Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Result
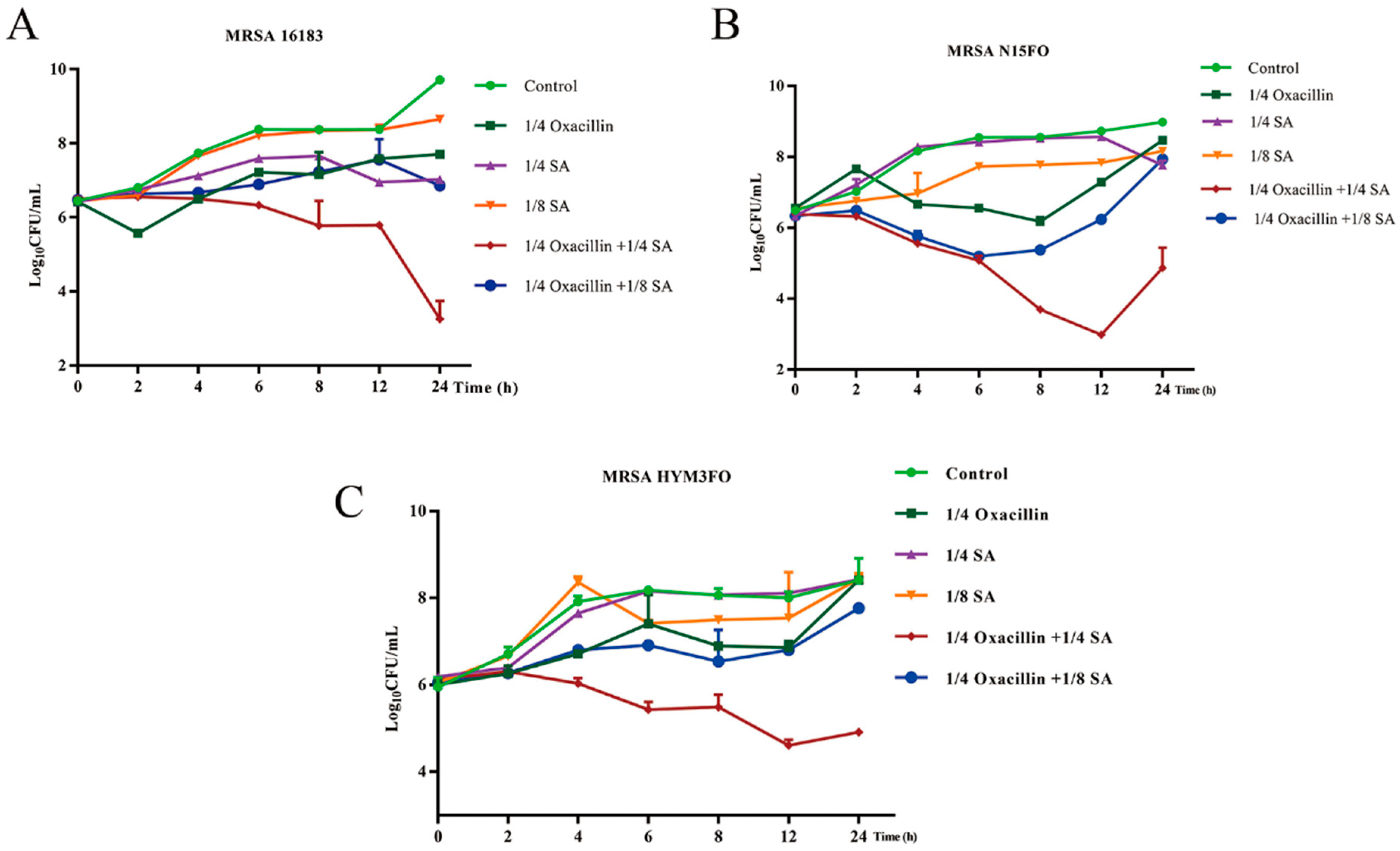
2.1. Drug Sensitivity Measurement Results
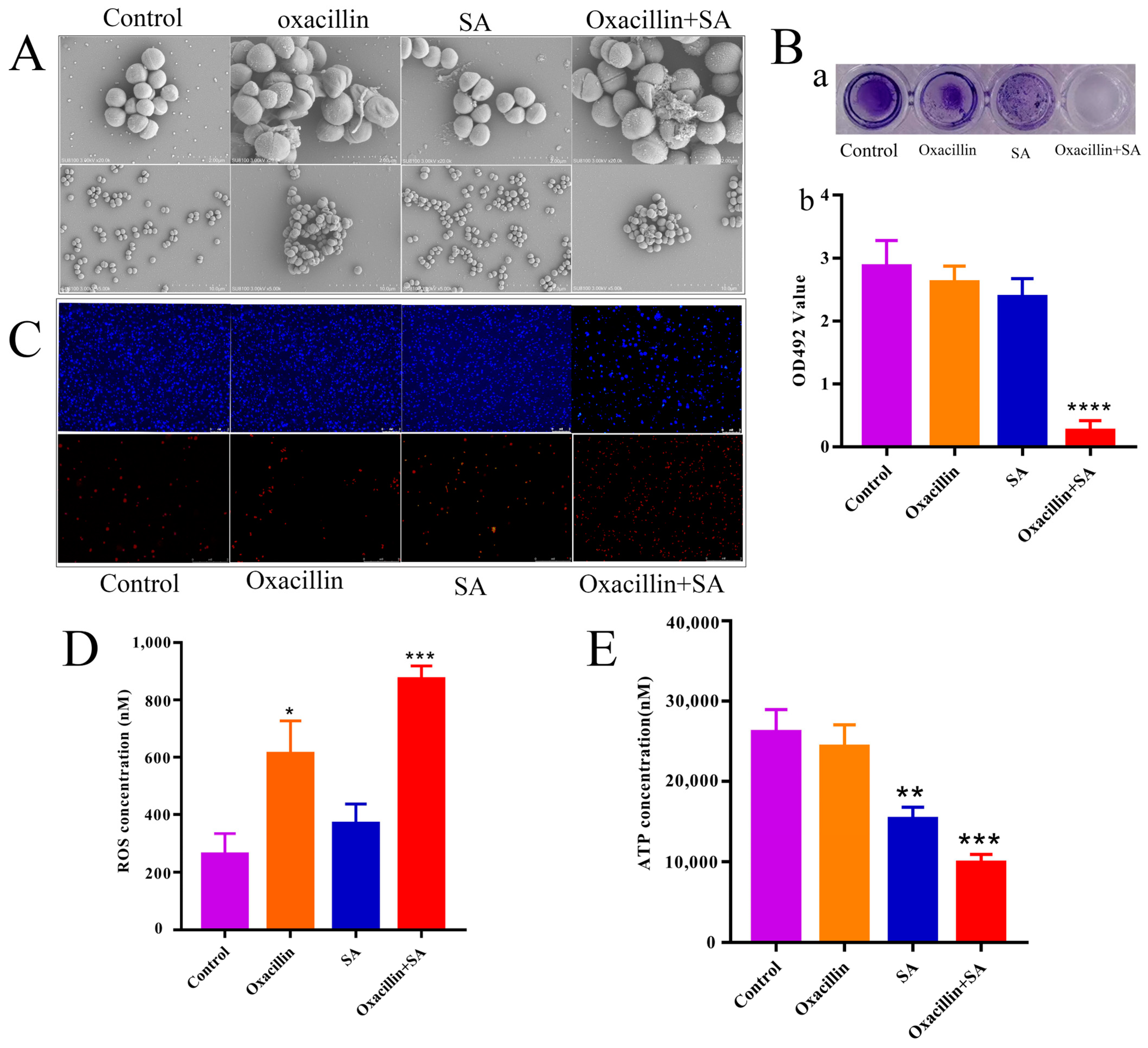
2.2. Effects on Cell Ultrastructure
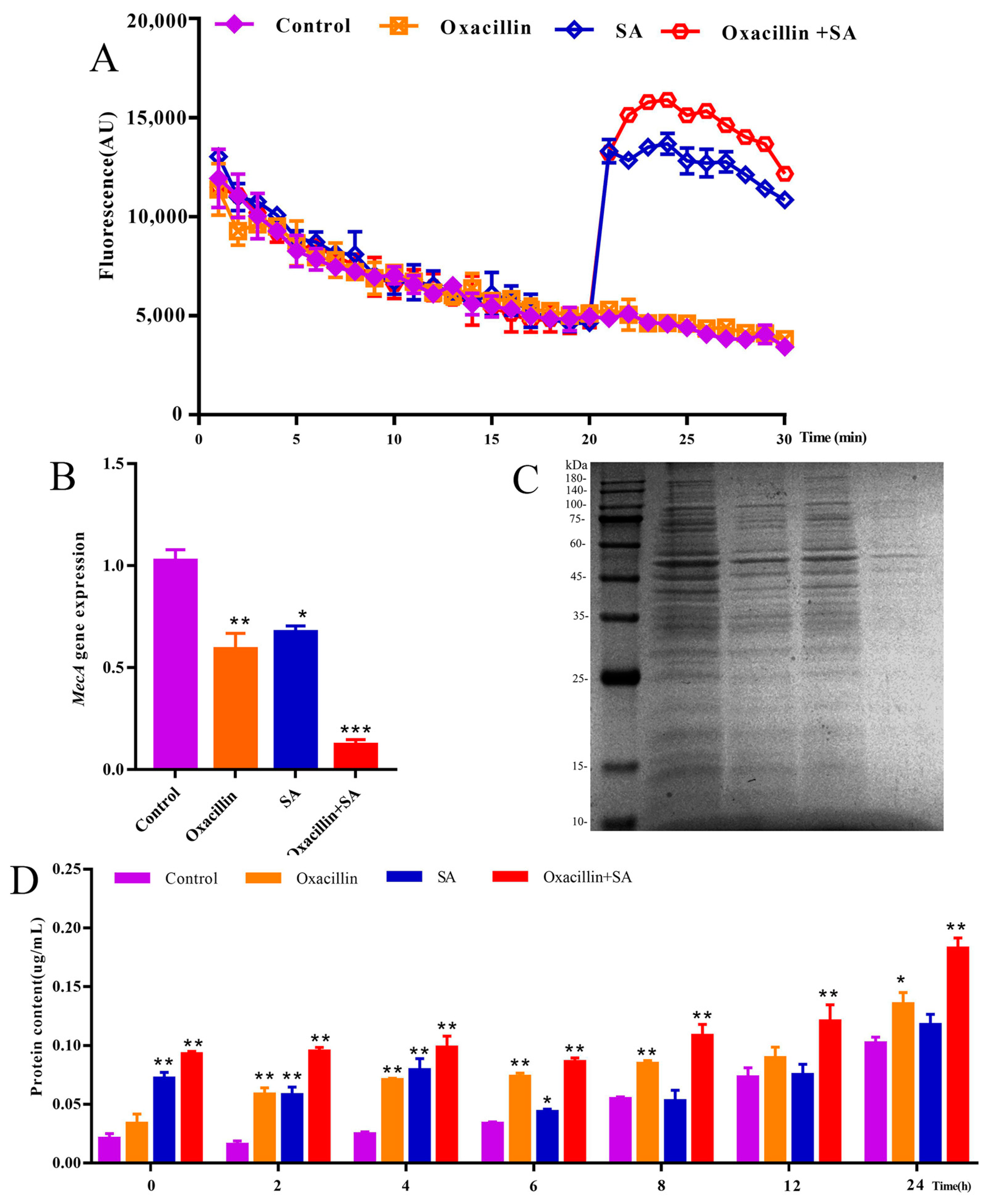
2.3. Effects of the MRSA Membrane and Expression of the mecA Gene
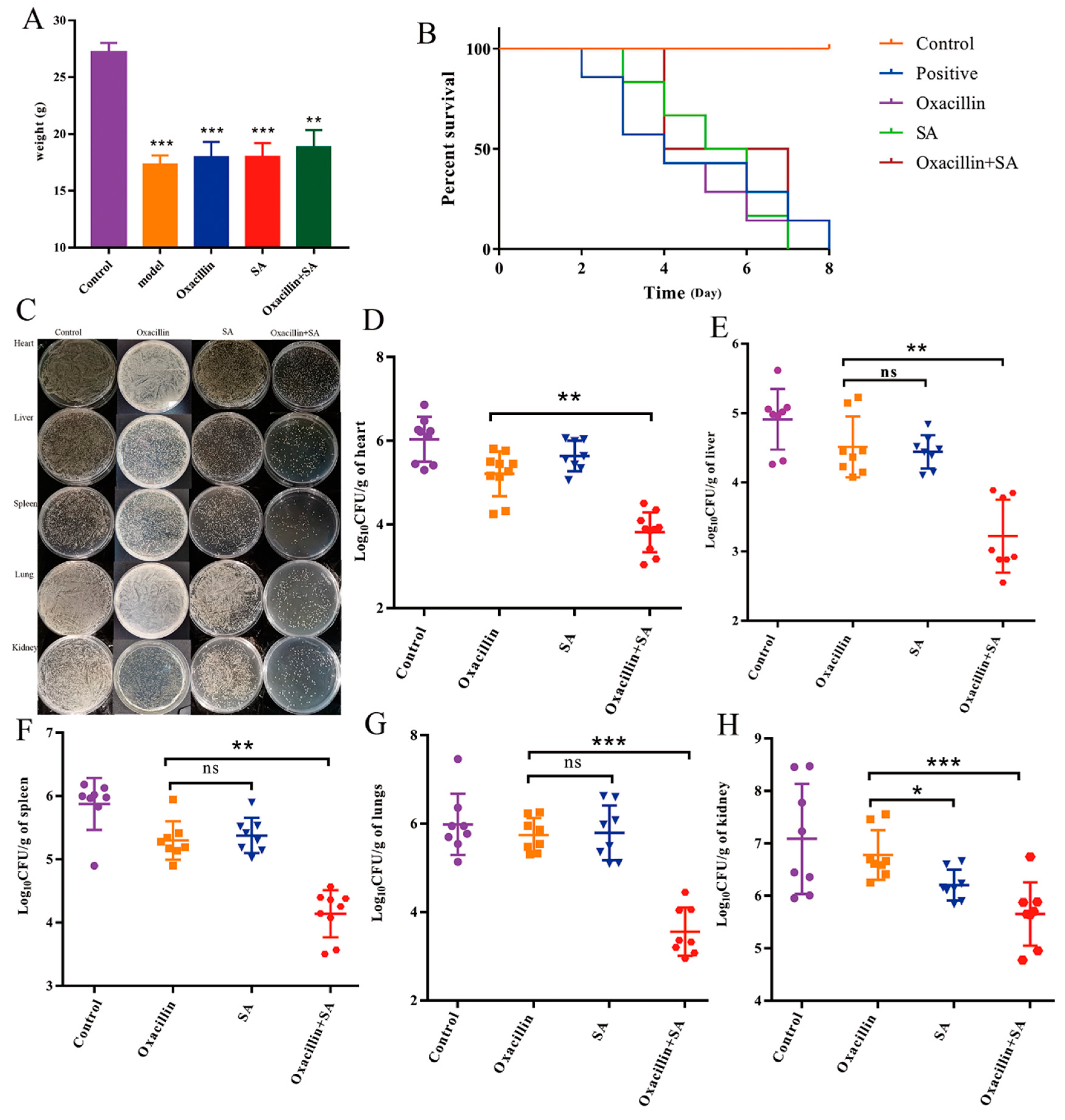
2.4. Oxacillin and SA Protect Mice from S. aureus Bacteremia
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Bacterial Strains
4.3. Minimal Inhibitory Concentration (MIC)
4.4. Synergy Assay
4.5. Time-Killing Growth Curve Assay
4.6. Scanning Electron Microscopy (SEM)
4.7. Live/Dead Bacterial Cell Viability Assay
4.8. Biofilm Formation Inhibition Assay
4.9. Reactive Oxygen Species (ROS) Measurement
4.10. Measurement of Extracellular–Intracellular ATP
4.11. mRNA Expression of Penicillin-Binding Protein (PBP)
4.12. Measurement of Protein Leakage
4.13. Membrane Permeability Assay
4.14. Murine Model of Bacteremia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.; Zeng, S.; Chen, Y.; Li, H.; Yoon, J. Prospects of coupled iron-based nanostructures in preclinical antibacterial therapy. Adv. Drug Deliv. Rev. 2023, 193, 114672. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Nunez-Garcia, J.; Kearns, A.M.; Doumith, M.; Butaye, P.R.; Argudín, M.A.; Lahuerta-Marin, A.; Pichon, B.; Abuoun, M.; Rogers, J.; et al. Livestock-associated methicillin resistant staphylococcus aureus (la-mrsa) clonal complex (cc) 398 isolated from uk animals belong to european lineages. Front. Microbiol. 2016, 7, 1741. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, H.; Pan, Y.; Zhai, Y.; Cai, T.; Yuan, X.; Gao, Y.; He, D.; Liu, J.; Yuan, L.; et al. Prevalence, resistance pattern, and molecular characterization of staphylococcus aureus isolates from healthy animals and sick populations in henan province, china. Gut Pathog. 2018, 10, 31. [Google Scholar] [CrossRef]
- Ferradas, C.; Cotter, C.; Shahbazian, J.H.; Iverson, S.A.; Baron; Misic, A.M.; Brazil, A.M.; Rankin, S.C.; Nachamkin, I.; Ferguson, J.M.; et al. Risk factors for antimicrobial resistance among staphylococcus isolated from pets living with a patient diagnosed with methicillin-resistant staphylococcus aureus infection. Zoonoses Public Health 2022, 69, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Founou, L.L.; Founou, R.C.; Allam, M.; Ismail, A.; Finyom, D.C.; Essack, S.Y. Genome analysis of methicillin-resistant staphylococcus aureus isolated from pigs: Detection of the clonal lineage st398 in cameroon and south africa. Zoonoses Public Health 2019, 66, 512–525. [Google Scholar] [CrossRef]
