exposure? J. Appl. Toxicol. 2009, 29, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kaonga, C.C.; Chidya, R.C.G.; Kosamu, I.B.M.; Abdel-dayem, S.M.; Mapoma, H.W.T.; Thole, B.; Mbewe, R.; Sakugawa, H. Trends in usage of selected fungicides in Japan between 1962 and 2014: A review. Int. J. Environ. Sci. Technol. 2018, 15, 1801–1814. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Mukherjee, A.; Milstein, D. Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes. ACS Catal. 2018, 8, 11435–11469. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef]
- Atobe, M. Organic electrosynthesis in flow microreactor. Curr. Opin. Electrochem. 2017, 2, 1–6. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Šljukić, B.; Santos, D.M.F.; Sequeira, C.A.C. Organic Electrosynthesis: From Laboratorial Practice to Industrial Applications. Org. Proc. Res. Dev. 2017, 21, 1213–1226. [Google Scholar] [CrossRef]
- Marken, F.; Cresswell, A.J.; Bull, S.D. Recent Advances in Paired Electrosynthesis. Chem. Rec. 2021, 21, 2585–2600. [Google Scholar] [CrossRef]
- Siu, J.C.; Fu, N.; Lin, S. Catalyzing Electrosynthesis: A Homogeneous Electrocatalytic Approach to Reaction Discovery. Acc. Chem. Res. 2020, 53, 547–560. [Google Scholar] [CrossRef]
- Yuan, Y.; Lei, A. Is electrosynthesis always green and advantageous compared to traditional methods? Nat. Commun. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zheng, Y.; Fang, T.; Chen, Y.; Zhu, Y.; Liang, Q.; Sheng, H.; Li, Z.; Chen, C.; Wang, X. Photocatalysis: An overview of recent developments and technological advancements. Sci. China Chem. 2020, 63, 149–181. [Google Scholar] [CrossRef]
- Gisbertz, S.; Pieber, B. Heterogeneous Photocatalysis in Organic Synthesis. ChemPhotoChem 2020, 4, 456–475. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Knappke, C.E.I.; Grupe, S.; Gärtner, D.; Corpet, M.; Gosmini, C.; von Wangelin, A.J. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J. 2014, 20, 6828–6842. [Google Scholar] [CrossRef] [PubMed]
- Noël, T.; Buchwald, S.L. Cross-coupling in flow. Chem. Soc. Rev. 2011, 40, 5010–5029. [Google Scholar] [CrossRef]
- So, C.M.; Kwong, F.Y. Palladium-catalyzed cross-coupling reactions of aryl mesylates. Chem. Soc. Rev. 2011, 40, 4963–4972. [Google Scholar] [CrossRef]
- Thapa, S.; Shrestha, B.; Gurung, S.K.; Giri, R. Copper-catalysed cross-coupling: An untapped potential. Org. Biomol. Chem. 2015, 13, 4816–4827. [Google Scholar] [CrossRef]
- Tobrman, T. Vinyl Esters and Vinyl Sulfonates as Green Alternatives to Vinyl Bromide for the Synthesis of Monosubstituted Alkenes via Transition-Metal-Catalyzed Reactions. Chemistry 2023, 5, 2288–2321. [Google Scholar] [CrossRef]
- Čubiňák, M.; Edlová, T.; Polák, P.; Tobrman, T. Indolylboronic Acids: Preparation and Applications. Molecules 2019, 24, 3523. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Ghanbarian, M.; Ghalavand, N.; Nazari, N. Current Applications of the Sonogashira Reaction in the Synthesis of Heterocyclic Compounds: An Update. Curr. Org. Chem. 2018, 22, 1420–1457. [Google Scholar] [CrossRef]
- Malapit, C.A.; Howell, A.R. Recent Applications of Oxetanes in the Synthesis of Heterocyclic Compounds. J. Org. Chem. 2015, 80, 8489–8495. [Google Scholar] [CrossRef] [PubMed]
- Oeser, P.; Koudelka, J.; Petrenko, A.; Tobrman, T. Recent Progress Concerning the N-Arylation of Indoles. Molecules 2021, 26, 5079. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Jain, S.C. “In Water” Syntheses of Heterocyclic Compounds. Mini-Rev. Org. Chem. 2011, 8, 455–464. [Google Scholar] [CrossRef]
- Veisi, H.; Ghorbani-Vaghei, R. Recent progress in the application of N-halo reagents in the synthesis of heterocyclic compounds. Tetrahedron 2010, 66, 7445–7463. [Google Scholar] [CrossRef]
- Volkova, Y.; Baranin, S.; Zavarzin, I. A3 Coupling Reaction in the Synthesis of Heterocyclic Compounds. Adv. Synth. Catal. 2021, 363, 40–61. [Google Scholar] [CrossRef]
- Buttard, F.; Sharma, J.; Champagne, P.A. Recent advances in the stereoselective synthesis of acyclic all-carbon tetrasubstituted alkenes. Chem. Commun. 2021, 57, 4071–4088. [Google Scholar] [CrossRef]
- Flynn, A.B.; Ogilvie, W.W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745. [Google Scholar] [CrossRef]
- Mukherjee, N.; Planer, S.; Grela, K. Formation of tetrasubstituted C–C double bonds via olefin metathesis: Challenges, catalysts, and applications in natural product synthesis. Org. Chem. Front. 2018, 5, 494–516. [Google Scholar] [CrossRef]
- Paek, S.M. Synthesis of tetrasubstituted alkenes via metathesis. Molecules 2012, 17, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Edlová, T.; Čubiňák, M.; Tobrman, T. Cross-Coupling Reactions of Double or Triple Electrophilic Templates for Alkene Synthesis. Synthesis 2021, 53, 255–266. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Huang, Z.; Wang, G.; Mohan, S.; Wang, C.; Hattori, H. Recent Advances in Efficient and Selective Synthesis of Di-, Tri-, and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation−Carbonyl Olefination Synergy. Acc. Chem. Res. 2008, 41, 1474–1485. [Google Scholar] [CrossRef]
- Polák, P.; Váňová, H.; Dvořák, D.; Tobrman, T. Recent progress in transition metal-catalyzed stereoselective synthesis of acyclic all-carbon tetrasubstituted alkenes. Tetrahedron Lett. 2016, 57, 3684–3693. [Google Scholar] [CrossRef]
- Reiser, O. Palladium-Catalyzed Coupling Reactions for the Stereoselective Synthesis of Tri- and Tetrasubstituted Alkenes. Angew. Chem. Int. Ed. 2006, 45, 2838–2840. [Google Scholar] [CrossRef]
- Krishnakumar, V.K.; Sharma, M.M. Synthesis of Triaryl Phosphates via Phase-Transfer Catalysis. Synthesis 1983, 1983, 558–559. [Google Scholar] [CrossRef]
- Zhong, C.; Huang, Y.; Zhang, H.; Zhou, Q.; Liu, Y.; Lu, P. Enantioselective Synthesis of 3-Substituted Cyclobutenes by Catalytic Conjugate Addition/Trapping Strategies. Angew. Chem. Int. Ed. 2020, 59, 2750–2754. [Google Scholar] [CrossRef]
