Study on the Antitumor Mechanism of Tanshinone IIA In Vivo and In Vitro through the Regulation of PERK-ATF4-HSPA5 Pathway-Mediated Ferroptosis
Abstract
1. Introduction
2. Results
2.1. Tanshinone IIA Inhibited the Proliferation and Migration of HepG2 Cells
2.2. Analysis of the Mechanisms and Molecular Targets of TIIA Treatment of Hepatocellular Carcinoma
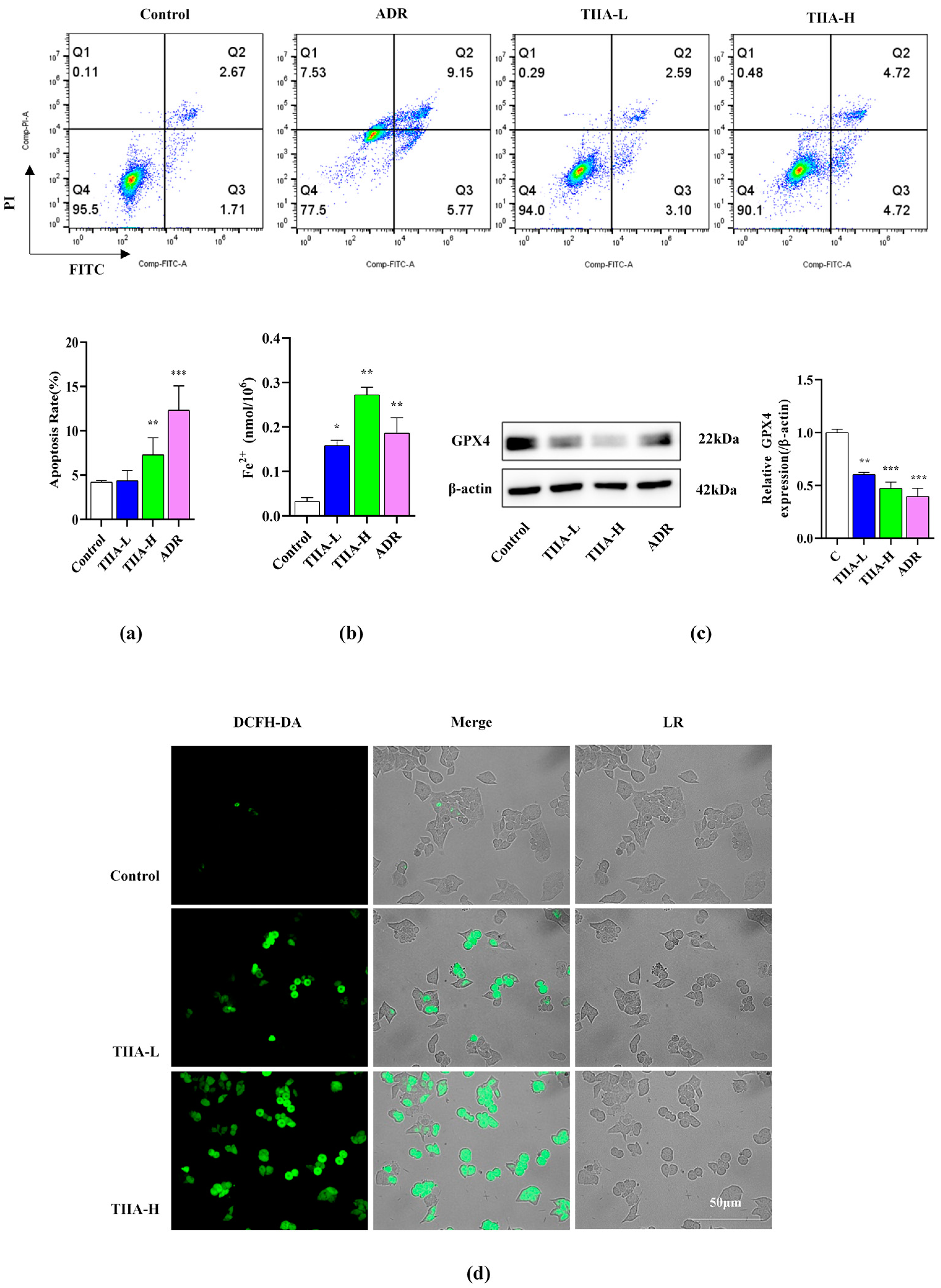
2.3. Tanshinone IIA-Promoted Ferroptosis of HepG2 Cells
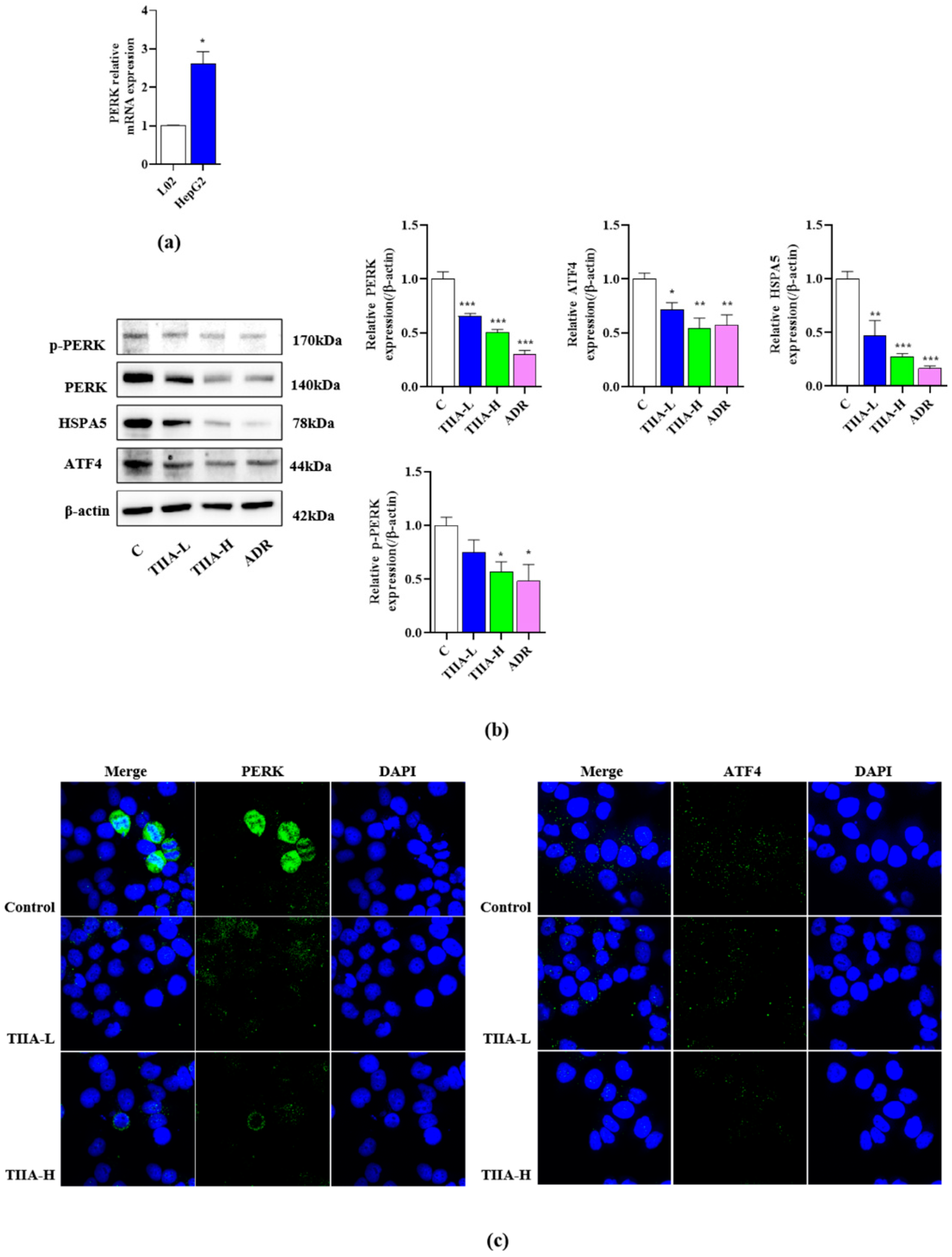
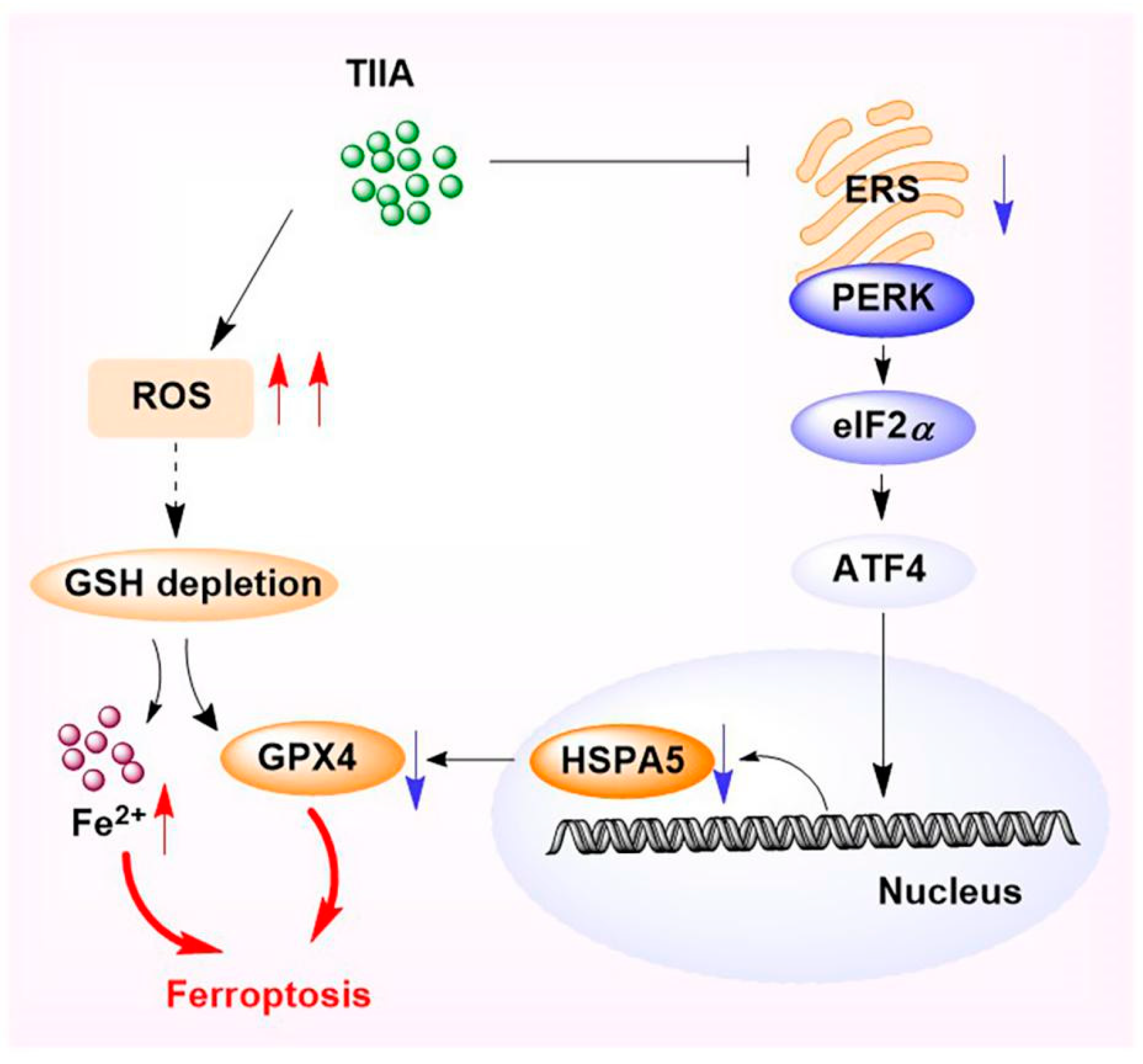
2.4. Inhibition of the Activation of the PERK-ATF4-HSPA5 Signaling Pathway in HepG2 Cells
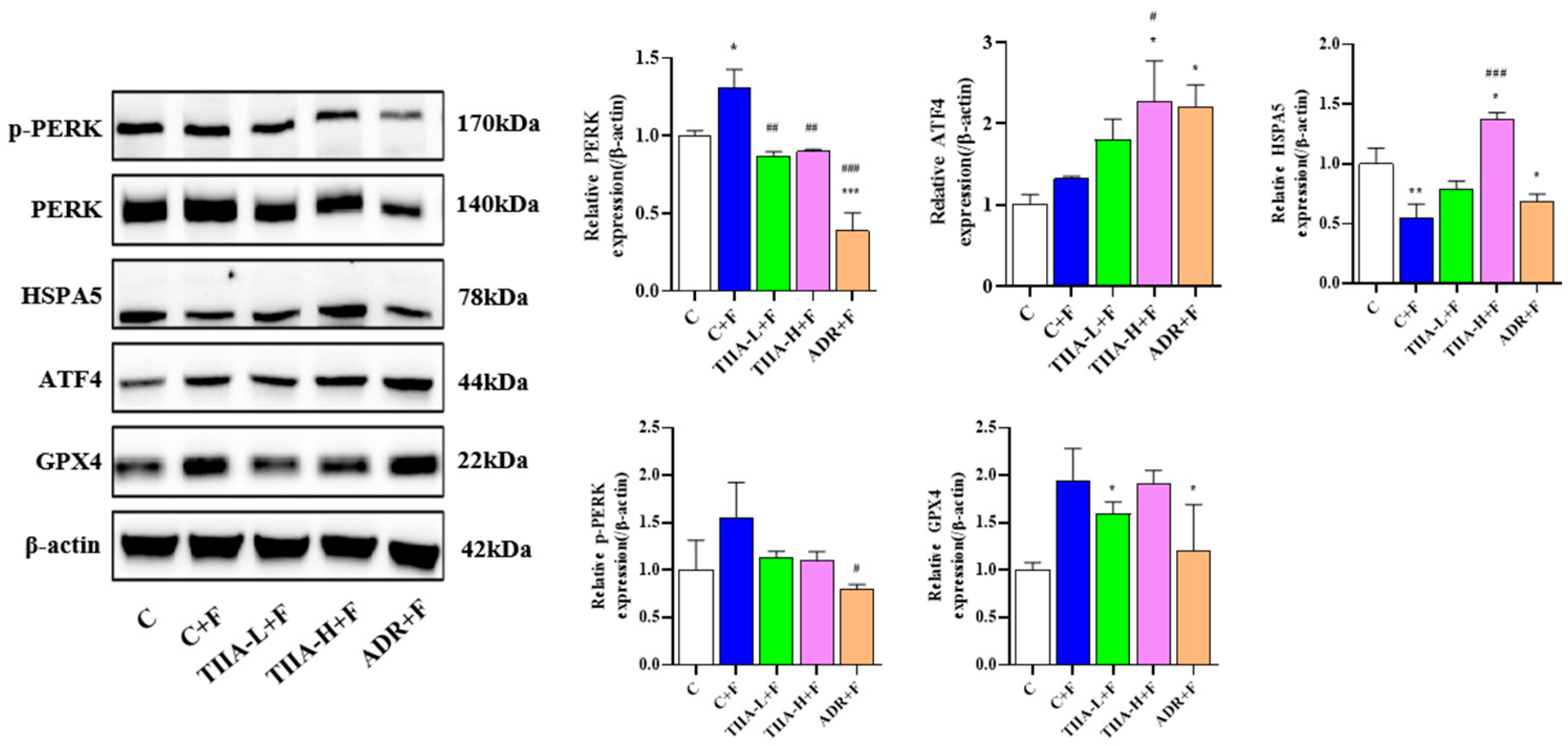
2.5. Docking and Cellular Thermal Shift Assay
2.6. Tanshinone IIA Inhibited Tumor Growth in H22 Tumor-Bearing Mice
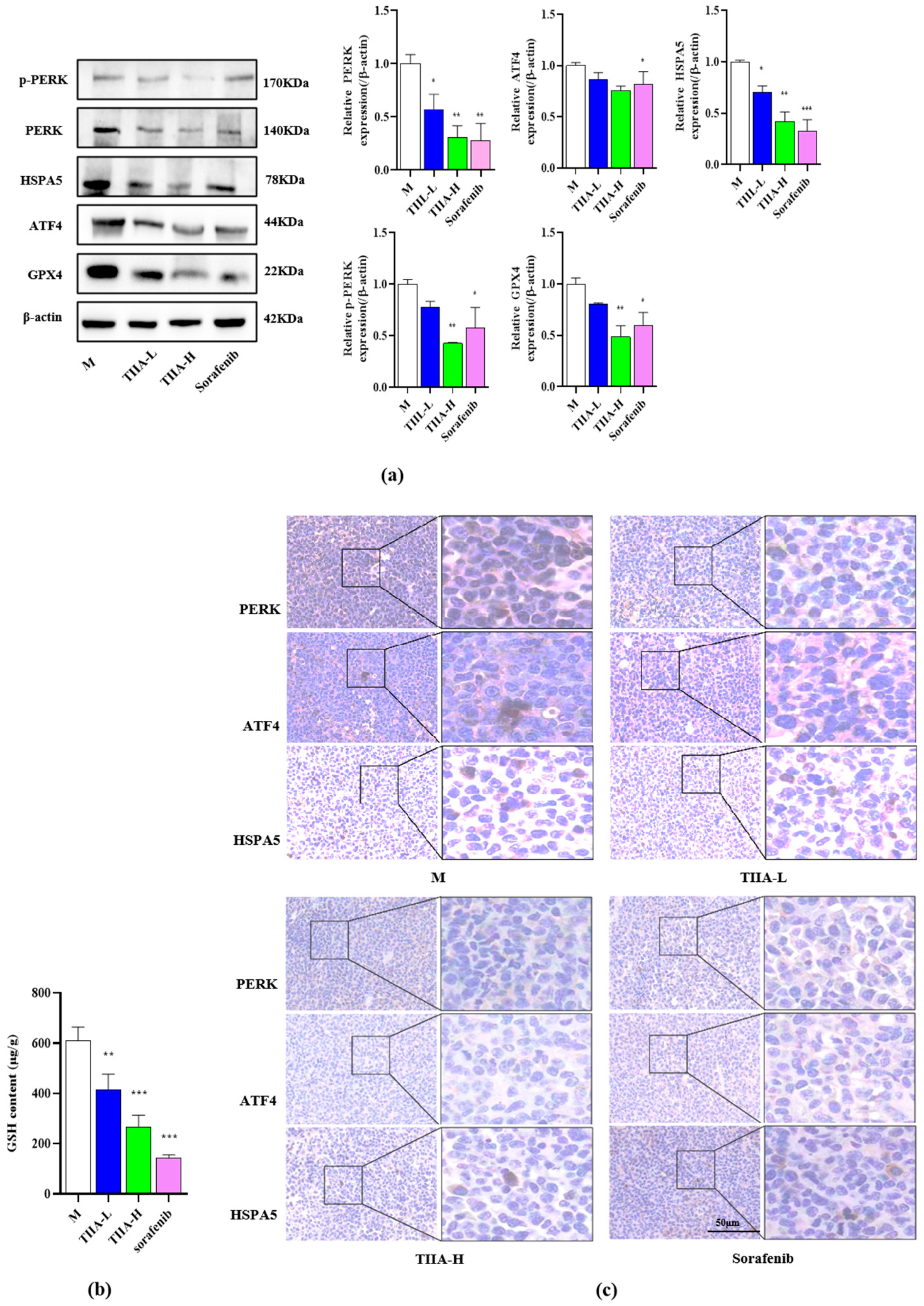
2.7. Tanshinone IIA Inhibited the PERK-ATF4-HSPA5 Signaling Pathway In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Network Pharmacology
4.3. Cells and Animal Model
4.4. Cell Viability Assay
4.5. Cell Cloning
4.6. Scratch and Transwell Testing
4.7. Annexin V/PI Stain
4.8. Real-Time Quantitative PCR Analysis
4.9. Western Blot
4.10. Fluorescence Staining1
4.11. Reactive Oxygen Species Assay
4.12. Molecular Docking
4.13. Cellular Thermal Shift Assay
4.14. Enzyme-Linked Immunosorbent Assay
4.15. Glutathione Assay Kit
4.16. H&E Staining
4.17. Immunohistochemical Analysis
