Abstract
The synthesis of hybrid molecules is one of the current strategies of drug discovery for the development of new lead compounds. The 1,2,3-triazole moiety represents an important building block in Medicinal Chemistry, extensively present in recent years. In this paper, we presented the design and the synthesis of new 1,2,3-triazole hybrids, containing both an isatine and a phenolic core. Firstly, the non-commercial azide and the alkyne synthons were prepared by different isatines and phenolic acids, respectively. Then, the highly regioselective synthesis of 1,4-disubstituted triazoles was obtained in excellent yields by a click chemistry approach, catalyzed by Cu(I). Finally, a molecular docking study was performed on the hybrid library, finding four different therapeutic targets. Among them, the most promising results were obtained on 5-lipoxygenase, an enzyme involved in the inflammatory processes.
1. Introduction
The hybrid molecules, obtained by the structural combination of different pharmacophoric sub-unities of two or more known bioactive derivatives, represent an excellent stratagem to design new pharmaceuticals with more potent properties while maintaining pre-selected characteristics of the original templates. The interest of researchers towards this class of molecules has undergone a large escalation in recent years with the idea that the hybrids can interact with more than one biological target, improving their activity, gaining higher selectivity, and possibly reducing drug resistance [1,2,3].
Among the myriad of compounds with biological profiles, 1,2,3-triazoles are some of the most extensively used scaffolds in pharmaceuticals for their special properties such as hydrogen bonding capability, rigidity, stability as well as a wide range of biological activities [4,5,6,7,8,9,10]. Moreover, their synthesis is very simple by the click chemistry approach [11,12]. In more detail, the concept of click chemistry (Azide-Alkyne Cycloaddition) was introduced by Huisgen in 1960 [13], who reported the 1,3-dipolar cycloaddition between a terminal alkyne and an azide to produce a mixture of 1,4- and 1,5-substituted triazole. Then, the introduction of a catalyst such as cuprum for the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and ruthenium for the Ru(II)-catalyzed reaction (RuAAC) produced 1,4- and 1,5-disubstituted regioisomers, respectively [14,15]. In the last years, a lot of other metal catalysts have been developed for the synthesis of substituted 1,2,3-triazoles in a regioselective manner [16].
In addition to triazoles, other nitrogen-containing molecular architectures have been widely utilized as versatile materials in pharmaceutical discovery. Oxindole derivatives and in particular isatin have received wide interest due to the presence of different functionalizable groups in their structure [17,18]. The isatin nucleus was explored in medicinal chemistry for the construction of compounds with antibacterial [19], anticancer [20], antiviral [21], and antioxidant properties [22].
Recently, the combination of two structurally different heterocycles in a single molecule to form hybrids, so-called molecules with “two heads”, have become very popular [23]. Generally, this typology of molecules is classified into three types: merged, fused, and conjugated molecular hybrids [24]. In particular, the latter are usually separated by a distinct linker group that is not found in either of the selective sub-units, and the 1,2,3-triazoles could be an ideal building block to this aim, due to its stability.
On the other hand, the combination of these heterocyclic moieties with an additional antioxidant portion on the backbone of the molecule could make the resulting hybrid compound even more potent. In fact, antioxidant activity underlies many biological properties, e.g., antiviral, anti-inflammatory, antibacterial, antiatherosclerotic, anticancer and neuroprotective [25,26], also considering that, for example, oxidative stress and imbalance of antioxidant properties are often the cause of cancer and cardiovascular diseases [27].
In general, the presence of more than one hydroxyl group bonded to a benzene ring, especially in the -ortho and -para positions, confers stronger antioxidant activity to the molecule. In particular, phenolic acids in which the aromatic ring is substituted with two or more hydroxyl groups, i.e., caffeic or gallic acid, have demonstrated more significant antioxidant properties than monophenols [28].
In this work, 1,2,3-triazoles conjugated with isatins and antioxidant molecules were synthesized by 1,3-dipolar cycloaddition in a regioselective manner by Cu(I) catalysis to promote the obtaining only of 1,4-disubstituted triazole derivatives. In this way, we obtained new conjugated molecular hybrids, containing the 1,2,3 triazole moiety as a linker between the two heads. This choice derived from the innumerable biological applications of 1,4-derivatives probably due to their spatial arrangement that permits them to form various secondary interactions with the active site of enzyme target (dipole-dipole interactions, hydrogen bonds, π–stacking, etc.) [29].
In addition, a series of molecular docking studies was performed on the full set of the synthesized products. Eight relevant targets were selected and studied to evaluate the potential biological activity of our hybrids.
2. Results and Discussion
2.1. Synthesis
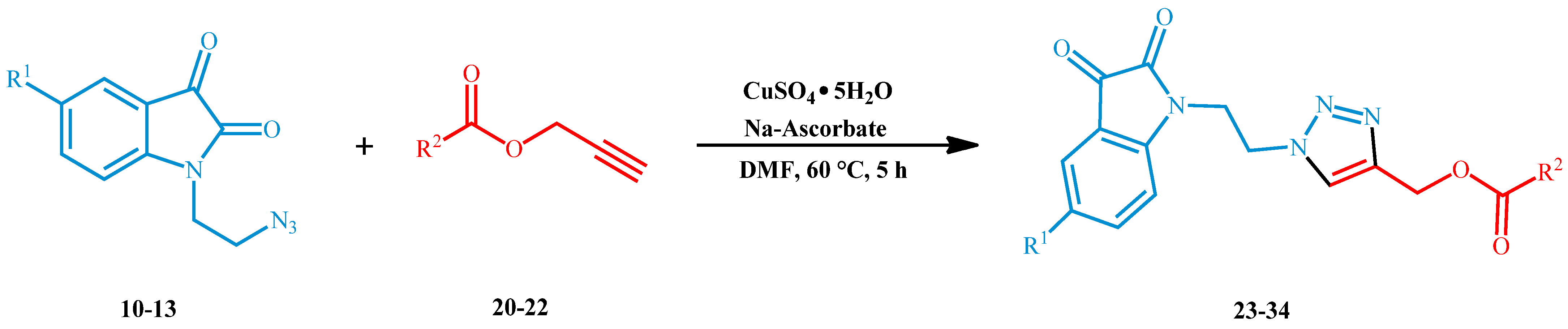
With the aim of realizing hybrids of 1,2,3-triazoles as the core between isatins and antioxidant molecules to enhance the sites interactivity with enzymatic tasks of biological targets (see Figure 1) and considering our great experience on the heterocycle synthesis by 1,3-dipolar cycloadditions [30,31,32,33,34,35], we selected the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) to perform 1,4-disubstituted triazole derivatives. Moreover, our molecules were projected with short spacers between the various active sub-units to make these compounds more flexible and adaptable in the enzymatic cavities (Figure 1).
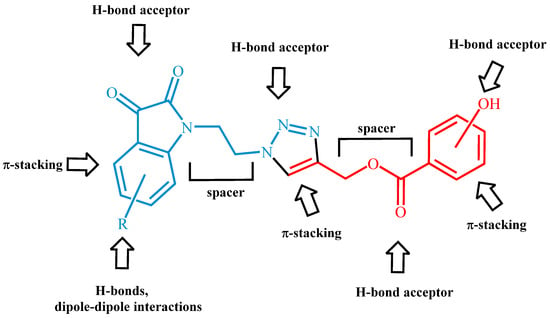
Figure 1.
Generic structure of synthesized 1,2,3 triazole hybrids.
In order to develop a regioselective copper(I)-catalyzed 1,3-dipolar cycloaddition reaction, a preventive preparation of opportune dipoles and dipolarophiles was necessary. For one, 1-(2-azidoethyl)isatins were used as 1,3-dipoles and were synthesized according to the reactions reported in Scheme 1.
