4-[(E)-2-(1-Pyrenyl)Vinyl]Pyridine Complexes: How to Modulate the Toxicity of Heavy Metal Ions to Target Microbial Infections
Abstract
1. Introduction
2. Results
2.1. Chemistry
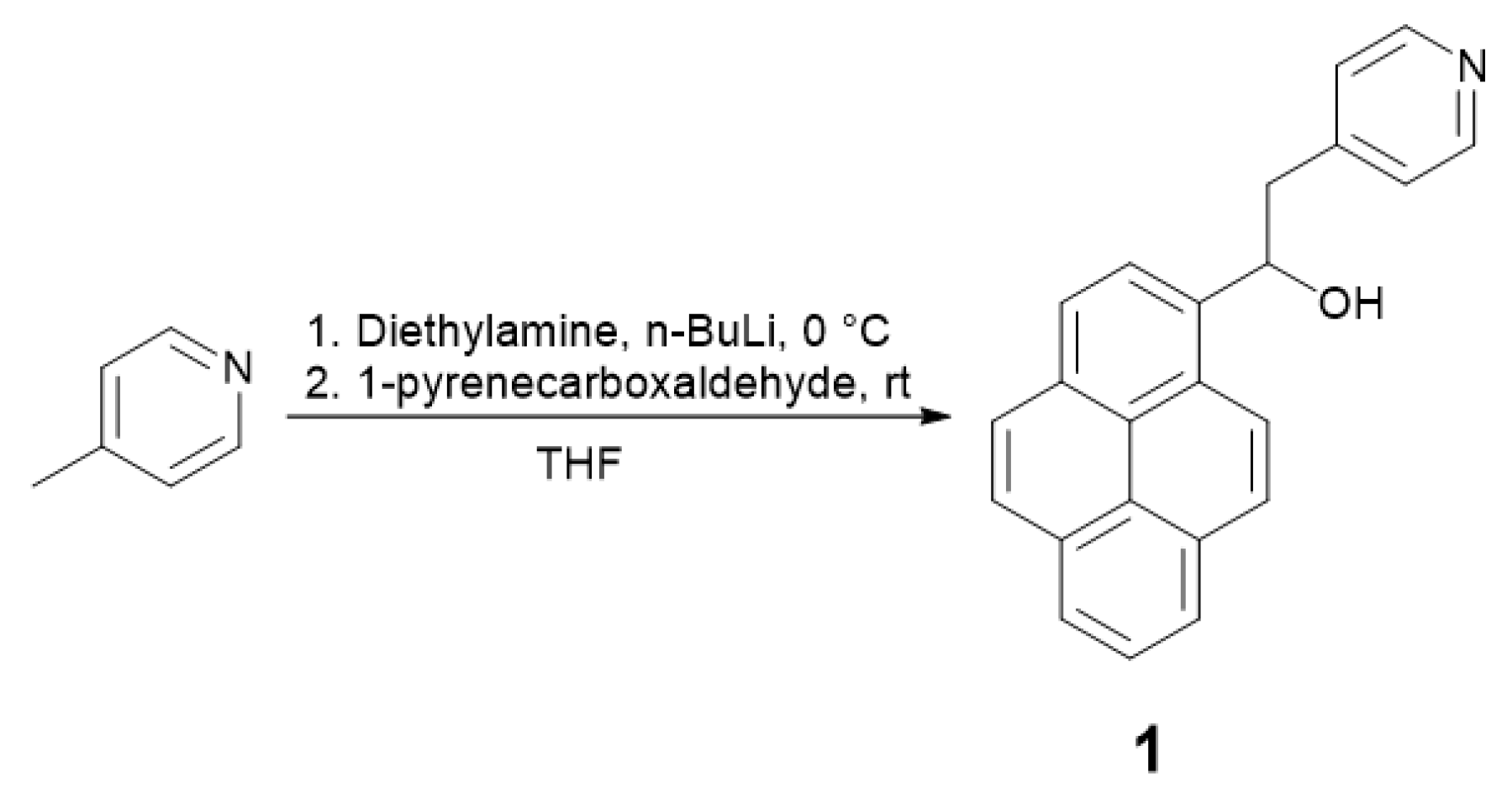
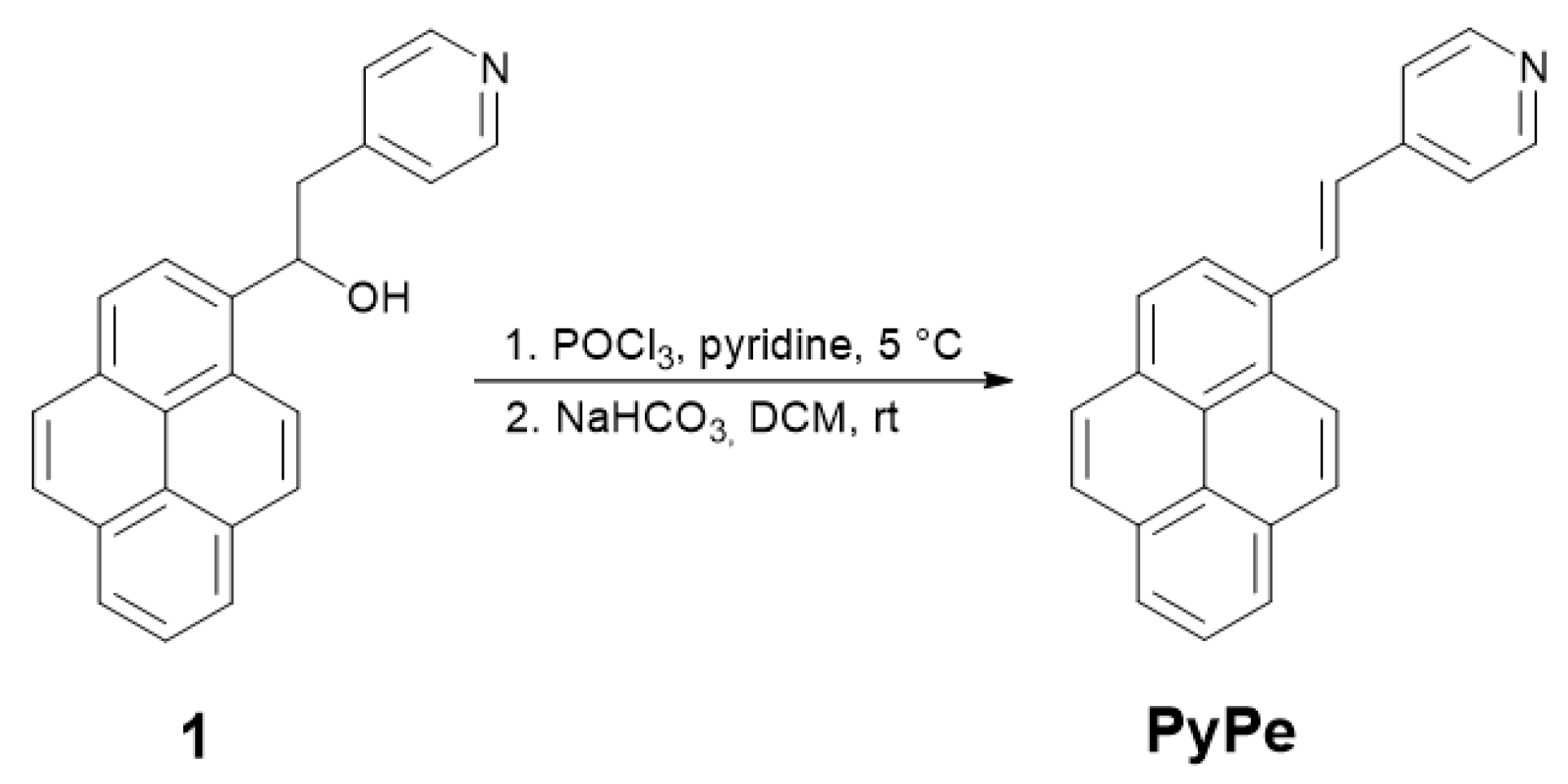
2.1.1. Synthesis of the Ligand
2.1.2. Synthesis of the Complexes
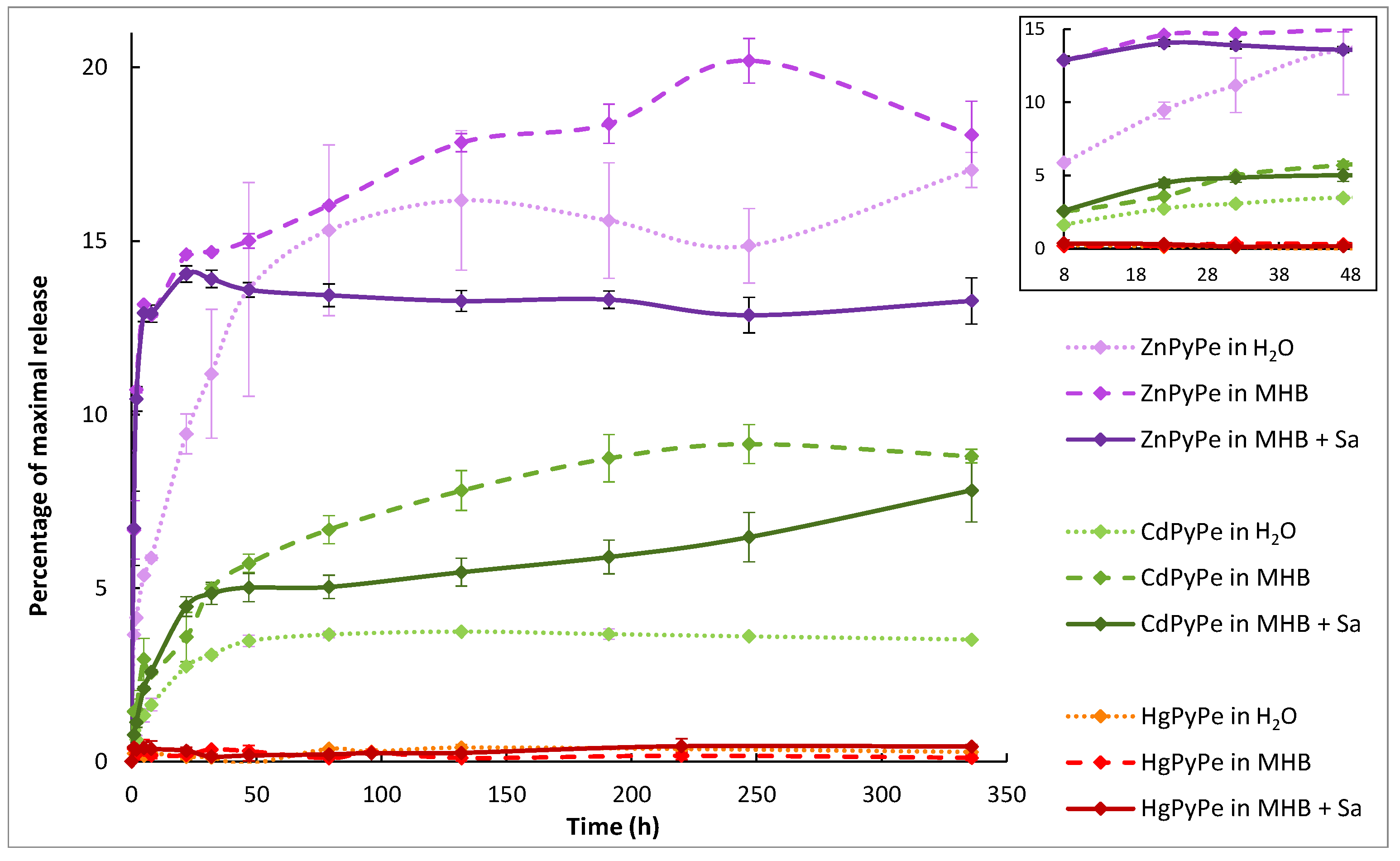
2.2. Compounds Stability
2.3. PyPe Solubility, Mixture and Complex Formation
2.4. Bioactivity of Metal-Organic Complexes
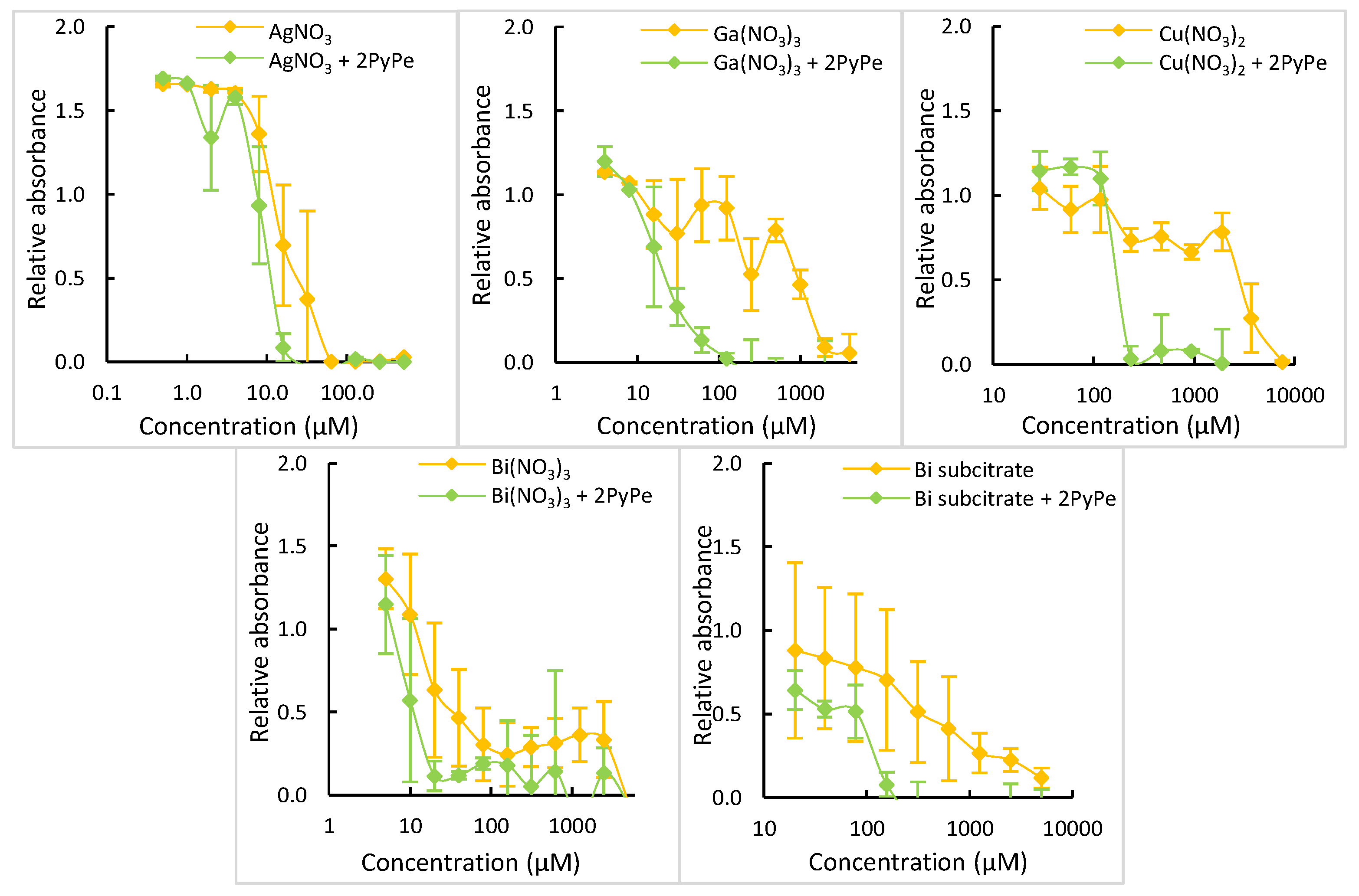
2.5. Bioactivity of Other Metal Ion-PyPe Mixtures
3. Discussion
4. Materials
4.1. 1-(Pyren-1-yl)-2-(Pyridin-4-yl)Ethan-1-ol (1) Synthesis
4.2. (E)-4-(2-(Pyren-1-yl)Vinyl)Pyridine (PyPe) Synthesis
4.3. Crystallization
4.4. Stability of the Complexes
4.5. Dynamic Light Scattering
4.6. Microdilution Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am. J. Public Health 2015, 105, 2409–2410. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Kumar, N.; Kumar, S.; Trigun, V. Antimicrobial Resistance: Progress in the Decade since Emergence of New Delhi Metallo-β-Lactamase in India. Indian J. Community Med. 2019, 44, 4. [Google Scholar] [PubMed]