4.18. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.A.-O.; Kelley, R.K.; Villanueva, A.A.-O.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.A.-O.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma A Review. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef]
- Dara, L.; Ji, C.; Kaplowitz, N. The Contribution of Endoplasmic Reticulum Stress to Liver Diseases. Hepatology 2011, 53, 1752–1763. [Google Scholar] [CrossRef]
- Lan, T.; Wang, W.; Zeng, X.X.; Tong, Y.H.; Mao, Z.J.; Wang, S.W. Saikosaponin A triggers cell ferroptosis in hepatocellular carcinoma by inducing endoplasmic reticulum stress-stimulated ATF3 expression. Biochem. Biophys. Res. Commun. 2023, 674, 10–18. [Google Scholar] [CrossRef]
- He, F.; Zhang, P.; Liu, J.L.; Wang, R.L.; Kaufman, R.J.; Yaden, B.C.; Karin, M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. 2023, 79, 362–377. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Sun, S.N.; Yi, Y.; Xiao, Z.X.J.; Chen, H. ER stress activates TAp73α to promote colon cancer cell apoptosis via the PERK-ATF4 pathway. J. Cancer 2023, 14, 1946–1955. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Q.H.; Sun, X.F.; Zeh, H.J.; Lotze, M.T.; Kang, R.; Tang, D.L. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017, 77, 2064–2077. [Google Scholar] [CrossRef]
