Abstract
There is little data on directly measured carboxymethyl lysine (CML) content in Indonesian foods. This study aimed to generate a database of CML values in foods commonly consumed in West Java and West Sumatra. The results were to be used to update our previous estimated CML values. CML values in food samples were measured using high-pressure liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). Food protein content was analyzed by Kjeldahl’s method or inferred from the nutrition facts’ label. A total of 210 food samples were examined, with the food groups of meat and poultry (1.06 mg CML/100 g edible food), and starchy foods (0.21 mg/100 g edible food) having the highest and lowest mean CML levels, respectively. We found that the foods with the top three highest CML content were fried starch dough (cimol), fried fish crackers, and chicken gulai. The mean of the estimated values (0.80 mg CML/100 g edible food) was higher than the directly measured values (0.66 mg CML/100 g edible food), [p < 0.035]. Conclusion: This database provides information on CML values in Indonesian foods, and can be further used to make a guide policy for the selection of foods to reduce non-communicable diseases. Further measurements are needed on Indonesian dishes to complete the database.
1. Introduction
Carboxymethyl lysine is one of several advanced glycation end products (AGEs) that is mostly used to measure the AGE content in foods or in the body [1,2,3,4,5] because of its stability and irreversible structure [6,7]. AGEs also known as glycotoxins, are harmful compounds [8,9] that are formed through nonenzymatic reactions between reducing sugars and foods, resulting in a brownish coloration that was first mentioned by Louis Camille Maillard in 1912 [10]. The identification of the CML-producing pathway through degradation of fructoselysine was subsequently described by Ahmed in 1986 [11]. CML is a product of the glycoxidation process through the oxidation of Amadori products or direct reaction of glyoxal with ε-amino lysine groups [12,13].
Circulatory AGEs in the body are associated with the balance of exogenous AGEs from dietary and cigarette smoke, the accumulation of AGEs in the body, endogenous AGEs synthesis, and the clearance of AGEs [7,14]. A correlation between high AGE consumption and circulatory CML was seen in several studies [15,16,17,18,19]; AGE is furthermore believed to be associated with insulin resistance [7,20], obesity [21,22], cardiovascular disease, and renal failure [1,7,23,24,25].
There are two mechanisms of CML-induced disease, namely a receptor-dependent and a receptor-independent pathway. In the receptor-dependent pathway, CML binds to the CML receptor and triggers some cascade reactions and serial signaling pathways [26]. In the receptor-independent pathway, covalent cross-linking reactions of CML with proteins, lipids, or extracellular matrices will occur, resulting in biochemical and cellular impairment [27]. Other models of the mechanism of action of CML were suggested by Chen and Guo, comprising (i) reactive oxygen species formation; (ii) mitochondrial dysfunction; (iii) AGEs acting as antigens in the immune system; and (iv) AGE-induced allergic reaction [5].
AGEs originating from foods contribute more to the AGE pool than endogenous AGEs do [17,28]. In healthy persons, about 10–30% of the amount of CML in the food consumed is absorbed in the intestinal epithelial cell membrane [28,29] as dipeptides by peptidase transporter PEPT1 [30]. The dipeptides are hydrolyzed into amino acids and penetrate into the membrane. CML is usually absorbed via simple diffusion [28,30]. Around 30% of AGEs are excreted in the urine in healthy people, but only 5% in patients with renal failure [10]. The remaining AGEs accumulate in the body [31]. One study has shown that CML consumed from foods in the long term is deposited mostly in the kidneys, colon, ileum, and lungs [5]. Deposits of CML are also found in the brain, skeletal muscle, testis, liver, heart, spleen, and body fat [5,27,32,33].
Food preparation has an influence on AGEs’ content through the browning effect and fluorescence formation. Dry-heat cooking techniques such as deep-frying, roasting, baking, and grilling increase the level of AGEs [34] up to 100-fold from that in untreated food [6,35]. High amounts of fat, animal protein, and cereals are known to result in higher AGEs content as compared to vegetables, fruits, and coffee [6,35,36]. Manufactured foods such as processed nuts and canned meat have high AGEs content [37]. Apart from the process of food preparation, it is known that the storage of foods also affects the AGEs content [35].
It is important to have a database of CML in foods if we want to investigate the as-sociation between dietary CML and NCDs. Uribarri et al. in the USA built a large CML database using enzyme-linked immunosorbent assay (ELISA). Hull et al. in the UK and Scheijen et al. in the Netherlands developed CML databases using liquid chroma-tography with tandem mass spectrometry (LC-MS/MS) [36,37,38]. In regard to Asian countries, Takeuchi et al. in Japan investigated various AGEs using ELISA and developed a CML database [39].
We recently developed a CML database of Indonesian foods by estimation from the results of existing studies that used the LC-MS/MS method [40]. The results of the study showed that in the food group of cereals, instant noodles had the highest estimated CML content and were the second largest contributor to CML intake after steamed white rice. Furthermore, we detected that there were twelve paths that involved dietary CML as a mediator on waist circumference (WC) and that most of the paths had a positive association [41]. A one-unit increment in dietary CML was associated with an increase in WC of 0.33 points. The close relationship between AGEs and obesity was also demonstrated through a bibliometric study [23]. This shows the importance of having a CML database of local foods. However, to date, there are no data on the CML content of Indonesian foods in the Indonesian database on food composition, particularly the CML content based on measurement. The primary objective of the present study was to obtain an Indonesian CML database of Indonesian foods using LCMS/MS. The secondary objective was to compare the estimated CML content of Indonesian foods with that measured by LCMS/MS.
2. Results
2.1. LCMS Optimization and Method Validation
Although CML is a compound with high polarity, it can still be separated by reversed-phase chromatography. In our study, the analyte was separated with a C18 column, at a column temperature of 40 °C, and gradient mobile phases A and B consisting of 0.1% formic acid in water and 0.1% formic acid in methanol, respectively. Mobile phase A was set up using gradient mode at 0–4 min 80% phase A, and 4–6 min 10% phase A. These conditions gave the most optimal separation results. The resulting LCMS chromatograms of the standard solution (upper chromatogram) and spiked CML in fried tilapia (lower chromatogram) are shown in Figure 1. Optimization of the MRM products was carried out from their highest abundance at m/z 84.1. The MRM product at m/z 84.1 was used as a quantification determinant in this study. The MRM internal standard CML-d4 was obtained at m/z 88.1. The retention times of the CML and CML-d4 standards were measured at the same time. The retention time of the CML separation remained consistent both in the standard solution and in the sample matrix.