- Butler, M.S.; Blaskovich, M.A.T.; Cooper, M.A. Antibiotics in the Pipeline at the End of 2015. J. Antibio. 2017, 70, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Biological Chemistry of Arsenic, Antimony and Bismuth; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Wang, R.; Lai, T.P.; Gao, P.; Zhang, H.; Ho, P.L.; Woo, P.C.Y.; Ma, G.; Kao, R.Y.T.; Li, H.; Sun, H. Bismuth Antimicrobial Drugs Serve as Broad-Spectrum Metallo-β-Lactamase Inhibitors. Nat. Commun. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Shareena Dasari, T.P.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. 2015, 4, 199. [Google Scholar]
- Abram, S.L.; Gagnon, J.; Priebe, M.; Hérault, N.; Fromm, K.M. Ag Nanoencapsulation for Antimicrobial Applications. Chimia 2018, 72, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Fromm, K.M. Give Silver a Shine. Nat. Chem. 2011, 3, 178. [Google Scholar] [CrossRef] [PubMed]
- Ben Miloud, S.; Ali, M.M.; Boutiba, I.; Van Houdt, R.; Chouchani, C. First Report of Cross Resistance to Silver and Antibiotics in Klebsiella Pneumoniae Isolated from Patients and Polluted Water in Tunisia. Water Environ. J. 2021, 35, 730–739. [Google Scholar] [CrossRef]
- Gómez-Sagasti, M.T.; Becerril, J.M.; Epelde, L.; Alkorta, I.; Garbisu, C. Early Gene Expression in Pseudomonas Fluorescens Exposed to a Polymetallic Solution. Cell Biol. Toxicol. 2015, 31, 39–81. [Google Scholar] [CrossRef] [PubMed]
- Delmar, J.A.; Su, C.C.; Yu, E.W. Bacterial Multidrug Efflux Transporters. Annu. Rev. Biophys. 2014, 43, 93–117. [Google Scholar] [CrossRef]
- Hobman, J.L.; Crossman, L.C. Bacterial Antimicrobial Metal Ion Resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Carver, P.L. Chapter 1 in Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in Clinic; De Gruyter, C.P., Ed.; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2019. [Google Scholar]