- MEIm, X.D.; Cao, Y.F.; Che, Y.Y.; Li, J.; Shang, Z.P.; Zhao, W.J.; Qiao, Y.J.; Zhang, J.Y. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.M.; Han, H.; Zhang, Y.; Liu, J.L.; Yu, J.Y.; Liu, H.M.; Liu, X.T.; Shan, H.; Wu, S.C. Tanshinone IIA protected against lipopolysaccharide-induced brain injury through the protective effect of the blood-brain barrier and the suppression of oxidant stress and inflammatory response. Food Funct. 2022, 13, 8304–8312. [Google Scholar] [CrossRef]
- Yuan, L.M.; Li, Q.H.; Zhang, Z.G.; Liu, Q.L.; Wang, X.C.; Fan, L.H. Tanshinone IIA inhibits the adipogenesis and inflammatory response in ox-LDL-challenged human monocyte-derived macrophages via regulating miR-130b/WNT5A. J. Cell. Biochem. 2020, 121, 1400–1408. [Google Scholar] [CrossRef]
- Tan, Y.L.; Ou, H.X.; Zhang, M.; Gong, D.; Zhao, Z.W.; Chen, L.Y.; Xia, X.D.; Mo, Z.C.; Tang, C.K. Tanshinone IIA Promotes Macrophage Cholesterol Efflux and Attenuates Atherosclerosis of apoE-/- Mice by Omentin-1/ABCA1 Pathway. Curr. Pharm. Biotechnol. 2019, 20, 422–432. [Google Scholar] [CrossRef]
- Chang, C.C.; Chu, C.F.; Wang, C.N.; Wu, H.T.; Bi, K.W.; Pang, J.H.S.; Huang, S.T. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 2014, 21, 207–216. [Google Scholar] [CrossRef]
- Sui, H.; Zhao, J.H.; Zhou, L.H.; Wen, H.T.; Deng, W.L.; Li, C.P.; Ji, Q.; Liu, X.; Feng, Y.Y.; Chai, N.; et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017, 403, 86–97. [Google Scholar] [CrossRef]