- Kotek, V.; Polák, P.; Tobrman, T. Efficient and simple preparation of functionalized 1,1-dibromoenol phosphates. Monat. Chem. 2016, 147, 405–412. [Google Scholar] [CrossRef]
- Kawada, H.; Ikoma, A.; Ogawa, N.; Kobayashi, Y. Activation of Marginally Reactive Boron Enolates by MeLi for the Formation of Enol Phosphates and Synthesis of the Δ9-THC Intermediate. J. Org. Chem. 2015, 80, 9192–9199. [Google Scholar] [CrossRef]
- Perkow, W. Umsetzungen mit Alkylphosphiten. I. Mitteil.: Umlagerungen bei der Reaktion mit Chloral und Bromal. Chem. Ber. 1954, 87, 755–758. [Google Scholar] [CrossRef]
- Adamek, J. Special Issue “Organophosphorus Chemistry: A New Perspective”. Molecules 2023, 28, 4752. [Google Scholar] [CrossRef]
- Keglevich, G. Organophosphorus Chemistry 2021. Molecules 2023, 28, 394. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.R. Organophosphorus chemistry. Beilstein J. Org. Chem. 2014, 10, 2087–2088. [Google Scholar] [CrossRef]
- Han, L.-B.; Yang, S.-D.; Waterman, R.; Weigand, J.J. Love in the Time of COVID. J. Org. Chem. 2020, 85, 14273–14275. [Google Scholar] [CrossRef]
- Fiorito, D.; Folliet, S.; Liu, Y.; Mazet, C. A General Nickel-Catalyzed Kumada Vinylation for the Preparation of 2-Substituted 1,3-Dienes. ACS Catal. 2018, 8, 1392–1398. [Google Scholar] [CrossRef]
- Braconi, E.; Cramer, N. Crossed Regio- and Enantioselective Iron-Catalyzed [4+2]-Cycloadditions of Unactivated Dienes. Angew. Chem. Int. Ed. 2022, 61, e202112148. [Google Scholar] [CrossRef] [PubMed]
- Braconi, E.; Götzinger, A.C.; Cramer, N. Enantioselective Iron-Catalyzed Cross-[4+4]-Cycloaddition of 1,3-Dienes Provides Chiral Cyclooctadienes. J. Am. Chem. Soc. 2020, 142, 19819–19824. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.R.; Zhong, H.; Macaulay, R.L.; Chirik, P.J. Regio- and Diastereoselective Iron-Catalyzed [4+4]-Cycloaddition of 1,3-Dienes. J. Am. Chem. Soc. 2019, 141, 8557–8573. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Ng, J.J.W.; Chiba, S. Generation of Allylmagnesium Reagents by Hydromagnesiation of 2-Aryl-1,3-dienes. Angew. Chem. Int. Ed. 2023, 62, e202217735. [Google Scholar] [CrossRef]
- Ohta, R.; Shio, Y.; Akiyama, T.; Yamada, M.; Harada, K.; Arisawa, M. Ligand-free reductive coupling of aldehydes with 1,3-dienes using a sulfur-modified Au-supported nickel nanoparticle catalyst. New J. Chem. 2023, 47, 7694–7700. [Google Scholar] [CrossRef]
- Zhao, H.; Caldora, H.P.; Turner, O.; Douglas, J.J.; Leonori, D. A Desaturative Approach for Aromatic Aldehyde Synthesis via Synergistic Enamine, Photoredox and Cobalt Triple Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202201870. [Google Scholar] [CrossRef]
- Li, C.; Shin, K.; Liu, R.Y.; Buchwald, S.L. Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem. Int. Ed. 2019, 58, 17074–17080. [Google Scholar] [CrossRef] [PubMed]
- Poisson, P.-A.; Tran, G.; Besnard, C.; Mazet, C. Nickel-Catalyzed Kumada Vinylation of Enol Phosphates: A Comparative Mechanistic Study. ACS Catal. 2021, 11, 15041–15050. [Google Scholar] [CrossRef]
- Fiorito, D.; Simon, M.; Thomas, C.M.; Mazet, C. Access to Highly Stereodefined 1,4-cis-Polydienes by a [Ni/Mg] Orthogonal Tandem Catalytic Polymerization. J. Am. Chem. Soc. 2021, 143, 13401–13407. [Google Scholar] [CrossRef] [PubMed]
- Desfeux, C.; Besnard, C.; Mazet, C. [n]Dendralenes as a Platform for Selective Catalysis: Ligand-Controlled Cu-Catalyzed Chemo-, Regio-, and Enantioselective Borylations. Org. Lett. 2020, 22, 8181–8187. [Google Scholar] [CrossRef] [PubMed]
- Saglam, M.F.; Fallon, T.; Paddon-Row, M.N.; Sherburn, M.S. Discovery and Computational Rationalization of Diminishing Alternation in [n]Dendralenes. J. Am. Chem. Soc. 2016, 138, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zhang, Z.; Da, Y.-X.; Quan, Z.-J.; Wang, X.-C. Iron-Catalyzed Kumada Cross-Coupling Reactions of Pyrimidin-2-yl Phosphates: An Efficient Approach to C2-Functionalized Pyrimidines. Asian J. Org. Chem. 2015, 4, 538–544. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Sun, H.-m.; Shen, Q.; Zhang, Y. Alkyl Grignard cross-coupling of aryl phosphates catalyzed by new, highly active ionic iron(ii) complexes containing a phosphine ligand and an imidazolium cation. Dalton Trans. 2016, 45, 17739–17747. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, B.; Sun, H.; Shen, Q.; Zhang, Y. Ionic iron(III) complexes bearing a dialkylbenzimidazolium cation: Efficient catalysts for magnesium-mediated cross-couplings of aryl phosphates with alkyl bromides. Appl. Organometal. Chem. 2017, 31, e3671. [Google Scholar] [CrossRef]
- Ren, J.-A.; Chen, X.; Gui, C.; Miao, C.; Chu, X.-Q.; Xu, H.; Zhou, X.; Ma, M.; Shen, Z.-L. Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Phosphates with Aryl Bromides. Adv. Synth. Catal. 2023, 365, 2511–2515. [Google Scholar] [CrossRef]
- Cui, M.; Oestreich, M. Synthesis of Silylated Cyclobutanone and Cyclobutene Derivatives Involving 1,4-Addition of Zinc-Based Silicon Nucleophiles. Chem. Eur. J. 2021, 27, 16103–16106. [Google Scholar] [CrossRef]
- Moinizadeh, N.; Klemme, R.; Kansy, M.; Zimmer, R.; Reissig, H.-U. Convenient Syntheses of Enantiopure 1,2-Oxazin-4-yl Nonaflates and Phosphates and Their Palladium-Catalyzed Cross-Couplings. Synthesis 2013, 45, 2752–2762. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, J.; Liu, Y.; Lu, P. Synthesis of Dibenzo[a,e]cyclooctene-5,11(6H,12H)-diones via the Elusive Benzocyclobutenone Anion. Synthesis 2021, 53, 4477–4483. [Google Scholar] [CrossRef]
- Kotek, V.; Dvořáková, H.; Tobrman, T. Modular and Highly Stereoselective Approach to All-Carbon Tetrasubstituted Alkenes. Org. Lett. 2015, 17, 608–611. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Li, Y.; Brown, M.K. Stereoselective Synthesis of All-Carbon Tetrasubstituted Alkenes from In Situ Generated Ketenes and Organometallic Reagents. Org. Lett. 2013, 15, 1610–1613. [Google Scholar] [CrossRef]