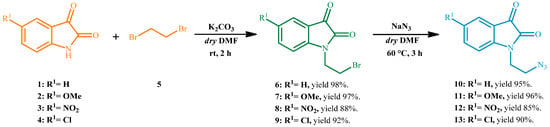
Scheme 1.
Generic synthesis of variously substituted 1-(2-azidoethyl)isatins 10–13.
The procedure for the synthesis of N-bromoalkyl derivatives was based on a modified method found in the literature [36]. In detail, opportune isatins 1–4 were reacted with 1,2-dibromoethane 5 and potassium carbonate in dry DMF at room temperature to give the corresponding 1-(2-bromoethyl)isatins 6–9 with excellent isolated yields. As observed in Scheme 1, the presence of different substituent groups in the 5-position does not seem to deeply affect the reactivity of the isatins 1–4 towards alkylation because comparable reaction yields were observed for all cases. The series of isolated bromoalkyl products 6–9 were subsequently reacted with NaN3 to form corresponding 1-(2-azidoethyl)isatin 10–13 with high reaction yield [37].
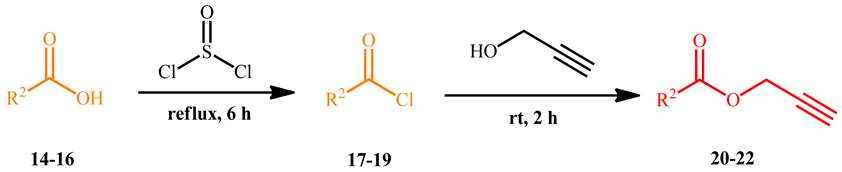
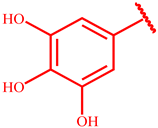
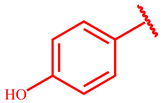
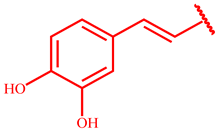
Successively, three phenolic acid propargyl esters 20–22 were chosen as dipolarophiles and their synthesis and results are illustrated in Table 1.

Table 1.
Synthesis of propargyl esters 20–22 starting from phenolic acids 14–16.
The synthetic procedure started from the chlorination of the phenolic acids 14–16 by thionyl chloride at reflux for six hours, obtaining the corresponding acyl chlorides 17–19, which were used, without isolation, with propargyl alcohol to give the desired propargyl esters 20–22 in quantitative yields.
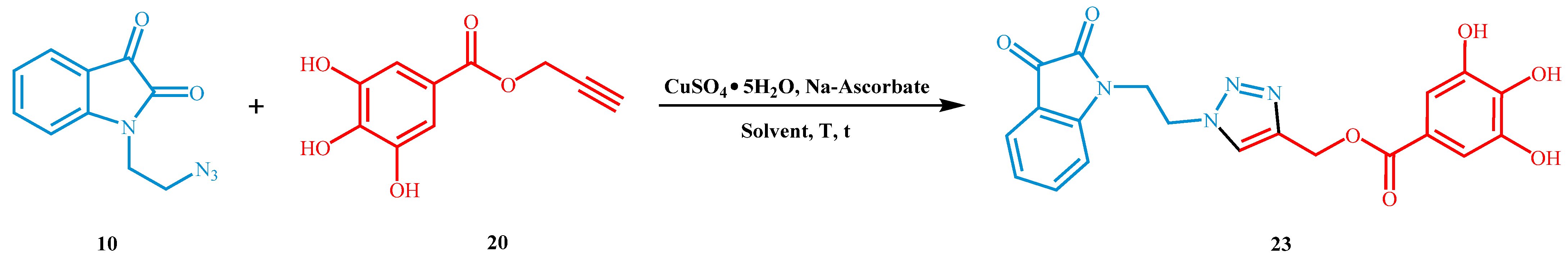
With the precursors in our hands, we selected the cycloaddition between 1-(2-azidoethyl)isatin 10 and propargyl 3,4,5-trihydroxybenzoate 20 as the model system to optimize the reaction conditions. We chose the Cu(II)sulfate pentahydrate/sodium ascorbate (CuSO4 5H2O/Na-Ascorbate) as the catalytic system due to its high compatibility with non-anhydrous organic solvents, as reported in the literature [38].
The changes in solvents, reagent molar ratios, catalyst molar ratios, temperature and time are summarized in Table 2.

Table 2.
Optimization of reaction conditions.
Initially, we decided to apply the reaction conditions used on triazole hybrids found in the literature [39], isolating 1,4-disubstituted 1,2,3-triazole 23. In this case, we observed only a 22% yield of the product after 48 h at room temperature with degradation of alkyne 20 (Table 2, entry 1). Then, we increased the temperature to 60 °C, resulting in a 67% yield of 23 after only 5 h (Table 2, entry 3). It is noteworthy that no significant variation was observed when the reaction was extended up to 24 h. Moreover, higher temperatures showed only the degradation of reagent 20. At this point, we decided to replace the DMF with EtOH, i-PrOH, and t-BuOH (Table 2, entries 4–6), classic conventional solvents commonly used in CuAAC. However, the reaction yields were lower (38–45%) for all three cases, probably due to the minor solubility of CuSO4 5H2O and Na-Ascorbate in the reaction medium. Instead, we performed a subsequent experiment (Table 2, entry 7) in which the reaction was carried out in DMF without added water provided product 23 with a yield comparable to that in the presence of water (Table 2, entry 3). Therefore, we decided to continue the investigation only in DMF as a solvent and by doubling the molar quantity of catalyst and co-catalyst, observing an increase in the reaction yield until 90% (Table 2, entry 8). Then, the amount of azide 10 was reduced to the stoichiometric ratio 1:1 (Table 2, entry 9), obtaining a result perfectly comparable to the previous one. To complete the control reaction we performed the reaction in the absence of a catalytic system (Table 2, entry 10) and we observed only traces of the final product.
Finally, to verify the goodness of the developed synthetic procedure, we reproduced the reaction on a gram-scale, obtaining similar reaction yields (Table 2, entry 11). Ultimately, the 1,3-dipolar cycloaddition was carried out in ionic liquid [mPy]OTf [40,41] (Table 2, entry 12), with the aim of recycling the solvent and catalytic system after the reaction. Unfortunately, in these conditions, only traces of product 23 were observed (Table 2, entry 12).
Once the reaction conditions were optimized (Table 2, entry 9), we extended the investigation by using the 1-(2-azidoethyl)isatins 10–13 and the propargyl esters 20–22 of phenolic acids to synthesize a new series of 1,4-disubstituted 1,2,3-triazoles hybridized molecules 23–34 (Table 3).

Table 3.
Synthesis of new hybrid molecules based on 1,4-disubstituted 1,2,3-triazoles by CuAAC.
As shown in Table 3, phenolic acid propargyl esters 20–22 exhibited high reactivity towards copper(I)-catalyzed 1,3-dipolar cycloaddition (CuAAC), resulting in high reaction yields in all cases. As you can see, the reactivity of the azides appears to be slightly dependent on the nature of the substituent in the 5-position. More specifically, when electron-withdrawing groups as -NO2 and -Cl were present on the backbone of the isatins, moderately lower yields were observed for reaction with all three dipolarophiles (Table 3, entries 3–4, 7–8, 11–12), if compared to the presence of the electron-donor group -OMe (Table 3, entries 2, 6, 10). It is worth noting that only a single 1,4-disubstituted 1,2,3-triazole regioisomer was obtained for all synthesized compounds, demonstrating the high regioselectivity of the well-known copper(I)-catalyzed azide-alkyne cycloaddition (see Supplementary Materials for the 1H NMR of reaction crude of 23) [42,43].
