Abstract
The Passiflora genus is recognised for its ethnopharmacological, sensorial, and nutritional significance. Yet, the screening of its dietary and bioactive molecules has mainly targeted hydrophilic metabolites. Following the PRISMA-P protocol, this review assessed the current knowledge on carotenoid composition and analysis within Passiflora, examining 968 records from seven databases and including 17 studies focusing on carotenoid separation and identification in plant parts. Those publications originated in America and Asia. P. edulis was the most frequently examined species of a total of ten, while pulp was the most studied plant part (16 studies). Carotenoid analysis involved primarily high-performance liquid chromatography separation on C18 columns and detection using diode array detectors (64.71%). Most studies identified the provitamin A β-carotene and xanthophylls lutein and zeaxanthin, with their geometric configuration often neglected. Only one study described carotenoid esters. Besides the methodology’s insufficient description, the lack of use of more accurate techniques and practices led to a high risk of bias in the carotenoid assignment in 17.65% of the articles. This review highlights the opportunity to broaden carotenoid studies to other species and parts within the diverse Passiflora genus, especially to wild, locally available fruits, which may have a strategic role in enhancing food diversity and security amidst climatic changes. Additionally, it urges the use of more accurate and efficient analytical methods based on green chemistry to better identify Passiflora carotenoids.
1. Introduction
The genus Passiflora is widely distributed in tropical and subtropical regions, with more than 500 species already identified in Central and South America, of which roughly 150 are native to Brazil and approximately 60 produce edible, morphologically diverse, and sensorially singular fruits called passion fruits [1,2,3,4]. Passion fruits from autochthonous and exotic varieties are regionally consumed in fresh form or in very traditional preparations, such as juices, and varied culinary recipes, besides processed products [5].
Benefits for human health have long been reported after consumption of different botanical parts of the Passiflora plants or their products. Sleep modulation and anxiolytic effects described in folk medicine and associated with administering leaf and flower infusions from Passiflora spp., primarily P. incarnata and P. alata, have been more recently attributed to their natural products such as β-carboline (harmala) alkaloids and flavonoids such as chrysin and vitexin, as well as benzoflavones [6,7,8]. In the context of herbal medicine, the use of aerial parts of P. incarnata L. for anxiety relief and control of mild insomnia has been documented in the Brazilian Pharmacopoeia [9], with the Medicines & Healthcare Product Regulatory Agency from the UK [10], among others, also contemplating Passiflora applications to human health [6].
Despite the well-recognised ethnopharmacological relevance of Passiflora spp., the nutritional, functional, and technological potentials of some species are still considered underexploited, especially when it comes to the wild ones [4]. Among the studies with these species, Duarte et al. [11,12] reported post-prandial (3 h) and chronic (for 14 days) health benefits associated with the respective consumption of P. setacea pulp juice (150 g pulp) and pulp (50 g) by overweight individuals. A reduction in HOMA-IR and insulinemia, an increase in HDL, as well as modulation of genes related to inflammatory mechanisms were noticed in the former study [11], while reduced IL-6 levels were observed in the latter [12]. Additionally, Santos et al. (2021) [1] evaluated the composition of bioactive dietary components in P. tenuifila and reported that this fruit is a good source of fibre, phenolic compounds, and carotenoids, suggesting interesting nutritional and functional properties. Indeed, the positive effects observed in epidemiological and intervention studies from the consumption of fruits such as passion fruit have been attributed to the presence of nutrients such as vitamins, minerals, and fibre in these foods and to their phytochemical composition [13,14].
The composition of phenolic compounds in Passiflora genus has been reviewed [4], highlighting the characteristic presence of C-glycosylflavones such as vitexin, isovitexin, orientin, and isoorientin [4]. Moreover, the peel and pulp of several varieties of passion fruits present colouration suggestive of the presence of carotenoids, but the lipophilic portion of components of Passiflora organs, especially carotenoids, overall do not receive as much attention as the hydrophilic fraction, as can be noticed in recent reviews convening data on the chemical characterisation of this plant [15,16,17,18].
Carotenoids constitute a family of more than 700 lipophilic compounds imparting yellow, orange, and red hues to a wide range of plant tissues, in which they play primary roles as part of the photosynthetic apparatus, especially in leaves, as well as secondary functions as antifungal agents and flavour components [19,20]. Structurally, they are characterised by a hydrocarbon backbone containing a series of conjugated double bonds (cdb, commonly in the more stable (all-E)-configuration) and can be classified as carotenes and xanthophylls, the latter presenting oxygenated groups in their molecule in contrast to the former. Hydroxylated xanthophylls can be naturally found esterified with fatty acids to constitute the carotenoid esters, which are often neglected given the increased complexity in analysis [21].
The carotenoid class is well known due to the provitamin A activity of some of its members, primarily the (all-E)-β-carotene, which displays the structural requirements to be bioconverted into two molecules of this indispensable nutrient after consumption. Furthermore, beneficial health effects of the dietary intake of provitamin A and non-provitamin A carotenoids have been reported and comprise particularly the decreased risk of developing age-related macular degeneration and other eye conditions [22,23], some types of cancer [24,25], and cardiovascular [26,27] and bone diseases [28]. Humans depend on their diet to obtain carotenoids and consequently be exposed to these health-promoting activities, with the chemical structure of these compounds determining their physicochemical properties; their behaviour through digestion, absorption, metabolism, and distribution; and ultimately, their biological efficacy [29,30].
Building on the above, it is relevant to evaluate individual carotenoids in different parts of Passiflora plants. The information on the chemical species present may be catalogued in databases and guide nutritional strategies, as well as be the basis for epidemiological research that seeks to elucidate the association between the consumption of phytochemicals and health outcomes. The carotenoid composition analysis is also the first step in bioavailability studies focusing on better retaining the health properties of these compounds. The knowledge about the main carotenoids present in Passiflora plants can further promote agronomic strategies for a greater production or accumulation of these compounds by the plant [31], as well as stimulate the consumption of locally available species as a source of bioactive compounds. Therefore, this study aimed to compile the available scientific data about the composition of carotenoids in organs of Passiflora plants, also providing a picture of the current scenario of the main methods of extraction, separation, identification, and sometimes quantification applied to this end.
2. Results and Discussion
2.1. Search Results
The database search, using different combinations of the chosen keywords, was blindly conducted by three of the co-authors and found a total of 968 documents (Figure 1). All the documents were arranged in the software Rayyan® [32] to perform the posterior selection stages. After removing duplicates (n = 82), titles and abstracts of the remaining 886 articles were blindly read by three of the co-authors, and 849 of them (95.82%) were excluded for not meeting the inclusion criteria. Of these, 552 articles (65.02%) were excluded for not addressing carotenoid composition analyses, and 258 (30.39%) for consisting of publication types not considered in this review, such as review articles, conference abstracts, case studies, editorials, letters or brief communications, and book chapters. Another 23 studies (2.71%) were excluded at this stage for offering only analysis of the total carotenoid content of the crude extracts without previous separation, while 16 papers (1.88%) were not included since they analysed several products obtained from parts of Passiflora plants, not the parts themselves. Therefore, 37 articles were selected for full-text reading, and 17 of them were included in this review (representing 4.18% and 1.92% of the initial number of articles, respectively, not considering the duplicate ones, Figure 1). Of the 20 articles excluded at the full-text reading stage, 19 analysed only total carotenoids, and one study focused on the functional groups found in carotenoids rather than the individual identification.
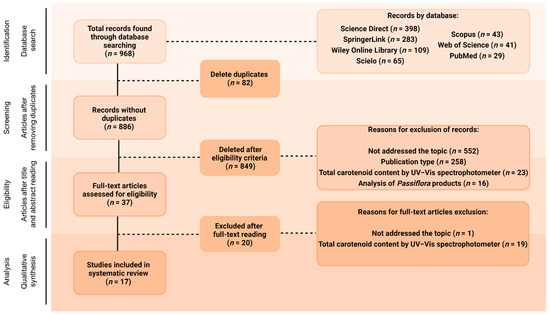
Figure 1.
Flow diagram of the search strategy. Created with BioRender.com.
First, the limited number of studies reporting the carotenoid profile of plant tissues of the numerous and widely distributed Passiflora genus draws attention. This subject has been considerably less explored than the phenolic compounds in the same botanical genus, as reviewed elsewhere [4]. For example, Gadioli et al. (2018) [4] found initially 2066 studies in their systematic review on phenolic compounds in different parts of Passiflora species, more than twice the number of studies found in the initial research of the present study (n = 886), and 82 out of those had, in fact, identified the polyphenols, against 17 studies presenting the carotenoid assignment included in the present review. As mentioned before, previous reviews on chemical composition of Passiflora plants indeed contain several papers that focus on hydrophilic fractions of compounds and relatively few ones with carotenoid analysis, most of which present only total carotenoid content or their analysis of products prepared from the plant parts rather than the plant organs themselves [15,16,17,18]. The same can be noticed in reviews summarizing the chemical composition of other plant species that included a section on carotenoids, which also reported few studies presenting the carotenoid profile of their samples, such as for buriti (Mauritia flexuosa) [33] and mango (Mangifera indica L.) [34], fruits with an expressive content of carotenoids.
In fact, many times researchers report the total content of carotenoids in their complex plant extracts as measured spectrophotometrically, using absorption coefficients of a single carotenoid present in the mixture. Reasons for choosing this method include either its relative simplicity and rapidity compared to chromatographic or other more complex analyses or limited expertise in these particular analytical procedures, constraints in equipment and supplies, or because such an estimate is considered satisfactory for accomplishing the study’s objectives. Markedly, 42 articles have been excluded in different stages of this review process by presenting only the total carotenoid content. In other words, only one-third of the articles that aim to analyse carotenoids in Passiflora present the individual carotenoid profile and in some cases, quantitation. While the estimation of total carotenoid content may remain a good response in specific scenarios, such as breeding experiments and for screening purposes, it is no longer sufficient for a broader scope of carotenoid research [35]. The characterisation of the bioactive compounds in plants not only expands the knowledge into its composition but also provides key information for a more comprehensive elucidation of the mechanisms of action underlying the post-prandial and chronic biological activities observed with its intake [4].
2.1.1. Time Period Covered in Papers Included and Their Origin
Another point of interest when considering the chemical profiling of Passiflora samples is that almost half of the papers included in this review, which included papers between 1990 and 2021, were published in the last 5 years (n = 7, 41.17%), notably in 2018 (n = 3, 17.65%) (Figure 2). Roughly 70% of the studies were published within the past decade, which may also reflect the technological development of analytical tools and research, as well as more wide distribution of chromatographic equipment and material, even in developing countries to which several Passiflora species are native. Further information about the techniques applied in the studies included will be discussed later in a dedicated section. Over the years, there has been also a growing interest in studying non-commercially explored species within the Passiflora genus, as well as other parts of Passiflora, including fruit peels and leaves, which is also addressed further in this review.
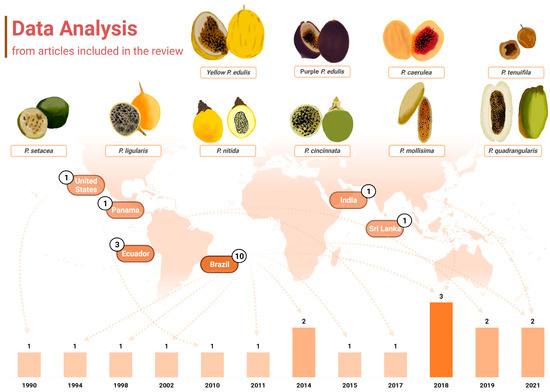
Figure 2.
Graphical representation of the data extracted from the articles containing carotenoid identification in Passiflora. The distribution of publications over the years is displayed at the bottom of the figure, where the years with higher numbers of publications are indicated by progressively darker bars. Additionally, the countries of origin of these studies are displayed, along with their respective publication counts. The fruits from the various Passiflora species analysed in the included studies are depicted through freehand digital illustrations by co-author M. L. B., created using Picsart® software (version 24.3.3). Created with BioRender.com.
It is also noteworthy that the publications included in this review were conducted in only six distinct countries across America and Asia (Figure 2). Interestingly, up to 2018, research on carotenoid identification in Passiflora organs was exclusively published by groups in America. Brazil was the leading contributor, accounting for 58.82% (n = 10) of the studies, followed by Ecuador, which represented 17.65% (n = 3) of the total. The United States, Panama, Sri Lanka, and India contributed one study each (5.88% of the total publications for either country). All the samples analysed were cultivated in the respective countries of publication.