- Wang, C.-S.; Tan, P.S.L.; Ding, W.; Ito, S.; Yoshikai, N. Regio- and Stereoselective Synthesis of Enol Carboxylate, Phosphate, and Sulfonate Esters via Iodo(III)functionalization of Alkynes. Org. Lett. 2022, 24, 430–434. [Google Scholar] [CrossRef]
- Bauer, A.; Maulide, N. A Stereoselective Reductive Hosomi–Sakurai Reaction. Org. Lett. 2018, 20, 1461–1464. [Google Scholar] [CrossRef]
- Meyer, D.; Renaud, P. Enantioselective Hydroazidation of Trisubstituted Non-Activated Alkenes. Angew. Chem. Int. Ed. 2017, 56, 10858–10861. [Google Scholar] [CrossRef]
- Simlandy, A.K.; Lyu, M.-Y.; Brown, M.K. Catalytic Arylboration of Spirocyclic Cyclobutenes: Rapid Access to Highly Substituted Spiro[3.n]alkanes. ACS Catal. 2021, 11, 12815–12820. [Google Scholar] [CrossRef]
- Mizuta, S.; Galicia-López, O.; Engle, K.M.; Verhoog, S.; Wheelhouse, K.; Rassias, G.; Gouverneur, V. Trifluoromethylation of Allylsilanes under Copper Catalysis. Chem. Eur. J. 2012, 18, 8583–8587. [Google Scholar] [CrossRef]
- Narita, K.; Fujisaki, N.; Sakuma, Y.; Katoh, T. A novel approach to oxazole-containing diterpenoid synthesis from plant roots: Salviamines E and F. Org. Biomol. Chem. 2019, 17, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Cahiez, G.; Guerret, O.; Moyeux, A.; Dufour, S.; Lefevre, N. Eco-Friendly and Industrially Scalable Synthesis of the Sex Pheromone of Lobesia botrana. Important Progress for the Eco-Protection of Vineyard. Org. Process Res. Dev. 2017, 21, 1542–1546. [Google Scholar] [CrossRef]
- Ikoma, A.; Ogawa, N.; Kondo, D.; Kawada, H.; Kobayashi, Y. Synthesis of (−)-Piperitylmagnolol Featuring ortho-Selective Deiodination and Pd-Catalyzed Allylation. Org. Lett. 2016, 18, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Oikawa, H.; Oguri, H. Biogenetically inspired synthesis and skeletal diversification of indole alkaloids. Nat. Chem. 2014, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Yang, Z.-K.; Minami, H.; Kojima, K.; Saito, T.; Wang, C.; Uchiyama, M. Revisitation of Organoaluminum Reagents Affords a Versatile Protocol for C–X (X = N, O, F) Bond-Cleavage Cross-Coupling: A Systematic Study. ACS Catal. 2017, 7, 3988–3994. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Ashida, Y.; Hori, H.; Sato, Y.; Honda, A.; Taira, M.; Tanabe, Y. (E)- and (Z)-stereodefined enol phosphonates derived from β-ketoesters: Stereocomplementary synthesis of fully-substituted α,β-unsaturated esters. Org. Biomol. Chem. 2015, 13, 8205–8210. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Wu, Q.; Bi, X.; Shi, E.; Xiao, J. Stereoselective synthesis of (E)-α,β-unsaturated esters: Triethylamine-catalyzed allylic rearrangement of enol phosphates. RSC Adv. 2023, 13, 13511–13515. [Google Scholar] [CrossRef]
- Kotek, V.; Polák, P.; Dvořáková, H.; Tobrman, T. Aluminum Chloride Promoted Cross-Coupling of Trisubstituted Enol Phosphates with Organozinc Reagents En Route to the Stereoselective Synthesis of Tamoxifen and Its Analogues. Eur. J. Org. Chem. 2016, 2016, 5037–5044. [Google Scholar] [CrossRef]
- Polák, P.; Tobrman, T. The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates. Org. Biomol. Chem. 2017, 15, 6233–6241. [Google Scholar] [CrossRef]
- Polák, P.; Tobrman, T. Novel Selective Approach to Terminally Substituted [n]Dendralenes. Eur. J. Org. Chem. 2019, 2019, 957–968. [Google Scholar] [CrossRef]
- Koudelka, J.; Tobrman, T. Synthesis of 2-Substituted Cyclobutanones by a Suzuki Reaction and Dephosphorylation Sequence. Eur. J. Org. Chem. 2021, 2021, 3260–3269. [Google Scholar] [CrossRef]
- Edlová, T.; Dvořáková, H.; Eigner, V.; Tobrman, T. Substrate-Controlled Regioselective Bromination of 1,2-Disubstituted Cyclobutenes: An Application in the Synthesis of 2,3-Disubstituted Cyclobutenones. J. Org. Chem. 2021, 86, 5820–5831. [Google Scholar] [CrossRef]
- Čubiňák, M.; Bigeon, J.; Galář, P.; Ondič, L.; Tobrman, T. The Synthesis of Tetrasubstituted Cycloalkenes Bearing π-Conjugated Substituents and Their Optical Properties. ChemistrySelect 2021, 6, 9904–9910. [Google Scholar] [CrossRef]
- Čubiňák, M.; Tobrman, T. Room-Temperature Negishi Reaction of Trisubstituted Vinyl Phosphates for the Synthesis of Tetrasubstituted Alkenes. J. Org. Chem. 2020, 85, 10728–10739. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Hashemi, E. Recent applications of the Suzuki reaction in total synthesis. Tetrahedron 2012, 68, 9145–9178. [Google Scholar] [CrossRef]
- Maluenda, I.; Navarro, O. Recent Developments in the Suzuki-Miyaura Reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Islam, M.M.; Islam, S.M. Suzuki–Miyaura reaction by heterogeneously supported Pd in water: Recent studies. RSC Adv. 2015, 5, 42193–42221. [Google Scholar] [CrossRef]
- Rossi, R.; Bellina, F.; Lessi, M. Selective Palladium-Catalyzed Suzuki–Miyaura Reactions of Polyhalogenated Heteroarenes. Adv. Synth. Catal. 2012, 354, 1181–1255. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Z.; Hu, X.; Tang, G.; Xu, P.; Zhao, Y.; Cheng, C.-H. Nickel-Catalyzed Cross-Coupling of Aryl Phosphates with Arylboronic Acids. J. Org. Chem. 2011, 76, 2338–2344. [Google Scholar] [CrossRef]
- Gigant, N.; Honraedt, A.; Gras, E.; Gillaizeau, I. Efficient Cross-Coupling of Dioxazaborocanes with α-Phosphate Enamides. Eur. J. Org. Chem. 2014, 2014, 7889–7894. [Google Scholar] [CrossRef]
- Senra, J.D.; Silva, A.C.; Santos, R.V.; Malta, L.F.B.; Simas, A.B.C. Palladium on Layered Double Hydroxide: A Heterogeneous System for the Enol Phosphate Carbon-Oxygen Bond Activation in Aqueous Media. J. Chem. 2017, 2017, 8418939. [Google Scholar] [CrossRef]
- Leidy, M.R.; Mason Hoffman, J.; Pongdee, R. Preparation of C-arylglycals via Suzuki–Miyaura cross-coupling of dihydropyranylphosphates. Tetrahedron Lett. 2013, 54, 6889–6891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mole, J.; Philip, R.M.; Anilkumar, G. Nickel-catalyzed (hetero)aryl borylations: An update. ARKIVOC 2022, 2022, 165–199. [Google Scholar] [CrossRef]