- Holten, M.C.; Andersson, M.; Rhod, L.A.; Petersen, A.; Mølbak, K.; Koch, A. Risk of hospitalization and death within 2 years after methicillin-resistant staphylococcus aureus (mrsa) diagnosis in persons colonized or infected with livestock and non-livestock-associated mrsa-a nationwide register-based cohort study. Zoonoses Public Health 2020, 67, 814–822. [Google Scholar] [CrossRef]
- Worthing, K.A.; Brown, J.; Gerber, L.; Trott, D.J.; Abraham, S.; Norris, J.M. Methicillin-resistant staphylococci amongst veterinary personnel, personnel-owned pets, patients and the hospital environment of two small animal veterinary hospitals. Vet. Microbiol. 2018, 223, 79–85. [Google Scholar] [CrossRef]
- Muzammil, I.; Ijaz, M.; Saleem, M.H.; Ali, M.M. Molecular characterization of vancomycin-resistant staphylococcus aureus isolated from bovine milk. Zoonoses Public Health 2023, 70, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Mekhloufi, O.A.; Chieffi, D.; Hammoudi, A.; Bensefia, S.A.; Fanelli, F.; Fusco, V. Prevalence, enterotoxigenic potential and antimicrobial resistance of staphylococcus aureus and methicillin-resistant staphylococcus aureus (mrsa) isolated from algerian ready to eat foods. Toxins 2021, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Miller, L.S. Host-pathogen interactions between the skin and staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 28–35. [Google Scholar] [CrossRef]
- Tascini, C.; Attanasio, V.; Ripa, M.; Carozza, A.; Pallotto, C.; Bernardo, M.; Francisci, D.; Oltolini, C.; Palmiero, G.; Scarpellini, P. Ceftobiprole for the treatment of infective endocarditis: A case series. J. Glob. Antimicrob. Resist. 2020, 20, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Kantecki, M.; Stone, G.G.; Hammond, J. Ceftaroline fosamil as a potential treatment option for staphylococcus aureus community-acquired pneumonia in adults. Int. J. Antimicrob. Agents 2019, 54, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Liu, F.; Zhu, K.; Shen, J.Z. Natural products that target virulence factors in antibiotic-resistant staphylococcus aureus. J. Agric. Food Chem. 2019, 67, 13195–13211. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Cohen, Y.; Poverenov, E.; Eltzov, E. Synergistic antimicrobial effect of the combination of beta-lactam antibiotics and chitosan derivative on multidrug-resistant bacteria. Int. J. Biol. Macromol. 2022, 223 Pt A, 1107–1114. [Google Scholar] [CrossRef]