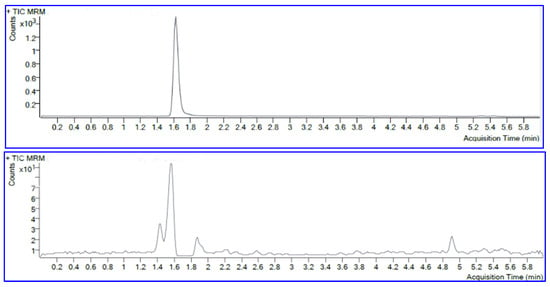
Figure 1.
Upper: chromatogram of CML standard. Lower: chromatogram of CML in fried tilapia.
2.1.1. Method Validation on Four Types of Food
The calibration equation was y = 3.55 + 3.39x, R2 = 0.999, while the linearity range was between 0.5 and 400 μg/L for CML. This is sufficient to determine most CML concentrations in foods. In case of higher CML concentrations in food, the extract can be diluted up to 10 times the targeted level. The limit of detection (LoD) was 0.5 µg/kg in fried tilapia and cornflakes, and 1.5 µg/kg in instant noodles and pangek sasau. The limit of quantification (LoQ) was 1.0 µg/kg in fried tilapia and cornflakes, and 5.0 µg/kg in instant noodles and pangek sasau. The LoD and LoQ were defined as the concentration (µg/kg) at which the signal-to-noise ratio of the peak of interest was 3 and 10, respectively.
2.1.2. The Recovery of CML-Spiked Samples
The recoveries of exogenous CML-spiked samples were determined at three concentrations, namely at the low concentration of 50 µg/kg, medium concentration of 100 µg/kg and high concentration of 300 µg/kg. The recovery of CML-spiked samples in the sample was almost 100%. The recovery tests were determined at three replications for each concentration. The recovery percentages of each food matrix are shown in Table 1.

Table 1.
The recovery percentages of CML spiked in fried tilapia, cornflakes, instant noodles and pangek sasau.
The precision test on the samples of fried tilapia, cornflakes, instant noodles, and pangek sasau was acceptable. Overall, the precision values obtained from almost all tested matrices are below 10% (Table 2). In the cornflake matrix, the interday precision is slightly above 10%. Sample homogenization is very important in precision and recovery testing.

Table 2.
The precision percentage of CML spiked in fried tilapia, cornflakes, instant noodles, and pangek sasau.
2.1.3. Quality Testing
The Reliability of the Measurement Was Analyzed with Duplicate Measurements
The reliability of the measurement was analyzed with the incurred sample analysis (ISR) using the same equipment, method, analyst, and laboratory, but on different days. About 15% of the total samples were selected by randomization. In our study, a strong correlation was found between the sample reanalysis of CML content in the foods (y = 0.02 + 0.97x, R = 0.928), with the mean difference between the measurement being 9.9%.
Quality Control
The quality control in every run day or batch is described in Figure 2. The mean CML concentration derived from 12 quality control food samples is shown by the red line. The yellow and soft blue lines refer to the mean ± 2 SD. The grey and green lines refer to the mean ± 3 SD. The quality control tests using CML spiked in fried tilapia do not exceed the mean ± 2 SD. The QC measurements are closer to the true value.
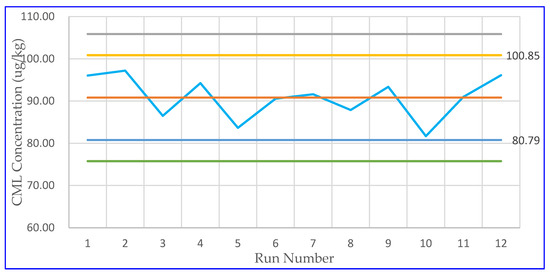
Figure 2.
Control chart displaying the variability in the measured concentration of CML.
2.2. Measurement of Indonesian Foods
The CML-in-foods database is presented by food group and in units of mg CML/100 g edible food, mg CML/kg protein, and mg CML/average portion, as shown in Table S1. The meat and poultry and starchy food groups have the highest and the lowest mean CML levels with 1.06 mg CML/100 g edible food and 0.21 mg/100 g edible food, respectively (Table 3). When expressed in mg CML/100 g edible food, kue talam and rengginang have the lowest CML content at 0.01 mg CML/100 g edible food, whereas cimol has the highest CML content at 5.35 mg CML/100 g edible food. When expressed in mg CML/kg protein, ikan maco goreng has the lowest CML content at 0.37 mg CML/kg protein and cimol the highest CML content at 7535.21 mg CML/kg protein.

Table 3.
Mean and range of the CML content of food groups, expressed per mg/100 g edible food, mg/kg protein, and mg/average portion size.
2.3. Estimated versus Directly Measured CML Content
We found a significant difference between the estimated (0.80 CML/100 g edible food) and directly measured (0.66 CML/100 g edible food) CML values, at p = 0.035. When viewed by food group, there was a difference between the estimated and measured CML content in the cereals (p < 0.001) and egg groups (p = 0.012), but not in the groups of starchy foods, legumes, meat and poultry, fish, shellfish and shrimp, and milk products and coffee (p > 0.05 in all groups), as shown in Figure 3.
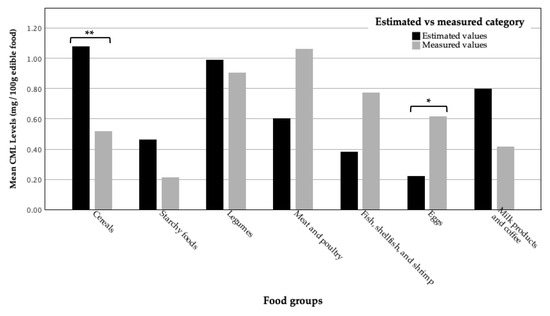
Figure 3.