Brazil is the largest producer and consumer of passion fruit worldwide [36], with a production of 697,859 tons of this fruit in 2022 [37]. In addition, at least 150 species of passion fruit are native to this country, factors that may be related to the greater interest in characterising their fruits. Two publications from Brazil are linked to a national research network called Passitec, and they used sample fruits from the experimental field of the Brazilian Agricultural Research Corporation (Embrapa-Cerrados, Planaltina, DF, Brazil), associated with this network. In recent years, Passitec has developed research with native and wild species of Passiflora spp. to establish their production chain and enable the insertion of these fruits from Brazilian biodiversity in the market. This network has the largest genetic collection of Passiflora spp. in the world, including varieties that have been associated with functional and medicinal effects [38]. The results obtained from research carried out within the framework of Passitec have already resulted in adaptable varieties being available in the market, including P. setacea BRS-PC (“Pérola do Cerrado”), P. cincinnata va BRS-SF (“Sertão Forte”), and P. alata va BRS-MC (“Mel do Cerrado”) [39]. This network has been recognised as one of the most relevant bioeconomy platforms established in Brazil [40].
The second largest country in the included publications, Ecuador is the main exporter of concentrated sour passion fruit juice (from P. edulis, 50° brix), P. edulis f. flavicarpa (yellow passion fruit) being the most cultivated species in this country, as it is in Brazil and Peru [41], while in South Africa and Australia, the purple passion fruit (P. edulis f. edulis) stands out in production [42,43]. In Colombia, the neighbouring country of Ecuador, besides P. edulis, five other species are grown commercially, namely P. ligularis, P. tripartita, P. edulis f. edulis, P. maliformis, and P. quadrangularis [42,44], but no study has been included from this country.
2.1.2. Passiflora Species and Parts Studied
This review encompassed analyses of then ten different species or varieties belonging to the Passiflora genus, namely yellow and purple P. edulis (sour passion fruit), P. cincinnata, P. nitida, P. setacea, P. mollissima (taxo or banana passion fruit), P. caerulea, P. quadrangularis (granadilla), P. ligularis, and P. tenuifila (passion fruits of these species are depicted in Figure 2). P. edulis was the most frequently studied species, as also reported elsewhere in terms of polyphenol composition [4] being analysed in 12 investigations of this review (70.59%). The yellow and purple cultivars were specified in eight and four of these studies, respectively. Studies with P. edulis have been published in research conducted in different countries, the USA, Brazil, Sri Lanka, and India, among the studies included in this review. Despite the great diversity of species of the Passiflora genus, the fruits of P. edulis in general are the most popular and widely distributed and consumed ones, mainly as an ingredient in juices, smoothies, jelly, jams, and sweets [45,46,47]. However, since 2011 an increase in research interest in other species in the genus has been identified, including wild and native species (Figure 2). In this context, Brazil and Ecuador stand out with publications that analyse native or exotic but still under-exploited species, such as the passion fruit from the Brazilian Cerrado (P. setacea), P. tenuifila [1], P. caerulea [48,49], P. mollissima [50,51], and P. quadrangularis [52].
The morphology of the passion fruits (as can be seen in Figure 2) and other plant organs varies depending on the formae, cultivar, variety, or species to which they belong. The taxonomy of the highly diverse genus Passiflora continues to be a subject of uncertainty, which explains why many research papers fail to identify the plant used at the infraspecific level or show inconsistency in the terminology for botanical forms of the species [16]. However, some traits are common, and an illustrative representation of general characteristics and morphological organization of yellow P. edulis Sims, the most studied cultivar in the papers of this review and one of the most commercialised passion fruits worldwide, can be found in Figure 3. A P. edulis fruit consists of a spherical to ovoid berry with a relatively tough and coloured peel (epicarp or exocarp), a middle white and spongy layer (mesocarp), and an inner peel layer (endocarp), which together are referred to as pericarp. The interior (locular cavity) is filled with the pulp, composed of numerous membranous sacs (aryl) that surround the seeds [16]. Overall, the leaves contain three to seven lobes, and the flowers are radially symmetrical with five petals and five sepals, often surrounded by a corona of brightly coloured filaments [53].
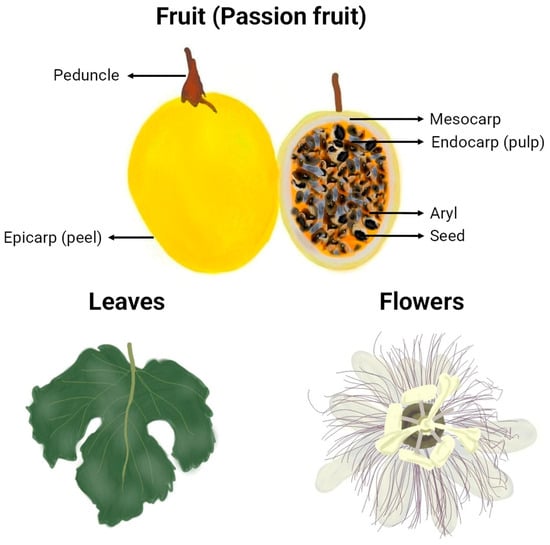
Figure 3.
Graphical representation of botanical parts of yellow P. edulis (va flavicarpa). Freehand digital drawing by the co-author M. L. B. using the software Picsart® (version 24.3.3).
Fruit pulp was the most studied part of the plant in terms of carotenoid composition. Only one study investigated carotenoids in leaves of P. edulis [54]. Peels were also examined in one study that analysed samples of P. caerulea and yellow and purple P. edulis [49]. In the case of peels of P. edulis fruits, colours in the spectrum from pale yellow to deep purple can be observed, so documenting this trait is absolutely relevant when it comes to the characterisation of natural pigments. The whole fruit of P. tenuifila, including peels, seeds, and pulp, was the material used in one study as reflects the way this fruit is commonly eaten. Three studies [51,52,55] denominated their sample material as “edible parts” of passion fruits (P. edulis, P. ligularis, P. quadrangularis, and P. mollissima), and although they did not specify it, it is reasonable to assume that seeds were included as they are commonly consumed along with the pulp.
2.2. Methods and Techniques Employed for Carotenoid Analysis in Passiflora
When investigating the carotenoid composition, it is critical to tailor the analytical procedure to the specific sample under investigation, adapting the protocol to its carotenoid composition as appropriated [35]. The selection of a proper method must also align closely with the objectives of the analysis. The analytical methods and approaches already adopted to examine the carotenoid composition in parts of Passiflora plants, along with their respective conditions, are summarised in Table 1. The sampling/preparation of the analytical sample is also of utmost importance and was considered in the quality analysis is explained in further sections.

Table 1.
Methods applied for extraction, separation, identification, and quantification of carotenoids from different botanical parts of Passiflora plants as described in the selected papers.
2.2.1. Carotenoid Extraction and Pre-Chromatographic Methods
Except when a direct method that does not require sample preparation is applied, carotenoid extraction is essential for their identification and quantification in foods and may affect the composition of those compounds as described in studies. This is because the final composition reported depends on whether the extraction was exhaustively performed with one or a sequence of solvents of different polarities or not, on the characteristics of the extraction solvent and how it can impact the stability of the compounds or promote the formation of extraction artifacts, on the extraction method efficiency and validation parameters, on the application of certain procedures used during the extraction such as the saponification, and on the adoption of precautions to avoid errors.
Since carotenoids are lipophilic molecules, organic solvents like acetone, hexane, ethyl acetate, and methanol are generally well suited for their extraction. Among the studies included in this review, 12 (70.59%) used acetone as the solvent in the extraction of carotenoids from: edible parts (1 fresh and 1 freeze-dried; n = 2); pulp (fresh; n = 7); pulp and peel (fresh; n = 1); leaves (freeze-dried; n = 1); and whole fruit (freeze-dried; n = 1). Acetone is generally the preferred organic solvent for extracting carotenoids, especially from fresh vegetable tissues, as its miscibility with water, besides the affinity for lipids, facilitates enhanced penetration into the sample, improving extraction efficiency, with satisfactory recoveries of both carotenes and xanthophylls [65,66]. However, carotenoids may be unstable in acetone, and a partitioning with a washing step is recommended to remove this solvent [66]. All those 12 studies carried out the partitioning step, transferring the carotenoids extracted with acetone to other organic solvents, with water washing to remove acetone residues. Only 3 [1,52,54] out of those 12 studies extracted carotenoids from freeze-dried samples using acetone, none of them describing whether the sample was reconstituted to its original moisture before the extraction or not. Carotenoids from freeze-dried samples are often extracted with solvents other than acetone as there is no need for a water-miscible solvent and also to avoid the partitioning and washing steps, as they are labour-intense, time consuming, and more prone to losses and errors as the analytical steps increase [66,67].
Another study was conducted using extraction from reconstituted freeze-dried pulp, applying a sequential extraction using a 1:2 mixture of hexane:NaCl (saturated sodium chloride solution in water), dichloromethane, and ethyl acetate, separately [61]. NaCl solutions can be used to prevent the formation of emulsions in the interface of organic and aqueous phases, with the higher ionic strength in the aqueous fraction favouring the migration of the extracted carotenoids to the organic fraction [68]. Also, the use of more than one solvent in sequence during carotenoid extraction is a strategy often employed to cover a wider range of polarity, which consequently allows carotenoids of different hydrophobicity levels to be solubilised and extracted, from the highly hydrophobic carotenoid esters and carotenes to the less hydrophobic xanthophylls during extraction [67,69]. However, analogous to what has been reported for samples of citrus pulp, in carotenoid analysis of passion fruit pulps, the combination of extracts from solvents of varying polarities, such as EtOAc and MeOH, may result in the precipitation of water-soluble substances, such as polysaccharides including pectin from the juice vesicle membranes, the aryls [70]. This would require a liquid–liquid partition or other separation step to remove the precipitates without losses of carotenoids, but such interference has not been observed or reported in any of the studies included in this review.
Two studies used methanol, pure [63] or in a solution with water (60:40, v/v), plus dichloromethane [50] as an extraction solvent for carotenoids from fresh passion fruit pulp. Methanol, as well as ethanol as a greener and safer option, are other examples of water-miscible organic solvents used to extract carotenoids with good penetration into the plant matrix, although there are concerns regarding the recovery of more hydrophobic molecules such as carotenes [35,71]. Still, from Passiflora pulp in natura, one study used chloroform [51], and one reported the use of hexane [56], low-polarity solvents that, in their turn, may not dissolve xanthophylls efficiently [35].
An important consideration is the toxicity of volatile organic solvents commonly used in carotenoid extraction, both to the analysts and to the environment. Consequently, there has been a shift towards the development and use of alternative, greener extraction methods and solvents [72,73]. These innovative approaches have also demonstrated good performance in carotenoid recovery and final extract quality in terms of bioactivities, showcasing their potential to improve analytical outcomes [74,75]. For instance, supercritical fluid extraction has been successfully applied in the extraction of carotenoids from orange peel [75]. Another strategy to eliminate the use of organic solvents involves the production of carotenoid-rich oils, as in the study by Chutia and Mahanta (2021) [70], which applied olive and sunflower oils as solvents in the extraction of carotenoids, assisted by ultrasounds and microwaves, from purple passion fruit peel. However, these studies, focusing, respectively, on carotenoids of a Passiflora residue and on the total carotenoid content, were not included in this review as no included study employed such innovative extraction techniques. Clearly, the use of innovative solvents and techniques, compatible with green chemistry, is a trend that demands more attention and application in Passiflora research.
A critical aspect in carotenoid extraction is avoiding an acidic environment or acid contact, so the natural acidity of passion fruit pulp may pose a challenge in carotenoid analysis of its matrix. Ramaiya et al. (2013) [76] measured the pH of pulp from various Passiflora species grown in Malaysia, and the values ranged from 3.16 to 3.76, while the total titratable acidity ranged from 0.88% to 3.03%. Citric acid was found to be the main organic acid present in P. edulis, P. maliformis, P. quadrangularis, and P. tenuifila fruits [1]. In Brazil, the standard of identity and quality for passion fruit pulp is pH = 2.7, and total acidity in citric acid is 2.5 g/100 g in fresh weight (fw) [77]. The release of acids from plant tissues and the resulting increase of the acidity of the medium during the extraction may promote E/Z isomerization of the carotenoids present in the sample, impacting the native carotenoid profile; thus, the use of a neutralizing agent can be useful to prevent this from occurring [67]. Interestingly, among the studies we reviewed, only one [55] utilised NaHCO3 to neutralise the organic acids in the sample.