- Roemer, T.; Schneider, T.; Pinho, M.G. Auxiliary factors: A chink in the armor of mrsa resistance to β-lactam antibiotics. Curr. Opin. Microbiol. 2013, 16, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, G.; Li, N.; Wang, X.; Kang, X.; Mao, Y.; Wang, G. Insights into the effects and mechanism of andrographolide-mediated recovery of susceptibility of methicillin-resistant staphylococcus aureus to β-lactam antibiotics. Microbiol. Spectr. 2023, 11, e297822. [Google Scholar] [CrossRef] [PubMed]
- Drawz, S.M.; Bonomo, R.A. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, e5. [Google Scholar]
- Lu, X.; Yang, X.; Li, X.; Lu, Y.; Ren, Z.; Zhao, L.; Hu, X.; Jiang, J.; You, X. In vitro activity of sodium new houttuyfonate alone and in combination with oxacillin or netilmicin against methicillin-resistant staphylococcus aureus. PLoS ONE 2013, 8, e68053. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Lee, W.X.; Basri, D.F.; Ghazali, A.R. Bactericidal effect of pterostilbene alone and in combination with gentamicin against human pathogenic bacteria. Molecules 2017, 22, 463. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.X.; Durham, D.G.; Richards, R.M. Baicalin synergy with beta-lactam antibiotics against methicillin-resistant staphylococcus aureus and other beta-lactam-resistant strains of S. Aureus. J. Pharm. Pharmacol. 2000, 52, 361–366. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, M.L.; Marqui, D.A.A.; Domingos, T.F.; Ortiz-Ramirez, F.; Cavalcanti, D.N.; Teixeira, V.L.; Fuly, A.L. Antiplatelet and anticoagulant effects of diterpenes isolated from the marine alga, dictyota menstrualis. Mar. Drugs 2014, 12, 2471–2484. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Zhang, L.; Liu, B.; Li, L.; Zhang, Y.; Zhao, H.; Ji, X.; Chen, Q.; Hu, M.; Bai, J.; et al. Synergistic effects of anti-mrsa herbal extracts combined with antibiotics. Future Microbiol. 