At this point, we propose a possible reaction mechanism for the reaction between 10 and 20 as illustrated in Scheme 2.
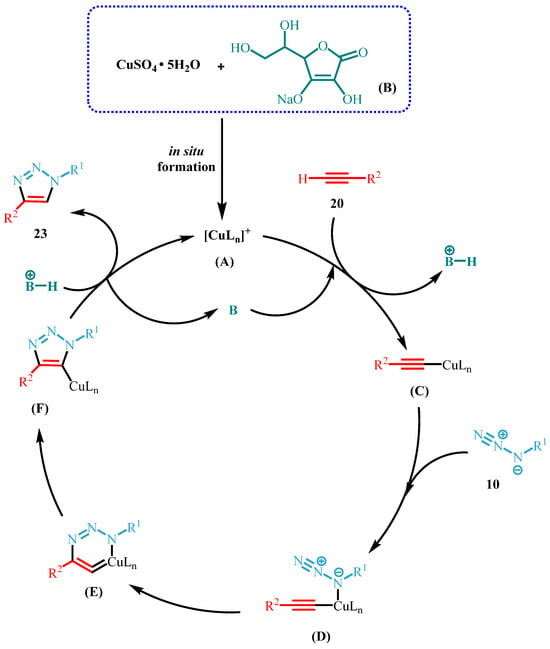
Scheme 2.
Proposed catalytic cycle for copper(I)-catalyzed azide-alkyne cycloaddition.
Initially, Cu(II) reacts with Na-ascorbate via a redox reaction to in situ form the Cu(I)-complex catalytic species (A), generically indicated as [CuLn]+. The catalytic cycle begins with the coordination of the Cu(I) (A) species to the triple bond of the alkyne 20, forming a π-complex. The base (B), consisting of the excess molar quantity of Na-ascorbate with respect to [CuLn]+, allows the alkyne deprotonation and the Cu(I) species binds to the sp hybridized carbon (C). Subsequently, azide 10 coordinates with the Cu-π-coordinated system with very specific regiochemistry (D), and the formation of a six-membered metallacycle occurs (E). The metallacycle undergoes a rearrangement whereby an aromatic five-membered cycle (F) is formed. Finally, protonation of the copper-linked 1,2,3-triazole causes the C-H bond formation, resulting in the desired 1,4-disubstituted 1,2,3-triazole product 23, and the catalyst is released [44,45].
2.2. Molecular Docking
With the aim of proposing new biological evaluation perspectives to the conjugates obtained, a series of molecular docking studies were carried out following a protocol from our recent study [46]. AutoDock Vina [47] was used, by keeping the docking parameters at default values. The geometries of the triazole conjugates were previously optimized quantum mechanically. The in silico studies were performed over eight targets retrieved from the Protein Data Bank (http://www.rcsb.org/accessed on 16 December 2023) for the full set of the compounds synthesized in this work. A total number of 104 simulations (96 for the newly synthesized compounds plus eight for reference inhibitors) were performed. The biological targets for the computational study were chosen on the basis of their relevance in medicinal chemistry, being involved in the etiopathogenesis of several diseases. Three specific aspects were taken into account: (1) evidence from the literature on the biological activity displayed by triazole-phenolic acids conjugates [48,49], (2) evidence on the biological activity displayed by triazole-isatin conjugates [42,50,51], (3) evidence on the activity of other relevant triazole hybrids [52,53].
The target 5-lipoxygenase (5-LOX) is a critical enzyme in arachidonic acid (ACD) breakdown and drives the formation of leukotrienes (LTs) [54]. These inflammatory mediators have far-reaching effects, contributing to conditions like asthma, allergic rhinitis, cardiovascular diseases, and even some cancers [55]. Cyclooxygenase-2 (COX-2) is another critical target, and its understanding offered alternatives for treating arthritis with reduced gastrointestinal risk, such as the discovery of celecoxib [56]. Angiotensin-converting enzyme (ACE) became a drug target in the 1970s and still represents a hot topic for specific ACE inhibitors for better blood pressure control and reduced side effects [57]. Type IIA topoisomerases (Top2), enzymes crucial for DNA organization, are targeted in cancer therapy. Humans have two isoforms of Type IIA topoisomerase: Topo IIα and Topo IIβ. The Topo IIα isoform is particularly sensitive to drugs that form complexes with the enzyme and DNA, disrupting cancer cell function [58]. Tubulin, crucial for many cellular functions, is a strategic target for antitumor drugs. By binding to tubulin, these drugs block essential processes, ultimately causing cell death [59]. The enzyme acetylcholinesterase (AChE) breaks down acetylcholine, a crucial brain chemical for memory and learning; its depletion in Alzheimer’s Disease leads to cognitive decline [60]. Lastly, the selected D-alanine-d-alanine ligase (Ddl) target is an enzyme that builds a crucial part of the bacterial cell wall and, for this reason, is a promising target for antibiotic development [61]. For all the computational simulations, a reference ligand from the literature was docked to the biological targets to give a reference binding score (Table 4).

Table 4.
Best docking affinity values of compounds 23–34 vs. co-crystalized ligands on selected receptors.
All the docked substrates showed strong potential affinity with the biological targets, which in most cases were superior or comparable to the reference ligands (as highlighted in bold text in Table 4). Moreover, for all the docked compounds, no particular difference in terms of affinity was revealed by varying the functional groups present on the structure. This might be due to the dimensions and the great conformational flexibility of the tested compounds, which mediate the detrimental or positive effect of the substituents.
In this regard, it can be instructive to calculate the average affinity values for all the conjugates 23–34 and compare them to the affinity values of the reference compounds (Table 5).

Table 5.
Percentage difference between the average affinity values of the triazole conjugates vs. the reference ligand.
On the basis of the differences in percentage between our substrates and the literature ligands, we extracted the best docking poses towards the four best resulting targets, namely 5-LOX/ACD, COX-2, ACE, and tubulin. For these cases, we represented the best docking substrates in accordance with the result reported in Table 4. For the 5-LOX/ACD system, substrate 26 interaction was represented, which showed an affinity of −9.0 kcal/mol (Figure 2A). In this case, the choice of substrate 26 was arbitrary since also substrates 29 and 30 gave the same affinity values. As for COX-2 inhibition, substrate 31 was the best-interacting substrate with an affinity of −10.3 kcal/mol (Figure 2B). Lastly, both for ACE and tubulin targets, compound 26 resulted in having the best affinity values of −9.8 (Figure 2C) and −9.7 kcal/mol (Figure 2D), respectively.
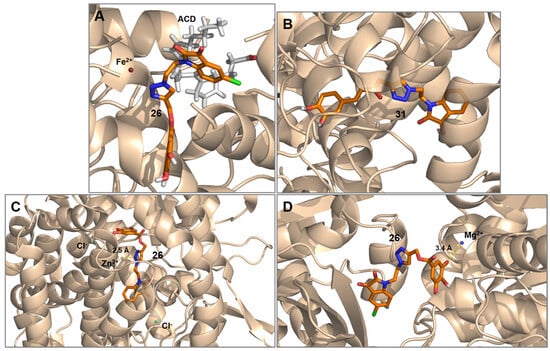
Figure 2.
Best docking poses of most interacting triazole conjugates with main four interacting receptors: (A) Compound 26 bound to 5-LOX in the presence of arachidonic acid (ACD) (PDB 3V99). (B) Compound 31 bound to COX-2 (PDB 4FM5). (C) Compound 26 bound to ACE (PDB 1O86). (D) Compound 26 bound to tubulin (PDB 1SA0). Ligands and binding residues are shown as sticks.