- Crisponi, G.; Nurchi, V.M. Metal Ion Toxicity. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–14. [Google Scholar]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjorklund, G.; Aaseth, J. Arsenic toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Vaughn, W.K. HSAB Theory and Acute Metal Ion Toxicity and Detoxification Processes. J. Inorg. Nucl. Chem. 1978, 40, 2081–2088. [Google Scholar] [CrossRef]
- Pahan, K.; Gachhui, R.; Ray, S.; Chaudhuri, J.; Mandal, A. Bacterial Degradation and Utilization of Merbromine and Fluorescein Mercuric Acetate. Bull. Environ. Contam. Toxicol. 1992, 48, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. The Role of Plasmids in Bacterial Resistance to Antiseptics, Disinfectants and Preservatives. J. Hosp. Infect. 1985, 6, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.J.; Ball, L.K.; Ball, R.; Pratt, D. Thiomersal in Vaccines. Lancet 2000, 355, 1279–1280. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Tracey, A.T.; Alvim, R.; Reisz, P.; Scherz, A.; Coleman, J.A.; Kim, K. Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies. Molecules 2020, 25, 5417. [Google Scholar] [CrossRef]
- Walker, J.; Saravia, N.G. Inhibition of Leishmania Donovani Promastigote DNA Topoisomerase I and Human Monocyte DNA Topoisomerases I and II by Antimonial Drugs and Classical Antitopoisomerase Agents. J. Parasitol. 2004, 90, 1155–1162. [Google Scholar] [CrossRef]
- Nadar, V.S.; Chen, J.; Dheeman, D.S.; Galván, A.E.; Yoshinaga-Sakurai, K.; Kandavelu, P.; Sankaran, B.; Kuramata, M.; Ishikawa, S.; Rosen, B.P.; et al. Arsinothricin, an arsenic-containing non-proteinogenic amino acid analog of glutamate, is a broad-spectrum antibiotic. Commun Biol. 2019, 2, 131. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Metzler-Nolte, N. The Potential of Organometallic Complexes in Medicinal Chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Sakaran, N.; Das, A.; Samanta, A. Interaction between a pyridyl and a naphthyl/pyrenyl moiety in covalently linked systems. Chem. Phys. Lett. 2002, 351, 61–70. [Google Scholar] [CrossRef]
- National Institute of Standards and Technologies. IR Spectra of Pyridine and Pyrene. Available online: https://webbook.nist.gov/ (accessed on 10 September 2023).