- Steven, A. Micelle-Mediated Chemistry in Water for the Synthesis of Drug Candidates. Synthesis 2019, 51, 2632–2647. [Google Scholar] [CrossRef]
- Jin, S.; Dang, H.T.; Haug, G.C.; He, R.; Nguyen, V.D.; Nguyen, V.T.; Arman, H.D.; Schanze, K.S.; Larionov, O.V. Visible Light-Induced Borylation of C–O, C–N, and C–X Bonds. J. Am. Chem. Soc. 2020, 142, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cheung, M.S.; Lin, Z.; Li, P. Metal-free borylation of electron-rich aryl (pseudo)halides under continuous-flow photolytic conditions. Org. Chem. Front. 2016, 3, 875–879. [Google Scholar] [CrossRef]
- Begliomini, S.; Sernissi, L.; Scarpi, D.; Occhiato, E.G. A Short, Chemo-Enzymatic Synthesis of Both Enantiomers of trans-3-Hydroxy pipecolic Acid. Eur. J. Org. Chem. 2014, 2014, 5448–5455. [Google Scholar] [CrossRef]
- Rey-Rodriguez, R.; Jestin, G.; Gandon, V.; Grelier, G.; Retailleau, P.; Darses, B.; Dauban, P.; Gillaizeau, I. Intermolecular Rhodium(II)-Catalyzed Allylic C(sp3)–H Amination of Cyclic Enamides. Adv. Synth. Catal. 2018, 360, 513–518. [Google Scholar] [CrossRef]
- Adamson, N.J.; Park, S.; Zhou, P.; Nguyen, A.L.; Malcolmson, S.J. Enantioselective Construction of Quaternary Stereogenic Centers by the Addition of an Acyl Anion Equivalent to 1,3-Dienes. Org. Lett. 2020, 22, 2032–2037. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, P.; Dong, L.; Wang, W.; Duan, S.; Wang, B.; Gong, X.; Ye, L.; Wang, H.; Tian, J. Discovery of the Next-Generation Pan-TRK Kinase Inhibitors for the Treatment of Cancer. J. Med. Chem. 2021, 64, 10286–10296. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Nasu, T.; Fujii, Y.; Asano, K.; Matsubara, S. Asymmetric Cycloetherification of in Situ Generated Cyanohydrins through the Concomitant Construction of Three Chiral Carbon Centers. Org. Lett. 2019, 21, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Muto, T.; Sekine, K.; Sasaki, M. Total Synthesis and Structure Revision of Didemnaketal B. Chem. Eur. J. 2014, 20, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Sakamoto, K.; Muto, T.; Sasaki, M. Concise synthesis of the C15–C38 fragment of okadaic acid, a specific inhibitor of protein phosphatases 1 and 2A. Tetrahedron 2015, 71, 6369–6383. [Google Scholar] [CrossRef]
- Sallio, R.; Lebrun, S.; Gigant, N.; Gillaizeau, I.; Deniau, E. Asymmetric Synthesis of 2-Heteroaryl Cyclic Amines: Total Synthesis of (–)-Anabasine. Eur. J. Org. Chem. 2014, 2014, 4381–4388. [Google Scholar] [CrossRef]
- Hu, X.-H.; Yang, X.-F.; Loh, T.-P. Selective Alkenylation and Hydroalkenylation of Enol Phosphates through Direct C–H Functionalization. Angew. Chem. Int. Ed. 2015, 54, 15535–15539. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.H.; Lee, T.S.; Kim, E.J.; Moon, B.; Kang, J. Palladium(II)-catalyzed ortho-arylation via phosphate-group-directed C–H activation. Tetrahedron 2013, 69, 5152–5159. [Google Scholar] [CrossRef]
- Chan, L.Y.; Cheong, L.; Kim, S. Pd(II)-Catalyzed ortho-Arylation of Aryl Phosphates and Aryl Hydrogen Phosphates with Diaryliodonium Triflates. Org. Lett. 2013, 15, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Moselage, M.; Sauermann, N.; Richter, S.C.; Ackermann, L. C–H Alkenylations with Alkenyl Acetates, Phosphates, Carbonates, and Carbamates by Cobalt Catalysis at 23 °C. Angew. Chem. Int. Ed. 2015, 54, 6352–6355. [Google Scholar] [CrossRef]
- Sauermann, N.; Loup, J.; Kootz, D.; Yatham, V.R.; Berkessel, A.; Ackermann, L. Triazolylidene Ligands Allow Cobalt-Catalyzed C–H/C–O Alkenyl ations at Ambient Temperature. Synthesis 2017, 49, 3476–3484. [Google Scholar] [CrossRef]
- Grosheva, D.; Cramer, N. Ketene Aminal Phosphates: Competent Substrates for Enantioselective Pd(0)-Catalyzed C–H Functionalizations. ACS Catal. 2017, 7, 7417–7420. [Google Scholar] [CrossRef]
- Lee, P.-S.; Xu, W.; Yoshikai, N. Directed C–H Alkenylation of Aryl Imines with Alkenyl Phosphates Promoted by a Cobalt–N-Heterocyclic Carbene Catalyst. Adv. Synth. Catal. 2017, 359, 4340–4347. [Google Scholar] [CrossRef]
- Xu, W.; Yoshikai, N. Cobalt-catalyzed directed C–H alkenylation of pivalophenone N–H imine with alkenyl phosphates. Beilstein J. Org. Chem. 2018, 14, 709–715. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yang, L.-M. Nickel-Catalyzed Amination of Aryl Phosphates through Cleaving Aryl C–O Bonds. Org. Lett. 2011, 13, 3750–3753. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, X.; So, C.M. Palladium-Catalyzed C(sp2)–N Bond Cross-Coupling with Triaryl Phosphates. J. Org. Chem. 2019, 84, 6366–6376. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; So, C.M. Exploration of Aryl Phosphates in Palladium-Catalyzed Mono-α-arylation of Aryl and Heteroaryl Ketones. J. Org. Chem. 2019, 84, 6337–6346. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Li, Y.-Y.; Zhang, S.-Q.; Hong, X.; Shi, S.-L. Unsymmetric N-heterocyclic carbene ligand enabled nickel-catalysed arylation of bulky primary and secondary amines. Chem. Sci. 2023, 14, 4390–4396. [Google Scholar] [CrossRef]
- Valiullina, Z.R.; Galeeva, A.M.; Gimalova, F.A.; Selezneva, N.K.; Khasanova, L.S.; Mavzyutov, A.R.; Miftakhov, M.S. Synthesis and In Vitro Antibacterial Activity of New C-3-Modified Carbapenems. Russ. J. Bioorg. Chem. 2019, 45, 398–404. [Google Scholar] [CrossRef]
- Lee, N.; Tan, C.-H.; Leow, D. Asymmetric Brook Rearrangement. Asian J. Org. Chem. 2019, 8, 25–31. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C. Radical-mediated 1,2-Brook rearrangements. Chem Catal. 2021, 1, 250–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.-J.; Huang, H.-M. Radical Brook Rearrangements: Concept and Recent Developments. Angew. Chem. Int. Ed. 2022, 61, e202205671. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Aita, K.; Ishikawa, S.; Terada, M. Synthesis of Tetrasubstituted Furans through One-Pot Formal [3 + 2] Cycloaddition Utilizing [1,2]-Phospha-Brook Rearrangement. Org. Lett. 2020, 22, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Ishikawa, S.; Aoki, T.; Terada, M. Synthesis of 2,3-allenylamides utilizing [1,2]-phospha-Brook rearrangement and their application to gold-catalyzed cycloisomerization providing 2-aminofuran derivatives. Chem. Commun. 2016, 52, 12513–12516. [Google Scholar] [CrossRef]