Mean differences between estimated and measured CML content. Wilcoxon test was used to compare continuous data between estimated and measured values of CML content between food groups. * Results were considered statistically significant at p < 0.05; and ** at p < 0.001.
3. Discussion
We measured 210 Indonesian food samples collected in West Sumatra and West Java, Indonesia. As far as we know, ours is the largest CML database with HPLC-MS/MS measurements of Indonesian foods. Foods from these two provinces are the most important focus of discussion about Indonesian foods [42]. One significant difference in the method of food processing between the two provinces that is interesting to explore is the prolonged cooking time by boiling and the dominance of coconut milk in West Sumatran foods as compared to the shorter cooking time by frying and the consumption of fresh vegetable dishes in the foods of West Java [43,44].
The method of CML determination in our study was adopted from the work of He et al. [45]. However, the sample preparation and extraction of CML from foods are different. Food is a sample matrix with a very diverse composition. Food shape, consistency, matrix complexity, protein and fat content, cooking process and analyte content will be challenges in testing CML in food. The processing samples included cutting into smaller pieces, grinding or crushing, defatted process, analyte reduction, hydrolysis, and analyte extraction. In some food samples with high fat content, n-hexane was used for an effective defatting process. Prior to hydrolysis, sodium borohydride was added to reduce the Amadori products (e.g., fructose–lysine) and lipid oxidation products. This step was very important to prevent the formation of CML during acid hydrolysis [46,47].
The cereals group had a higher mean CML/100 g edible food if compared to the starchy group, which is in line with the study of Hull et al. [36]. The mean CML value in the meat and poultry group (1.06 mg CML/100 g edible food) was higher than in the study of Hull et al., who showed that the meat and fish group had a mean CML value of 0.9 mg/100 g edible food [36]. These differences in CML values between the groups may have been due to the differences in food processing, sampling variability, stability of food matrices, and analytical variability.
We found a significant difference between the estimated CML content and the directly measured CML content in the cereal group (p < 0.001). The mean estimated CML value in the cereal group was 1.09 mg CML/100 g edible food, which is higher than the mean CML value by direct measurement (0.52 mg CML/100 g edible food). The CML contents of Indonesian sweet snacks in the previous database were estimated with reference to European sweet snacks, which are mostly processed at a high temperature. In contrast, traditional Indonesian snacks such as soft glutinous rice flour cake filled with sweet grated coconut (bugis) or coconut cakelets (bandros) are prepared at a lower temperature; therefore, the differences in CML values between these food items were large (CML value by estimation for bugis and bandros was 1.81 mg CML/100 g edible food, and by measurement, for bugis, it was 0.67 mg CML/100 g edible food, and for bandros, it was 0.14 mg CML/100 g edible food).
Interestingly, the mean CML content of foods in the group of fish, shellfish and shrimp was found to be higher than in Scheijen et al.’s study [37]. The difference could be due to the thickness of fish that was exposed to heat. Chen and Smith [48] measured the CML levels in meat samples, including fried fish fillet of tilapia and salmon, in approximately 2 mm of the outer layer and 2 mm of the middle layer of the fish fillet. Their study showed that all CML values of the outer layer samples were four to sixteen times higher than the CML values of the middle layer. Tilapia fillet had high CML values in the outer layer and even higher values in the middle layer if compared to salmon fillet. The fish samples used in this study were mostly of small-size fish, such as tilapia, ikan bilis, ikan kembung, and processed fish, such as salted fish, processed milkfish (bandeng pindang), and fish crackers. Salt could increase the CML content through glucose dehydration [35].
The cooking time of the foods apparently increased the CML content during the preparation of beef-based foods. Gulai, kalio, and rendang are the three foods typical of Minangkabau, West Sumatra, that are cooked using identical spices but different cooking times. The first type of Minangkabau food is gulai, which is cooked until the total moisture content is reduced and the sauce becomes thin and yellowish in color. The second type is kalio, which is cooked for around 1–2 h at a temperature of 90–93 °C, so that the sauce thickens to a brown color. The cooking of rendang needs 3–4 h to be complete and sometimes even takes up until 6–7 h at a temperature of 80–93 °C so that the sauce thickens and the meat becomes dry and dark brown in color [43,49]. Our study showed that gulai has the lowest CML content in mg CML/100 g edible food, followed by kalio and rendang with 1.12 mg/100 g, 1.21 mg/100 g, and 1.72 mg/100 g, respectively.
Adding flour into food processing may increase the CML content of food. Cod fish processed by baking had a CML content of about 0.06 mg/100 g, while the CML content in battered cod fish processed by baking increased almost ten times to 0.59 mg/100 g [36]. The increase was also seen in breaded cod fish prepared by baking, which had a CML value of 1.09 mg/100 g. This study also found that fried chicken with added flour had a higher CML content (1.81 mg CML/100 g edible food) than fried chicken without added flour (0.99 mg CML/100 g edible food). Different results were obtained with tempe mendoan (lightly fried battered tempeh), which had the same level of CML content (0.63 mg CML/100 g edible food) as tempe goreng (tempeh, fried without added flour) with a CML content of 0.67 mg CML/100 g edible food). This may be due to the fact that even though flour is added to the tempeh, tempe mendoan has a shorter cooking time than tempe goreng (fried tempeh). Besides high heating temperature and low water content, longer cooking time contributes to AGE formation in foods [4,15,20,35].
Protein content has a positive correlation with CML content with r = 0.301 (p < 0.001). This is in line with the study of Wu et al., who investigated the influence of shrimp-processing methods on AGE content and showed a positive correlation of protein and oil contents with CML content (p < 0.05). Water content had a negative correlation with CML content (p < 0.01) [34]. The study of Zhao S et al. [50] also showed a strong positive correlation between protein content and CML content for canned fish with r = 0.46. Differing results were obtained by Fu S. et al. for plant-based food analogs [31] and by Niu L. et al. for commercial fish products [35], both of which did not find a significant correlation between protein and CML content. However, although the correlation between protein and CML content is still subject to controversy, modifications in dietary CML can still be performed without affecting the total protein intakes of the subjects [51,52]. This shows the importance of a CML database as a guideline for selecting foods that are low in AGEs in modifying unhealthy dietary patterns.