2020, 15, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Rhee, M.H.; Yun, B.S.; Lim, Y.H.; Song, H.G.; Shin, K.S. Synergistic anti-bacterial effects of phellinus baumii ethyl acetate extracts and β-lactam antimicrobial agents against methicillin-resistant staphylococcus aureus. Ann. Lab. Med. 2016, 36, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kuok, C.F.; Hoi, S.O.; Hoi, C.F.; Chan, C.H.; Fong, I.H.; Ngok, C.K.; Meng, L.R.; Fong, P. Synergistic antibacterial effects of herbal extracts and antibiotics on methicillin-resistant staphylococcus aureus: A computational and experimental study. Exp. Biol. Med. 2017, 242, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Kirubakari, B.; Chen, Y.; Sasidharan, S. Synergistic effect of polyalthia longifolia leaf and antibiotics against clinical isolates of methicillin-resistant staphylococcus aureus (mrsa) by microscopic technique. Anti Inflamm. Anti Allergy Agents Med. Chem. 2020, 19, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Akilandeswari, K.; Ruckmani, K. Synergistic antibacterial effect of apigenin with β-lactam antibiotics and modulation of bacterial resistance by a possible membrane effect against methicillin resistant staphylococcus aureus. Cell. Mol. Biol. 2016, 62, 74–82. [Google Scholar] [CrossRef]
- Mun, S.H.; Lee, Y.S.; Han, S.H.; Lee, S.W.; Cha, S.W.; Kim, S.B.; Seo, Y.S.; Kong, R.; Kang, D.H.; Shin, D.W.; et al. In vitro potential effect of morin in the combination with β-lactam antibiotics against methicillin-resistant staphylococcus aureus. Foodborne Pathog. Dis. 2015, 12, 545–550. [Google Scholar] [CrossRef]
- Chen, X.B.; Wang, Z.L.; Yang, Q.Y.; Zhao, F.Y.; Qin, X.L.; Tang, X.E.; JL, D.; Chen, Z.H.; Zhang, K.; Huang, F.J. Diosgenin glucoside protects against spinal cord injury by regulating autophagy and alleviating apoptosis. Int. J. Mol. Sci. 2018, 19, 2274. [Google Scholar] [CrossRef]
- Bai, J.; Wu, Y.; Liu, X.; Zhong, K.; Huang, Y.; Gao, H. Antibacterial activity of shikimic acid from pine needles of cedrus deodara against staphylococcus aureus through damage to cell membrane. Int. J. Mol. Sci. 2015, 16, 27145–27155. [Google Scholar] [CrossRef] [PubMed]
- Dyrda-Terniuk, T.; Sugajski, M.; Pryshchepa, O.; Śliwiak, J.; Buszewska-Forajta, M.; Pomastowski, P.; Buszewski, B. The study of protein-cyclitol interactions. Int. J. Mol. Sci. 2022, 23, 2940. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.J.; Kim, S.H. Hypolipogenic effect of shikimic acid via inhibition of mid1ip1 and phosphorylation of ampk/acc. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef] [PubMed]