- Kondoh, A.; Iino, A.; Ishikawa, S.; Aoki, T.; Terada, M. Efficient Synthesis of Polysubstituted Pyrroles Based on [3+2] Cycloaddition Strategy Utilizing [1,2]-Phospha-Brook Rearrangement under Brønsted Base Catalysis. Chem. Eur. J. 2018, 24, 15246–15253. [Google Scholar] [CrossRef]
- Kondoh, A.; Aoki, T.; Terada, M. Synthesis of Phenanthrene Derivatives by Intramolecular Cyclization Utilizing the [1,2]-Phospha-Brook Rearrangement Catalyzed by a Brønsted Base. Chem. Eur. J. 2015, 21, 12577–12580. [Google Scholar] [CrossRef]
- Kondoh, A.; Koda, K.; Kamata, Y.; Terada, M. Synthesis of Indolizine Derivatives Utilizing [1,2]-Phospha-Brook Rearrangement/Cycloisomerization Sequence. Chem. Lett. 2017, 46, 1020–1023. [Google Scholar] [CrossRef]
- Kondoh, A.; Ojima, R.; Terada, M. Formal Fluorinative Ring Opening of 2-Benzoylpyrrolidines Utilizing [1,2]-Phospha-Brook Rearrangement for Synthesis of 2-Aryl-3-fluoropiperidines. Org. Lett. 2021, 23, 7894–7899. [Google Scholar] [CrossRef]
- Kondoh, A.; Takei, A.; Terada, M. Novel Methodology for the Efficient Synthesis of 3-Aryloxindoles: [1,2]-Phospha-Brook Rearrangement–Palladium-Catalyzed Cross-Coupling Sequence. Synlett 2016, 27, 1848–1853. [Google Scholar] [CrossRef]
- Kondoh, A.; Terada, M. Synthesis of 2,2-Disubstituted 2H-Chromenes through Carbon-Carbon Bond Formation Utilizing a [1,2]-Phospha-Brook Rearrangement under Brønsted Base Catalysis. Chem. Eur. J. 2022, 28, e202201198. [Google Scholar] [CrossRef]
- Kondoh, A.; Aoki, T.; Terada, M. Intramolecular Cyclization of Alkynyl α-Ketoanilide Utilizing [1,2]-Phospha-Brook Rearrangement Catalyzed by Phosphazene Base. Org. Lett. 2014, 16, 3528–3531. [Google Scholar] [CrossRef]
- Kondoh, A.; Aoki, T.; Terada, M. Generation and Application of Homoenolate Equivalents Utilizing [1,2]-Phospha-Brook Rearrangement under Brønsted Base Catalysis. Chem. Eur. J. 2017, 23, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Aoki, T.; Terada, M. Organocatalytic Arylation of α-Ketoesters Based on Umpolung Strategy: Phosphazene-Catalyzed SNAr Reaction Utilizing [1,2]-Phospha-Brook Rearrangement. Chem. Eur. J. 2018, 24, 13110–13113. [Google Scholar] [CrossRef]
- Kondoh, A.; Hirozane, T.; Terada, M. Formal Umpolung Addition of Phosphites to 2-Azaaryl Ketones under Chiral Brønsted Base Catalysis: Enantioselective Protonation Utilizing [1,2]-Phospha-Brook Rearrangement. Chem. Eur. J. 2022, 28, e202201240. [Google Scholar] [CrossRef]
- Kondoh, A.; Ozawa, R.; Aoki, T.; Terada, M. Intramolecular addition of benzyl anion to alkyne utilizing [1,2]-phospha-Brook rearrangement under Brønsted base catalysis. Org. Biomol. Chem. 2017, 15, 7277–7281. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Tasato, N.; Aoki, T.; Terada, M. Brønsted Base-Catalyzed Transformation of α,β-Epoxyketones Utilizing [1,2]-Phospha-Brook Rearrangement for the Synthesis of Allylic Alcohols Having a Tetrasubstituted Alkene Moiety. Org. Lett. 2020, 22, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Terada, M. Brønsted base-catalyzed α-oxygenation of carbonyl compounds utilizing the [1,2]-phospha-Brook rearrangement. Org. Chem. Front. 2015, 2, 801–805. [Google Scholar] [CrossRef]
- Ranga, S.; Chakravarty, M.; Chatterjee, T.; Ghosal, S. Mechanistic insights into n-BuLi mediated phospha-Brook rearrangement. New J. Chem. 2019, 43, 9886–9890. [Google Scholar] [CrossRef]
- Tan, Q.; Guo, N.; Yang, L.; Wang, F.; Feng, X.; Liu, X. Asymmetric Organocatalytic 1,6-Conjugate Addition of para-Quinone Methides Using [1,2]-Phospha-Brook Rearrangement. J. Org. Chem. 2023, 88, 9332–9342. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Pandey, C.B.; Kumar, S.; Tiwari, B. Carbene-Catalyzed Tandem [1,2]-Phospha-Brook/[1,4]-Phosphate Rearrangement: Access to α-Ketophosphates via Controlled Cross-Acyloin Condensation. J. Org. Chem. 2018, 83, 9478–9483. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ishida, Y.; Takamizu, Y.; Yasui, T. Synthesis of (Difluoromethyl)cycloalkenes from 2-Cycloalkenones by Utilizing Phospha-Brook Rearrangement. Adv. Synth. Catal. 2019, 361, 3739–3743. [Google Scholar] [CrossRef]
- Cheibas, C.; Fincias, N.; Casaretto, N.; Garrec, J.; El Kaïm, L. Passerini–Smiles Reaction of α-Ketophosphonates: Platform for Phospha-Brook/Smiles Embedded Cascades. Angew. Chem. Int. Ed. 2022, 61, e202116249. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh, R.P. Stereoselective Reductive Coupling Reactions Utilizing [1,2]-Phospha-Brook Rearrangement: A Powerful Umpolung Approach. J. Org. Chem. 2023, 88, 10325–10338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Miao, Z. Research Progress in [1,2]-Phospha-Brook Rearrangement Reaction. Univ. Chem. 2021, 36, 2008082. [Google Scholar] [CrossRef]
- Melvin, L.S. An efficient synthesis of 2-hydroxyphenylphosphonates. Tetrahedron Lett. 1981, 22, 3375–3376. [Google Scholar] [CrossRef]
- Delgado Rosario, E.; Rectenwald, M.F.; Gaffen, J.R.; Rheingold, A.L.; Protasiewicz, J.D. Organophosphorus decorated lithium borate and phosphate salts with extended π-conjugated backbone. Dalton Trans. 2021, 50, 6667–6672. [Google Scholar] [CrossRef] [PubMed]
- Placidi, M.P.; Botta, M.; Kálmán, F.K.; Hagberg, G.E.; Baranyai, Z.; Krenzer, A.; Rogerson, A.K.; Tóth, I.; Logothetis, N.K.; Angelovski, G. Aryl-Phosphonate Lanthanide Complexes and Their Fluorinated Derivatives: Investigation of Their Unusual Relaxometric Behavior and Potential Application as Dual Frequency 1H/19F MRI Probes. Chem. Eur. J. 2013, 19, 11644–11660. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.Y.; Baulina, T.Y.V.; Pasechnik, M.P.; Matveev, S.V.; Matveeva, A.G. Synthesis and Coordination Properties of Tripodal Ligand on the Triphenylphosphine Oxide Platform with Carbamoyl Side Arms. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 946–962. [Google Scholar] [CrossRef]