Weight per portion has no correlation with CML content, at r = −0.049 (p = 0.504). The cereal group, starchy food group, and meat and poultry group that had nearly identical mean weights per portion (79.5 g, 73 g and 64.7 g, respectively) showed very large differences in mean CML contents. The meat and poultry group had the highest mean CML content, namely 1.06 mg CML/100 g edible food, being more than twice the mean CML in the cereal group (0.52 mg CML/100 g edible food), as well as more than five times the mean CML in the group of starchy foods (0.21 mg CML/100 g edible food).
In addition, we examined 14 food items that were taken from the two provinces (Table 4). The mean CML values from West Sumatra and West Java were 0.59 mg/100 g edible food and 0.65 mg/100 g edible food, respectively, at p-value 0.290. Although these means were not statistically significantly different, significant differences in CML content were seen in several food items, such as fried chicken breast and boiled noodle.

Table 4.
Comparison of CML content of foods from two provinces.
The CML contents of foods also differed from those in the databases of other countries (Table 5). In our study, the CML content of white rice from West Sumatra was highest at 0.73 mg CML/100 g edible food, whereas that from European countries [37] was lowest at 0.07 mg CML/100 g edible food. White rice samples from West Java had a CML content of 0.24 mg CML/100 g edible food, which was slightly higher than that from the UK [36] at 0.20 mg CML/100 g edible food.

Table 5.
CML content of Indonesian foods compared with European and UK food databases.
This study recommends the standardization of CML examination procedures that may be used by researchers in the determination of CML levels. Reducing cooking times and refraining from adding flour in the processing of foods may minimize the CML content of these foods. This food database may be used as a reference in estimating CML intakes, and, in turn, may be used to evaluate the relationship between CML intake and disease. Recommendations for the selection of foods may be formulated for the Indonesian communities, particularly those in the two aforementioned provinces.
A limitation of this study is that some food samples were very difficult to homogenize in the preparation process. To date, there is no standard procedure for performing CML measurements; so, each laboratory should carry out CML procedures taken from publications and modify and revalidate these procedures. To our knowledge, this is the first study on CML measurements conducted in Indonesia. Therefore, to minimize bias, the validity and reliability of the measurements were determined with internal standards and CML standards, and performed ISR. One of the objectives of ISR is quality control of the components of measurement to support assay reproducibility. The number of samples to reanalyze for ISR assessments is at least 5% of the study samples in applicable studies. However, we randomly performed an analysis on 15% of all examined samples. The measurements were carried out on different days but in the same laboratory, using the same equipment, method, and analyst. The difference between the measurements was relatively small, 9.9%, which is in accordance with the consensus recommendation that the difference between the concentrations obtained in the initial analysis and the concentrations measured during ISR should be within ±20% [53].
Another limitation is that the food samples were obtained solely from two Indonesian provinces. The present study also did not evaluate in detail the recipes for food processing and the histories of food storage before and after processing, such that these may have resulted in differences in CML content.
4. Materials and Methods
4.1. Selection of Foods
Food selection was based on the foods most consumed in the study of Liman et al. [40]. The foods were grouped according to the Indonesian Food Composition Table (TKPI), as described in that study [40]. The food items were listed and ranked according to the highest consumption and included those that were assumed to have a high CML value per 100 g edible food. A total of 224 food samples were selected, consisting of 192 prepared food samples that were obtained from the two provinces and 32 samples of manufactured foods, as shown in Figure 4.
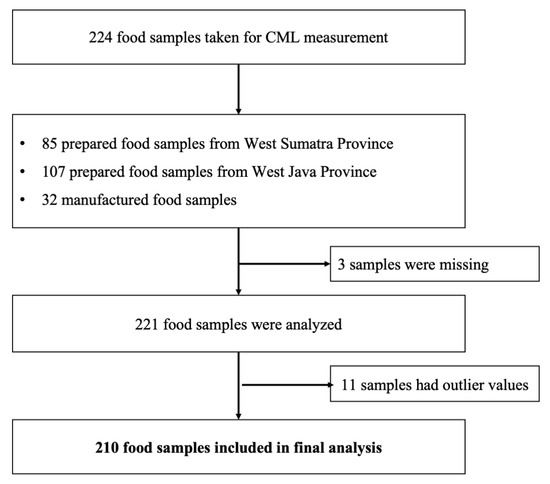
Figure 4.
Food sample selection for CML measurement.
There were three missing food samples and eleven food samples that had extreme values were excluded; so, the total number of food samples included in the final analysis was 210. Average food portions are based on the weight of the food at food sampling or the portion weight stated on the package. Mixed food dishes were examined separately for each food item; for example, chicken porridge was examined separately for rice porridge, shredded boiled chicken, fried cakwe, fried soy beans, and chips. The CML calculation for chicken porridge was based on the CML content of each type of food, the food weight, and the total food weight.
4.2. Storage of Samples
Prepared foods were collected from respondents’ homes, stallholders, street food vendors, and from traditional markets. The fast food samples were collected from fast food restaurants, while the manufactured foods were collected from groceries, convenience stores, or minimarkets. Each of the prepared food samples was put in a cooler box provided with Blue Ice packs to prevent oxidation and immediately registered and weighed using calibrated digital scales (Tanita KD-811, Tokyo, Japan). Before handling the samples, the samplers washed their hands with antiseptic soap and handled the foods with clean gloves. Prepared foods were separated into consumable and non-consumable parts using clean spoons and forks, or knives. The non-consumable parts (i.e., bone and sauces) were removed and not measured in this study. The samples were weighed twice to the nearest 0.1 g, each time before and after the non-consumable parts were removed, and the average of those weights was used. Manufactured foods (i.e., cereals, biscuit, chocolate) were kept in their sealed packages. Instant noodles were prepared according to the pack’s instructions at the designated central location. The food samples were placed in dry plastic vacuum containers (Kris vacuum plastic sealer 22 × 500 cm, Hong Kong, China) that did not easily leak. The air in the plastic container was expelled by means of a double-seal vacuum sealer machine (Kris vacuum sealer VS200, China). Each collected food sample was labeled using a permanent marker by stating the sample number, the name of the sample, and the location and time of sampling. The information on the sampler’s name, food type, and cooking method was collected and recorded on a file. The sample was then repacked in a plastic bag to prevent leakage and stored as soon as possible in a freezer at −20 °C until required for measurement. Dry ice was used in transporting the samples from the central location to the laboratory to maintain the temperature.