- Shiu, W.K.; Gibbons, S. Anti-staphylococcal acylphloroglucinols from hypericum beanii. Phytochemistry 2006, 67, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- ENishio, K.; Ribeiro, J.M.; Oliveira, A.G.; Andrade, C.G.; Proni, E.A.; Kobayashi, R.K.; Nakazato, G. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata lepeletier, 1836, and S. Postica latreille, 1807. Sci. Rep. 2016, 6, 21641. [Google Scholar]
- Sun, X.; Shang, J.; Wu, A.; Xia, J.; Xu, F. Identification of dynamic signatures associated with smoking-related squamous cell lung cancer and chronic obstructive pulmonary disease. J. Cell. Mol. Med. 2020, 24, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Di, K.N.; Tay, S.T.; Ponnampalavanar, S.S.S.; Pham, D.T.; Wong, L.P. Socio-demographic factors associated with antibiotics and antibiotic resistance knowledge and practices in vietnam: A cross-sectional survey. Antibiotics 2022, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lyu, X.; Guo, X.; Yang, H.; Duan, L.; Zhu, H.; Pan, H.; Gong, F.; Wang, L. Distinct ampk-mediated fas/hsl pathway is implicated in the alleviating effect of nuciferine on obesity and hepatic steatosis in hfd-fed mice. Nutrients 2022, 14, 1898. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.Y.; Liou, J.M.; Cheng, W.C.; Hsu, J.T.; Hsu, T.L.; Wu, M.S.; Wong, C.H. Combating multidrug-resistant helicobacter pylori with moenomycin a in combination with clarithromycin or metronidazole. Front. Chem. 2022, 10, 897578. [Google Scholar] [CrossRef]
- Shang, Y.; Guo, J.; Zhao, Y.; Chen, J.; Meng, Q.; Qu, D.; Zheng, J.; Yu, Z.; Wu, Y.; Deng, Q. Clemastine inhibits the biofilm and hemolytic of staphylococcus aureus through the gdpp protein. Microbiol. Spectr. 2022, 10, e54121. [Google Scholar] [CrossRef]
- Ljoljić, B.V.; Gašić, U.M.; Milojković-Opsenica, D.; Rimac, H.; Vuković, R.J.; Vlainić, J.; Brlek-Gorski, D.; Kosalec, I. Antibacterial fractions from erodium cicutarium exposed-clinical strains of staphylococcus aureus in focus. Antibiotics 2022, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Nadafi, R.; Koning, J.J.; Veninga, H.; Stachtea, X.N.; Konijn, T.; Zwiers, A.; Malmström, A.; den Haan, J.; Mebius, R.E.; Maccarana, M.; et al. Dendritic cell migration to skin-draining lymph nodes is controlled by dermatan sulfate and determines adaptive immunity magnitude. Front. Immunol. 