Moreover, during the carotenoid extraction procedure, a clean-up step of saponification is traditionally employed to remove interfering compounds, such as triacylglycerols and chlorophylls, simultaneously extracted from plant matrices. This procedure was carried out in 11 (64.71%, [2,48,49,51,52,55,56,58,59,60,62]) of the 17 studies included. A 10% (w/v) solution of KOH in methanol (n = 7, 63.64%, [2,48,49,52,58,59,60]) and a saturated ethanolic solution of KOH (n = 2, 18.18%, [56,62]) were applied, in both cases, with an overnight duration. The other two studies used a 5% (w/v) methanolic solution of KOH, with durations of 4 h at 50 °C [51] and 2 h at room temperature [55]. Whereas this procedure is more commonly carried out with the non-saponified carotenoid extract [2,48,49,51,52,55,58,59,60,62], some research groups perform it with the homogenised plant matrix before the addition of extraction solvents [56], the vegetal tissues being likely softened in this procedure, which might facilitate the extraction. Although producing a cleaner extract, saponification increases the extraction time and may lead to the degradation of carotenoids, especially lutein and violaxanthin, as well as the formation of extraction artifacts [66]. Hot and long saponification procedures and those carried out with high concentrations of alkali may intensify such reactions, which can result in a misleading final interpretation of the qualitative and quantitative data [78]. As the impact of the saponification procedure on the quality of the saponified extract obtained and extraction efficiency depends also on the sample matrix and the type of carotenoid species present, preliminary tests and method validation are recommended to balance variables such as time, temperature, and alkali concentration and define the mildest conditions sufficient to hydrolyse the esters, which can be monitored in a straightforward way by TLC, and then by HPLC [79,80]. In addition, when the sample presents carotenoid esters in its composition, the ester bonds between xanthophylls and fatty acids are also cleaved during saponification. Although this is a choice made by several researchers because the complexity of analysis is reduced, the information on the native carotenoid profile of the sample is also lost, and the relatively new notion that the esterification may impact the bioavailability of carotenoids make this examination of relevance in carotenoid research. The six remaining studies (35.29%) [1,50,54,57,63] did not perform the saponification step, but only one [1] identified and quantified carotenoid esters in its Passiflora sample.
2.2.2. Carotenoid Separation and Identification
All separation and identification strategies applied by the studies reviewed are also summarised in Table 1. Carotenoid separation is traditionally performed by using liquid chromatography techniques, which was true for the set of papers included in this review. Among them, carotenoid separation was achieved by using thin-layer chromatography (TLC, n = 1), open column chromatography (OCC, n = 2), high-performance liquid chromatography (HPLC, n = 15), and its fast and more efficient variants ultra-fast liquid chromatography (UFLC, n = 1; 5.88%) and rapid-resolution liquid chromatography (RRLC, n = 1; 5.88%), employed alone or combined. Two studies [57,58], both from the 1990s, performed an OCC procedure, associated with TLC or not, before injection into HPLC.
Godoy and Rodriguez-Amaya (1994) [57] investigated the presence of (Z)-isomers of provitamin A carotenoids in several Brazilian fruits, including P. edulis. To do so, they performed a two-step OCC separation, first on a MgO (magnesium oxide)/Hyflo Supercel (Celite®, a form of diatomaceous earth, SiO2) phase, where they achieved the separation of the provitamin A carotenoids, followed by a calcium hydroxide (Ca(OH)2) column to separate the carotenoid isomers. To identify the carotenoid profile of yellow P. edulis, Mercadante, Britton, and Rodriguez-Amaya (1998) [58] applied the separation using OCC containing neutral alumina (a treated aluminium oxide—grade III) having separated the carotenoids into three fractions according to their polarity (i. carotenes and epoxy-carotenes; ii. monohydroxy- and keto-carotenoids; iii. polyhydroxy-carotenoids). Then, each band was re-chromatographed by TLC on silica gel, and the bands obtained were again purified by TLC (MgO/Kieselguhr—a form of diatomaceous earth). TLC is a chromatographic separation technique well suited for qualitative analysis and is the method performed in the latter study since the final recovery of carotenoids may not be quantitative [69].
Among the normal-phase stationary phases employed in those classical liquid chromatography studies, silica gel separates carotenoids mainly according to their polarity (the less non-polar carotenoids show higher retention), while MgO presents different adsorption affinities depending on the number and arrangement of cdb in the molecule, with carotenoids with a higher number of cdb showing greater retention [68]. Hyflo supercel® also helps to control adsorption of compounds in MgO, preventing polar carotenoids from irreversibly adhering to the column [66]. On the other hand, chromatography on Ca(OH)2 is widely used and necessary for separation of the isomers, which would not be achieved using the previous columns. Those methods applied by both studies were considered the best way to separate and study carotenoid isomers in combination with HPLC using a reversed phase C18 column at that time, when a C30 column was not available yet.
Regarding the analysis in liquid chromatographs, studies by García-Ruiz et al. (2017) [50] and Gunathilake, Ranaweera, and Rupasinghe (2018) [54] used RRLC and UFLC equipment, respectively. García-Ruiz et al. (2017) [50] performed the chromatographic run of their carotenoids in 12 min, which indeed represents important time savings compared to the studies that used HPLC equipment analysed in this review. The average run time in studies employing HPLC was 57.22 min (nine studies reported this information), reaching up to 130 min. Gunathilake, Ranaweera, and Rupasinghe (2018) [54] did not report the total run time of their samples. As for the resolution quality of the chromatograms, we cannot compare it with other studies present in this review as they were not available in the respective publications. However, Stinco et al. (2014) [81] developed and validated the method applied by García-Ruiz et al. (2017) [50], demonstrating that there was good quality of separation of nine carotenoids present in their samples.
The LC stationary phases more commonly applied to separate carotenoids are reverse-phase C18 and C30 columns [82]. In this review, 11 studies (64.71%, [2,50,51,52,55,56,57,58,59,62,63]) used a C18 column for carotenoid separation, one [58] of them also using a nitrile column for xanthophyll separation; five studies (29.41%, [1,48,49,60,61] applied a C30 column; and one study used a C8 column (5.88%, [54]. The development and use of the C30 column to separate carotenoids is relatively recent, with the first study being published in 1994 [83], so studies before this data, such as those of Homnava, Rogers, and Eitenmiller (1990) [56] and Godoy and Rodriguez-Amaya (1994) [57], did not have this option at that time. This column was developed to specifically meet the demands corresponding to carotenoid analysis and allowed for more efficient separation of geometric and structural isomers in contrast to the C18 column, as well as superior selectivity for both polar and non-polar carotenoids [84,85]. Whereas the retention time of carotenoids in HPLC analyses is influenced by multiple factors related to mobile and stationary phases, such as the composition of solvents and temperature in which the column is kept, overall studies have been demonstrating higher relative retention times of these compounds in C30 columns in contrast to C18 phases, related to the higher hydrophobicity of the former (longer C-chain) [86,87,88]. Moreover, due to the differences in retention capacities, it is also possible to observe certain changes in the elution order of some carotenoids when the separations are carried out on C18 or C30 columns, since retention occurs basically according to polarity in the C18 column, and in the C30 column other factors such as the size of the molecule, the polyene chain length, and hydrophobicity are also to be considered [89].
Additionally, the solvents used in the mobile phases and the C30 columns are usually more expensive and selective, which may lead to the use of the C18 column in several studies [82]. Column temperature is another relevant factor to be considered in combination with other method parameters in the separation of carotenoids by HPLC, since it influences the selectivity and the retention time of carotenoids and may lead to biases in the interpretation of results [66]. Among the 17 studies included in this review, only 6 specified the temperature applied on the column during the separation of carotenoids, which ranged from 22 °C to 33 °C. The management of column temperature during carotenoid separation was discussed in the review by Rivera and Canela-Garayoa (2012) [85].
Several organic solvents and their combinations can be used to compose the mobile phases applied in HPLC separation of carotenoids. In the studies included in this review, eight different solvents were employed as mobile phase constituents, namely: acetonitrile (ACN) (n = 8; 40.06%), dichloromethane (CH2Cl2) (n = 3; 17.65%), methanol (MeOH) (n = 17; 100%), ethyl acetate (EtOAc) (n = 5; 29.41%), hexane (n = 1; 5.88%), methyl tert-butyl ether (MTBE) (n = 5; 29.41%), isopropanol (n = 2; 11.76%), and water (n = 4; 23.53%). MeOH was used in all studies included in this review, regardless of the stationary phase. All five studies that used a C30 column as stationary phase applied MeOH and MTBE as mobile phases. Three of these studies added water to their mobile phases, and one added 0.1% triethylamine (TEA) to the MeOH:MTBE solution; the latter was also applied in two other studies using C18 columns. TEA is an organic compound used at low concentrations (due to possible pH changes) to compose mobile phases in carotenoid analysis to improve symmetry and reduce peak tailing, as it influences compound retention [87].
The LC detectors used in the carotenoid identification of Passiflora parts comprised those traditionally employed in carotenoid research. These comprise detection based on UV–Vis light absorption, either at a specific wavelength (UV–Vis detector) or across a wavelength range (diode array detector, DAD), and the more sensitive mass spectral detection in mass spectrometers (MS) to obtain insights on the compounds’ molecular mass. Five studies (29.41%) [48,49,62,63] reported the use of UV–Vis detectors, thirteen (76.47%) [1,2,50,51,52,54,55,56,57,58,59,60,61] used DAD, and only three (17.65%) [1,58,60] employed MS, in series. Four studies [48,49,62,63] applied only UV–Vis detectors, and Homnava, Rogers, and Eitenmiller (1990) [56] applied DAD, in addition to UV–Vis, only for the purple P. edulis samples.
The identification of carotenoids that have been separated by chromatographic techniques was generally performed according to parameters of the chromatographic elution in combination with spectral characteristics of each peak provided by UV–Vis, DAD, and MS detectors (Table 1). The retention time on a reverse-phase column under the applied chromatographic conditions, where the compounds typically elute in order of polarity along with the elution order may provide insights on the type of compound, were indeed observed by many of the authors during identification, in combination with UV–Vis (wavelength of maximum absorption, fine spectral structure; cis peak intensity) and MS spectral characteristics (often in positive mode, so protonated molecule ([M + H]+); and MS/MS fragments). The combination and interpretation of this information offer important structural clues on the carotenoid molecule and allow comparison with authentic standards or with data present in the scientific literature for the identification of the carotenoids in the sample, especially those also using more accurate and orthogonal responses such as nuclear magnetic resonance spectroscopy (NMR) and molecular dichroism, which remain not frequently used in laboratory routines.
Mercadante, Britton, and Rodriguez-Amaya (1998) [58] applied NMR to identify prolycopene, a molecule that cannot be properly distinguished from lycopene by only using mass spectrometry, due to the isomerization promoted by the high ionization temperature. The application of NMR in this study allowed the differentiation and the unequivocal identification of the prolycopene molecule. Oliveira et al. (2014) [61] used near-infrared (NIR) and mid-infrared (MIR) spectroscopy techniques for predicting ripening parameters in yellow P. edulis, including β-carotene content. In this study, it was observed that NIR and MIR techniques were not suitable for the β-carotene, and the authors justify the low concentration of these compounds compared to other components of the food. Infrared spectroscopy techniques are known for being best suited for macroconstituents (≥0.5%), and its application for the quantification of bioactive compounds in foods, including carotenoids, was recently reviewed by Johnson et al. (2023) [90]. The use of these direct, non-destructive analysis techniques would facilitate real-time sample monitoring without extensive preparation, presenting a significant advantage over traditional methods by reducing chemical and time consumption [91,92].
It is important to note that the identification of carotenoid esters remains a challenge. Besides the presence of interfering compounds in non-saponified extracts, there are two major difficulties in the carotenoid ester analysis: (1) the variety of molecules that can be formed by the various combinations of xanthophylls and fatty acids, which also increases the number of chromatographic peaks and often reduce the peak resolution and (2) the fact that there is no difference in the chromophore among the different esters of a given xanthophyll and between them and the free xanthophyll, so the utilization of either UV–Vis or DAD detectors does not provide enough information for molecule identification. In these cases, the application of at least mass spectrometry becomes indispensable to obtain a sufficient response for the tentative identification of the molecule. This technique, particularly with high-resolution analysers like QTOF and Orbitrap, plays a crucial role in the assignment of carotenoids in general as it provides more sensitive and accurate molecular mass information, assisting the structural assignment [93,94].