- Kudryavtsev, I.Y.; Bykhovskaya, O.V.; Matveeva, A.G.; Baulina, T.V.; Pasechnik, M.P.; Matveev, S.V.; Vologzhanina, A.V.; Turanov, A.N.; Karandashev, V.K.; Brel, V.K. New tripodal ligand on the triphenylphosphine oxide platform with 1,2,3-triazole side arms: Synthesis, structure, coordination, and extraction properties. Monatsh. Chem. 2020, 151, 1705–1713. [Google Scholar] [CrossRef]
- Alessi, M.; Patel, J.J.; Zumbansen, K.; Snieckus, V. The Tetraethylphosphorodiamidate (OP(O)(NEt2)2) Directed Metalation Group (DMG). Directed ortho and Lateral Metalation and the Phospha Anionic Fries Rearrangement. Org. Lett. 2020, 22, 2147–2151. [Google Scholar] [CrossRef]
- Patel, J.J.; Blackburn, T.; Alessi, M.; Sawinski, H.; Snieckus, V. Tetraethylphosphorodiamidate-Directed Metalation Group: Directed Ortho and Remote Metalation, Cross Coupling, and Remote Phospha Anionic Fries Rearrangement Reactions. Org. Lett. 2020, 22, 3860–3864. [Google Scholar] [CrossRef]
- Taylor, C.; Watson, A. The Anionic Phospho-Fries Rearrangement. Curr. Org. Chem. 2004, 8, 623–636. [Google Scholar] [CrossRef]
- Wu, S.; Deligonal, N.; Protasiewicz, J.D. An unusually unstable ortho-phosphinophenol and its use to prepare benzoxaphospholes having enhanced air-stability. Dalton Trans. 2013, 42, 14866–14874. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Li, M.; Liu, Y.; Zhou, Y.; Zhao, C.; Goto, M.; Yin, S.-F.; Han, L.-B. Stereoselective Synthesis of Phosphoryl-Substituted Phenols. Adv. Synth. Catal. 2014, 356, 781–794. [Google Scholar] [CrossRef]
- Korb, M.; Schaarschmidt, D.; Lang, H. Anionic Phospho-Fries Rearrangement at Ferrocene: One-Pot Approach to P,O-Substituted Ferrocenes. Organometallics 2014, 33, 2099–2108. [Google Scholar] [CrossRef]
- Herd, O.; Heßler, A.; Hingst, M.; Tepper, M.; Stelzer, O. Water soluble phosphines VII. Palladium-catalyzed P–C cross coupling reactions between primary or secondary phosphines and functional aryliodides—A novel synthetic route to water soluble phosphines. J. Organomet. Chem. 1996, 522, 69–76. [Google Scholar] [CrossRef]
- Korb, M.; Lang, H. Planar Chirality from the Chiral Pool: Diastereoselective Anionic Phospho-Fries Rearrangements at Ferrocene. Organometallics 2014, 33, 6643–6659. [Google Scholar] [CrossRef]
- Korb, M.; Lehrich, S.W.; Lang, H. Reactivity of Ferrocenyl Phosphates Bearing (Hetero-)Aromatics and [3]Ferrocenophanes toward Anionic Phospho-Fries Rearrangements. J. Org. Chem. 2017, 82, 3102–3124. [Google Scholar] [CrossRef]
- Kakimoto, N.; Ogura, Y.; Watanabe, H.; Takikawa, H. Total synthesis of both enantiomers of clavigerins B and C. Tetrahedron 2020, 76, 131297. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, W.; Tian, H.; Sun, S.; Li, X.; Tian, W.; Gui, J. Facile Access to Bridged Ring Systems via Point-to-Planar Chirality Transfer: Unified Synthesis of Ten Cyclocitrinols. J. Am. Chem. Soc. 2019, 141, 5021–5033. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, W.; Tian, H.; Tian, W.; Gui, J. Scalable Synthesis of Cyclocitrinol. J. Am. Chem. Soc. 2018, 140, 9413–9416. [Google Scholar] [CrossRef]
- Kaabi, A.; Besbes, R. Amino Phosphate Monoesters: A Convenient Source of 2-Alkylamino-3-methoxy-3-phenylpropionates via Aziridinium Ions. Synth. Commun. 2013, 43, 1587–1593. [Google Scholar] [CrossRef]
- Shinohara, R.; Kawashima, H.; Ogawa, N.; Kobayashi, Y. Substitution of Secondary Benzylic Phosphates with Diarylmethyl Anions. Tetrahedron 2019, 75, 2717–2725. [Google Scholar] [CrossRef]
- Shinohara, R.; Ogawa, N.; Kawashima, H.; Wada, K.; Saito, S.; Yamazaki, T.; Kobayashi, Y. SN2 Reaction of Diarylmethyl Anions at Secondary Alkyl and Cycloalkyl Carbons. Eur. J. Org. Chem. 2019, 2019, 1461–1478. [Google Scholar] [CrossRef]
- Pallikonda, G.; Chakravarty, M. Benzylic Phosphates in Friedel–Crafts Reactions with Activated and Unactivated Arenes: Access to Polyarylated Alkanes. J. Org. Chem. 2016, 81, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sakai, M.; Ishida, Y.; Yasui, T. Synthesis of 1-(Difluoromethyl)alk-1-enes via Palladium-Catalyzed SN2′-Type Substitution Reaction of Difluoromethylated Allylic Phosphates with 1,3-Dicarbonyl Compounds and Imides. J. Org. Chem. 2021, 86, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takase, T.; Kuroyanagi, E.; Yasui, T. Synthesis of difluoromethylated diarylmethanes via Fe(OTf)3-catalyzed Friedel–Crafts reaction of 2,2-difluoro-1-arylethyl phosphates. Chem. Commun. 2021, 57, 3877–3880. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Ohzono, A.; Shirota, K. Phosphinative cyclopropanation of allyl phosphates with lithium phosphides. Chem. Commun. 2020, 56, 11851–11854. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.M.; Li, Q.; Rötheli, A.R.; Jacobsen, E.N. Catalytic activation of glycosyl phosphates for stereoselective coupling reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 35–39. [Google Scholar] [CrossRef]
- Li, Q.; Levi, S.M.; Jacobsen, E.N. Highly Selective β-Mannosylations and β-Rhamnosylations Catalyzed by Bis-thiourea. J. Am. Chem. Soc. 2020, 142, 11865–11872. [Google Scholar] [CrossRef]
- Li, Y.; Jie, J.; Li, H.; Yang, H.; Fu, H. Synthesis of Spirotetrahydrofuran Oxindoles via Palladium-Catalyzed [4 + 1] Cycloaddition of Diphenyl 2-Oxoindolin-3-yl Phosphates and 2-Methylidenetrimethylene Carbonate. Org. Lett. 2021, 23, 6499–6503. [Google Scholar] [CrossRef]
- Chen, Q.; Teng, Y.; Xu, F. Lanthanide Silylamide-Catalyzed Synthesis of Pyrano[2,3-b]indol-2-ones. Org. Lett. 2021, 23, 4785–4790. [Google Scholar] [CrossRef] [PubMed]
- Rokade, B.V.; Guiry, P.J. Synthesis of α-Aryl Oxindoles by Friedel–Crafts Alkylation of Arenes. J. Org. Chem. 2020, 85, 6172–6180. [Google Scholar] [CrossRef]