2018, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Liu, S.; Yu, Y.; Guo, L.; Wang, Y.; Feng, Y.; Fei, P.; Kang, H.; Ali, M.A. Antibacterial mode of eucommia ulmoides male flower extract against staphylococcus aureus and its application as a natural preservative in cooked beef. Front. Microbiol. 2022, 13, 846622. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Shaukat, I.; Rajput, S.A.; Shukat, R.; Hanif, S.; Huang, S.; Aleem, M.T.; Li, K.; Li, Q.; Chen, C.; et al. Icariin alleviates escherichia coli lipopolysaccharide-mediated endometritis in mice by inhibiting inflammation and oxidative stress. Int. J. Mol. Sci. 2022, 23, 10219. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chu, Y.; Chen, H.; Li, X.; Wu, Q.; Jin, L.; Wang, G.; Huang, J. Rice transcription factor osmads25 modulates root growth and confers salinity tolerance via the aba-mediated regulatory pathway and ros scavenging. PLoS Genet. 2018, 14, e1007662. [Google Scholar] [CrossRef]
- Fu, Y.; Jin, Y.; Shan, A.; Zhang, J.; Tang, H.; Shen, J.; Zhou, C.; Yu, H.; Fang, H.; Zhao, Y.; et al. Polydatin protects bovine mammary epithelial cells against zearalenone-induced apoptosis by inhibiting oxidative responses and endoplasmic reticulum stress. Toxins 2021, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Lu, P.; Liu, S.; Zhang, S.; Gong, Z.; Cai, X.; Zhou, B.; Lin, Q.; Liu, J. Disruption of the chitin biosynthetic pathway results in significant changes in the cell growth phenotypes and biosynthesis of secondary metabolites of monascus purpureus. J. Fungi 2022, 8, 910. [Google Scholar] [CrossRef]
- Thiriard, A.; Raze, D.; Locht, C. Development and standardization of a high-throughput bordetella pertussis growth-inhibition assay. Front. Microbiol. 2020, 11, 777. [Google Scholar] [CrossRef]
- Wilson, S.E.; Graham, D.R.; Wang, W.; Bruss, J.B.; Castaneda-Ruiz, B. Telavancin in the treatment of concurrent staphylococcus aureus bacteremia: A retrospective analysis of atlas and attain studies. Infect. Dis. Ther. 2017, 6, 413–422. [Google Scholar] [CrossRef]
- Woziwodzka, A.; Krychowiak-Maśnicka, M.; Gołuński, G.; Bosiewska, A.; Borowik, A.; Wyrzykowski, D.; Piosik, J. New life of an old drug: Caffeine as a modulator of antibacterial activity of commonly used antibiotics. Pharmaceuticals 2022, 15, 872. [Google Scholar] [CrossRef]