The overall displayed affinity results highlight two main aspects. (1) As said, our triazole conjugates show notable conformational flexibility, which results in a better adaptation to the target’s pocket. The presence of versatile spacers like triazoles allows for an easier clickable synthesis of large conjugates containing useful functional groups such as carboxyl (as in the isatin portion) and hydroxyl (as in the phenolic acid portion) groups, capable of taking part in hydrogen bond interactions with target’s backbones. It must be noted that the presence of the carboxyl groups and nitrogen, in particular, can trigger the formation of H-bonds [62]. Larger ligand sizes generally ensure stronger interactions with the target [63,64], but might pose significant problems related to the ligand’s rigidity precluding access to the target’s interacting pocket [65]. Hence, the presence of 1,4-disubstituted spacing triazoles as in our case might solve such rigidity-related issues allowing for more hydrogen bond interactions in the three-dimensional space. This evidence also is in line with the use of triazoles as spacers in the design of new bioactive compounds [66].
(2) The presence of the triazole group not only allows for an extent of conformational flexibility but also prompts the coordination when a metal is present in the active site. In our docking analysis, for three cases over the four high-affinity cases considered, it is worth noting that our substrates showed higher affinity towards active sites wherein a metal was present (5-LOX, ACE, and tubulin). This is related to the capacity of the interacting ligand to offer a strong coordinating site represented by the triazole core, as in the case of substrate 26 with ACE (Figure 2C), or the phenolic acid portion as in the case of substrate 26 with tubulin (Figure 2D). For the ACE interaction, zinc coordination takes place with an interaction distance of 2.5 Å, in line with the recent evidence on the Zn-coordination-related inhibition of β-lactames by triazole ligands [67]. Such theoretical evidence highlights the importance of the coordinating capabilities of the triazole core in medicinal chemistry, which were also discussed in our previous work on the synthesis and docking studies over a new series of pyrimidine-containing 1,5-disubstituted triazoles [31].
As for the coordination of the phenolic acid portion of substrate 26 with Mg2+ ion in the tubulin active site, a coordination distance of 3.4 Å suggests a weaker kind of interaction, but results in line with the literature evidence on the phenolic acid’s capabilities to exert a biological activity on the basis of their coordinating capabilities [68].
3. Materials and Methods
Commercial starting materials were purchased from Merck (Milano, Italy) or Alfa Aesar (Karlsruhe, Germany) and were used without further purification. Reactions were monitored by TLC using silica plates 60-F264, commercially available from Merck (Milano, Italy). Additionally, 1H and 13C-NMR spectra and two-dimensional NMR spectra were recorded at 300 and 500 MHz and 125.7 MHz, respectively, in DMSO-d6 using tetramethylsilane (TMS) as internal standard (Bruker ACP 300 MHz and Bruker Avance 500 MHz with a 5 mm TBO probe, Rheinstetten, Germany). Chemical shifts are given in parts per million and coupling constants in Hertz. The purity and the regiochemistry were established by NMR spectra (1H NMR experiments). High-resolution mass spectra (HRMS) were recorded with a Bruker Compact QTOF instrument (Bruker, Billerica, MA, USA). HRMS spectra were acquired in positive ion mode, with a mass resolution of 30,000. Mass calibration was performed with a solution of sodium formate clusters and processed in HPC mode. Spectra acquisition was performed in flow injection, with a full scan mode in the range of 30 to 1000 m/z. N2 was the source of dry gas (V = 4 L/min, T = 180 °C). The ion formula of each compound was calculated with the Smart Formula tool of the Bruker software platform (Compass data analysis 4.4), analyzing the isotopic pattern ratio with 4 mDa mass confidence. Additionally, for HRMS analysis, all samples were dissolved in MeOH.
Synthesis and characterization of variously substituted 1-(2-bromoethyl)isatins 6–9 and variously substituted 1-(2-azidoethyl)isatins 10–13 are realized by modified literature procedures [36,37], respectively, and reported in the Supplementary Materials. Phenolic acid propargyl esters 20–22 were prepared with the procedures reported in the Supplementary Materials.
3.1. General Procedure for Synthesis of 1,4-Disubstituted 1,2,3-Triazoles 23–34
In a 50 mL two-necked round-bottomed flask, equipped with a bubble condenser and a magnetic stir bar, the appropriate phenolic acid propargyl ester 20–22 (1.20 mmol) and the opportune 1-(2-azidoethyl)isatin 10–13 (1.20 mmol) in DMF (20 mL) were dissolved. Subsequently, copper sulfate pentahydrate (0.24 mmol) and sodium ascorbate (0.96 mmol) were added. The reaction mixture was heated at 60 °C for 5 h under stirring and monitored by TLC. The mixture was evaporated under reduced pressure by azeotrope with toluene and then with ethanol, and the obtained oily crude was purified by flash chromatography using CHCl3:MeOH (8:2 v/v) to furnish the solid products 23–34 in good yields.
(1-(2-(isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 3,4,5-trihydroxybenzoate (23). Orange solid, yield = 91%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.13 (t, J = 5.60 Hz, 2H, CH2), 4.66 (t, J = 5.60 Hz, 2H, CH2), 5.20 (s, 2H, CH2), 6.81–6.88 (m, 1H, Ar), 6.89–6.96 (m, 2H, Ar), 6.99–7.08 (m, 1H, Ar), 7.45–7.55 (m, 2H, Ar), 8.29 (s, 1H, CH), 9.00 (bs, 1H, OH), 9.30 (bs, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 40.98, 47.92, 58.04, 109.62, 111.12, 118.29, 119.94, 124.23, 125.47, 126.61, 139.03, 143.22, 146.54, 151.15, 159.11, 166.42, 175.96, 183.89. ESI(+)-MS: m/z [M + H]+ calcd for [C20H17N4O7]+, 425.1092, found 425.1098.
(1-(2-(5-methoxy-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 3,4,5-trihydroxybenzoate (24). Violet solid, yield = 92%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 3.71 (s, 1H, CH3), 4.10 (t, J = 5.40 Hz, 2H, CH2), 4.64 (t, J = 5.40 Hz, 2H, CH2), 5.21 (s, 2H, CH2), 6.80–6.88 (m, 1H, Ar), 6.90–6.96 (m, 2H, Ar), 7.06–7.15 (m, 2H, Ar), 8.29 (s, 1H, CH), 9.00 (bs, 1H, OH), 9.30 (bs, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.00, 47.87, 56.79, 58.12, 109.58, 112.32, 118.81, 119.93, 124.80, 126.50, 139.60, 143.26, 144.96, 146.56, 156.70, 159.15, 166.46, 175.97, 184.14. ESI(+)-MS: m/z [M + H]+ calcd for [C21H19N4O8]+, 455.1197, found 455.1216.
(1-(2-(5-nitro-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 3,4,5-trihydroxybenzoate (25). Yellow solid, yield = 89%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.13–4.35 (m, 2H, CH2), 4.67 (s, 2H, CH2), 5.19–5.25 (m, 2H, CH2), 6.85–7.17 (m, 3H, Ar), 8.14–8.45 (m, 3H, Ar and CH), 9.22 (bs, 3H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 47.86, 58.16, 67.04, 109.52, 121.21, 126.75, 129.30, 133.80, 139.66, 143.36, 144.24, 146.58, 149.81, 155.38, 159.68, 166.46, 173.81, 181.77. ESI(+)-MS: m/z [M + H]+ calcd for [C20H16N5O9]+, 470.0943, found: 470.0936.