4.3. Determination of CML in Foods
There are four topics that should receive attention in the determination of CML content, namely preparation of samples, instrumentation, LC-MS/MS analytical parameters, and validation.
4.3.1. Preparation of Samples
Before preparing the food samples, the reducing solution was made up. The first step was to make up the borate buffer solution at pH 9.2 and concentration of 400 mmol/L by mixing the first solution consisting of 4.024 g sodium tetraborate in 50 mL ultrapure water with the second solution consisting of 1.2366 g of boric acid in 50 mL ultrapure water, with each solution being stored in a volumetric flask of 50 mL (Pyrex brand, Iwaki, Bandung, Indonesia. To speed up the dissolution process, the mixture was sonicated for five minutes. After both solutions were thoroughly mixed, they were combined and their pH adjusted to 9.2, as determined with S400 digital pH meter (Mettler Toledo, Greifensee, Switzerland), by the addition of NaOH or HCl. After preparing the borate buffer, the sodium borohydrate solution was made up as the reducing solution at a concentration of 100 mmol/L. A quantity of 0.19 g sodium borohydrate was dissolved in 50 mL of the borate buffer solution at pH 9.2.
The next step was the preparation of the food samples to be extracted. The food samples were removed from storage at −20 °C and left to stand at room temperature. Solid food was ground to a paste in a mortar. Then, 100 mg was weighed on scales (Mettler Toledo, type newClassic MF, model MS204S, Columbus, OH, USA, with an accuracy of 0.1 mg–220 mg) and placed in a tube.
Fatty foods with an estimated fat content of more than 20% were defatted in 1 mL n-hexane (Merck KGaA, Darmstadt, Germany, CAS No. 110-54-3). The 20% fat content was obtained from the Indonesian Food Composition Table (TKPI) [54], from Nutrisurvey, from food composition data of ASEAN countries [55], or from the USDA food list [56]. The defatted food mixture was separated by centrifugation (Eppendorf 5702 centrifuge, serial no. 5702BG929940, from Eppendorf AG, 22331 Hamburg, Germany), at 3700 rpm for 5 min, and the precipitate was collected for CML extraction.
The sample was then reduced with the addition of 1 mL of reducing solution, as prepared previously, and left to stand for two hours at a temperature at 23–25 °C (room temperature). After that, 1 mL HCl 6N/12% was added to the sample in the reducing solution; the mixture was then incubated for 30 min in a hot digestion system (ROCKER, Rocker Scientific Co., Ltd., COD reactor, model CR25, cat. no. 179200-22, Taiwan, China) at 110 °C. The purpose of the COD reaction is to determine the amount of organic matter, in this case, originating from the fat content of the food.
After incubation, 990 μL methanol (Tedia Company Inc, Fairfield, OH, USA, lot no. 18080199) and 10 μL internal standard (iSTD) comprising Nε-(1-Carboxymethyl)-L-Lysine-(4,4,5,5-d4) from Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA, were added. The solution was then sonicated (Bransonic 3510E-DTH, ultrasonic cleaner, Branson Ultrasonics Corporation, Danbury, CT, USA, made in Mexico) for 10–20 min at 23–25 °C, and homogenized by vortexing (approximately 10–20 s). The sample mixture was separated by centrifugation at 3700 rpm for 10 min. Then, 500 μL of the supernatant was taken with a micropipette and inserted into the HPLC vial, at which the samples are ready to be analyzed with HPLC-MS/MS.
The CML contents in the foods were calculated with the following formula:
where the following definitions apply:
- c: CML content from LC MS/MS detection;
- df: dilution factor;
- v: sample volume (L);
- W: sample weight (kg).
The expression of CML in food as CML mg/100 g edible food was calculated by the formula:
The expression of CML in food as CML mg/kg protein was calculated by the following formula:
Similarly, the expression of CML in food as CML mg/average portion size was calculated by the formula:
4.3.2. Instrumentation
The LC method was carried out on an Agilent 1260 Infinity II system (Santa Clara, CA, USA), Agilent SB-C18 column with dimension of 2.1 × 50 mm, 1.7 μm (Agilent Technologies, CA, USA), and Agilent Ultivo Triple Quadrupole Mass Spectrometer (Santa Clara, CA, USA).
4.3.3. LC-MS/MS Analytical Parameters
A 10 μL aliquot of sample extract was injected into the HPLC MS/MS system at a column temperature of 40 °C. The composition of the mobile phase was 0.1% formic acid in water (mobile phase A), and 0.1% formic acid in methanol (mobile phase B). The mobile phase was set up using gradient mode at 0–4 min 80% phase A and 4–6 min 10% phase A. The LC setting for accurate and reliable results was developed with a 1260 Infinity II system (Santa Clara, CA, USA), using Agilent Jet Stream (AJS) Positive ESI mode as ion source. The drying gas temperature was 350 °C with a gas flow of 8 L/minute, nebulizer pressure was 35 psi with sheath gas temperature of 350 °C and gas flow of 8 L/minute. Capillary voltage, nozzle voltage, and delta EMV were 3000 V, 0 V, and 500 V, respectively. The target analyte structure was Nε-carboxymethyl lysine. Multiple Reaction Monitoring (MRM) transition parameters for the analytes are described in Table 6.

Table 6.
Multiple Reaction Monitoring parameters of CML.
4.3.4. Method Validation
CML contents in foods were determined in the Prodia Industrial Toxicology Laboratory, Cikarang, West Java, Indonesia. This method protocol was validated for selected foods, including fried tilapia, ikan pangek sasau (processed traditional Minangkabau fish), cornflakes, and fried instant noodles.