- Qian, J.; Cao, Y.; Zhang, J.F.; Li, L.C.; Wu, J.; Yu, J.L.; Huo, J.G. Tanshinone IIA Alleviates the Biological Characteristics of Colorectal Cancer via Activating the ROS/JNK Signaling Pathway. Anti-Cancer Agents Med. Chem. 2023, 23, 227–236. [Google Scholar]
- Wang, Y.X.; Wu, H.J.; Niu, W.; Chen, J.; Liu, M.L.; Sun, X.; Li, Z.C. Tanshinone IIA attenuates paraquat-induced acute lung injury by modulating angiotensin-converting enzyme 2/angiotensin-(1-7) in rats. Mol. Med. Rep. 2018, 18, 2955–2962. [Google Scholar] [CrossRef]
- Yu, W.C.; Yu, Y.; Wang, C.; Zhang, Z.J.; Xue, Z.H. Mechanism by which salt stress induces physiological responses and regulates tanshinone synthesis. Plant Physiol. Biochem. 2021, 164, 10–20. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Lin, R.; Yuan, B.X.; Yang, G.D.; Liu, Y.; Zhang, H. Tanshinone IIA downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbit. J. Ethnopharmacol. 2008, 115, 217–222. [Google Scholar] [CrossRef]
- Fu, K.Q.; Feng, C.; Shao, L.Z.; Mei, L.; Cao, R.F. Tanshinone IIA exhibits anti-inflammatory and antioxidative effects in LPS-stimulated bovine endometrial epithelial cells by activating the Nrf2 signaling pathway. Res. Vet. Sci. 2021, 136, 220–226. [Google Scholar] [CrossRef]