Furthermore, two studies applied specific chemical reactions to obtain structural information on the carotenoid molecules of their studies. Godoy and Rodriguez-Amaya (1994) [57] applied acetylation and methylation reactions of the extracts obtained to investigate the presence of hydroxycarotenoids. In both cases, the presence of the hydroxyl group was characterised by an increase in the retention factor in TLC. Reduction with NaBH4 was also applied to investigate the presence of apocarotenals, the positive reaction enabling the observation of a characteristic three-peak carotenoid spectrum, referring to the hydroxycarotenoid formed in the reaction. In addition, the iodine-catalysed isomerization was carried out for E/Z isomer differentiation, also in the study by Mercadante, Britton, and Rodriguez-Amaya (1998) [58]. This reaction is based on bathochromic and hypsochromic effects in the wavelength of maximum absorption for (Z)- and (all-E)-carotenoids, respectively [66].
Finally, using the techniques presented and data analysis tools, comprehensive metabolomic research may offer further insights into the formation of chemical structures such as apocarotenoids and oxidative products [95].
2.2.3. Carotenoid Quantification
Whereas the analysis of carotenoid quantity was not an inclusion factor in the present review, 16 articles of the total 17 presented the individual carotenoid quantification in their samples. Most of the studies employed DAD or UV–Vis detector responses after chromatographic separation, thirteen using external [1,2,48,49,50,51,52,54,55,56,59,60,62] and one using internal standardization [61], while one study [57] estimated the concentration of isolated carotenoids with a UV–Vis spectrophotometer using their absorption coefficients. In the latter, authors stated that the quantitation was not performed by HPLC-DAD given the lack of chromatographic resolution of the geometric isomers and the difficulty in the acquisition of (Z)-isomer standards [57]. In one study, it was not clearly stated how the carotenoids were quantified [63], and none of the 17 carotenoids were quantified by using mass spectrometry. For the external calibration, solutions with known concentrations of carotenoid standards of high purity were prepared within the linear working range and separately injected under the same conditions as the carotenoid extract of interest [96]. Although commonly used for quantification in carotenoid analysis by HPLC-DAD, external standardization does not account for any potential losses of analytes during sample preparation and analysis. The internal standardisation, i.e., the addition of a known quantity of internal standards to the sample at the beginning of the analytical process, might help to minimise this bias and ensure greater measurement accuracy [97]. However, the choice of an internal standard can be challenging since, to guarantee the accuracy of the measurement, the compound must exhibit similar chemical properties to the carotenoid of interest, but at the same time, it must be absent in the sample matrix and not overlap carotenoid peaks during chromatographic elution [96]. The study included in this review that carried out quantification using an internal standard used β-apo-8′-carotenal [61], a naturally occurring apocarotenoid originated from the β-carotene and one of the most applied internal standards for carotenoid quantification [98,99].
Among the papers included in which external curves were applied, carotenoids were generally quantified using their specific authentic standards [48,49,50,51,52,54,55,56,59,60,62]. However, for those studies in which a broader identification was presented, standard curves of a carotenoid with a similar chromophore were applied to quantify those compounds for which a standard was not available, or the (all-E)-carotenoid standard curve was applied to quantify its respective (Z)-isomer [1,2,61]. As it is not always possible to obtain all the necessary standards, it is a common practice the use a carotenoid standard that shares UV–Vis spectral characteristics with the carotenoid of interest for quantification [96]. The same strategy is generally applied for the quantification of carotenoid esters, given the low commercial availability of their standards [100]. In this sense, external calibration curves of free xanthophylls were applied for the carotenoid ester quantification in whole fruit of P. tenuifila [1]. However, this is not free from bias, and under ideal conditions, the first choice should be using the specific carotenoid for quantification [35,101]. This is particularly true when considering a gradient method, since carotenoid light absorption is affected by the solvent composition, and, for instance, free xanthophylls elute in different chromatographic regions than their ester forms [96]. Other methods that have also been applied for carotenoid ester quantification include: (i) the calculation of the result as a percentage of area (area obtained from the peak of the carotenoid ester as a function of the total area of the chromatogram); (ii) the carotenoid ester standard synthesised in the laboratory; and (iii) the application of molecular weight correction factors (when utilizing a calibration curve of a free carotenoid to measure a carotenoid ester with an identical chromophore) [96,100].
Additionally, an exhaustive extraction of carotenoids was reported only in three studies [1,57,60] included in this review. When the characterisation of a sample is the purpose of the analysis, the adoption of a quantitative approach is important to guarantee the accuracy, reliability, and relevance of the findings. For an important discussion on quality assurance for carotenoid analysis, readers are referred to the work of Rodriguez-Amaya (2010) [102].
2.3. Carotenoid Composition of Parts of Passiflora Plants
A total of 29 different carotenoid molecules were detected in different organs of Passiflora plants of different species, in the articles analysed (Table 2, Figure 4). This estimate is a result of the following assumptions: (i.) most of the articles included in this review did not report the geometric isomerism of carotenoids (see Table 2), and in this case, when not specified, carotenoids were computed as (all-E)-isomers since it is the most common form of the majority of carotenoids in nature; (ii.) (Z)-isomers reporting the number of the carbon with (Z)-unsaturation in some studies and those not reporting this number were considered once during counting, as well as cryptoxanthin and β-cryptoxanthin. Moreover, it should be noticed that 11 of the 17 studies (64.71%) included in this review were interested in identifying a fraction of the carotenoids of their samples rather than the total profile. Four studies [50,54,60,61] stated the aim of determining only two or three major carotenoids in their samples, while the study by Murillo et al. (2010) [55] examined only the macular xanthophylls lutein and zeaxanthin in passion fruits from Panama. In addition, Homnava et al. (1990) [56] and Godoy and Rodriguez-Amaya (1994) [57] exclusively identified carotenoids with provitamin A activity. Only a single study included in this review investigated and reported the whole native carotenoid profile including the xanthophyll esters [1].

Table 2.
Carotenoids identified and quantified in different parts and species of Passiflora plants as reported by the papers selected in this review.
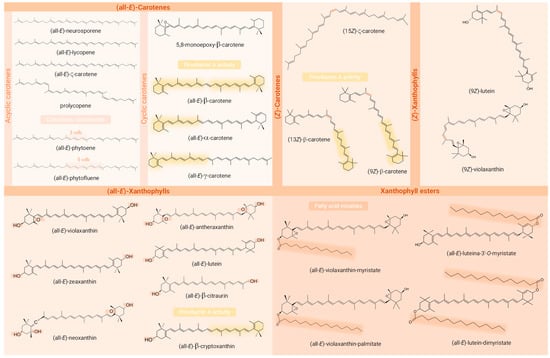
Figure 4.
Chemical structures of the carotenoids identified in the Passiflora samples analysed in the studies included in the review. Structural requirements for provitamin A activity (presence of a β-ring and a C11 polyenic chain), functional groups containing oxygen, the (Z)-bond, and the fatty acids acylated are highlighted in the structures of provitamin A carotenoids, xanthophylls, (Z)-geometric isomers, and carotenoid esters, respectively. Created with ChemSketch® (V14.00, FREE).
Among the identified compounds, 14 were classified as carotenes, 9 as free xanthophylls, 5 as xanthophyll esters, and 1 as an apocarotenoid. An exception to the article that analysed only macular xanthophylls, β-carotene was identified in all the studies, being the only carotenoid common to all of them. Moreover, among the most frequently described carotenoids in Passiflora parts, the xanthophylls lutein (53% of studies), zeaxanthin, and β-cryptoxanthin (41% each) stood out, being followed by the carotenes lycopene (29%) and α- and ζ-carotene (24% each). Interestingly, whereas β-carotene, β-cryptoxanthin, lutein, and lycopene are often characteristic compounds in many fruits, ζ-carotene is more commonly detected in Passiflora samples, even being the major compound found in some samples. Regarding the geometrical isomers, (13Z)- (or (13Z)- or (15Z)-) and (9Z)-isomers of β-carotene were reported in three [1,2,58] and one [1] study, respectively, whereas in the article intended to identify (Z)-isomers of provitamin A carotenoids, they were absent in the sample analysed [57]. The compounds (Z)-ζ-carotene, (Z)-violaxanthin, and (9Z)- and (13Z or 13′Z)-lutein, besides an unidentified poly-(Z)-carotene, were also reported in these very limited studies (n = 4, 23.53 % of the total) included in the present review that differentiated the geometric isomers of at least one of the compounds identified, most not describing the carbon where the cis bond occurs. The identification of carotenoid constitutional and diasteroisomers can be challenging due to various factors [103]. These include the large number of possible isomers that can be found in nature and in crude plant extracts for a single carotenoid molecule, given their long system of cdb (geometric isomers) and the structural complexity of molecules within the carotenoid class. Many of these compounds have similar physicochemical properties such as polarity, which can lead to peak co-elutions. The UV–Vis spectra of mixtures often complicate the analysis of variables such as spectral fine structure (%III/II) and may preclude compound identification [104]. Another point that makes it difficult to identify carotenoid isomers is the low commercial availability of standards that cover all possible isomers of a given carotenoid. Also, the possibility of their formation as analysis artefacts can be a confusing factor in relation to the actual composition of the sample [67]. Whereas isomers sharing the same chromophore cannot be differentiated by DAD, UV–Vis spectra characteristics are generally very useful for distinguishing geometric isomers of carotenoids, the (Z)-forms showing a reduction in absorbance and fine spectral structure in contrast to their (all-E)- counterparts. In addition, the cis peak emerges (142 nm below the λ max), although it is not always clearly visible as the cis bond approaches the extremities of the molecule [105]. In the case of constitutional isomers, combining UV–Vis or DAD with MS responses provides complementary information and may allow their correct identification, since these isobaric molecules may present different absorption spectra or MS/MS fragmentation patterns despite the having same molecular weight [99,104]. Although MS provides important structural information that contributes to the identification of carotenoid peaks, it still only allows tentative identification and is unable to distinguish between E/Z-isomers and between 5,6- and 5,8-epoxides [35]. Thus, NMR stands out as a particularly useful tool for the unequivocal identification of carotenoid isomers. This technique allows analysis of the environment of each hydrogen atom using 1H-NMR spectra, as well as the type of each carbon atom using 13C spectra, enabling the identification of the precise location of the cis bonds and the full elucidation of the structures [105,106].
The only study reporting the geometric isomers of all the compounds identified with clarity was also the only study to report the carotenoid ester assignment. The analysis of the carotenoid esters and the geometry of the molecule including the configuration of cdb is of high relevance, since carotenoids with different structural forms exhibit different properties and functions including their interaction with different enzymes, and even their provitamin A activity, when this is the case [30].
Gunathilake, Ranaweera, and Rupasinghe (2018) [54] did not perform a comprehensive analysis of the carotenoids present in P. edulis leaves but focused only on β-carotene and lutein as they are the most common in leaves, where they play a role as primary carotenoids in the plant’s photosynthetic apparatus, and due to the important antioxidant activity of these compounds. In this study, the lutein content found in P. edulis leaves was the lowest among the other leaves analysed (Sesbania grandiflora; Cassia auriculata; Gymnema lactiferum; Olax zeylanica; Centella asiatica). Since this was the only study included that evaluated carotenoids from Passiflora leaves, we therefore highlight the gap in the identification of the carotenoids that constitute the leaves of Passiflora plants.
Out of the 12 studies analysing passion fruit pulp, 10 (83.33%) focused on P. edulis, including both purple [49,56,59,63] and yellow varieties [2,49,56,58,59,60,61,62], while 3 studies [54,55,57] did not specify the variety. Of these, only three provided a detailed identification of carotenoids in yellow P. edulis [2,58,59], with one of these studies also examining purple P. edulis [59]. In the purple variety, 8 carotenoids were identified [59], whereas 11 to 13 carotenoids were found in the yellow variety [58,59]. β-carotene, ranging from 2.39 µg/g to 13.35 µg/g, and the colourless phytofluene (not quantified) were the most common in both varieties across all three studies. In the other studies, β-carotene and ζ-carotene were the most abundant carotenoids of P. edulis pulp, in their (all-E)- and (Z)-forms [2,59]. Notably, Konta et al. (2014) [60] identified only these two carotenes as the major ones in yellow P. edulis pulp, with average contents of 13.8 µg/g and 16.7 µg/g (fw) for β-carotene and ζ-carotene, respectively. Additionally, two studies [2,59] reported (13Z)-β-carotene, varying from trace amounts to 0.40 µg/g (fw).
When comparing conventional and organic growing systems, Pertuzatti et al. (2015) [62] found β-cryptoxanthin as the major carotenoid in yellow P. edulis pulps, its concentration being higher in the conventional (249.90 µg/g, fw) than in the organic cultivation system (139.40 µg/g, fw). The authors attributed this result to the difference in light exposure or fruit ripening control between the two treatments analysed. In this study, β-carotene levels varied from 0.56 µg/g, fw to 0.77 µg/g, fw, which are lower than the values found in the studies reported above for the same species and organ.