(1-(2-(5-chloro-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 3,4,5-trihydroxybenzoate (26). Red solid, yield = 88%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.13 (t, J = 5.88 Hz, 2H, CH2), 4.64 (t, J = 5.88 Hz, 2H, CH2), 5.21 (s, 2H, CH2), 6.87–6.97 (m, 3H, Ar), 7.48–7.65 (m, 2H, Ar), 8.29 (s, 1H, CH), 9.00 (bs, 1H, OH), 9.30 (bs, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.07, 47.83, 58.09, 109.60, 112.94, 119.69, 119.92, 125.01, 126.60, 128.56, 137.90, 139.62, 143.29, 146.57, 149.68, 158.89, 166.46, 182.81. ESI(+)-MS: m/z [M + H]+ calcd for [C20H16ClN4O7]+, 459.0702, found 459.0704.
(1-(2-(isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 4-hydroxybenzoate (27). Orange solid, yield = 92%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.13 (t, J = 6.03 Hz, 2H, CH2), 4.66 (t, J = 6.03 Hz, 2H, CH2), 5.24 (s, 2H, CH2), 6.78–6.89 (m, 3H, Ar), 7.03 (t, J= 7.45 Hz, 1H, Ar), 7.45–7.55 (m, 2H, Ar), 7.76 (d, J = 8.82 Hz, 2H, Ar), 8.31 (s, 1H, CH), 10.36 (bs, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.00, 47.94, 58.19, 111.09, 116.28, 118.27, 120.86, 124.16, 125.41, 126.60, 132.51, 138.98, 143.21, 151.14, 159.07, 163.08, 166.11, 183.85. ESI(+)-MS: m/z [M + H]+ calcd for [C20H17N4O5]+, 393.1193, found 393.1196.
(1-(2-(5-methoxy-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 4-hydroxybenzoate (28). Violet solid, yield = 94%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 3.71 (s, 3H, CH3), 4.11 (t, J = 5.77 Hz, 2H, CH2), 4.65 (t, J = 5.77 Hz, 2H, CH2), 5.25 (s, 2H, CH2), 6.76–6.88 (m, 3H, Ar), 7.05–7.14 (m, 2H, Ar), 7.76 (d, J = 8.81 Hz, 2H, Ar), 8.30 (s, 1H, CH), 10.36 (bs, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.02, 47.94, 56.75, 58.24, 110.01, 112.23, 116.28, 118.73, 120.85, 124.84, 126.54, 132.47, 143.20, 144.98, 156.62, 159.11, 163.07, 166.12, 184.09. ESI(+)-MS: m/z [M + H]+ calcd for [C21H19N4O6]+, 423.1299, found 423.1292.
(1-(2-(5-nitro-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 4-hydroxybenzoate (29). Orange solid, yield = 87%. 1H NMR (DMSO-d6, 500 MHz): δ (ppm) 4.23 (t, J = 5.64 Hz, 2H, CH2), 4.68 (t, J = 5.64 Hz, 2H, CH2), 5.24 (s, 2H, CH2), 6.79–6.85 (m, 2H, Ar), 6.96 (d, J = 8.84 Hz, 1H, Ar), 7.69–7.75 (m, 2H, Ar), 8.19 (d, J = 2.50 Hz, 1H, Ar), 8.32 (s, 1H, CH), 8.36 (dd, J = 8.78 Hz, 2.50 Hz, 1H, Ar), 10.35 (bs, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.48, 48.02, 58.24, 111.45, 116.26, 118.63, 120.14, 120.76, 126.72, 132.36, 133.65, 143.37, 143.89, 155.39, 159.61, 163.08, 166.07, 181.71. ESI(+)-MS: m/z [M + H]+ calcd for [C20H16N5O7]+, 438.1044, found 438.1046.
(1-(2-(5-chloro-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl 4-hydroxybenzoate (30). Red solid, yield = 89%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.14 (t, J = 5.92 Hz, 2H, CH2), 4.65 (t, J = 5.92 Hz, 2H, CH2), 5.25 (s, 2H, CH2), 6.80–6.90 (m, 3H, Ar), 7.50–7.59 (m, 2H, Ar), 7.76 (d, J = 8.70 Hz, 2H, Ar), 8.31 (s, 1H, CH), 10.36 (bs, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.13, 47.92, 58.21, 112.83, 116.32, 119.64, 120.85, 124.93, 126.63, 128.44, 132.47, 137.81, 143.25, 149.68, 158.86, 163.08, 166.13, 182.77. ESI(+)-MS: m/z [M + H]+ calcd for [C20H16ClN4O5]+, 427.0804, found 427.0806.
(1-(2-(isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl (E)-3-(3,4-dihydroxyphenyl)acrylate (31). Orange solid, yield = 90%. 1H NMR (DMSO-d6, 500 MHz): δ (ppm) 4.13 (t, J = 5.61 Hz, 2H, CH2), 4.66 (t, J = 5.61 Hz, 2H, CH2), 5.15 (s, 2H, CH2), 6.23 (d, J = 15.92 Hz, 1H, CH), 6.76 (d, J = 8.15 Hz, 1H, Ar), 6.87 (d, J = 8.18 Hz, 1H, Ar), 6.95–7.17 (m, 3H, Ar), 7.40–7.64 (m, 3H, Ar and CH), 8.27 (s, 1H, CH), 9.18 (bs, 1H, OH), 9.64 (bs, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 40.99, 47.89, 57.74, 111.16, 114.38, 115.74, 116.70, 118.29, 122.49, 124.20, 125.45, 126.37, 126.61, 139.08, 143.19, 146.54, 146.59, 149.50, 151.14, 159.07, 167.09, 183.86. ESI(+)-MS: m/z [M + H]+ calcd for [C22H19N4O6]+, 435.1299, found 435.1292.
(1-(2-(5-methoxyisatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl (E)-3-(3,4-dihydroxyphenyl)-acrylate (32). Red solid, yield = 93%. 1H NMR (DMSO-d6, 500 MHz): δ (ppm) 3.71 (s, 3H, CH3), 4.10 (t, J = 5.95 Hz, 2H, CH2), 4.65 (t, J = 5.95 Hz, 2H, CH2), 5.15 (s, 2H, CH2), 6.23 (d, J = 15.89 Hz, 1H, CH), 6.77 (d, J = 8.32 Hz, 1H, Ar), 6.81 (d, J = 8.61 Hz, 1H, Ar), 7.00 (dd, J = 8.34 Hz, 2.07 Hz, 1H, Ar), 7.05 (d, J = 2.07 Hz, 1H, Ar), 7.10 (d, J = 2.68 Hz, 1H, Ar), 7.14 (dd, J = 8.61 Hz, 2.68 Hz, 1H, Ar), 7.47 (d, J = 15.89 Hz, 1H, CH), 8.25 (s, 1H, CH), 9.16 (bs, 1H, OH), 9.63 (bs, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.06, 47.97, 56.83, 57.84, 110.08, 112.35, 114.38, 115.84, 116.72, 118.78, 122.55, 125.03, 126.44, 126.63, 143.24, 145.04, 146.58, 146.68, 149.55, 156.71, 159.16, 167.17, 184.16. ESI(+)-MS: m/z [M + H]+ calcd for [C23H21N4O7]+, 465.1405, found 465.1413.