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper (I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ4. Dalt. Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Purkayastha, D.D.; Das, G.; Bhattacharjee, C.R.; Mondal, P.; Prasad, S.K.; Rao, D.S.S. Photoluminescent tetrahedral d(10)-metal Schiff base complexes exhibiting highly ordered mesomorphism. Polyhedron 2016, 105, 150–158. [Google Scholar] [CrossRef]
- Fisher Scientific, Zinc(II) Iodide Pure 98+%. Available online: https://www.fishersci.ch/shop/products/zinc-iodide-98-pure-thermo-scientific/10686782#?keyword=zinc%20iodide (accessed on 10 September 2023).
- Fisher Scientific, Cadmium(II) Iodide Acros Organics 99%. Available online: https://www.fishersci.ch/shop/products/cadmium-iodide-99-acros-organics/10402855/en&usg=AOvVaw0xD1K2FeabOfppJDdB7tHJ&opi=89978449 (accessed on 17 December 2023).
- Lide, D.R. (Ed.) Section 8 Analytical Chemistry. In Handbook of Chemistry and Physics, 77th ed.; CRC Press: Boca Raton, FL, USA, 1996; p. 8.92. [Google Scholar]
- Hantke, K. Bacterial Zinc Uptake and Regulators. Curr. Opin. Microbiol. 2005, 8, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Turner, R.J. Silver Antibacterial Synergism Activities with Eight Other Metal(Loid)-Based Antimicrobials against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.R.; Midolo, P. The Actions of Bismuth in the Treatment of Helicobacter Pylori Infection. Aliment. Pharmacol. Ther. 1997, 11, 27–33. [Google Scholar] [CrossRef]
- Fisher Scientific, Summary of Key Physical Data for Solvents. Available online: https://www.fishersci.co.uk/gb/en/scientific-products/technical-tools/summary-key-physical-data-solvents.html (accessed on 7 January 2024).
- Yang, K.; Kotak, H.A.; Haynes, C.J.E. Metal-Organic Ion Transport Systems. Coord. Chem. Rev. 2022, 470, 214705. [Google Scholar] [CrossRef]
- Booth, I.R.; Edwards, M.D.; Miller, S. Bacterial Ion Channels. Biochemistry 2003, 42, 10045–10053. [Google Scholar] [CrossRef]
- Fritsch, S.; Ivanov, I.; Wang, H.; Cheng, X. Ion Selectivity Mechanism in a Bacterial Pentameric Ligand-Gated Ion Channel. Biophys. J. 2011, 100, 390–398. [Google Scholar] [CrossRef]
- Hantke, K. Bacterial Zinc Transporters and Regulators. In Zinc Biochemistry, Physiology, and Homeostasis: Recent Insights and Current Trends; Springer: Dordrecht, The Netherlands, 2001; pp. 53–63. [Google Scholar]
- Vermaas, J.V.; Crowley, M.F.; Beckham, G.T. Molecular Simulation of Lignin-Related Aromatic Compound Permeation through Gram-Negative Bacterial Outer Membranes. J. Biol. Chem. 2022, 298, 102627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Li, H.; Tian, X.; Li, X. Tryptophan-Based Self-Assembling Peptides with Bacterial Flocculation and Antimicrobial Properties. Langmuir 2020, 36, 11316–11323. [Google Scholar] [CrossRef]
- Espeche, J.C.; Varas, R.; Maturana, P.; Cutro, A.C.; Maffía, P.C.; Hollmann, A. Membrane Permeability and Antimicrobial Peptides: Much More than Just Making a Hole. Pept. Sci. 2023, 116, e24305. [Google Scholar] [CrossRef]