- Chiu, T.L.; Su, C.C. Tanshinone IIA increases protein expression levels of PERK, ATF6, IRE1α, CHOP, caspase-3 and caspase-12 in pancreatic cancer BxPC-3 cell-derived xenograft tumors. Mol. Med. Rep. 2017, 15, 3259–3263. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Sun, J.; Shen, J.; Liu, F.; Ning, B.; Lu, Z.; Wei, L.; Jiang, X. Tanshinone IIA alleviates NLRP3 inflammasome-mediated pyroptosis in Mycobacterium tuberculosis-(H37Ra-) infected macrophages by inhibiting endoplasmic reticulum stress. J. Ethnopharmacol. 2022, 282, 114595. [Google Scholar] [CrossRef]
- He, Y.; Ruganzu, J.B.; Lin, C.; Ding, B.; Zheng, Q.; Wu, X.; Ma, R.; Liu, Q.; Wang, Y.; Jin, H.; et al. Tanshinone IIA ameliorates cognitive deficits by inhibiting endoplasmic reticulum stress-induced apoptosis in APP/PS1 transgenic mice. Neurochem. Int. 2020, 133, 104610. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, J.; Li, X.; Dong, N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci. Rep. 2020, 40, BSR20201807. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Kim, S.; Woo, C.H. Laminar Flow Inhibits ER Stress-Induced Endothelial Apoptosis through PI3K/Akt-Dependent Signaling Pathway. Mol. Cells 2018, 41, 964–970. [Google Scholar]
- Yan, Y.; Huang, J.; Huan, C.; Li, L.; Li, C. Non-Esterified Fatty Acid Induces ER Stress-Mediated Apoptosis via ROS/MAPK Signaling Pathway in Bovine Mammary Epithelial Cells. Metabolites 2022, 12, 803. [Google Scholar] [CrossRef]