(1-(2-(5-nitro-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl (E)-3-(3,4-dihydroxyphenyl)- acrylate (33). Orange solid, yield = 86%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.23 (t, J = 5.87 Hz, 2H, CH2), 4.67 (t, J = 5.88 Hz, 2H, CH2), 5.15 (s, 2H, CH2), 6.20 (d, J = 15.86 Hz, 1H, CH), 6.76 (d, J = 8.16 Hz, 1H, Ar), 6.99 (dd, J = 8.40 Hz, 2.15 Hz, 1H, Ar), 7.01 (d, J = 8.85 Hz, 1H, Ar), 7.04 (d, J = 2.15 Hz, 1H, Ar), 7.45 (d, J = 15.87 Hz, 1H, CH), 8.22 (d, J = 2.40 Hz, 1H, Ar), 8.28 (s, 1H, CH), 8.41 (dd, J = 8.81 Hz, 2.45 Hz, 1H, Ar), 9.36 (bs, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.38, 47.93, 57.80, 111.54, 114.18, 115.83, 116.64, 118.64, 120.18, 122.40, 126.32, 126.70, 133.75, 143.31, 143.95, 146.50, 146.64, 149.49, 155.36, 159.58, 167.05, 181.71. ESI(+)-MS: m/z [M + H]+ calcd for [C22H18N5O8]+, 480.1150, found 480.1145.
(1-(2-(5-chloro-isatin-1-yl)ethyl)-1,2,3-triazol-4-yl)methyl (E)-3-(3,4-dihydroxyphenyl) acrylate (34). Red solid, yield = 88%. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 4.13 (t, J = 5.66 Hz, 2H, CH2), 4.64 (t, J = 5.65 Hz, 2H, CH2), 5.15 (s, 2H, CH2), 6.23 (d, J = 15.95 Hz, 1H, CH), 6.76 (d, J = 8.09 Hz, 1H, Ar), 6.85 (d, J = 8.27 Hz, 1H, Ar), 6.95–7.10 (m, 2H, Ar), 7.48 (d, J = 15.94 Hz, 1H, CH), 7.54–7.64 (m, 2H, Ar), 8.26 (s, 1H, CH), 9.38 (bs, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ (ppm) 41.14, 47.94, 57.82, 112.92, 114.35, 115.84, 116.71, 119.66, 122.56, 124.98, 126.42, 126.71, 128.52, 137.94, 143.27, 146.56, 146.69, 149.54, 149.71, 158.89, 167.16, 182.82. ESI(+)-MS: m/z [M + H]+ calcd for [C22H18ClN4O6]+, 469.0909, found 469.0900.
3.2. Molecular Docking Calculations
Geometry optimizations and frequency calculations for stationary point characterization were carried out with Gaussian16 using the M06–2X hybrid functional [69], the 6–31 G (d, p) basis set, and ultrafine integration grids. Bulk solvent effects in water were considered implicitly through the IEF-PCM polarizable continuum model [70]. The protein structure was processed with AutodockTools-1.5.6rc3 [71] to toggle problems of incomplete structures due to missing atoms or water molecules. Ligands and protein structure files were converted to PDBQT (Protein Data Bank Partial Charge and Atom Type) and the docking was executed using the Lamarckian algorithm. The grid box consisted of 30 grid points in all three dimensions (X, Y, and Z) separated by a distance of 1 Å between each one. The crystallographic ligands were docked after adding hydrogen atoms with AutodockTools-1.5.6rc3 and without optimizing their geometry. For all the simulations, the protein was kept rigid allowing for flexibilization of the ligands around their rotatable bonds.
4. Conclusions
In conclusion, in this work, we designed a new library of hybrids containing a 1,2,3-triazole moiety as a linker between an isatin and a phenolic acid. Firstly, four different 1-(2-azidoethyl)isatines and three phenolic acid propargyl esters were prepared in good to excellent yields. Then, after the optimization of the reaction condition, a CuAAC was performed to obtain twelve different 1,4-disubstituted-1,2,3-triazoles, in a highly regioselective manner and in excellent yields (86–92%). The influence of the substituents on the aromatic rings was evaluated and all the hybrids were purified and characterized. Finally, a molecular docking study was carried out to hypothesize potential therapeutic targets for these new compounds. Eight different potential enzymatic substrates were found considering the previous affinities reported in the literature for the three building blocks (isatine, 1,2,3-triazole, and phenolic acid) as a research parameter. All the hybrids showed strong potential affinity with them, if compared to the reference ligands reported in the literature. The most interesting results, in term of affinity, were found for all the hybrids on the enzyme 5-lipoxygenase (5-LOX), in the presence of its natural substrate, the arachidonic acid (ACD), thus confirming the possibility to potentially develop a new class of anti-inflammatory agents starting from these compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29071556/s1. Characterization spectra of the 1-(2-bromoethyl) isatins 6–9 (1H NMR; 13C NMR; COSY NMR; HRMS); Characterization spectra of the 1-(2-azidoethyl) isatins 10–13 (1H NMR; 13C NMR; COSY NMR; HRMS); Characterization spectra of the propargyl esters 20–22 (1H NMR; 13C NMR; COSY NMR; HRMS); 1H NMR (300 MHz) spectra of crude of the model reaction for synthesis of 23; Characterization spectra of the hybrid molecules 23–34 (1H NMR; 13C NMR; COSY NMR; HRMS).
Author Contributions
Conceptualization, V.A., L.M. and P.C.; methodology, V.A. and A.J.; validation, V.A., M.A.T. and A.M.; formal analysis, V.A., G.F. and A.J.; investigation, V.A., L.M., M.A.T. and P.C.; resources, L.M., A.D.N. and P.C.; data curation, V.A., M.A.T., A.M., G.F. and A.J.; writing—original draft preparation, L.M., V.A., P.C. and M.A.T.; writing—review and editing, A.D.N., V.A. and P.C.; supervision, L.M., A.D.N. and P.C.; funding acquisition, L.M., A.D.N. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and Supplementary Materials.