- Sajid, M.I.; Lohan, S.; Kato, S.; Tiwari, R.K. Combination of amphiphilic cyclic peptide [r(4)w(4)] and levofloxacin against multidrug-resistant bacteria. Antibiotics 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Flamm, R.K.; Rhomberg, P.R.; Lindley, J.M.; Sweeney, K.; Ellis-Grosse, E.J.; Shortridge, D. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against gram-negative bacterial strains by using time-kill curves. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhong, Y.; He, T.; Peng, S.; Yang, L. A codispersed nanosystem of silver-anchored mos(2) enhances antibacterial and antitumor properties of selective laser sintered scaffolds. Int. J. Bioprinting 2022, 8, 577. [Google Scholar] [CrossRef]
- Wang, S.; Wei, X.; Sun, X.; Chen, C.; Zhou, J.; Zhang, G.; Wu, H.; Guo, B.; Wei, L. A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-rnai delivery system. Int. J. Nanomed. 2018, 13, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Oleinikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Pelageev, D.N.; Rasin, A.B.; Menshov, A.S.; Popov, R.S.; Kim, N.Y.; Chingizova, E.A.; et al. New antibacterial chloro-containing polyketides from the alga-derived fungus Asteromyces cruciatus kmm 4696. J. Fungi 2022, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, E.; Zhang, Z.; Yang, D.; Li, G.; Cao, H.; Huang, H. A novel indolizine derivative induces apoptosis through the mitochondria p53 pathway in hepg2 cells. Front. Pharmacol. 2019, 10, 762. [Google Scholar] [CrossRef]
- Sun, T.; Yang, W.; Toprani, S.M.; Guo, W.; He, L.; Deleo, A.B.; Ferrone, S.; Zhang, G.; Wang, E.; Lin, Z.; et al. Induction of immunogenic cell death in radiation-resistant breast cancer stem cells by repurposing anti-alcoholism drug disulfiram. Cell Commun. Signal. 2020, 18, 36. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, K.; Liu, Y.; Fu, J.; Zong, G.; Ma, X.; Cao, G. Deletion of the response regulator phop accelerates the formation of aerial mycelium and spores in actinosynnema pretiosum. Front. Microbiol. 2022, 13, 845620. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; Ye, X.; Yang, L.; Hu, R.; Ma, J.; Ma, S.; Li, D.; Zhou, J.; Nie, G.; et al. Unraveling cadmium toxicity in trifolium repens l. Seedling: Insight into regulatory mechanisms using comparative transcriptomics combined with physiological analyses. Int. J. Mol. Sci. 2022, 23, 4612. [Google Scholar] [CrossRef]