Reis et al. (2018) [48,49] characterised the pulp of P. caerulea passion fruit and reported lycopene as the most abundant carotenoid, with values varying from 44.05 µg/g [49] to 108.39 [48] µg/g, both in dry basis. This result is consistent with the fact that this species exhibits a more reddish coloured pulp compared to other Passiflora species (Figure 2). In comparison to the pulp of purple and yellow P. edulis, passion fruit pulp from P. caerulea presented higher content of lutein, zeaxanthin, and lycopene, while yellow P. edulis showed higher content of β-carotene [49]. This study was the only one that also analysed a peel from passion fruits and found that the P. caerulea sample presented higher contents of all five carotenoids identified (lutein, zeaxanthin, cryptoxanthin, α-carotene, and β-carotene) [49]. In addition, lutein and β-carotene were the major carotenoids found in the passion fruit peel, regardless of the species.
Wondraceck et al. (2011) [59] analysed the carotenoid profile of pulps from different passion fruits cultivated in the Brazilian Savannah (P. cincinnata, P. nitida, P. setacea, native P. edulis (yellow), native P. edulis (purple) and commercial P. edulis). The major carotenoids varied according to the species studied, being (all-E)-β-carotene the predominant in P. setacea, (Z)-ζ-carotene the predominant in yellow and purple native P. edulis samples, whereas (all-E)-ζ-carotene and (all-E)-β-carotene were the major compounds in commercial P. edulis samples. These results demonstrate the variety of carotenoid composition that can be found in passion fruits, which makes the complete characterisation of the carotenoid profiles of the most diverse species of passion fruit of great relevance.
P. mollissima pulp [50] and edible parts [51] were evaluated in studies from Ecuador. Both studies did not perform a complete analysis of the carotenoid profile of P. mollissima. García-Ruiz et al. (2017) [50] reported the three major carotenoids, α-carotene (1.64 µg/g, dw), β-carotene (79.74 µg/g, dw), and zeaxanthin (1.86 µg/g, dw), while Pérez-Balladares et al. (2019) [51] reported β-carotene (16.25 µg/g, fw) and lutein (45.37 µg/g, fw). Neither of the two studies reported the moisture content of their samples; thus, considering the passion fruit moisture content around 85% [16], we can estimate the β-carotene content of P. mollissima pulp from García-Ruiz et al. (2017) [50] in fresh weight to be around 11.96 µg/g, which is slightly lower than that found by Pérez-Balladares et al. (2019) [51]. However, it is important to highlight that the studies might have analysed different parts, as edible parts might include seeds.
Pérez-Balladares et al. (2019) [51] also analysed edible parts of P. ligularis pulp, and theirs was the only study analysing this species. They found that P. ligularis presented lower values of β-carotene and lutein when compared to P. mollissima analysed in the same study. But both species presented higher values for lutein when compared to P. edulis (variety not specified) edible parts (0.1 µg/g, fw) [55]. Guevara et al. (2019) [52] detected only β-carotene (<0.5 mg/100 g) in edible parts of P. quadrangularis. Lycopene and lutein were also analysed in this study but not detected in their sample. To the best of our knowledge, no other studies have evaluated the complete carotenoid profile in P. quadrangularis, P. mollissima, or P. ligularis, so the composition of these species warrants further investigation.
The study of Santos et al. (2021) [1] was the only one presenting a detailed carotenoid profile. Four carotenoid esters were identified in the whole fruit of P. tenuifila grown in the Brazilian Savannah, which corresponded to the monoesters (all-E)-violaxanthin-myristate, (all-E)-violaxanthin-palmitate, (all-E)-luteina-3′-O-myristate, and the diester (all-E)-lutein-dimyristate. This study analysed ripe and mature green fruits and found that for ripe fruits the major carotenoid was (all-E)-β-carotene, ranging from 8.38 µg/g, fw to 11.10 µg/g, fw, values that are within the range found for other passion fruits analysed in this review. As for mature green fruits, (all-E)-lutein was the major carotenoid (11.86 to 13.24 µg/g, fw). The study also highlighted that the variation in the composition of carotenoids between ripe and mature green stages, especially among the two major compounds, contributed to the discrimination of P. tenuifila fruit according to the stage of fruit ripeness.
This review underlines the remarkable qualitative and quantitative variation in composition of carotenoids from Passiflora parts among the studies and among different samples analysed in the same study. This diversity is expected as it reflects the complex interaction of genetic (including species and varieties to which they belong) and environmental (cultivation conditions, climate, temperature, soil type, water availability, maturity stage, and post-harvest handling, among others) factors influencing the synthesis and accumulation of these metabolites in different vegetable tissues and organs. Plants grown in different locations, or at different times of the year, may present different carotenoid content and profile, as observed [29]. Moreover, the analytical technique employed largely impacts the magnitude and accuracy of the result. Therefore, it is of particular importance to analyse the results with caution. On the other hand, these results are a good indication that Passiflora organs can be further exploited as a source of carotenoids, primarily provitamin A ones.
Despite not being part of this study to evaluate different applications of Passiflora in industry, it is relevant to emphasise that some studies considered these applications in their analyses, which demonstrates this potential. García-Ruiz et al. (2017) [50] considered the use of P. mollissima as additive in the food, pharmaceutical, and cosmetic industries and applied a spray-dried technique to produce microencapsulate P. mollissima, which showed good stability in the bioactive compounds analysed and maintenance of their functional properties. Samyor, Deka, and Das (2021) [63] produced a foam mat-dried passion fruit powder from P. edulis Sims pulp and found greater content of β-carotene in the powder, demonstrating that there was good carotenoid retention even after processing. The powder produced is suitable for use in the food industry, replacing artificial additives and incorporating nutritional value into the food.
The analysis of the studies included in this review allows reinforcing the nutritional potential of edible Passiflora fruits, which, added to its pleasant sensory characteristics, may represent an excellent option for consumption in natura and application in the food industry. However, it is necessary to expand the studies about the comprehensive identification of carotenoids from Passiflora, using adequate methods that allow the good identification of the molecules present in the samples, especially when it comes to unexplored and underused wild species. Increasing knowledge about the composition of Passiflora carotenoids can open space for optimizing its production and make greater use of the species, resulting in increased consumption by the population (edible parts), and explore the possibilities of their application in the industry.
Carotenoids identified in Passiflora samples, as examined in this review, were shown to exhibit promising health benefits. Provitamin A activity was associated with carotenoids such as β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin found in Passiflora plants, crucial for fighting vitamin A deficiency, particularly in developing countries and for people with vegetarian or vegan diets [107]. Passiflora extracts also contain lutein and zeaxanthin, known for promoting eye health by acting as antioxidants and protecting against blue light, showing efficacy in enhancing eye health for individuals with and without eye diseases [108]. Additionally, colourless carotenoids, phytoene and phytofluene, found in Passiflora plants have been associated with decreased risk of developing cancers, antioxidant activity, and skin-damage prevention [109]. Given the health potential of carotenoids described Passiflora, further studies that specifically address the bioactivities of these compounds from plants of this genus are encouraged.
2.4. Analysis of the Risk of Bias in Carotenoid Identification from Passiflora
The main purpose of this review was to verify the carotenoids already identified in Passiflora, although quantitative data was also included when the included study provided it. Therefore, aspects related to carotenoid identification parameters were carefully considered when analysing the risk of bias in the included studies. The proper identification of carotenoids depends on the appropriate interpretation of combined information provided by different analytical tools, so the less information available about the carotenoid structural characteristics, the less assertive the identification [110]. Minimum parameters that should be analysed for carotenoid identification are the following: “(i) UV–Vis absorption agreement with the suggested chromophore. (ii) Chromatographic data on two different systems, preferably Rf (TLC) and Rt (HPLC), including co-chromatography with an authentic sample. (iii) Mass spectrum with at least one quality allowing confirmation of the molecular mass” [65,111]. In cases where this minimum information cannot be obtained, it is recommended to use the terms characterisation or tentative identification; also, the more parameters one can combine for carotenoid determination, the more likely it is that the identification will be assertive [105].
According to the parameters determined for the risk of bias in the identification of carotenoids, eight studies (47.06%) showed low risk of bias (LRQ), six studies (35.29%) showed moderate risk of bias (MRB), and three studies (17.65%) showed high risk of bias (HRB) (Figure 5). Some studies did not present the methods applied for the carotenoid analysis in a way that they could be reproduced. In cases where it was not possible to clearly identify whether or how some step was performed, the parameter was considered as “unclear”. In addition, for articles that referenced to another study without a brief or explicit description, it was considered that the authors performed the experiment under the same conditions as described in the cited reference.
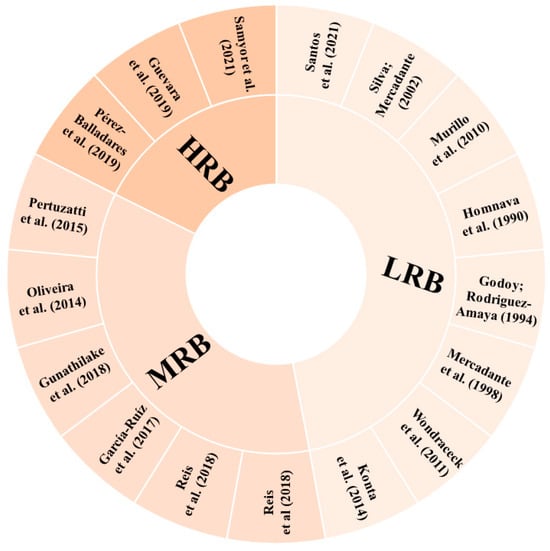
Figure 5.
Risk of bias in each included study. LRB: low risk of bias; HRB: high risk of bias; MRB: medium risk of bias [1,2,48,49,50,51,52,54,55,56,57,58,59,60,61,62,63].
The studies classified as HRB did not present, including either methodology or results, any of the parameters considered for carotenoid identification in their samples. One of these studies [63] mentioned the monitoring of the wavelengths 292 and 325 nm for β-carotene detection, not typically employed for this purpose. This lack of essential details makes it challenging for readers to replicate the study with confidence and fully understand the research.
Four studies classified as MRB [48,49,54,62] compared the compounds’ retention times with those of the respective standards available to identify the molecules. Three [48,49,62] of these studies reported the use of an UV–Vis detector, which in fact limits the number of parameters that can be applied, and one [54] had an HPLC-DAD detector, which also allows the analysis of the UV–Vis spectra characteristics. The last two studies classified as MRB [50,61] applied HPLC-DAD for carotenoid separation and identification and used the comparison with the retention time and UV–Vis spectra characteristics of the respective standards to identify the carotenoid molecules. Phytoene and phytofluene were tentatively identified by Oliveira et al. [61] according to their UV–Vis spectra characteristics. The identification of carotenoids using only the retention time or the UV–Vis spectrum and the elution order on the HPLC column can lead to misidentification, since these parameters can vary, and more than one carotenoid can present the same values, leading to confusion in the peak identification. Thus, when other parameters, such as mass spectra, are not available, it is important to carry out co-chromatography with standards [105].
A relevant and common point for all the studies classified as MRB and HRB, influencing their final score in relation to the risk of bias, was the absence of geometric isomer differentiation in the molecules tentatively identified. Moreover, none of these studies presented figures of chromatograms or spectra, and whereas there is no intention to discuss herein the total researchers’ autonomy to choose the better way to present their data and to question the peer review process, this type of figure does provide readers with valuable insights on the compound characterisation in papers intended to do so. Thus, it was considered a quality parameter in this review, even though the authors agree that it may be somehow subjective.
In the studies that demonstrated a low risk of bias, it is noteworthy that they provided details about the parameters employed for compound identification, offering a more extensive range of parameter combinations (retention time and UV–Vis and MS spectra characteristics compared with standards and available literature), besides making the information of chromatographic and spectral characteristics accessible to the readers. Besides identification tables and the description in the text of the characteristics used for carotenoid assignment, among the eight studies classified as LRB, only two [58,60] did not present pictures of their chromatograms or spectra. Three studies classified as LRB [55,56,60] did not differentiate between E/Z molecules.