Acknowledgments
We thank the University of Calabria and the “PNRR-MAD-2022-12376295—Next Generation EU—PNRR M6C2—Investimento 2.1 Valorizzazione e potenziamento della ricerca biomedica del SSN”. We also acknowledge the Italian Ministry of University and Scientific Research (MUR) for a doctoral grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef]
- Beruvé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as important antibacterial agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, S.; Li, P.; Xie, W.; Jin, Y.; Sun, Q.; Wu, Q.; Sun, P.; Zhang, Y.; Yang, X. Synthesis and SAR studies of biaryloxy-substituted triazoles as antifungal agents. Bioorg. Med. Chem. Lett. 2008, 18, 3261–3265. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Gao, C.; Ren, Q.-C.; Chang, L.; Lv, Z.-S.; Feng, L.-S. Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 138, 501–513. [Google Scholar] [CrossRef]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3-Triazole Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef]
- Kumbhare, R.M.; Kosurkar, U.B.; Ramaiah, M.J.; Dadmal, T.L.; Pushpavalli, S.; Pal-Bhadra, M. Synthesis and biological evaluation of novel triazoles and isoxazoles linked 2-phenyl benzothiazole as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 5424–5427. [Google Scholar] [CrossRef]
- Algieri, V.; Algieri, C.; Maiuolo, L.; De Nino, A.; Pagliarani, A.; Tallarida, M.A.; Trombetti, F.; Nesci, S. 1,5-Disubstituted-1,2,3-triazoles as inhibitors of the mitochondrial Ca2+-activated F1FO-ATP(hydrol)ase and the permeability transition pore. Ann. N. Y. Acad. Sci. 2021, 1485, 43–55. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Marchi, S.; Forte, M.; Tallarida, M.A.; Bianchi, F.; La Mantia, D.; Algieri, V.; Stanzione, R.; Cotugno, M.; et al. 1,5-disubstituted-1,2,3-triazoles counteract mitochondrial dysfunction acting on F1FO-ATPase in models of cardiovascular diseases. Pharmacol. Res. 2023, 187, 106561. [Google Scholar] [CrossRef]
- De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst. Catalysts 2018, 8, 364. [Google Scholar] [CrossRef]
- De Nino, A.; Algieri, V.; Tallarida, M.A.; Costanzo, P.; Pedrón, M.; Tejero, T.; Merino, P.; Maiuolo, L. Regioselective Synthesis of 1,4,5-Trisubstituted-1,2,3-Triazoles from Aryl Azides and Enaminones. Eur. J. Org. Chem. 2019, 33, 5725–5731. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar cycloadditions. 76. Concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J. Org. Chem. 1976, 41, 403–419. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Empting, M.; Avrutina, O.; Meusinger, R.; Fabritz, S.; Reinwarth, M.; Biesalski, M.; Voigt, S.; Buntkowsky, G.; Kolmar, H. “Triazole bridge”: Disulfide-bond replacement by ruthenium-catalyzed formation of 1,5-disubstituted 1,2,3-triazoles. Angew. Chem. Int. Ed. 2011, 50, 5207–5211. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida, M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Nath, R.; Pathania, S.; Grover, G.; Akhtar, M.J. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020, 1222, 128900. [Google Scholar] [CrossRef]
- Tallarida, M.A.; Olivito, F.; Navo, C.D.; Algieri, V.; Jiritano, A.; Costanzo, P.; Poveda, A.; Moure, M.J.; Jiménez-Barbero, J.; Maiuolo, L.; et al. Highly Diastereoselective Multicomponent Synthesis of Spirocyclopropyl Oxindoles Enabled by Rare-Earth Metal Salts. Org. Lett. 2023, 25, 3001–3006. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Gao, M.; Zhang, X.; Liu, Z.; Zhao, S.; Lv, Z.; Xiao, J. Benzofuran-isatin-imine hybrids tethered via different length alkyl linkers: Design, synthesis and in vitro evaluation of anti-tubercular and anti-bacterial activities as well as cytotoxicity. Eur. J. Med. Chem. 2019, 165, 323–331. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El Hassab, M.A.; Abo-Ashour, M.F.; Al-Warhi, T.; Elaasser, M.M.; Safwat, N.A.; Suliman, H.; Ahmed, M.F.; SAl-Rashood, T.; Abdel-Aziz, H.A.; et al. Development of isatin-thiazolo[3,2-a]benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity: Synthesis, biological and molecular dynamics investigations. Bioorg. Chem. 2021, 110, 104748. [Google Scholar] [CrossRef]
- Sevinçli, Z.Ş.; Duran, G.N.; Özbil, M.; Karalı, N. Synthesis, molecular modeling and antiviral activity of novel 5-fluoro-1H-indole-2,3-dione 3-thiosemicarbazones. Bioorg. Chem. 2020, 104, 104202. [Google Scholar] [CrossRef]
- Sonmez, F.; Gunesli, Z.; Kurt, B.Z.; Gazioglu, I.; Avci, D.; Kucukislamoglu, M. Synthesis, antioxidant activity and SAR study of novel spiro-isatin-based Schiff bases. Mol. Divers. 2019, 23, 829–844. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Mubarak, M.S. Hybrid Drugs as Potential Combatants Against Drug-Resistant Microbes: A Review. Curr. Top Med. Chem. 2017, 17, 895–906. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Cos, P.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship of flavonoids as antioxidant and pro-oxidant compounds. Stud. Nat. Prod. Chem. 2000, 22, 307–341. [Google Scholar] [CrossRef]
- Costanzo, P.; Oliverio, M.; Maiuolo, J.; Bonacci, S.; De Luca, G.; Masullo, M.; Arcone, R.; Procopio, A. Novel Hydroxytyrosol-Donepezil Hybrids as Potential Antioxidant and Neuroprotective Agents. Front. Chem. 2021, 9, 741444. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 707. [Google Scholar]
- Hsieh, C.L.; Yen, G.C.; Chen, H.Y. Antioxidant activities of phenolic acids on ultraviolet radiation-induced erythrocyte and low density lipoprotein oxidation. J. Agric. Food Chem. 2005, 53, 6151–6155. [Google Scholar] [CrossRef]
- Corredor, M.; Solà, J.; Alfonso, I. Disubstituted 1,2,3-triazoles as amide bond mimetics. Targets Heterocycl. Syst. 2017, 21, 1–22. [Google Scholar] [CrossRef]
- Leggio, A.; Liguori, A.; Maiuolo, L.; Napoli, A.; Procopio, A.; Siciliano, C.; Sindona, G. Model studies towards the synthesis of 4′-azaerythrofuranosyl adenines as analogues of the antiviral drug 2′,3′-dideoxyadenosine (ddA). J. Chem. Soc. Perkin Trans. 1997, 20, 3097–3099. [Google Scholar] [CrossRef]
- Algieri, V.; Costanzo, P.; Tallarida, M.A.; Olivito, F.; Jiritano, A.; Fiorani, G.; Peccati, F.; Jiménez-Osés, G.; Maiuolo, L.; De Nino, A. Regioselective Synthesis and Molecular Docking Studies of 1,5-Disubstituted 1,2,3-Triazole Derivatives of Pyrimidine Nucleobases. Molecules 2022, 27, 8467. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, L.; Algieri, V.; Russo, B.; Tallarida, M.A.; Nardi, M.; Di Gioia, M.L.; Merchant, Z.; Merino, P.; Delso, I.; De Nino, A. Synthesis, Biological and In Silico Evaluation of Pure Nucleobase-Containing Spiro (Indane-Isoxazolidine) Derivatives as Potential Inhibitors of MDM2-p53 Interaction. Molecules 2019, 24, 2909. [Google Scholar] [CrossRef]
- Maiuolo, L.; Russo, B.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Tallarida, M.A.; De Nino, A. Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles by 1,3-dipolar cycloaddition: Role of Er(OTf)3, ionic liquid and water. Tetrahedron Lett. 2019, 60, 672–674. [Google Scholar] [CrossRef]
- Maiuolo, L.; De Nino, A.; Algieri, V.; Nardi, M. Microwave-Assisted 1,3-Dipolar Cyclo-addition: Recent Advances in Synthesis of Isoxazolidines. Mini-Rev. Org. Chem. 2017, 14, 136–142. [Google Scholar] [CrossRef]