Fried tilapia, cornflakes, instant noodles, and pangek sasau were chosen to represent a variety of food types. Fried tilapia and ikan pangek sasau represent foods rich in protein and fat but low in carbohydrates. The difference between these two foods lies in the processing method. Fried tilapia is processed by frying in hot oil, but ikan pangek sasau is processed by being steamed with spices and coconut milk. Cornflakes and instant noodles represent carbohydrate-rich foods and are processed at high temperature. Cornflakes are served straight away without any further processing. Instant noodles are processed through boiling with the addition of oil, soy sauce, and spices. For the selection of food matrices, the CML content and cooking process should be taken into consideration.
Optimization of LCMS/MS Conditions
CML is a polar agent and is difficult to separate in a non-polar column. The analytical method was adopted from the work of He et al. [45]. The C18 column (Agilent Zorbax Eclipse plus C18; 4.6 × 10 mm, 5 μm) was evaluated for the separation of CML. The solutions of formic acid in water and formic acid in methanol were used as mobile phase at a flow rate of 0.5 mL/minute with gradient condition. The column temperature was set at 40 °C. The chosen mobile phases in gradient mode consisting of 0–4 min 80% phase A and 4–6 min 10% phase B showed a better chromatogram. MRM optimization was conducted using MassHunter software v.1.1 for Ultivo LC/TQ C.01.00 2018.
Preparation of Calibration Standard and Linearity of the Calibration Curve
The stock solution was made by solubilizing 1 mg of CML and CML-d4 in water, aliquoting and storing at −20 °C. The stock solutions of CML were freshly diluted using mobile phase A concentrations 0.5, 2.5, 5, 10, 25, 100, 200 and 400 μg/L. The internal standard CML-d4 was prepared in the same way at a concentration of 50 μg/L. The calibration curves were determined in duplicate on three consecutive days and their linearity was evaluated. The intensity ratio of CML to CML-d4 and the concentration of CML were determined to make a standard curve. The deviation of the calculated concentrations was within ±15% of the nominal concentration. The limit of quantification was determined with six replicates.
Precision
Intraday and interday precision were determined by testing three replicates of three levels in three consecutive days. It is expressed as a percentage and is obtained by multiplying the standard deviation by 100 and dividing this product by the average. The precision was described as a percentage of relative standard deviation (% RSD). The %RSD was acceptable within ±15% of the nominal values.
Extraction Recovery
Recovery was evaluated by comparing and spiking the food samples with CML standard (50, 100 and 300 mg/kg). The extraction recovery was determined with the following formula:
C: level of CML CML spiking;
C1: level of CML in food;
C2: level of CML spiking and CML in food.
Limit of Detection and Limit of Quantification (LoD and LoQ)
The LoD and LoQ were determined by the signal-to-noise ratio of the peak of the analytical target at 3 and 10, respectively. LoD and LoQ are expressed in ug/kg.
4.3.5. Quality Testing
Reliability of Measurement
The reliability of the measurement was analyzed with the sample reanalysis of 15% of the total samples of the prepared and manufactured foods that were selected by randomization.
Quality Control
Fried tilapia was used as a sample base for quality control. Fried tilapia was obtained and selected around 200 g of fish meat. The fish meat was homogenized with a chopper, and the CML content was measured. The CML content of fish meat was used as the baseline CML value. Some of the fish meat was taken to be used in sample-based QC. The CML solution was added with a concentration of about 1 mg/kg and homogenized until it was a porridge-like mass. The mixture should produce a final spiked concentration of 100 µg/kg. The slurry mass obtained was 50 g, which was then aliquoted into plastic containers of 1 g each for QC testing. Before being used, these QC samples were stored in the freezer at −20 °C. Sample-based QC was taken every running day. Storage, preparation, and extraction up to CML content analysis were carried out by the same method as the test sample. QC sample testing was carried out in the middle of the sample testing series. In 1 running day, around 20 samples were analyzed for their CML content. The results of testing of the QC samples were calculated and documented using a control chart.
4.4. Determination of Protein Content
Protein levels in foods were determined in the other accredited and standardized laboratory in West Java, Indonesia. The protein content from prepared foods was measured by the Kjeldahl method, which refers to the standard procedure SNI 01-2891-1992, point 7.1. in the Indonesian national standards guideline on food and drinks testing. The protein content of manufactured foods was read from the nutrition facts’ label on the packaging or measured by the Kjeldahl method if there was no label. Briefly, 1 g sample (for high protein content 0.3–0.5 g of sample was used) was put in the Kjeltec tube, and 1 g selenium and 12 mL concentrated H2SO4 were added. The homogenate was then heated in a block digester (Kjel Digester K-446, Buchi Labortechnik AG, Flawil, Switzerland) at 420 °C for 2 h. Then, the Kjel Digester was turned off and the homogenate removed and left to cool at room temperature. After cooling, 3 drops of phenolphthalein (PP), 50 mL 40% NaOH, and 25 mL distilled water were added. Distillation in a steam distillation system (Buchi distillation K-355, Buchi Labortechnik AG, Switzerland) was then carried out for around 10 min, using 4% boric acid as the absorbing solution to three times its initial volume of 50 mL. The distillate was titrated with a solution of 0.2 N HCl to a red endpoint. Then, the blanks were titrated (Kjeltec system 2020 digestor, Tecator Inc., Herndon, VA, USA).
The following formula was used to calculate the protein content:
where Vs = volume of sample, Vb = volume of blank, N = normality of titrating solution, m = sample weight, and fk = species-specific nitrogen-to-protein conversion factor, with the following values: food in general = 6.25, milk and dairy products = 6.38, butter and nuts = 5.46, UHT milk = 7.0, peanuts = 5.46, soybeans = 5.71, coconut = 5.30, wheat = 5.38, rice = 5.95.
4.5. Statistical Analysis
SPSS program version 28.0.1.1. was used for analyzing the data, with the following details: the Kolmogorov–Smirnov test was used for testing the normality of the data, while the Wilcoxon test was used to determine the correlation between the estimated and measured CML content, the Mann–Whitney test was used to analyze the between-group differences in CML content from the two provinces, and Spearman’s rank correlation test was used to analyze correlation between protein content and weight per portion with CML content. The significance level was set at p < 0.05.