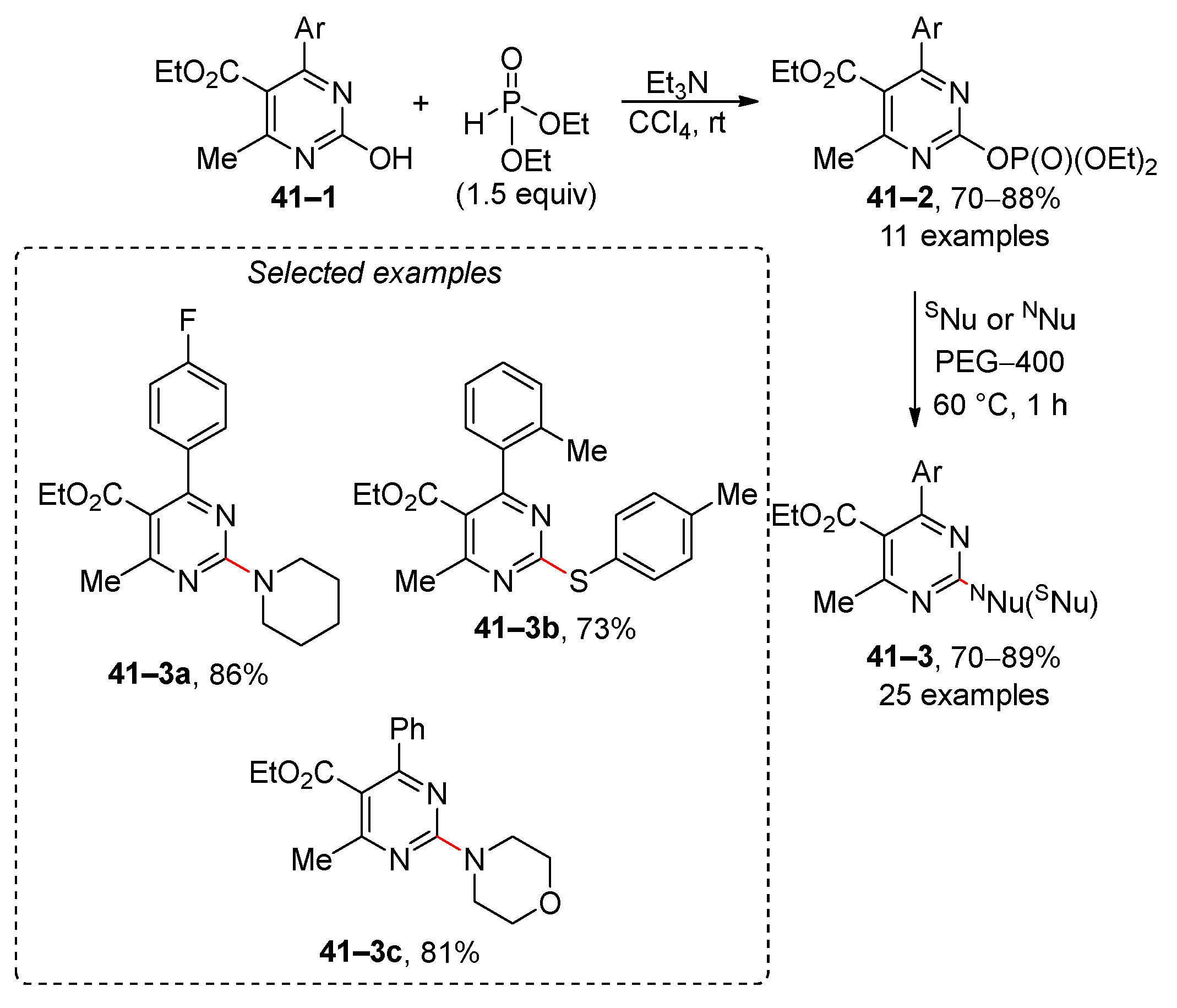
- Xing, T.; Wei, K.-J.; Quan, Z.-J.; Wang, X.-C. Nucleophilic Substitution Reaction of Pyrimidin-2-yl Phosphates Using Amines and Thiols as Nucleophiles Mediated by PEG-400 as an Environmentally Friendly Solvent. Synthesis 2015, 47, 3925–3935. [Google Scholar] [CrossRef]
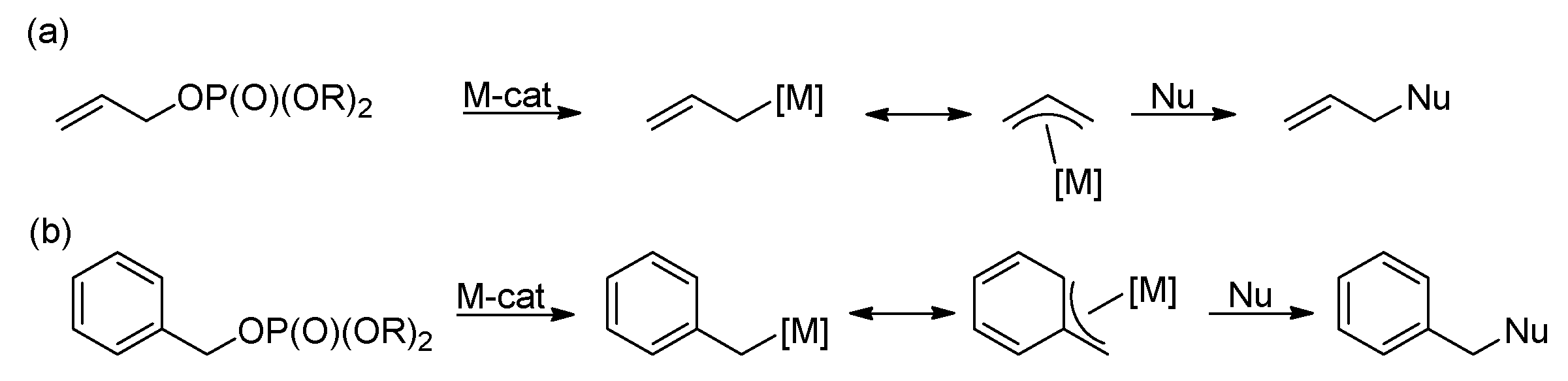
- Butt, N.A.; Zhang, W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015, 44, 7929–7967. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, L.; Heravi, M.M. Applications of Transition-Metal-Catalyzed Asymmetric Allylic Substitution in Total Synthesis of Natural Products: An Update. Chem. Rec. 2021, 21, 29–68. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Evans, P.A. Transition-Metal-Catalyzed Allylic Substitution Reactions: Stereoselective Construction of α- and β-Substituted Carbonyl Compounds. Synthesis 2013, 45, 3179–3198. [Google Scholar] [CrossRef]
- Qu, J.; Helmchen, G. Applications of Iridium-Catalyzed Asymmetric Allylic Substitution Reactions in Target-Oriented Synthesis. Acc. Chem. Res. 2017, 50, 2539–2555. [Google Scholar] [CrossRef] [PubMed]
- Sundararaju, B.; Achard, M.; Bruneau, C. Transition metal catalyzed nucleophilic allylic substitution: Activation of allylic alcohols via π-allylic species. Chem. Soc. Rev. 2012, 41, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
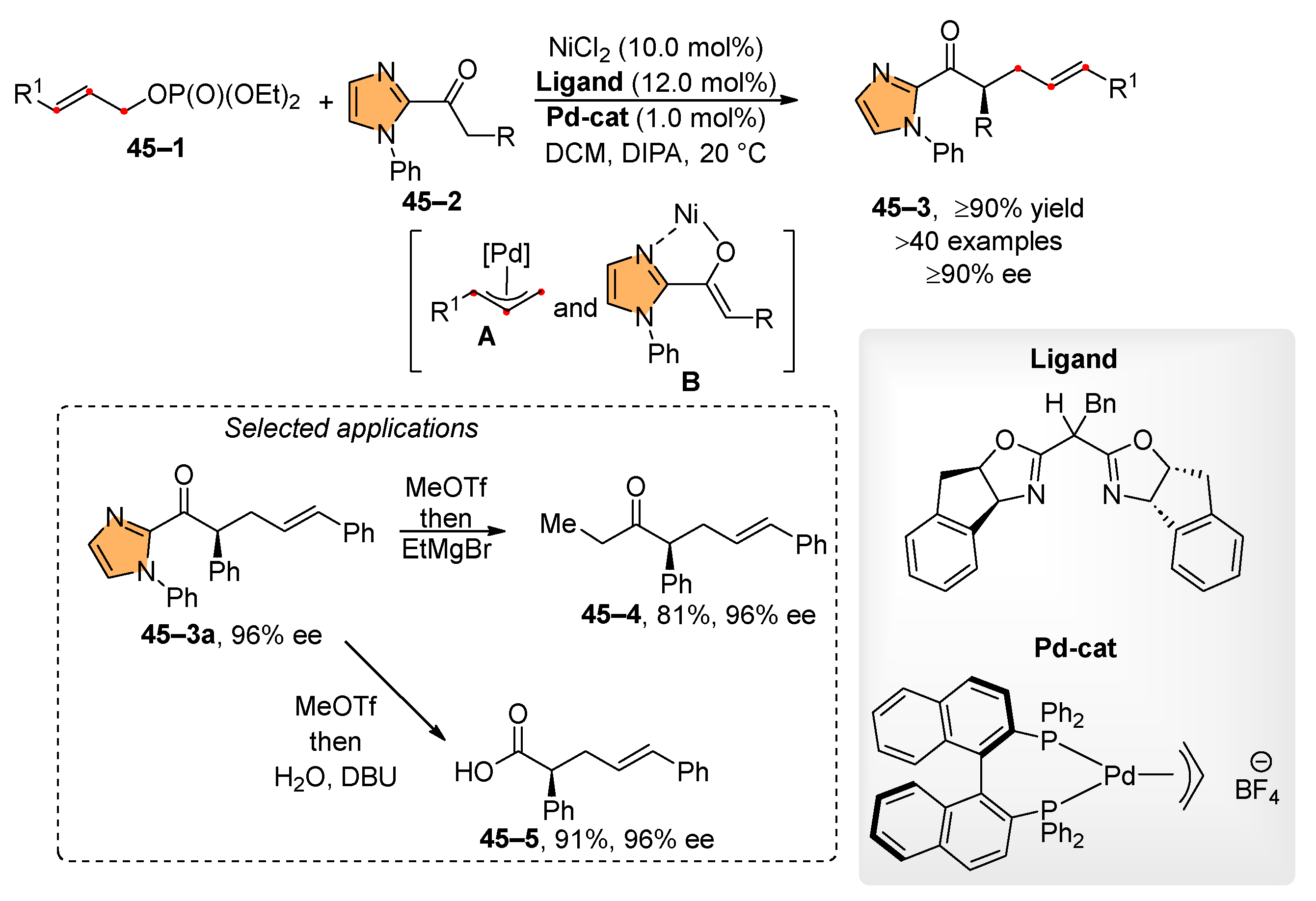
- Gao, F.; Carr, J.L.; Hoveyda, A.H. A Broadly Applicable NHC–Cu-Catalyzed Approach for Efficient, Site-, and Enantioselective Coupling of Readily Accessible (Pinacolato)alkenylboron Compounds to Allylic Phosphates and Applications to Natural Product Synthesis. J. Am. Chem. Soc. 2014, 136, 2149–2161. [Google Scholar] [CrossRef]
- Lee, J.; Torker, S.; Hoveyda, A.H. Versatile Homoallylic Boronates by Chemo-, SN2′-, Diastereo- and Enantioselective Catalytic Sequence of Cu−H Addition to Vinyl-B(pin)/Allylic Substitution. Angew. Chem. Int. Ed. 2017, 56, 821–826. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Y.; Torker, S.; Hoveyda, A.H. SN2″-Selective and Enantioselective Substitution with Unsaturated Organoboron Compounds and Catalyzed by a Sulfonate-Containing NHC-Cu Complex. J. Am. Chem. Soc. 2018, 140, 16842–16854. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jung, B.; Torker, S.; Hoveyda, A.H. N-Heterocyclic Carbene–Copper-Catalyzed Group-, Site-, and Enantioselective Allylic Substitution with a Readily Accessible Propargyl(pinacolato)boron Reagent: Utility in Stereoselective Synthesis and Mechanistic Attributes. J. Am. Chem. Soc. 2015, 137, 8948–8964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Zhang, B.; Lu, X.; Liu, J.-H.; Lu, X.-Y.; Xiao, B.; Fu, Y. Copper-Catalyzed SN2′-Selective Allylic Substitution Reaction of gem-Diborylalkanes. Org. Lett. 2016, 18, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hoveyda, A.H. Catalytic SN2′- and Enantioselective Allylic Substitution with a Diborylmethane Reagent and Application in Synthesis. Angew. Chem. Int. Ed. 2016, 55, 3455–3458. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, Z.; Fu, C.; Wang, G.; Zheng, C.; Wu, X. Synergistic Ni/Pd Catalysis for Asymmetric Allylic Alkylation of 2-Acyl Imidazoles. Org. Lett. 2023, 25, 5448–5453. [Google Scholar] [CrossRef] [PubMed]
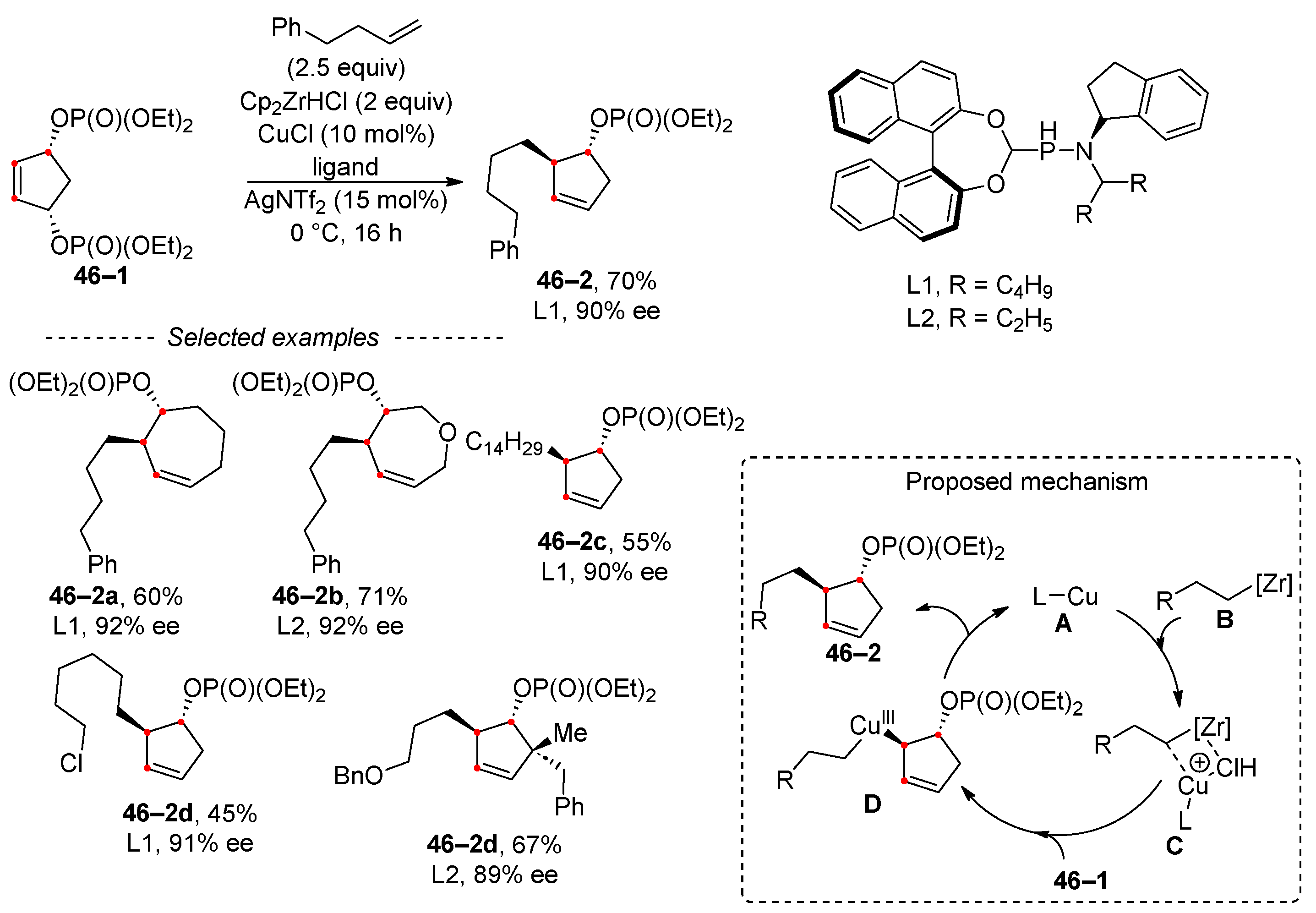
- Jacques, R.; Pullin, R.D.C.; Fletcher, S.P. Desymmetrization of meso-bisphosphates using copper catalysis and alkylzirconocene nucleophiles. Nat. Commun. 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Niu, J.; Guo, X.; Xiong, T.; Zhang, Q. Copper-Catalyzed Asymmetric Hydroallylation of Vinylsilanes. Eur. J. Org. Chem. 2022, 2022, e202101575. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Buchwald, S.L. Enantioselective CuH-Catalyzed Hydroallylation of Vinylarenes. J. Am. Chem. Soc. 2016, 138, 5024–5027. [Google Scholar] [CrossRef] [PubMed]
- Yurino, T.; Tani, R.; Ohkuma, T. Pd-Catalyzed Allylic Isocyanation: Nucleophilic N-Terminus Substitution of Ambident Cyanide. ACS Catal. 2019, 9, 4434–4440. [Google Scholar] [CrossRef]
- Yurino, T.; Tange, Y.; Ohkuma, T. Palladium-Catalyzed Nucleophilic Isocyanation for the Synthesis of α-Aryl-α-Isocyanoacetoamide Derivatives. Bull. Chem. Soc. Jpn. 2021, 94, 2155–2161. [Google Scholar] [CrossRef]
- Yurino, T.; Tange, Y.; Tani, R.; Ohkuma, T. Ag2O-catalysed nucleophilic isocyanation: Selective formation of less-stable benzylic isonitriles. Org. Chem. Front. 2020, 7, 1308–1313. [Google Scholar] [CrossRef]
- Takise, R.; Itami, K.; Yamaguchi, J. Cyanation of Phenol Derivatives with Aminoacetonitriles by Nickel Catalysis. Org. Lett. 2016, 18, 4428–4431. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Zheng, B.-H.; Ding, C.-H.; Hou, X.-L. Enantioselective Synthesis of 2,3-Disubstituted Indanones via Pd-Catalyzed Intramolecular Asymmetric Allylic Alkylation of Ketones. Org. Lett. 2013, 15, 6086–6089. [Google Scholar] [CrossRef]
- Spoehrle, S.S.M.; West, T.H.; Taylor, J.E.; Slawin, A.M.Z.; Smith, A.D. Tandem Palladium and Isothiourea Relay Catalysis: Enantioselective Synthesis of α-Amino Acid Derivatives via Allylic Amination and [2,3]-Sigmatropic Rearrangement. J. Am. Chem. Soc. 2017, 139, 11895–11902. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Zhang, G.; Xu, M.; Qi, X. ProPhenol Derived Ligands to Simultaneously Coordinate a Main-Group Metal and a Transition Metal: Application to a Zn−Cu Catalyzed Reaction. Chem. Eur. J. 2022, 28, e202104268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, J.; Gao, Y.; Li, X.; Tang, G.; Zhao, Y. Synthesis of Diarylmethanes through Palladium-Catalyzed Coupling of Benzylic Phosphates with Arylsilanes. Synlett 2014, 25, 2928–2932. [Google Scholar] [CrossRef]
- Zhang, Y.; Raugh, N.; Koert, U. Fluorotrifluoromethyl Group Installation: Tetrasubstituted Tertiary Stereocenters Containing C–F and C–CF3 Bonds via Copper-Mediated Allylic Substitution. Org. Lett. 2023, 25, 5641–5645. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Sarlah, D. Arenophile-Mediated Dearomative Functionalization Strategies. Synlett 2018, 29, 845–855. [Google Scholar] [CrossRef]