- Gao, H.; Wu, M.; Liu, H.; Zhang, T.; Zhang, X. Cell Toxic Damages during Polycyclic Aromatic Hydrocarbons Biodegradation by Pseudomonas Aeruginosa G24. J. Water Process Eng. 2023, 54, 103992. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbio. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Thurman, R.; Gerba, C.P. The molecular mechanisms of copper and silver ions disinfection of bacteria and viruses. Crit. Rev. Environ. Sci. Technol. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as Disinfectant. Rev. Environ. Cont. Toxicol. 2007, 191, 23. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]




| Features 1 | ZnPyPe | CdPyPe | HgPyPe |
|---|---|---|---|
| Space group | P21/c | P21/c | P21/c |
| Coordination | Distorted tetrahedral (τ4 = 0.88) | Distorted tetrahedral (τ4 = 0.82) | Distorted tetrahedral (τ4 = 0.77) |
| N1−M−N2 | 91.8° | 85.5° | 80.0° |
| M−N1 | 2.069(7) Å | 2.289(10) Å | 2.418(4) Å |
| M−N2 | 2.085(7) Å | 2.300(10) Å | 2.424(4) Å |
| M−I1 | 2.5384(11) Å | 2.6804(12) Å | 2.6343(4) Å |
| M−I2 | 2.5440(11) Å | 2.6904(11) Å | 2.6452(4) Å |
| Torsion angle L1 | 11.7° | 9.7° | 9.5° |
| Torsion angle L2 | 23.6° | 21.7° | 20.5° |
| π-π interaction between two complexes | 3.3–3.5 Å | 3.3–3.4 Å | 3.3–3.4 Å |
| More Active Compounds | Less Active Compounds | |||
|---|---|---|---|---|
| ZnI2 + 2PyPe | ZnI2 | ZnPyPe | ||
| 250–500 | ×4 | 1000–2000 | solubility issue | |
| CdI2 + 2PyPe | CdI2 | CdPyPe | ||
| 4–8 | ×2 | 8–16 | = | 8–16 |
| HgI2 + 2PyPe | HgI2 | HgPyPe | ||
| 0.3–0.5 | ×10 | 2–4 | ×4 | 8–16 |
| AgNO3 + 2PyPe | AgNO3 | |||
| 8–16 | ×4 | 32–64 | ||
| Ga(NO3)3 + 2PyPe | Ga(NO3)3 | |||
| 64–128 | ×16 | 1024–2048 | ||
| Cu(NO3)2 + 2PyPe | Cu(NO3)2 | |||
| 120–240 | >×20 | MIC > 2500 | ||
| Bi(NO3)3 + 2PyPe | Bi(NO3)3 | |||
| 10–20 | >×250 | MIC > 2500 | ||
| Bi(subcitrate)2 + 2PyPe | Bi(subcitrate)2 | |||
| 80–160 | >×30 | MIC > 2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarte, J.V.; Crochet, A.; Fromm, K.M. 4-[(E)-2-(1-Pyrenyl)Vinyl]Pyridine Complexes: How to Modulate the Toxicity of Heavy Metal Ions to Target Microbial Infections. Molecules 2024, 29, 1565. https://doi.org/10.3390/molecules29071565
Schwarte JV, Crochet A, Fromm KM. 4-[(E)-2-(1-Pyrenyl)Vinyl]Pyridine Complexes: How to Modulate the Toxicity of Heavy Metal Ions to Target Microbial Infections. Molecules. 2024; 29(7):1565. https://doi.org/10.3390/molecules29071565
Chicago/Turabian StyleSchwarte, Justine V., Aurélien Crochet, and Katharina M. Fromm. 2024. "4-[(E)-2-(1-Pyrenyl)Vinyl]Pyridine Complexes: How to Modulate the Toxicity of Heavy Metal Ions to Target Microbial Infections" Molecules 29, no. 7: 1565. https://doi.org/10.3390/molecules29071565
APA StyleSchwarte, J. V., Crochet, A., & Fromm, K. M. (2024). 4-[(E)-2-(1-Pyrenyl)Vinyl]Pyridine Complexes: How to Modulate the Toxicity of Heavy Metal Ions to Target Microbial Infections. Molecules, 29(7), 1565. https://doi.org/10.3390/molecules29071565