- Wang, S.S.; Sun, L.F.; Zhu, Z.J.; Liu, J.Y.; Ge, W.; Li, B.Y.; Wang, B. Cryptotanshinone alleviates myocardial ischemia and reperfusion injury in rats to mitigate ER stress-dependent apoptosis by modulating the JAK1/STAT3 axis. Am. J. Transl. Res. 2022, 14, 5024–5039. [Google Scholar]
- Alannan, M.; Fayyad-Kazan, H.; Trézéguet, V.; Merched, A. Targeting Lipid Metabolism in Liver Cancer. Biochemistry 2020, 59, 3951–3964. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Gao, S.Q.; Lei, M.; Li, Z.N. Hesperidin suppresses ERS-induced inflammation in the pathogenesis of non-alcoholic fatty liver disease. Aging 2022, 14, 1265–1279. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.Y.; Xiong, Y.H.; Fang, B.; Lin, X.; Huang, Q.F. Didymin Ameliorates Liver Fibrosis by Alleviating Endoplasmic Reticulum Stress and Glycerophospholipid Metabolism: Based on Transcriptomics and Metabolomics. Drug Des. Dev. Ther. 2022, 16, 1713–1729. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Kaplowitz, N.; Lebeaupin, C.; Kroemer, G.; Kaufman, R.J.; Malhi, H.A.-O.; Ren, J.A.-O. Endoplasmic reticulum stress in liver diseases. Hepatology 2023, 77, 619–639. [Google Scholar] [CrossRef]
- Nakagawa, H.; Umemura, A.; Taniguchi, K.; Font-Burgada, J.; Dhar, D.; Ogata, H.; Zhong, Z.; Valasek, M.A.; Seki, E.; Hidalgo, J.; et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 2014, 26, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Mi, Y.J.; Zhang, X.F.; Ma, Q.; Song, Y.C.; Zhang, L.W.; Wang, D.D.; Xing, J.L.; Hou, B.X.; Li, H.L.; et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 402. [Google Scholar] [CrossRef]