The poor description of the methodology mentioned above increased the associated risk in the carotenoid assignment as assessed in this review and shown in Figure 5. Besides the description of sample preparation, extraction, identification, and quantification parameters, another important aspect of data reliability and quality that was overall overlooked in the included papers involves method validation. The validation of analytical methods refers to the application of a set of tests that analyses and documents the performance attributes of a method, demonstrating whether the method in question is suitable for obtaining the desired analytical response. It is therefore recommended that studies prioritise the use of validated methods [112].
As previously described, most of the studies included in this review used classical methods, previously validated and consolidated for carotenoid analysis. Although the long practice of successful use of a given analytical method on a variety of analytes and matrices allows for certain reliability, it is reasonable to assume the necessity of verifying the validated method when implementing it in a specific laboratory [112]. It is important to note that the specific requirements for partial validation may vary depending on local regulatory guidelines or other specific needs [113]. For instance, the Brazilian regulatory guidelines [114] advocate partial validation including at least the parameters of precision, accuracy, and selectivity. Thompson et al. (2002), in the harmonised guidelines from IUPAC [112], considered that the extent to which a laboratory needs to perform validation of a method depends on the method’s current status and the laboratory’s competence. This document recommends the reporting of precision, matrix variation, ruggedness, and linearity in the case of when the “method has been published in the scientific literature together with some analytical characteristics” [112]. The Harmonised Tripartite Guideline [115] recommends the analysis of selectivity, range and linearity, accuracy, precision, LOD, and LOQ as minimum parameters for method validation. According to Petry and Mercadante (2019), for carotenoid analysis by HPLC-DAD-MS, the following validation parameters are the most commonly applied: accuracy, precision, limit of detection, and limit of quantification [96].
The papers included presented only specific figures of validation, many of them not associated with the minimum requirements of the test according to validation guides or references. Since losses can occur during all the steps of carotenoid analysis, from extraction onwards, it is important to present the precision parameter to demonstrate the congruence of the values found [116]. In the included studies, precision was mostly verified as repeatability, i.e., by intra-assay precision with standard deviations (in general n = 3, Table 2), with only two studies presenting the coefficient of variation (CV) of their assays [2,62]. The CV values were below 3.7% and 8% for extractions of Silva and Mercadante (2002) [2] and Pertuzatti et al. (2015) [62], respectively. For accuracy assessment, recovery rates that are also applied for accuracy purposes [116] were reported in a single study by Homnava et al. (1990) [56]. These authors found recovery rates for β-carotene and α-carotene of 87 ± 21.4% and 82 ± 22.0%, respectively, after adding these standards to the sample matrix before extraction and saponification.
Considering the analysis of carotenoids in complex matrices like foods, matrix effect analysis can be potentially significative, but none of the studies included in this review reported this data. The matrix effect occurs due to the presence of components in the food matrix that can hamper the analysis of the desired analyte [113], which can have qualitative or quantitative consequences [117]. In analysis of non-saponified carotenoid extracts by LC-MS, triacylglycerols of the matrix can co-elute with carotenoid molecules and suppress their ionisation in MS, hampering their correct identification [96] and potentially the quantification. This is one of the reasons why LC-DAD has been more applied for carotenoid ester quantification than LC-MS, as exemplified by the single article included in this review analysing non-saponified extracts [1]. Nonetheless, when the matrix effect is not known, the method of standard addition to a blank matrix can be more satisfactory for quantification [117,118]. A close concept to the matrix effect is selectivity, which can be assessed through the interplay between the analyte and the matrix, along with an analytical curve generated without the matrix [96]. If these curves run in parallel, indicating no deviation, the determination of the substance of interest is unaffected by matrix effects, thus rendering the method quantitatively selective [96]. None of the studies included presented such data. In terms of qualitative analysis, the reliability of peak assignment and its purity, the identity verification can be further analysed by other means, including confirmatory techniques, such as derivatization reactions; also, the peak purity can be determined by tandem MS analysis, which enables the identification of impurities within the peak [106]. In the studies included in this review, derivatization reactions were applied in three studies [57,58,59], and three studies applied MS analysis [1,58,60], as can be seen in Table 1 and Section 2.2.2.
The Limit of Detection (LOD) was reported by four studies [48,49,55,62], and Limit of Quantification (LOQ) measurements were reported in three studies [48,49,62]. Finally, linearity was assessed in the studies that quantified their carotenoids using external calibration curves, none of them presenting any parameter of linearity testing besides the determination coefficient.
Although impacting the overall quality, the absence of information on the validation of the methods applied in the included papers does not mean that it was not carried out. It is possible that this detailed information was not provided for reasons of text length, prioritising the information to be presented in the paper. Nonetheless, the description of at least a few parameters of method validation or verification is warranted and another gap identified by the present research in carotenoid analysis in Passiflora.
Many studies included in this review reported only a few carotenoid molecules rather than a complete profile. Some characteristics of these studies may have contributed to not reporting the complete carotenoid profile of their Passiflora samples: 1. The identification of carotenoids was only one of the aims of the paper, as in the study by Konta et al. (2014) [60], which aimed to evaluate the antihypertensive effect of P. edulis pulp in rats; 2. Passiflora was only one of their samples, as with Guevara et al. (2019) [52] and Pérez-Balladares et al. (2019) [51], who provided analysis of 19 fruits and 13 different foods, respectively. Major carotenoids were likely highlighted, but it is worth noting that, although the activities are generally attributed to compounds more concentrated in plant samples, minor compounds could also have significant potential and potentially high efficiencies, either individually or through their synergistic interactions with other molecules. Overall, the average number of carotenoids identified per study included in this review was low, pointing to a need for a more detailed exploration of carotenoids in future research. It is known that the identification of carotenoids is a complex task that requires time and the combined information provided by specific tools and that some studies had equipment limitations, which limits the amount of information available for peak identification and increases the dependence on standards for comparison. Nonetheless, more attention should be directed to the comprehensive identification of carotenoids of various parts of Passiflora plants from different species, with the observation of the analytical steps and the use of more sensitive and accurate techniques.
3. Methodology
3.1. Search Strategy and Eligibility Criteria
The review was based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [119]. An automated literature search, no time restriction to May 2022, on seven scientific databases, namely MEDLINE (PubMed), Web of Science, Scielo, Science Direct, SCOPUS, SpringerLink, and Wiley Online Library was performed. The search terms applied in the research with the Boolean operator “or” were i. “carotenoids” or “carotenes” or “xanthophylls”, ii. “composition” or “identification” or “characterization”, and iii. “Passiflora” or “passion fruit”. Three reviewers conducted searches on databases using different combinations of the chosen keywords and, after removing the duplicates, independently analysed the title and abstracts to exclude the studies that did not meet the inclusion criteria. The coordinator resolved the disagreements. All procedures were conducted blindly in the software Rayyan® [32] used for systematic reviews.
Complete and original articles that carried out the separation and identification of one or more carotenoids in different parts of plants of the genus Passiflora, written in English, Portuguese, or Spanish were included in this review. The quantification of carotenoids could or could not have been performed. Books, book chapters, conference abstracts, review articles, and dissertations and theses were excluded, as well as original articles that did not separate or isolate carotenoids.
3.2. Data Synthesis
The following information was collected from the studies included for analysis: Passiflora species, plant part analysed, methods used for carotenoid extraction, separation, identification and quantification, carotenoid profile, and individual content, when available. Two reviewers summarised the collected information in a standardised table, in which the content was checked by experts.
3.3. Risk of Bias
For the analysis of the risk of bias in carotenoid identification in the studies included in this review, we used as a model the criteria applied by Gadioli et al. (2018) [4], which was adapted from the Metanalysis of Statistics Assessment and Review Instrument (MASTARI) protocol [120]. In this sense, nine criteria were established, comprising (1) Does the study describe the sampling procedure? (2) Is the extraction method described so that it can be reproduced? (3) Does the study describe the methods applied for carotenoid separation, identification, and/or quantification? (4) Were the chromatographic characteristics used as a parameter for carotenoid identification? (5) Were the UV–Vis spectra characteristics measured and used as a parameter for carotenoid identification? (6) Were the MS characteristics determined and used as a parameter for carotenoid identification? (7) Were co-chromatography or comparison with available standards applied in the carotenoid identification process? (8) Does the study present chromatograms and/or mass spectra figures? (9) Does the peak assignment contemplate the geometric configuration of cdb (E/Z isomerism)? Each criterion was evaluated as “yes”, “no”, or “unclear”, applied when the parameter was not clearly stated in the text. The frequency of “yes” was applied as a criterion to analyse the risk of bias, classifying the studies as low risk of bias (LRB) (≥70% yes), moderate risk of bias (MRB) (50–69% yes), and high risk of bias (HRB) (<50% yes) [4].
4. Conclusions
In conclusion, this review showed that research on carotenoids in the Passiflora genus remains limited, highlighting a knowledge gap. The predominant focus on P. edulis, despite the vast diversity within the genus, underlines a need to broaden research to include wild, and underutilised species. Such expansion is vital not only for understanding the potential health benefits of these fruits and their sustainable use but also for exploring their role in climate resilience and food security.
The analytical approach in existing studies has often lacked specificity and accuracy, with advanced techniques like MS/MS and NMR employed in only a minority of cases. This calls for a shift towards more advanced methods to accurately identify the wide array of carotenoids in Passiflora. The pulp has been the most examined part, with β-carotene, lutein, β-cryptoxanthin, and zeaxanthin being the most frequently identified carotenoids. Moreover, the high frequency of ζ-carotene identification suggests that it is a significant component of Passiflora chemical profile. However, a more comprehensive profiling, encompassing the native carotenoid profile and the analysis of other botanical parts and diverse species, is essential.
Future research should not only aim to delineate the carotenoid profiles more thoroughly but also consider the broader implications of these findings. Expanding our understanding of Passiflora carotenoids can lead to optimised cultivation and utilization of these species, offering economic benefits to producers and productive regions; contributing to public health; and enhancing industry applications, such as their use as food additives, in the design of functional foods, and incorporation in nutraceuticals and cosmetics.
Author Contributions
Conceptualization: M.d.M.R.L., D.B.R. and L.d.L.d.O.; Methodology: M.d.M.R.L., R.B., F.N. and L.d.L.d.O.; Validation: D.B.R. and L.d.L.d.O.; Formal analysis: M.d.M.R.L., F.N. and R.B.; Investigation: M.d.M.R.L. and D.B.R.; Writing—original draft: M.d.M.R.L., R.B. and M.L.B.; writing—review and editing: M.d.M.R.L., D.B.R. and L.d.L.d.O.; Visualization: M.d.M.R.L., D.B.R. and L.d.L.d.O.; Supervision: D.B.R. and L.d.L.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the Ph.D. scholarship provided to M.d.M.R.L. (88887.509475/2020-00). D.B.R. is grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2020) and would like to acknowledge the Fundo Europeu de Desenvolvimento Regional (FEDER) and PRR, within the scope of VIIAFOOD Agenda (Valorization, Industrialization and Commercial Innovation Platform for Agri-Food, n.º C644929456-00000040), for her contract. R.B. (código 387 (PIBIC-FAPDF)) and M.L.B. (código 384 (PIBIC-FAPDF)) are grateful to Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) and to Programa de Iniciação Científica—ProIC (UnB) for financial support (Editais ProIC/DPG/UnB—PIBIC/FAPDF 2022/2023—Programa de Iniciação Científica da Universidade de Brasília (ProIC/DPG/UnB)).
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors dec1are no conflicts of interest.