- Petrenko, A.; Mrkobrada, S.; Tobrman, T. State-or-the-Art Approaches to the Synthesis of 2H-Pyrroles. Targets Heterocycl. Syst. 2021, 25, 308–325. [Google Scholar] [CrossRef]
- Polák, P.; Tobrman, T. Dearomatization Strategy for the Synthesis of Arylated 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles. Org. Lett. 2017, 19, 4608–4611. [Google Scholar] [CrossRef]
- Huang, G.; Yin, B. Recent Developments in Transition Metal-Catalyzed Dearomative Cyclizations of Indoles as Dipolarophiles for the Construction of Indolines. Adv. Synth. Catal. 2019, 361, 405–425. [Google Scholar] [CrossRef]
- Komatsuda, M.; Muto, K.; Yamaguchi, J. Pd-Catalyzed Dearomative Allylation of Benzyl Phosphates. Org. Lett. 2018, 20, 4354–4357. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, A.; Komatsuda, M.; Muto, K.; Yamaguchi, J. Dearomative Allylation of Naphthyl Cyanohydrins by Palladium Catalysis: Catalyst-Enhanced Site Selectivity. Org. Lett. 2020, 22, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Czabaniuk, L.C. Palladium-Catalyzed Asymmetric Benzylation of Azlactones. Chem. Eur. J. 2013, 19, 15210–15218. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.J.; Yang, C.; Fyfe, J.W.B.; Snaddon, T.N. Enantioselective α-Benzylation of Acyclic Esters Using π-Extended Electrophiles. Angew. Chem. Int. Ed. 2018, 57, 12102–12105. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, T.; Yokoyama, Y.; Inamura, K.; Katakura, S.-i.; Komoriya, S.; Yamaguchi, H.; Hara, T.; Iwamoto, M. Dibasic (Amidinoaryl)propanoic Acid Derivatives as Novel Blood Coagulation Factor Xa Inhibitors. J. Med. Chem. 1994, 37, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Toyomasu, T.; Nishio, H.; Shishido, T. Gold-catalyzed thioetherification of allyl, benzyl, and propargyl phosphates. Catal. Sci. Technol. 2022, 12, 1109–1116. [Google Scholar] [CrossRef]
- Zhang, K.; Provot, O.; Alami, M.; Tran, C.; Hamze, A. Pd-Catalyzed Coupling of N-Tosylhydrazones with Benzylic Phosphates: Toward the Synthesis of Di- or Tri-Substituted Alkenes. J. Org. Chem. 2022, 87, 1249–1261. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Amberg, W.; Bennani, Y.L.; Crispino, G.A.; Hartung, J.; Jeong, K.S.; Kwong, H.L.; Morikawa, K.; Wang, Z.M. The osmium-catalyzed asymmetric dihydroxylation: A new ligand class and a process improvement. J. Org. Chem. 1992, 57, 2768–2771. [Google Scholar] [CrossRef]
- Krawczyk, E.; Mielniczak, G.; Owsianik, K.; Łuczak, J. Asymmetric oxidation of enol phosphates to α-hydroxy ketones using Sharpless reagents and a fructose derived dioxirane. Tetrahedron Asymmetry 2012, 23, 1480–1489. [Google Scholar] [CrossRef]
- Owsianik, K.; Krawczyk, E.; Mielniczak, G.; Koprowski, M.; Sieroń, L. Three-step synthesis of chiral and sterically hindered amino alcohols based on cyclic enol phosphates. Tetrahedron 2018, 74, 7343–7350. [Google Scholar] [CrossRef]
- Krawczyk, E.; Koprowski, M.; Mielniczak, G.; Owsianik, K. Asymmetric synthesis of 5,7-O-dimethyleucomols via enantioselective oxidation of enol phosphates. Tetrahedron Asymmetry 2015, 26, 876–883. [Google Scholar] [CrossRef]
- Bulman Page, P.C.; Almutairi, S.M.; Chan, Y.; Stephenson, G.R.; Gama, Y.; Goodyear, R.L.; Douteau, A.; Allin, S.M.; Jones, G.A. Asymmetric Oxidation of Enol Derivatives to α-Alkoxy Carbonyls Using Iminium Salt Catalysts: A Synthetic and Computational Study. J. Org. Chem. 2019, 84, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Q.; Qian, C.; Liu, C.; Liu, W.; Chen, K.; Lei, A. Solvent-Enabled Radical Selectivities: Controlled Syntheses of Sulfoxides and Sulfides. Angew. Chem. Int. Ed. 2016, 55, 1094–1097. [Google Scholar] [CrossRef]
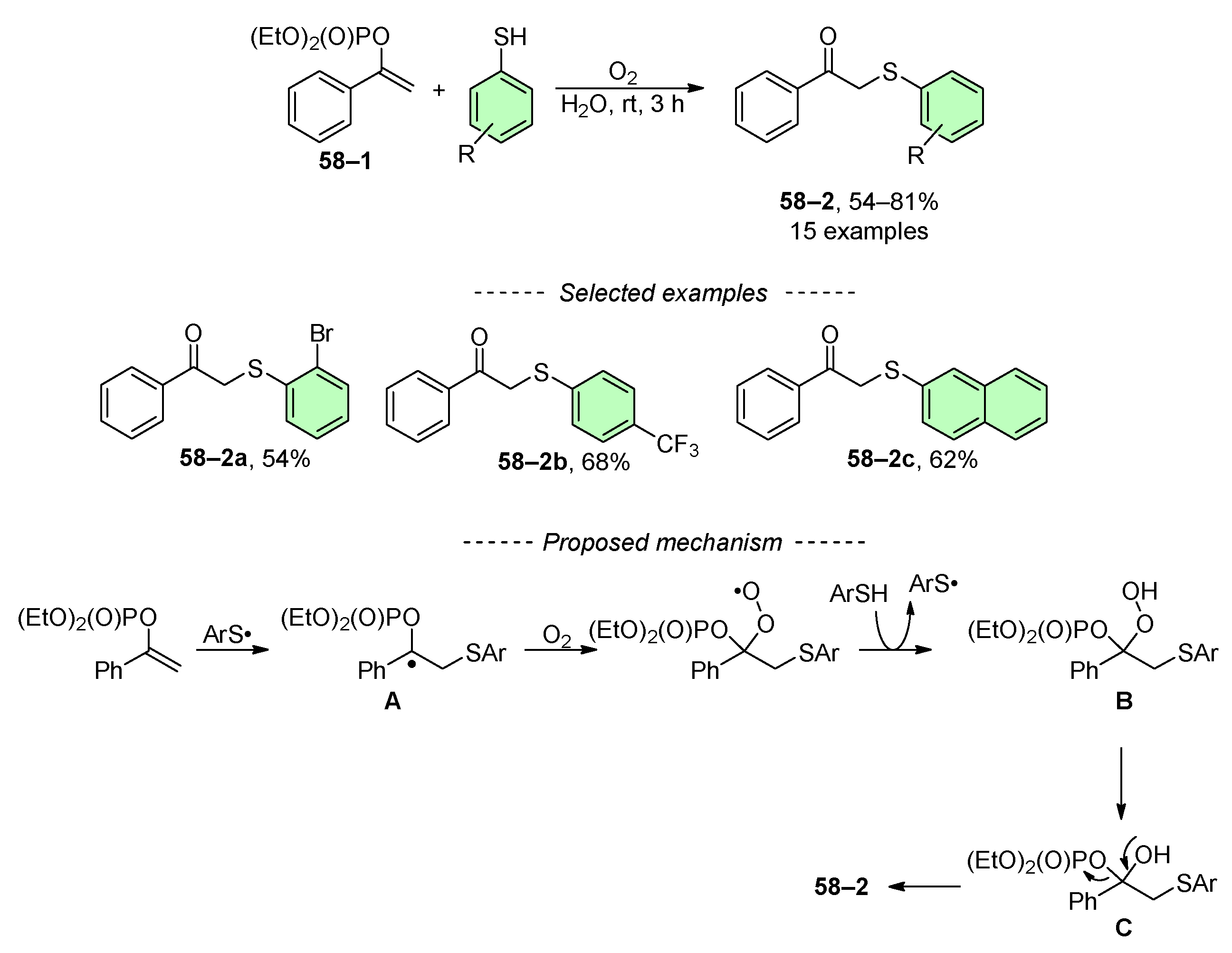
- Wang, H.; Wang, G.; Lu, Q.; Chiang, C.-W.; Peng, P.; Zhou, J.; Lei, A. Catalyst-Free Difunctionalization of Activated Alkenes in Water: Efficient Synthesis of β-Keto Sulfides and Sulfones. Chem. Eur. J. 2016, 22, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Wang, F.; Ning, R.; Kong, D.; Wu, M. A new synthetic approach to oxindoles (1,3-dihydro-2H-indol-2-ones) by reductive dephosphorylation with hydroiodic acid of 3-(diethylphosphoryloxy)- oxindoles, derived from isatins (1H-Indole-2,3-diones). ARKIVOC 2022, 2022, 135–146. [Google Scholar] [CrossRef]
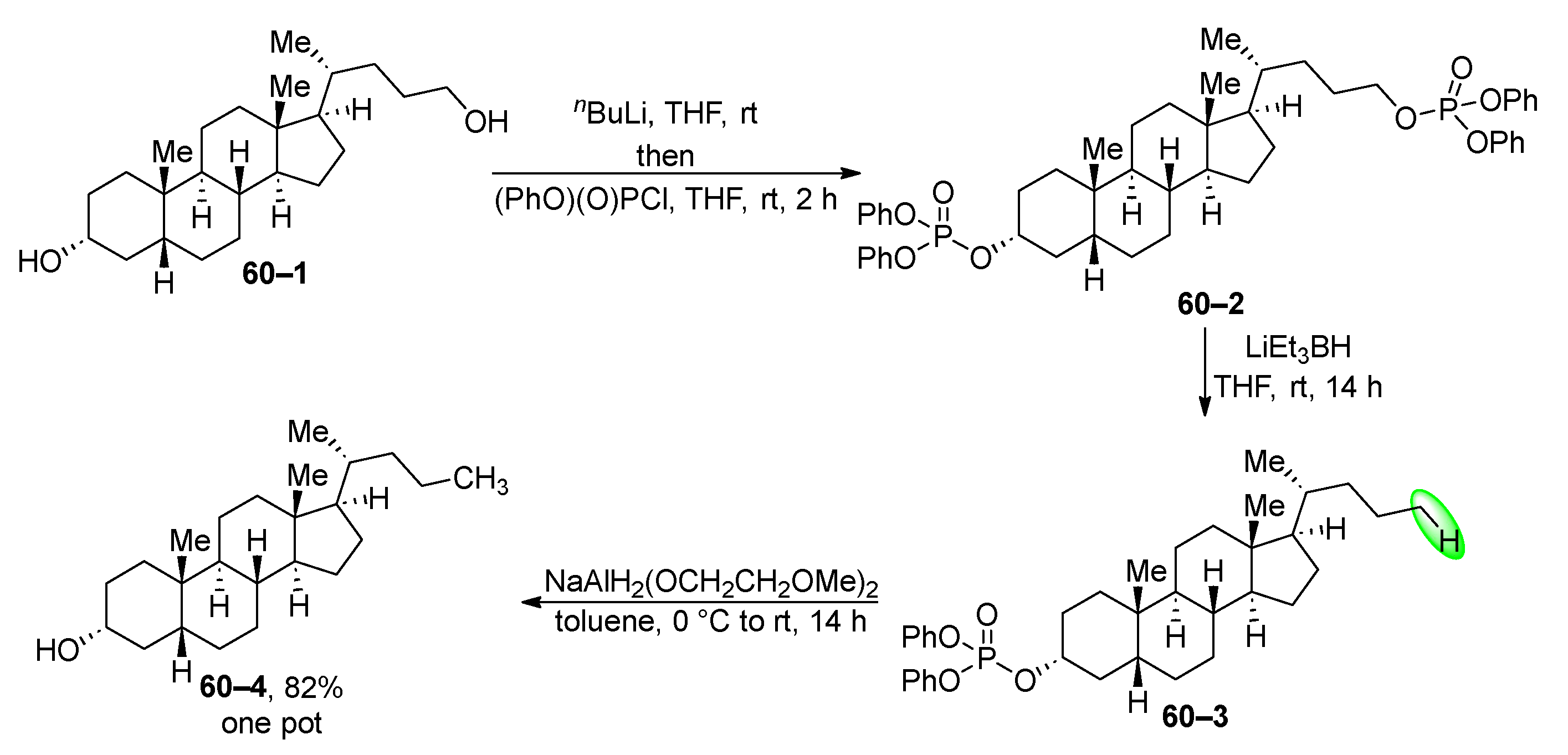
- Chowdhury, S.; Standaert, R.F. Deoxygenation of Unhindered Alcohols via Reductive Dealkylation of Derived Phosphate Esters. J. Org. Chem. 2016, 81, 9957–9963. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Zhao, G.; Ramadoss, V.; Tian, L.; Wang, Y. Electrochemical Deoxygenative Barbier-Type Reaction. Org. Lett. 2022, 24, 3668–3673. [Google Scholar] [CrossRef]
- Tomkiel, A.M.; Siergiejczyk, L.; Naróg, D.; Płoszyńska, J.; Sobkowiak, A.; Morzycki, J.W. Electrochemical cholesterylation of sugars with cholesteryl diphenylphosphate. Steroids 2017, 117, 44–51. [Google Scholar] [CrossRef]




















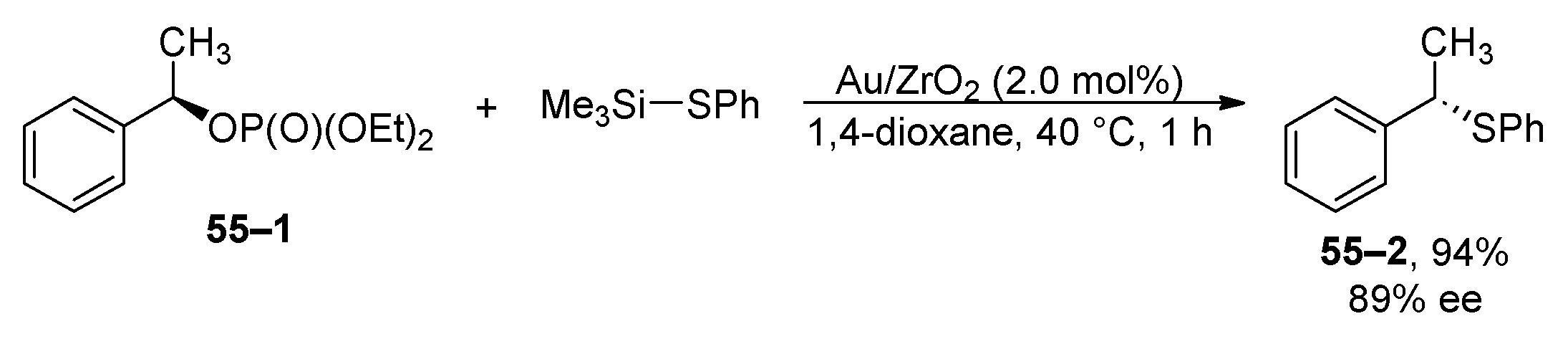


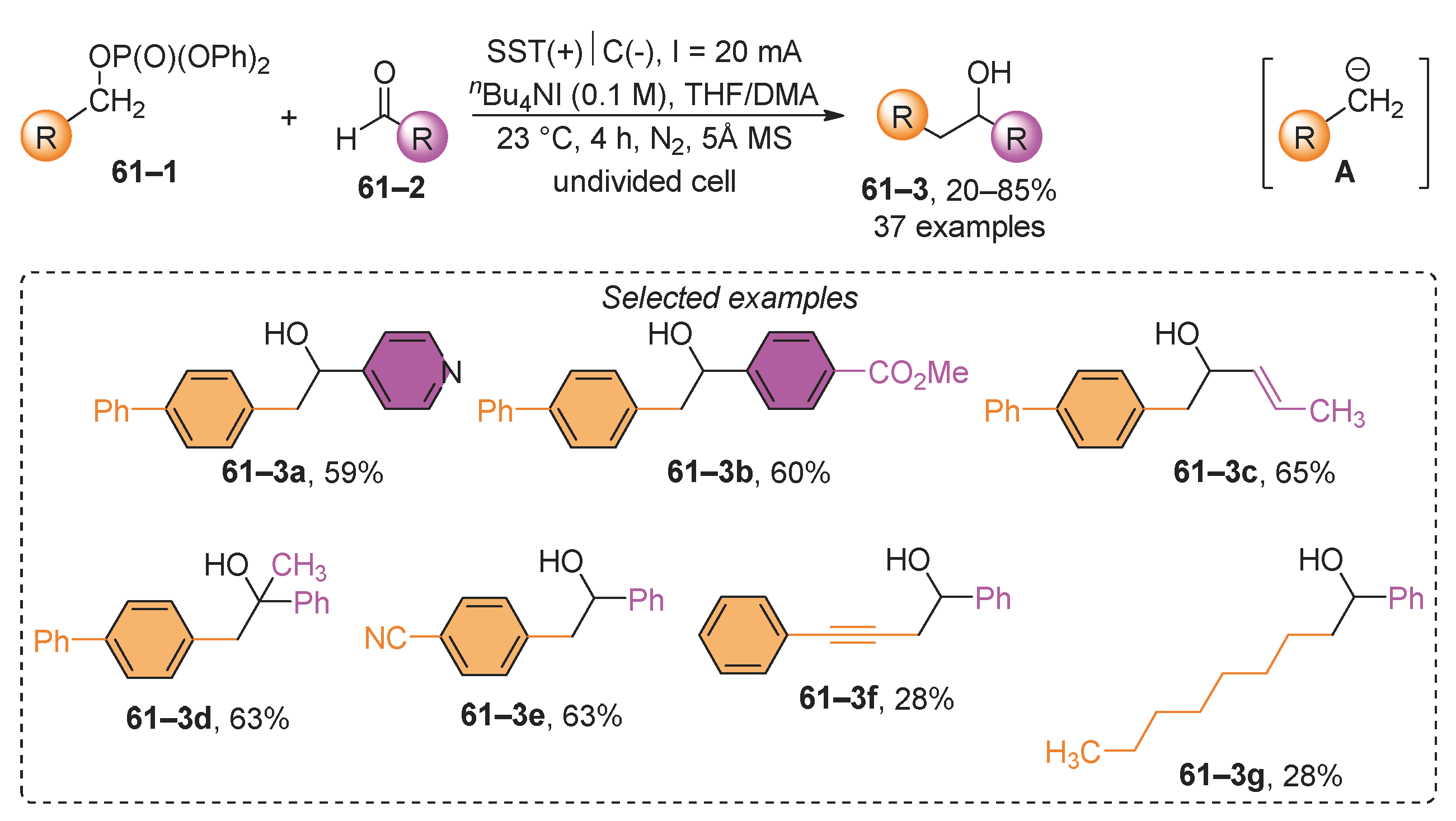
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oeser, P.; Tobrman, T. Organophosphates as Versatile Substrates in Organic Synthesis. Molecules 2024, 29, 1593. https://doi.org/10.3390/molecules29071593
Oeser P, Tobrman T. Organophosphates as Versatile Substrates in Organic Synthesis. Molecules. 2024; 29(7):1593. https://doi.org/10.3390/molecules29071593
Chicago/Turabian StyleOeser, Petr, and Tomáš Tobrman. 2024. "Organophosphates as Versatile Substrates in Organic Synthesis" Molecules 29, no. 7: 1593. https://doi.org/10.3390/molecules29071593
APA StyleOeser, P., & Tobrman, T. (2024). Organophosphates as Versatile Substrates in Organic Synthesis. Molecules, 29(7), 1593. https://doi.org/10.3390/molecules29071593