5. Conclusions
We present our CML database of Indonesian foods, which can be further used to make a guide policy for the selection of foods and their processing or for designing intervention studies with restricted CML intake to reduce non-communicable disease complications. The mean of the estimated values was statistically higher than that of the directly measured values. Therefore, future studies are required to measure the CML content of a larger number of food items from different locations with specific food-processing procedures to complete the database.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29061304/s1, Table S1: CML content of selected Indonesian foods.
Author Contributions
Conceptualization, P.B.L.; supervised the fieldwork and data collection, R.D.; methodology, P.B.L., M. and R.D.; analyzed and validated data on carboxymethyl lysine content in laboratory analysis, M.; analyzed the data, P.B.L., M. and Y.; writing of original draft preparation, P.B.L.; interpreted the data and drafted the manuscript, P.B.L., M. and R.D.; reviewed and revised the manuscript to ensure the quality of the content, R.D. and Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Faculty of Medicine, University of Indonesia (0019/UN2.F1/ETIK/2018 on 8 January 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available in article and Supplementary Materials.
Acknowledgments
The authors would like to thank all enumerators of this study for their assistance and Andi Wijaya for his support in realizing the measurements. Thanks are also due to Richard Tjan for proofreading this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galiniak, S.; Biesiadecki, M. Influence of food-derived advanced glycation end products on health. Eur. J. Clin. Exp. Med. 2018, 16, 330–334. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M.T. Dietary advanced glycation end products: Digestion, metabolism and modulation of gut microbial ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhuang, Y.; Zou, X.; Chen, M.; Cui, B.; Jiao, Y.; Cheng, Y. Advanced Glycation End Products: A comprehensive review of their detection and occurrence in food. Foods 2023, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, T.L. Dietary advanced glycation end-products elicit toxicological effects by disrupting gut microbiome and immune homeostasis. J. Immunotoxicol. 2021, 18, 93–104. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A role for advanced glycation end products in molecular ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Blood and tissue advanced glycation end products as determinants of cardiometabolic disorders focusing on human studies. Nutrients 2023, 15, 2002. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, X.; Shi, L.; Liu, W. Comprehensive analyses of advanced glycation end products and heterocyclic amines in peanuts during the roasting process. Molecules 2023, 28, 7012. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Matafome, P. Gut metabolism of sugars: Formation of glycotoxins and their intestinal absorption. Diabetology 2022, 3, 596–605. [Google Scholar] [CrossRef]
- Pinto, R.S.; Minanni, C.A.; de Araújo Lira, A.L.; Passarelli, M. Advanced Glycation End Products: A sweet flavor that embitters cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 2404. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Thorpe, S.R.; Baynes, J.W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 1986, 261, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Assar, S.H.; Ames, J.M. Formation of N(epsilon)-(carboxymethyl)lysine and loss of lysine in casein glucose-fatty acid model systems. J. Agric. Food Chem. 2010, 58, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Ikeda, K.; Higashi, T.; Sano, H.; Jinnouchi, Y.; Araki, T.; Horiuchi, S. Hydroxyl radical mediates N epsilon-(carboxymethyl)lysine formation from Amadori product. Biochem. Biophys. Res. Commun. 1997, 234, 167–172. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.J.M.; Hanssen, N.M.J.; van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef]
- Semba, R.D.; Ang, A.; Talegawkar, S.; Crasto, C.; Dalal, M.; Jardack, P.; Traber, M.G.; Ferrucci, L.; Arab, L. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: The Energetics Study. Eur. J. Clin. Nutr. 2012, 66, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Deo, P.; Clifton, P.M. Differential effects of dietary patterns on advanced glycation end products: A randomized crossover study. Nutrients 2020, 12, 1767. [Google Scholar] [CrossRef]
- Guilbaud, A.; Niquet-Leridon, C.; Boulanger, E.; Tessier, F.J. How Can Diet Affect the Accumulation of Advanced Glycation End-Products in the Human Body? Foods 2016, 5, 84. [Google Scholar] [CrossRef]
- Ottum, M.S.; Mistry, A.M. Advanced glycation end products: Modifiable environmental factors profoundly mediate insulin resistance. J. Clin. Biochem. Nutr. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Sayej, W.N.; Knight, P.R., 3rd; Guo, W.A.; Mullan, B.; Ohtake, P.J.; Davidson, B.A.; Khan, A.; Baker, R.D.; Baker, S.S. Advanced glycation end products induce obesity and hepatosteatosis in CD-1 wild-type mice. BioMed Res. Int. 2016, 2016, 7867852. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Sharifi-Zahabi, E.; Lari, A.; Fatahi, S.; Shidfar, F. The impact of low advanced glycation end products diet on obesity and related hormones: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 22194. [Google Scholar] [CrossRef]
- Liman, P.B.; Anastasya, K.S.; Salma, N.M.; Yenny, Y.; Faradilla, M.A. Research trends in advanced glycation end products and obesity: Bibliometric analysis. Nutrients 2022, 14, 5255. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, S.; Shi, Y.; Wang, P.; Wu, Y.; Li, G. Dietary advanced glycation end products (dAGEs): An insight between modern diet and health. Food Chem. 2023, 415, 135735. [Google Scholar] [CrossRef]