References
- Santos, J.T.d.C.; Petry, F.C.; Tobaruela, E.d.C.; Mercadante, A.Z.; Gloria, M.B.A.; Costa, A.M.; Lajolo, F.M.; Hassimotto, N.M.A. Brazilian Native Passion Fruit (Passiflora Tenuifila Killip) Is a Rich Source of Proanthocyanidins, Carotenoids, and Dietary Fiber. Food Res. Int. 2021, 147, 110521. [Google Scholar] [CrossRef]
- Silva, S.R.; Mercadante, A.Z. Composição de Carotenóides de Maracujá-Amarelo (Passiflora edulis Flavicarpa) in Natura. Ciência Tecnol. Aliment. 2002, 22, 254–258. [Google Scholar] [CrossRef]
- Ramaiya, S.; Bujang, J.; Zakaria, M. Nutritive Values of Passion Fruit (Passiflora Species) Seeds and Its Role in Human Health. J. Agric. Food Dev. 2018, 4, 23–30. [Google Scholar] [CrossRef]
- Gadioli, I.L.; da Cunha MD, S.B.; de Carvalho MV, O.; Costa, A.M.; Pineli, L.D.L.D.O. A Systematic Review on Phenolic Compounds in Passiflora Plants: Exploring Biodiversity for Food, Nutrition, and Popular Medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Lucas-González, R.; Capanoglu, E.; Pateiro, M.; Mousavi Khaneghah, A.; Hano, C.; Lorenzo, J.M. Current Trends in Passiflora Genus Research: Obesity and Fermented Foods Systematic Review. Trends Food Sci. Technol. 2022, 127, 143–155. [Google Scholar] [CrossRef]
- da Fonseca, L.R.; Rodrigues, R.d.A.; Ramos, A.d.S.; Da Cruz, J.D.; Ferreira, J.L.P.; Silva, J.R.d.A.; Amaral, A.C.F. Herbal Medicinal Products from Passiflora for Anxiety: An Unexploited Potential. Sci. World J. 2020, 2020, 6598434. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-windy, S.; Al-Gareeb, A.I. Beneficial Neuro-Pharmacological Effect of Passionflower (Passiflora incarnate L.). Online J. Neurol. Brain Disord. 2020, 3, 285–289. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária (ANVISA). Formulário de Fitoterápicos, 2nd ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2021. [Google Scholar]
- Government of United Kingdom; Medicines and Healthcare Products Regulatory Agency (MHRA). Herbal Medicines Granted a Traditional Herbal Registration (THR). Available online: https://www.gov.uk/government/publications/herbal-medicines-granted-a-traditional-herbal-registration-thr/herbal-medicines-granted-a-traditional-herbal-registration (accessed on 9 September 2023).
- Duarte, I.d.A.E.; Milenkovic, D.; Borges, T.K.d.S.; Rosa, A.J.d.M.; Morand, C.; de Oliveira, L.d.L.; Costa, A.M. Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans. Nutrients 2020, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.; de Souza, M.C.M.; Curinga, R.M.; Mendonça, H.M.; de Lacerda de Oliveira, L.; Milenkovic, D.; Hassimotto, N.M.A.; Costa, A.M.; Malaquias, J.V.; dos Santos Borges, T.K. Effect of Passiflora setacea Juice and Its Phenolic Metabolites on Insulin Resistance Markers in Overweight Individuals and on Microglial Cell Activity. Food Funct. 2022, 13, 6498–6509. [Google Scholar] [CrossRef] [PubMed]
- Denny, A.; Buttriss, J. Plant Foods and Health: Focus on Plant Bioactives; Norwich: Norfolk, UK, 2007. [Google Scholar]
- Pan, W.H.; Yeh, N.H.; Yang, R.Y.; Lin, W.H.; Wu, W.C.; Yeh, W.T.; Sung, M.K.; Lee, H.S.; Chang, S.J.; Huang, C.J.; et al. Vegetable, Fruit, and Phytonutrient Consumption Patterns in Taiwan. J. Food Drug Anal. 2018, 26, 145–153. [Google Scholar] [CrossRef]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.M.A.; Geraldi, M.V.; Junior, M.R.M.; Silvestre, A.J.D.; Rocha, S.M. Purple Passion Fruit (Passiflora edulis f. Edulis): A Comprehensive Review on the Nutritional Value, Phytochemical Profile and Associated Health Effects. Food Res. Int. 2022, 160, 111665. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Z.C.; Cruz, J.M.d.A.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.d.A. Passion Fruit (Passiflora Spp.) Pulp: A Review on Bioactive Properties, Health Benefits and Technological Potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, S.; Hou, G.; Zhao, F.; Meng, Q.; Tan, S. Phytochemistry, Nutritional Composition, Health Benefits and Future Prospects of Passiflora: A Review. Food Chem. 2023, 428, 136825. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Carotenoids: Handbook; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2004; ISBN 978-3-7643-6180-8. [Google Scholar]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold Carotenoids: Much More than Lutein Esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential Health Benefits of Carotenoid Lutein: An Updated Review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef]
- Kim, J.A.; Jang, J.H.; Lee, S.Y. An Updated Comprehensive Review on Vitamin a and Carotenoids in Breast Cancer: Mechanisms, Genetics, Assessment, Current Evidence, and Future Clinical Implications. Nutrients 2021, 13, 3162. [Google Scholar] [CrossRef]
- Konecki, T.; Juszczak, A.; Cichocki, M. Can Diet Prevent Urological Cancers? An Update on Carotenoids as Chemopreventive Agents. Nutrients 2022, 14, 1367. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and Anti-Inflammatory Mechanisms of Action of Astaxanthin in Cardiovascular Diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Riaz, S.; Shahzaib Nadeem, M.; Mubeen, U.; Maham, K. Role of Carotenoids in Cardiovascular Disease. In Carotenoids; Martínez-Espinosa, R.M., Brzozowski, T., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Kan, B.; Guo, D.; Yuan, B.; Vuong, A.M.; Jiang, D.; Zhang, M.; Cheng, H.; Zhao, Q.; Li, B.; Feng, L.; et al. Dietary Carotenoid Intake and Osteoporosis: The National Health and Nutrition Examination Survey, 2005–2018. Arch Osteoporos 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Carotenoid Research: History and New Perspectives for Chemistry in Biological Systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.D. A Simple HPLC Method for the Comprehensive Analysis of Cis/Trans (Z/E) Geometrical Isomers of Carotenoids for Nutritional Studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.d.L.; Sanchez, B.A.O.; Celestino, I.C.; Costa Celestino, S.M.C.; de Alencar, E.R.; Costa, A.M. Shelf Life and Retention of Bioactive Compounds in Storage of Pasteurized Passiflora setacea Pulp, an Exotic Fruit from Brazilian Savannah. LWT 2022, 159, 113202. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- do Nascimento Silva, N.R.R.; Cavalcante, R.B.M.; da Silva, F.A. Nutritional Properties of Buriti (Mauritia flexuosa) and Health Benefits. J. Food Compos. Anal. 2023, 117, 105092. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera Indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Structures and Analysis of Carotenoid Molecules. In Carotenoids in Nature; Rodriguez-Amaya, D.B., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 71–108. [Google Scholar]
- Altendorf, S. Minor Tropical Fruits: Mainstreaming a Niche Market. 2018. Available online: https://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Tropical_Fruits/Documents/Minor_Tropical_Fruits_FoodOutlook_1_2018.pdf (accessed on 15 August 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE) Produção de Maracujá. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/maracuja/br (accessed on 20 February 2024).
- de O Pineli, L.d.L.; Rodrigues, J.d.S.Q.; Costa, A.M.; de Lima, H.C.; Chiarello, M.D.; Melo, L. Antioxidants and Sensory Properties of the Infusions of Wild Passiflora from Brazilian Savannah: Potential as Functional Beverages. J. Sci. Food Agric. 2015, 95, 1500–1506. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Rede Passitec: Desenvolvimento Tecnológico Para Uso Funcional e Medicinal Das Passifloras Silvestres. Available online: https://www.cpac.embrapa.br/Passitec/ (accessed on 27 August 2023).
- Jaramillo, E.H. de Bioeconomía: El Futuro Sostenible. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2018, 42, 188. [Google Scholar] [CrossRef]
- Viera, W.; Shinohara, T.; Samaniego, I.; Sanada, A.; Terada, N.; Ron, L.; Suárez-Tapia, A.; Koshio, K. Phytochemical Composition and Antioxidant Activity of Passiflora Spp. Germplasm Grown in Ecuador. Plants 2022, 11, 328. [Google Scholar] [CrossRef]
- Faleiro, F.G.; Junqueira, N.T.V.; Costa, A.M. Ações de Pesquisa e Desenvolvimento para o Uso Diversificadode Espécies Comerciais e Silvestres de Maracujá (Passiflora Spp.); Embrapa Cerrados: Planaltina, DF, Brazil, 2015. [Google Scholar]
- Castillo, N.R.; Ambachew, D.; Melgarejo, L.M.; Blair, M.W. Morphological and Agronomic Variability among Cultivars, Landraces, and Genebank Accessions of Purple Passion Fruit, Passiflora edulis f. Edulis. HortScience 2020, 55, 768–777. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Desarrollo Rural. Cadena Del Pasifloras. Indicadores e Instrumentos. Primer Trimestre 2021. Available online: https://sioc.minagricultura.gov.co/Pasifloras/Documentos/2021-03-31%20Cifras%20Sectoriales.pdf (accessed on 15 August 2023).
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Revisão de Literatura Completa. Available online: http://www.cpac.embrapa.br/passitec/revisaodeliteraturacompleta/ (accessed on 27 August 2023).
- Ramaiya, S.D.; Bujang, J.B.; Zakaria, M.H.; Saupi, N. Nutritional, Mineral and Organic Acid Composition of Passion Fruit (Passiflora Species). Food Res. 2019, 3, 231–240. [Google Scholar] [CrossRef]
- Biswas, S.; Mishra, R.; Bist, A.S. Passion to Profession: A Review of Passion Fruit Processing. Aptisi Trans. Technopreneurship 2021, 3, 48–56. [Google Scholar] [CrossRef]
- dos Reis, L.C.R.; Facco, E.M.P.; Flôres, S.H.; Rios, A.d.O. Stability of Functional Compounds and Antioxidant Activity of Fresh and Pasteurized Orange Passion Fruit (Passiflora caerulea) during Cold Storage. Food Res. Int. 2018, 106, 481–486. [Google Scholar] [CrossRef]
- dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant Potential and Physicochemical Characterization of Yellow, Purple and Orange Passion Fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Girones-Vilaplana, A.; León, P.; Moreno, D.A.; Stinco, C.M.; Meléndez-Martínez, A.J.; Ruales, J. Banana Passion Fruit (Passiflora mollissima (Kunth) L.H. Bailey): Microencapsulation, Phytochemical Composition and Antioxidant Capacity. Molecules 2017, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balladares, D.; Castañeda-Terán, M.; Granda-Albuja, M.G.; Tejera, E.; Iturralde, G.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits, Tubers and Legumes Traditionally Consumed in the Andean Regions of Ecuador as a Source of Health-Promoting Compounds. Plant Foods Hum. Nutr. 2019, 74, 350–357. [Google Scholar] [CrossRef]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits Consumed in the Western Coastal Region of Ecuador as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef]
- Carlosama, A.R.; Faleiro, F.G.; Morera, M.P.; Costa, A.M. Pasifloras: Especies Cultivadas En El Mundo, 1st ed.; Carlosama, A.R., Faleiro, F.G., Morera, M.P., Costa, A.M., Eds.; ProImpress—Gráfica e Comunicação Visual: Brasília, DF, Brazil, 2020. [Google Scholar]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Analysis of Rutin, Β-carotene, and Lutein Content and Evaluation of Antioxidant Activities of Six Edible Leaves on Free Radicals and Reactive Oxygen Species. J. Food Biochem. 2018, 42, e12579. [Google Scholar] [CrossRef]
- Murillo, E.; Meléndez-Martínez, A.J.; Portugal, F. Screening of Vegetables and Fruits from Panama for Rich Sources of Lutein and Zeaxanthin. Food Chem. 2010, 122, 167–172. [Google Scholar] [CrossRef]
- Homnava, A.; Rogers, W.; Eitenmiller, R.R. Provitamin A Activity of Specialty Fruit Marketed in the United States. J. Food Compos. Anal. 1990, 3, 119–133. [Google Scholar] [CrossRef]
- Godoy, H.T.; Rodriguez-Amaya, D.B. Occurrence of Cis-Isomers of Provitamin A in Brazilian Fruits. J. Agric. Food Chem. 1994, 42, 1306–1313. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Britton, G.; Rodriguez-Amaya, D.B. Carotenoids from Yellow Passion Fruit (Passiflora edulis). J. Agric. Food Chem. 1998, 46, 4102–4106. [Google Scholar] [CrossRef]
- Wondraceck, D.C.; Faleiro, F.G.; Sano, S.M.; Vieira, R.F.; Agostini-Costa, T.d.S. Composição de Carotenoides Em Passifloras Do Cerrado. Rev. Bras. Frutic. 2011, 33, 1222–1228. [Google Scholar] [CrossRef]
- Konta, E.M.; Almeida, M.R.; Do Amaral, C.L.; Darin, J.D.C.; De Rosso, V.V.; Mercadante, A.Z.; Antunes, L.M.G.; Bianchi, M.L.P. Evaluation of the Antihypertensive Properties of Yellow Passion Fruit Pulp (Passiflora edulis Sims f. Flavicarpa Deg.) in Spontaneously Hypertensive Rats. Phytother. Res. 2014, 28, 28–32. [Google Scholar] [CrossRef]