- Jiang, Y.; Bi, Y.; Zhou, L.; Zheng, S.; Jian, T.; Chen, J. Tanshinone IIA inhibits proliferation and migration by downregulation of the PI3K/Akt pathway in small cell lung cancer cells. BMC Complement. Med. Ther. 2024, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tang, H.; Wang, G.; Li, P.; Song, Z.; Li, W.; Sun, X.; Zhong, X.; Yu, Q.; Zhu, S.; et al. Targeting survivin with Tanshinone IIA inhibits tumor growth and overcomes chemoresistance in colorectal cancer. Cell Death Discov. 2023, 9, 351. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Huang, J.; Jiang, S.; Qin, Z.; Cao, L.; Tan, G.; Zhao, Z.; Huang, M.; Jin, J.A.-O. Tanshinone IIA reverses gefitinib resistance in EGFR-mutant lung cancer via inhibition of SREBP1-mediated lipogenesis. Phytother. Res. 2024, 38, 1574–1588. [Google Scholar] [CrossRef]
- Shen, L.; Lou, Z.; Zhang, G.; Xu, G.; Zhang, G. Diterpenoid Tanshinones, the extract from Danshen (Radix Salviae Miltiorrhizae) induced apoptosis in nine human cancer cell lines. J. Tradit. Chin. Med. 2016, 36, 514–521. [Google Scholar]
- Pinto, B.A.S.; França, L.M.; Laurindo, F.R.M.; Paes, A.M.A. Unfolded Protein Response: Cause or Consequence of Lipid and Lipoprotein Metabolism Disturbances? Adv. Exp. Med. Biol. 2019, 1127, 67–82. [Google Scholar] [PubMed]