- Maiuolo, L.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Delso, I.; Tallarida, M.A.; De Nino, A. Nitrones and Nucleobase-Containing Spiro-Isoxazolidines Derived from Isatin and indanone: Solvent-Free Microwave-Assisted Stereoselective Synthesis and Theoretical Calculations. RSC Adv. 2017, 7, 48980–48988. [Google Scholar] [CrossRef]
- Shibinskaya, M.O.; Lyakhov, S.A.; Mazepa, A.V.; Andronati, S.A.; Turov, A.V.; Zholobak, N.M.; Spivak, N.Y. Synthesis, cytotoxicity, antiviral activity and interferon inducing ability of 6-(2-aminoethyl)-6H-indolo[2,3-b]quinoxalines. Eur. J. Med. Chem. 2010, 45, 1237–1243. [Google Scholar] [CrossRef]
- Kumar, K.; Sagar, S.; Esau, L.; Kaur, M.; Kumar, V. Synthesis of novel 1H-1,2,3-triazole tethered C-5 substituted uracil-isatin conjugates and their cytotoxic evaluation. Eur. J. Med. Chem. 2012, 58, 153–159. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide-alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Yadav, M.; Lal, K.; Kumar, A.; Singh, P.; Vishvakarma, V.K.; Chandra, R. Click reaction inspired synthesis, antimicrobial evaluation and in silico docking of some pyrrole-chalcone linked 1,2,3-triazole hybrids. J. Mol. Struct. 2023, 1273, 134321. [Google Scholar] [CrossRef]
- Algieri, V.; Algieri, C.; Costanzo, P.; Fiorani, G.; Jiritano, A.; Olivito, F.; Tallarida, M.A.; Trombetti, F.; Maiuolo, L.; De Nino, A.; et al. Novel Regioselective Synthesis of 1,3,4,5-Tetrasubstituted Pyrazoles and Biochemical Valuation on F1FO-ATPase and Mitochondrial Permeability Transition Pore Formation. Pharmaceutics 2023, 15, 498. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-López, D.; Russo, B.; Algieri, V. Efficient Organocatalyst Supported on a Simple Ionic Liquid as a Recoverable System for the Asymmetric Diels–Alder Reaction in the Presence of Water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Singh, H.; Singh, J.V.; Gupta, M.K.; Saxena, A.K.; Sharma, S.; Nepali, K.; Bedi, P.M.S. Triazole tethered isatin-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies. Bioorg. Med. Chem. Lett. 2017, 27, 3974–3979. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Nesci, S.; Algieri, C.; Tallarida, M.A.; Stanzione, R.; Marchi, S.; Pietrangelo, D.; Trombetti, F.; D’Ambrosio, L.; Forte, M.; Cotugno, M.; et al. Molecular mechanisms of naringenin modulation of mitochondrial permeability transition acting on F1FO-ATPase and counteracting saline load-induced injury in SHRSP cerebral endothelial cells. Eur. J. Cell Biol. 2024, 103, 151398. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- De Lucia, D.; Lucio, O.M.; Musio, B.; Bender, A.; Listing, M.; Dennhardt, S.; Koeberle, A.; Garscha, U.; Rizzo, R.; Manfredini, S.; et al. Design, synthesis and evaluation of semi-synthetic triazole-containing caffeic acid analogues as 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 2015, 101, 573–583. [Google Scholar] [CrossRef]
- Cai, H.; Huang, X.; Xu, S.; Shen, H.; Zhang, P.; Huang, Y.; Jiang, J.; Sun, Y.; Jiang, B.; Wu, X.; et al. Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur. J. Med. Chem. 2016, 108, 89–103. [Google Scholar] [CrossRef]
- Bhagat, K.; Vir Singh, J.; Sharma, A.; Kaur, A.; Kumar, N.; Gulati, H.K.; Singh, A.; Singh, H.; Bedi, P.M.S. Novel series of triazole containing coumarin and isatin based hybrid molecules as acetylcholinesterase inhibitors. J. Mol. Struct. 2021, 1245, 131085. [Google Scholar] [CrossRef]
- El Malaha, T.; Farag, H.; Hemdanc, B.A.; Mageida, R.E.A.; Abdelrahmand, M.T.; El-Manawatye, M.A.; Nour, H.F. Synthesis, in vitro antimicrobial evaluation, and molecular docking studies of new isatin-1,2,3-triazole hybrids. J. Mol. Struct. 2022, 1250, 131855. [Google Scholar] [CrossRef]
- Basappa, V.C.; Kameshwar, V.H.; Kumara, K.; Kumar Achutha, D.; Krishnappagowda, L.N.; Kariyappa, A.K. Design and synthesis of coumarin-triazole hybrids: Biocompatible anti-diabetic agents, in silico molecular docking and ADME screening. Heliyon 2020, 6, e05290. [Google Scholar] [CrossRef]
- Kummari, B.; Polkam, N.; Ramesh, P.; Anantaraju, H.; Yogeeswari, P.; Anireddy, J.S.; Guggilapud, S.D.; Babu, B.N. Design and synthesis of 1,2,3-triazole–etodolac hybrids as potent anticancer molecules. RSC Adv. 2017, 7, 23680–23686. [Google Scholar] [CrossRef]
- Samuelsson, B.; Dahlén, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef]
- Steinhilber, D.; Hofmann, B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin. Pharmacol. Toxicol. 2014, 114, 70–77. [Google Scholar] [CrossRef]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and Biological Evaluation of the 1,5-Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)-3- (trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef]
- Wong, M.K.S. Angiotensin Converting Enzymes. In Handbook of Hormones; Academic Press: Cambridge, MA, USA, 2016; pp. 263–265, e29D-1–e29D-4. [Google Scholar] [CrossRef]
- Broeck, A.V.; Lotz, C.; Drillien, R.; Haas, L.; Bedez, C.; Lamour, V. Structural basis for allosteric regulation of Human Topoisomerase IIα. Nat. Commun. 2021, 12, 2962. [Google Scholar] [CrossRef]
- Honore, S.; Pasquier, E.; Braguer, D. Understanding microtubule dynamics for improved cancer therapy. Cell. Mol. Life Sci. 2005, 62, 3039–3056. [Google Scholar] [CrossRef]
- da Silva, V.B.; de Andrade, P.; Kawano, D.F.; Morais, P.A.B.; de Almeida, J.R.; Carvalho, I.; Taft, C.A.; de Paula da Silva, C.H.T. In silico design and search for acetylcholinesterase inhibitors in Alzheimer’s disease with a suitable pharmacokinetic profile and low toxicity. Future Med. Chem. 2011, 3, 947–960. [Google Scholar] [CrossRef]
- Pederick, J.L.; Thompson, A.P.; Bell, S.G.; Bruning, J.B. d-Alanine–d-alanine ligase as a model for the activation of ATP-grasp enzymes by monovalent cations. J. Biol. Chem. 2020, 295, 7894–7904. [Google Scholar] [CrossRef]
- Calandra, P.; Mandanici, A.; Liveri, V.T. Self-assembly in surfactant-based mixtures driven by acid-base reactions: Bis(2-ethylhexyl) phosphoric acid-n-octylamine systems. RSC Adv. 2013, 3, 5148–5155. [Google Scholar] [CrossRef]
- Lawson, D.M.; Williams, C.E.; Mitchenall, L.A.; Pau, R.N. Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: The 1.2 A resolution crystal structure of Azotobacter vinelandii ModA. Structure 1998, 6, 1529–1539. [Google Scholar] [CrossRef]
- Biessen, E.A.; Bakkeren, H.F.; Beuting, D.M.; Kuiper, J.; Van Berkel, T.J. Ligand size is a major determinant of high-affinity binding of fucose- and galactose-exposing (lipo)proteins by the hepatic fucose receptor. Biochem. J. 1994, 299, 291–296. [Google Scholar] [CrossRef]
- Caron, G.; Digiesi, V.; Solaro, S.; Ermondi, G. Flexibility in early drug discovery: Focus on the beyond-Rule-of-5 chemical space. Drug Discov. Today 2020, 25, 621–627. [Google Scholar] [CrossRef]
- Patil, P.C.; Tan, J.; Demuth, D.R.; Luzzio, F.A. ‘Second-generation’ 1,2,3-triazole-based inhibitors of Porphyromonas gingivalis adherence to oral streptococci and biofilm formation. MedChemComm 2019, 10, 268–279. [Google Scholar] [CrossRef]
- Sevaille, L.; Gavara, L.; Bebrone, C.; De Luca, F.; Nauton, L.; Achard, M.; Mercuri, P.; Tanfoni, S.; Borgianni, L.; Guyon, C.; et al. 1,2,4-Triazole-3-thione Compounds as Inhibitors of Dizinc Metallo-β-lactamases. ChemMedChem 2017, 12, 972–985. [Google Scholar] [CrossRef]
- Fedenko, V.S.; Landi, M.; Shemet, S.A. Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions. Int. J. Mol. Sci. 2022, 23, 11370. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comp. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).