- Wang, G.; Brunel, J.M.; Preusse, M.; Mozaheb, N.; Willger, S.D.; Larrouy-Maumus, G.; Baatsen, P.; Häussler, S.; Bolla, J.M.; Van Bambeke, F. The membrane-active polyaminoisoprenyl compound nv716 re-sensitizes pseudomonas aeruginosa to antibiotics and reduces bacterial virulence. Commun. Biol. 2022, 5, 871. [Google Scholar] [CrossRef]

| Antibiotic | Strain | MICantibiotic (μg/mL) | FIC | MICSA (μg/mL) | FIC | FICI | Interpretation |
|---|---|---|---|---|---|---|---|
| Amoxicillin | N21 | 512 | 0.25 | 4000 | 0.25 | 0.5 | Synergistic |
| 43,300 | 32 | 0.0078 | 8000 | 0.25 | 0.2578 | Synergistic | |
| N30 | 512 | 0.25 | 8000 | 0.5 | 0.75 | Additive | |
| N24 | 512 | 0.25 | 8000 | 0.25 | 0.5 | Synergistic | |
| N22 | 512 | 0.5 | 4000 | 0.5 | 1 | Irrelevant | |
| Oxacillin | 16,183 | 128 | 0.0625 | 4000 | 0.25 | 0.3125 | Synergistic |
| N27FO | 4 | 0.25 | 4000 | 0.25 | 0.5 | Synergistic | |
| N15FO | 2 | 0.25 | 4000 | 0.25 | 0.5 | Synergistic | |
| HYM3FO | 4 | 0.25 | 4000 | 0.25 | 0.5 | Synergistic | |
| Ampicillin | S16-5′ | 16 | 0.5 | 4000 | 0.25 | 0.75 | Additive |
| 43,300 | 8 | 0.125 | 4000 | 0.25 | 0.375 | Synergistic | |
| S14-19 | 128 | 0.25 | 4000 | 0.25 | 0.5 | Synergistic | |
| Ceftriaxone | S14-19 | 4 | 0.25 | 4000 | 0.125 | 0.375 | Synergistic |
| Ceftiofur | 16,183 | 128 | 0.5 | 4000 | 0.25 | 0.5 | Synergistic |
| Cefoxitin | 16,183 | 64 | 0.25 | 4000 | 0.25 | 0.5 | Synergistic |
| Cefovecin | 16,183 | 1024 | 0.5 | 4000 | 0.25 | 0.75 | Additive |
| Ceftazidime | 16,183 | 128 | 0.5 | 4000 | 0.125 | 0.625 | Additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Ye, M.; Wang, X.; Zhu, Y.; Sun, X.; Gu, R.; Chen, L.; Fang, B. Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus. Molecules 2024, 29, 1528. https://doi.org/10.3390/molecules29071528
Hou L, Ye M, Wang X, Zhu Y, Sun X, Gu R, Chen L, Fang B. Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus. Molecules. 2024; 29(7):1528. https://doi.org/10.3390/molecules29071528
Chicago/Turabian StyleHou, Limin, Minqi Ye, Xiaoyu Wang, Yifan Zhu, Xueyan Sun, Ruiheng Gu, Liangzhu Chen, and Binghu Fang. 2024. "Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus" Molecules 29, no. 7: 1528. https://doi.org/10.3390/molecules29071528
APA StyleHou, L., Ye, M., Wang, X., Zhu, Y., Sun, X., Gu, R., Chen, L., & Fang, B. (2024). Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus. Molecules, 29(7), 1528. https://doi.org/10.3390/molecules29071528