- Zawada, A.; Machowiak, A.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Accumulation of advanced glycation end-products in the body and dietary habits. Nutrients 2022, 14, 3982. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Yao, H.P.; Sun, Z. N(ε)-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products. World J. Diabetes 2023, 14, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Cleland, T.P.; Sroga, G.E.; Vashishth, D. Accumulation of carboxymethyl-lysine (CML) in human cortical bone. Bone 2018, 110, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; He, C.-J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of free and peptide-bound glycated amino acids: Synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins. Chembiochem 2011, 12, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ma, Y.; Wang, Y.; Sun, C.; Chen, F.; Cheng, K.W.; Liu, B. Contents and correlations of N(ε)-(carboxymethyl)lysine, N(ε)-(carboxyethyl)lysine, acrylamide and nutrients in plant-based meat analogs. Foods 2023, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Baylan, U.; Baidoshvili, A.; Simsek, S.; Schalkwijk, C.G.; Niessen, H.; Krijnen, P. Increased accumulation of the advanced glycation endproduct Ne(carboxymethyl) lysine in the intramyocardial vasculature in patients with epicarditis. Int. J. Exp. Pathol. 2023, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Chen, J. Increased accumulation of protein-bound N(ε)-(carboxymethyl)lysine in tissues of healthy rats after chronic oral N(ε)-(carboxymethyl)lysine. J. Agric. Food Chem. 2015, 63, 1658–1663. [Google Scholar] [CrossRef]
- Wu, R.; Jia, C.; Rong, J.; Xiong, S.; Liu, R. Effect of Pretreatment Methods on the Formation of Advanced Glycation End Products in Fried Shrimp. Foods 2023, 12, 4362. [Google Scholar] [CrossRef]
- Niu, L.; Kong, S.; Chu, F.; Huang, Y.; Lai, K. Investigation of advanced glycation end-products, alpha;-dicarbonyl compounds, and their correlations with chemical composition and salt levels in commercial fish products. Foods 2023, 12, 4324. [Google Scholar] [CrossRef]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Ne-(carboxymethyl) lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Scheijen, J.L.J.M.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.-I.; Furuno, S.; Shirai, H.; Kawakami, M.; Muramatsu, M.; Kobayashi, Y.; Yamagishi, S.-I. Assessment of the concentrations of various advanced glycation end-products in beverages and foods that are commonly consumed in Japan. PLoS ONE 2015, 10, e0118652. [Google Scholar] [CrossRef]
- Liman, P.B.; Djuwita, R.; Agustina, R. Database development of carboxymethyl lysine content in foods consumed by Indonesian women in two selected provinces. J. Int. Dent. Med. Res. 2019, 12, 268–277. [Google Scholar]
- Liman, P.B.; Agustina, R.; Djuwita, R.; Umar, J.; Permadhi, I.; Helmizar; Hidayat, A.; Feskens, E.J.M.; Abdullah, M. Dietary and plasma carboxymethyl lysine and tumor necrosis factor-α as mediators of body mass index and waist circumference among women in Indonesia. Nutrients 2019, 11, 3057. [Google Scholar] [CrossRef]
- Wijaya, S. Indonesian food culture mapping: A starter contribution to promote Indonesian culinary tourism. J. Ethn. Foods 2019, 6, 9. [Google Scholar] [CrossRef]
- Lipoeto, N.I.; Agus, Z.; Oenzil, F.; Masrul, M.; Wattanapenpaiboon, N.; Wahlqvist, M.L. Contemporary Minangkabau food culture in West Sumatra, Indonesia. Asia Pac. J. Clin. Nutr. 2001, 10, 10–16. [Google Scholar] [CrossRef]
- Budiningsih, S.; Ohnot, Y.; Prihartono, J.; Dillon, D.S.; Tjahjadi, G.; Soetrisno, E.; Hardjolukito, E.; Ramli, M.; Darwis, I.; Tjindarbumi, D.; et al. Breast cancer risk factors among Sundanese and other ethnic groups in Indonesia. Med. J. Indones. 1999, 8, 128–132. [Google Scholar] [CrossRef]
- He, J.; Zeng, M.; Zheng, Z.; He, Z.; Chen, J. Simultaneous determination of Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine in cereal foods by LC–MS/MS. Eur. Food Res. Technol. 2013, 238, 367–374. [Google Scholar] [CrossRef]
- Hartkopf, J.; Pahlke, C.; Lüdemann, G.; Erbersdobler, H. Determination of N-carboxymethyllysine by a reversed-phase high-performance liquid chromatography method. J. Chromatogr. A 1994, 672, 242–246. [Google Scholar] [CrossRef]
- Assar, S.H.; Moloney, C.; Lima, M.; Magee, R.; Ames, J.M. Determination of Nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 2009, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Smith, J.S. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Rini; Azima, F.; Sayuti, K.; Novelina. The evaluation of nutritional value of rendang Minangkabau. Agric. Agric. Sci. Procedia 2016, 9, 335–341. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, H.; Xie, J.; Shen, M. Investigation into the contents of nutrients, Nε-carboxymethyllysine and Nε-carboxyethyllysine in various commercially canned fishes to find the correlation between them. J. Food Compos. Anal. 2021, 96, 103737. [Google Scholar] [CrossRef]
- Yacoub, R.; Nugent, M.; Cai, W.; Nadkarni, G.N.; Chaves, L.D.; Abyad, S.; Honan, A.M.; Thomas, S.A.; Zheng, W.; Valiyaparambil, S.A.; et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS ONE 2017, 12, e0184789. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Gebauer, S.K.; Baer, D.J.; Sun, K.; Turner, R.; Silber, H.A.; Talegawkar, S.; Ferrucci, L.; Novotny, J.A. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J. Nutr. 2014, 144, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Fluhler, E.; Vazvaei, F.; Singhal, P.; Vinck, P.; Li, W.; Bhatt, J.; de Boer, T.; Chaudhary, A.; Tangiuchi, M.; Rezende, V.; et al. Repeat analysis and incurred sample reanalysis: Recommendation for best practices and harmonization from the global bioanalysis consortium harmonization team. AAPS J. 2014, 16, 1167–1174. [Google Scholar] [CrossRef]
- Mahmud, M.K.; Hermana; Zulfianto, N.A.; Apriyantoro, R.R.; Ngadiarti, I.; Hartati, B.; Bernadus; Tinexcelly. Tabel Komposisi Pangan Indonesia [Indonesian Food Composition Table]; Mahmud, M.K., Zulfianto, N.A., Eds.; PT Elex Media Komputindo: Jakarta, Indonesia, 2009. [Google Scholar]
- Institute of Nutrition Mahidol University. ASEAN Food Composition Database, Electronic Version 1. February 2014. Available online: http://www.inmu.mahidol.ac.th/aseanfoods/composition_data.html (accessed on 10 February 2019).
- United States Departement of Agriculture, Beltsville Human Nutrition Research Center. USDA Food Composition Databases v.3.8.6.4 2017-10-02. Available online: https://fdc.nal.usda.gov (accessed on 25 February 2019).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).