| Target Gene | Primer Sequences (5′-3′) |
|---|---|
| PERK (forward) | GATCGCAGAGGCAGTGGAGTTTC |
| PERK (reverse) | TTGTCCTGTGTGTCTGGCATAAGC |
| GAPDH (forward) | TTGCCCTCAACGACCACTTT |
| GAPDH (reverse) | TGGTCCAGGGGTCTTACTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhao, W.; Wang, W.; Yao, Z.; Chen, W.; Feng, X. Study on the Antitumor Mechanism of Tanshinone IIA In Vivo and In Vitro through the Regulation of PERK-ATF4-HSPA5 Pathway-Mediated Ferroptosis. Molecules 2024, 29, 1557. https://doi.org/10.3390/molecules29071557
Guo C, Zhao W, Wang W, Yao Z, Chen W, Feng X. Study on the Antitumor Mechanism of Tanshinone IIA In Vivo and In Vitro through the Regulation of PERK-ATF4-HSPA5 Pathway-Mediated Ferroptosis. Molecules. 2024; 29(7):1557. https://doi.org/10.3390/molecules29071557
Chicago/Turabian StyleGuo, Chunxiang, Wei Zhao, Wei Wang, Zheng Yao, Wenhui Chen, and Xiaoyi Feng. 2024. "Study on the Antitumor Mechanism of Tanshinone IIA In Vivo and In Vitro through the Regulation of PERK-ATF4-HSPA5 Pathway-Mediated Ferroptosis" Molecules 29, no. 7: 1557. https://doi.org/10.3390/molecules29071557
APA StyleGuo, C., Zhao, W., Wang, W., Yao, Z., Chen, W., & Feng, X. (2024). Study on the Antitumor Mechanism of Tanshinone IIA In Vivo and In Vitro through the Regulation of PERK-ATF4-HSPA5 Pathway-Mediated Ferroptosis. Molecules, 29(7), 1557. https://doi.org/10.3390/molecules29071557