- de Oliveira, G.A.; de Castilhos, F.; Renard, C.M.-G.C.; Bureau, S. Comparison of NIR and MIR Spectroscopic Methods for Determination of Individual Sugars, Organic Acids and Carotenoids in Passion Fruit. Food Res. Int. 2014, 60, 154–162. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Sganzerla, M.; Jacques, A.C.; Barcia, M.T.; Zambiazi, R.C. Carotenoids, Tocopherols and Ascorbic Acid Content in Yellow Passion Fruit (Passiflora edulis) Grown under Different Cultivation Systems. LWT 2015, 64, 259–263. [Google Scholar] [CrossRef]
- Samyor, D.; Deka, S.C.; Das, A.B. Physicochemical and Phytochemical Properties of Foam Mat Dried Passion Fruit (Passiflora edulis Sims) Powder and Comparison with Fruit Pulp. J. Food Sci. Technol. 2021, 58, 787–796. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Mercadante, A.Z. Identification and Quantification of Carotenoids, By HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Schiedt, K.; Liaaen-Jensen, S. Isolation and Analysis. In Carotenoids: Isolation and Analysis; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser: Basel, Switzerland, 1995; Volume 1A, pp. 81–103. [Google Scholar]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI PRESS: Washington, DC, USA, 2001. [Google Scholar]
- Petry, F.C.; Mercadante, A.Z. New Method for Carotenoid Extraction and Analysis by HPLC-DAD-MS/MS in Freeze-Dried Citrus and Mango Pulps. J. Braz. Chem. Soc. 2018, 29, 205–215. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology and Technology, 1st ed.; Rodriguez-Amaya, D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9781118733301. [Google Scholar]
- Mercadante, A.Z. Chromatographic Separation of Carotenoids. Arch. Latinoam. Nutr. 1999, 49, 52S–57S. [Google Scholar]
- Chutia, H.; Mahanta, C.L. Green Ultrasound and Microwave Extraction of Carotenoids from Passion Fruit Peel Using Vegetable Oils as a Solvent: Optimization, Comparison, Kinetics, and Thermodynamic Studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Menezes Silva, J.V.; Silva Santos, A.; Araujo Pereira, G.; Campos Chisté, R. Ultrasound-Assisted Extraction Using Ethanol Efficiently Extracted Carotenoids from Peels of Peach Palm Fruits (Bactris Gasipaes Kunth) without Altering Qualitative Carotenoid Profile. Heliyon 2023, 9, e14933. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods 2023, 12, 4080. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.d.A.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; de Rosso, V.V. Ionic Liquid Associated with Ultrasonic-Assisted Extraction: A New Approach to Obtain Carotenoids from Orange Peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Shaffiq Sahrir, M.A. Sugars, Ascorbic Acid, Total Phenolic Content and Total Antioxidant Activity in Passion Fruit (Passiflora) Cultivars. J. Sci. Food Agric. 2013, 93, 1198–1205. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento—MAPA/Secretaria de Defesa Agropecuária. Instrução Normativa No 37, de 1o de Outubro de 2018; Ministério da Agricultura, Pecuária e Abastecimento—MAPA/Secretaria de Defesa Agropecuária: Brasília, DF, Brazil, 2018. [Google Scholar]
- Kimura, M.; Rodriguez-Amaya, D.B.; Godoy, H.T. Assessment of the Saponification Step in the Quantitative Determination of Carotenoids and Provitamins A. Food Chem. 1990, 35, 187–195. [Google Scholar] [CrossRef]
- Scotter, M. Review and Evaluation of Available Methods of Extraction and Analysis for Approved Natural Colours in Food and Drink; DEFRA Food and Environment Research Agency: London, UK, 2010. [Google Scholar]
- Hong, H.T.; Takagi, T.; O’Hare, T.J. An Optimal Saponification and Extraction Method to Determine Carotenoids in Avocado. Food Chem. 2022, 387, 132923. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Benítez-González, A.M.; Hernanz, D.; Vicario, I.M.; Meléndez-Martínez, A.J. Development and Validation of a Rapid Resolution Liquid Chromatography Method for the Screening of Dietary Plant Isoprenoids: Carotenoids, Tocopherols and Chlorophylls. J. Chromatogr. A 2014, 1370, 162–170. [Google Scholar] [CrossRef]
- Nunes, I.L.; Mercadante, A.Z.; Mercadante, A.Z. Vantagens e Desvantagens Das Colunas C 18 e C 30 Para a Separação de Carotenóides Por CLAE. Rev. Bras. De Ciências Farm. 2006, 42, 539–546. [Google Scholar] [CrossRef]
- Sander, L.C.; Epler Sharpless, K.; Craft, N.E.; Wise, S.A. Development of Engineered Stationary Phases for the Separation of Carotenoid Isomers. Anal. Chem. 1994, 66, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Emenhiser, C.; Englertb, G.; Sanderc, L.C.; Ludwigd, B.; Schwartzaq, S.J. Isolation and Structural Elucidation of the Predominant Geometrical Isomers of Cy-Carotene. J. Chromatogr. Ai 1996, 719, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.M.; Canela-Garayoa, R. Analytical Tools for the Analysis of Carotenoids in Diverse Materials. J. Chromatogr. A 2012, 1224, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, M.-S.; Yang, F.-L.; Wang-Hsu, G.-S.; Chen, B.-H. Determination of Major Carotenoids in Human Serum by Liquid Chromatography. J. Food Drug Anal. 2004, 12, 79–83. [Google Scholar] [CrossRef]
- Ligor, M.; Kováčová, J.; Gadzała-Kopciuch, R.M.; Studzińska, S.; Bocian, S.; Lehotay, J.; Buszewski, B. Study of RP HPLC Retention Behaviours in Analysis of Carotenoids. Chromatographia 2014, 77, 1047–1057. [Google Scholar] [CrossRef]
- Tai, C.-Y.; Chen, B.H. Analysis and Stability of Carotenoids in the Flowers of Daylily ( Hemerocallis d Isticha ) as Affected by Various Treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef]
- Turcsi, E.; Nagy, V.; Deli, J. Study on the Elution Order of Carotenoids on Endcapped C18 and C30 Reverse Silica Stationary Phases. A Review of the Database. J. Food Compos. Anal. 2016, 47, 101–112. [Google Scholar] [CrossRef]
- Johnson, J.B.; Walsh, K.B.; Naiker, M.; Ameer, K. The Use of Infrared Spectroscopy for the Quantification of Bioactive Compounds in Food: A Review. Molecules 2023, 28, 3215. [Google Scholar] [CrossRef]
- Cebi, N.; Bekiroglu, H.; Erarslan, A. Nondestructive Metabolomic Fingerprinting: FTIR, NIR and Raman Spectroscopy in Food Screening. Molecules 2023, 28, 7933. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR Spectroscopy Combined with Multivariate Regression Modeling as a Preliminary Approach for Carotenoids Determination in Cucurbita Spp. Appl. Sci. 2020, 10, 3722. [Google Scholar] [CrossRef]
- Giuffrida, D.; Zoccali, M.; Mondello, L. Recent Developments in the Carotenoid and Carotenoid Derivatives Chromatography-Mass Spectrometry Analysis in Food Matrices. TrAC Trends Anal. Chem. 2020, 132, 116047. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Acquisition of Mass Spectrometry Data of Carotenoids: A Focus on Big Data Management. In Plant and Food Carotenoids: Methods and Protocols, Methods in Molecular Biology; Rodríguez-Concepción, M., Welsch, R., Eds.; Springer Science+Business Media, LLC, part of Springer Nature: New York, NY, USA, 2020; Volume 2083, pp. 135–144. [Google Scholar]
- Arathi, B.P.; Sowmya, P.R.-R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of Carotenoids: The Challenges and Prospects—A Review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Quantification and Method Validation. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; Mercadante, A.Z., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 351–372. [Google Scholar]
- de Oliveira, E.C.; Muller, E.I.; Abad, F.; Dallarosa, J.; Adriano, C. Internal Standard versus External Standard Calibration: An Uncertainty Case Study of a Liquid Chromatography Analysis. Quim. Nova 2010, 33, 984–987. [Google Scholar] [CrossRef][Green Version]
- Durojaye, B.O.; Riedl, K.M.; Curley, R.W.; Harrison, E.H. Uptake and Metabolism of β-Apo-8′-Carotenal, β-Apo-10′-Carotenal, and β-Apo-13-Carotenone in Caco-2 Cells. J. Lipid Res. 2019, 60, 1121–1135. [Google Scholar] [CrossRef]
- Rodríguez-Suárez, C.; Requena-Ramírez, M.D.; Hornero-Méndez, D.; Atienza, S.G. The Breeder’s Tool-Box for Enhancing the Content of Esterified Carotenoids in Wheat: From Extraction and Profiling of Carotenoids to Marker-Assisted Selection of Candidate Genes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2022; Volume 671, pp. 99–125. [Google Scholar]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid Esters in Foods—A Review and Practical Directions on Analysis and Occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Liaan-Jensen, S. Basic Carotenoid Chemistry. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 1–30. [Google Scholar]
- Rodriguez-Amaya, D.B. Quantitative Analysis, in Vitro Assessment of Bioavailability and Antioxidant Activity of Food Carotenoids—A Review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- O’Neil, C.A.; Schwartz, S.J. Chromatographic Analysis of Cis/Trans Carotenoid Isomers. J. Chromatogr. A 1992, 624, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, X.; Lu, Y.; Xu, J.; Chen, J.; Chen, H. An Improved Method for the Separation of Carotenoids and Carotenoid Isomers by Liquid Chromatography–Mass Spectrometry. J. Sep. Sci. 2021, 44, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.Z. Identificación de Carotenoides. In Carotenoides en Agroalimentación y Salud; Meléndez-Martínez, A.J., Ed.; Editorial Terracota: Mexico City, Mexico, 2017; pp. 78–94. [Google Scholar]
- Furr, H.C. Analysis of Retinoids and Carotenoids: Problems Resolved and Unsolved. J. Nutr. 2004, 134, 281S–285S. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Grune, T. The Contribution of Β-carotene to Vitamin A Supply of Humans. Mol. Nutr. Food Res. 2012, 56, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Shankar, P.; Yao, Y.; Su, X.; Kim, J.E. Effect of Xanthophyll-Rich Food and Supplement Intake on Visual Outcomes in Healthy Adults and Those with Eye Disease: A Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Controlled Trials. Nutr. Rev. 2024, 82, 34–46. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A Comprehensive Review on the Colorless Carotenoids Phytoene and Phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef]
- Liaaen-Jensen, S. Combined Approach: Identification and Structure Elucidation of Carotenoids. In Carotenoids Volume 1B: Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 343–354. [Google Scholar]
- Pfander, H.; Riesen, R.; Niggli, U. HPLC and SFC of Carotenoids: Scope and Limitations. Pure Appl. Chem. 1994, 66, 947–954. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized Guidelines for Single Laboratory Validation of Methods of Analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Marson, B.M.; Concentino, V.; Junkert, A.M.; Fachi, M.M.; Vilhena, R.O.; Pontarolo, R. Validation of Analytical Methods in a Pharmaceutical Quality System: An Overview Focused on HPLC Methods. Quim. Nova 2020, 43, 1190–1203. [Google Scholar] [CrossRef]
- Ministério da Saúde—MS; Agência Nacional de Vigilância Sanitária—ANVISA. Resolução da Diretoria Colegiada—RDC NO 166, 24 July 2017; Ministério da Saúde—MS: Brasília, Brazil; Agência Nacional de Vigilância Sanitária—ANVISA: Brasília, Brazil, 2017. [Google Scholar]
- ICH Harmonised Tripartite Guideline Validation of Analitical Procedures: Text and Methodology Q2(R1). 2005. Available online: https://somatek.com/wp-content/uploads/2014/06/sk140605h.pdf (accessed on 19 February 2024).
- Ribani, M.; Beatriz, C.; Bottoli, G.; Collins, C.H.; Sales, I.C.; Jardim, F. Validação Em Métodos Cromatográficos e Eletroforéticos. Qímica Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.-L.; Evard, H.; Herodes, K.; Ravio, P.; Leito, I. Tutorial Review on Validation of Liquid Chromatography–Mass Spectrometry Methods: Part II. Anal. Chim. Acta 2015, 870, 8–28. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. 2002. Available online: https://s27415.pcdn.co/wp-content/uploads/2020/01/64ER20-7/Validation_Methods/d-AOAC_Guidelines_For_Single_Laboratory_Validation_Dietary_Supplements_and_Botanicals.pdf (accessed on 28 February 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute. The Joanna Briggs Institute Reviewers’ Manual—2014; The Joanna Briggs Institute: Adelaide, Australia, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).