Coal-Derived Humic Substances: Insight into Chemical Structure Parameters and Biomedical Properties
Abstract
1. Introduction
2. Results and Discussion
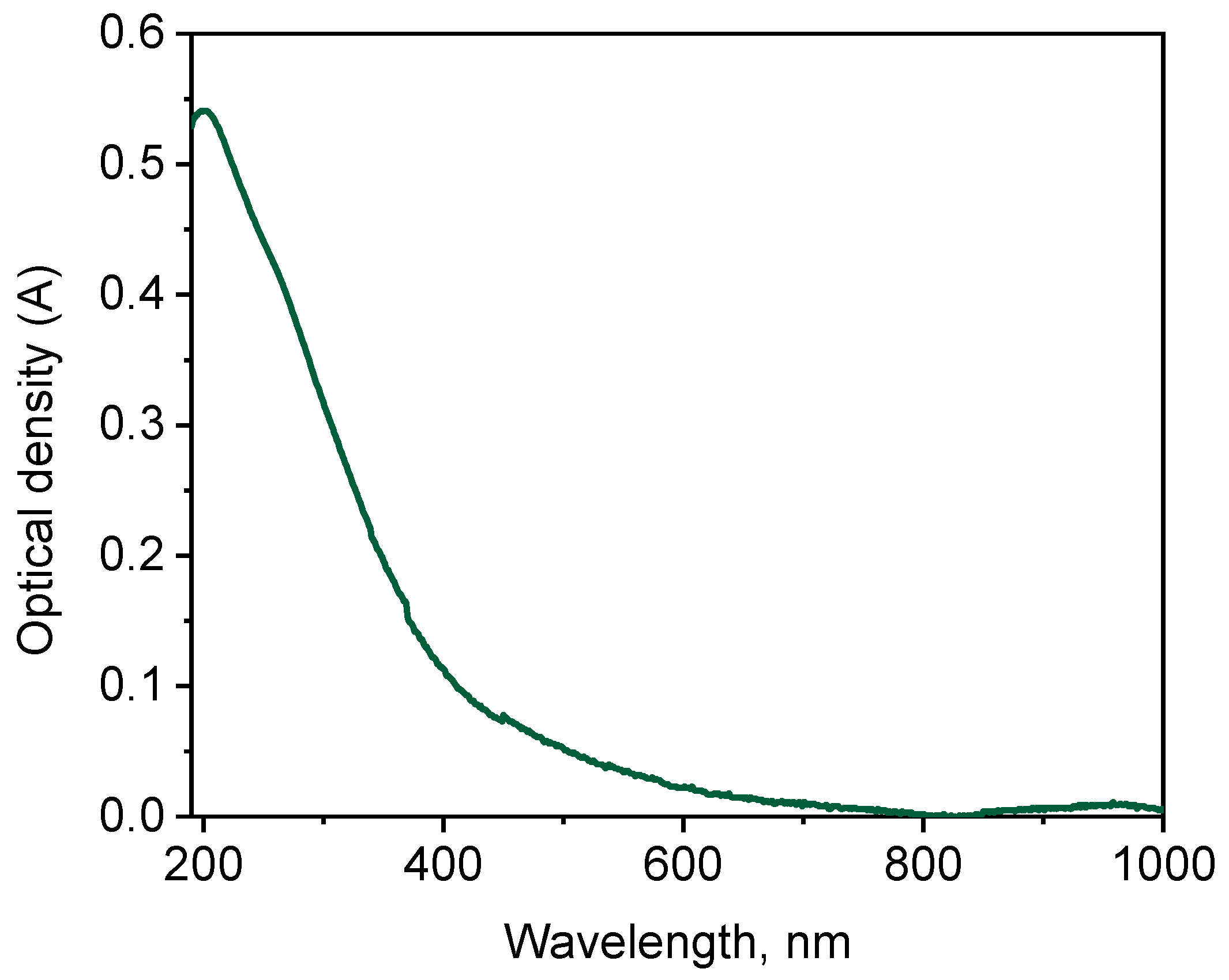
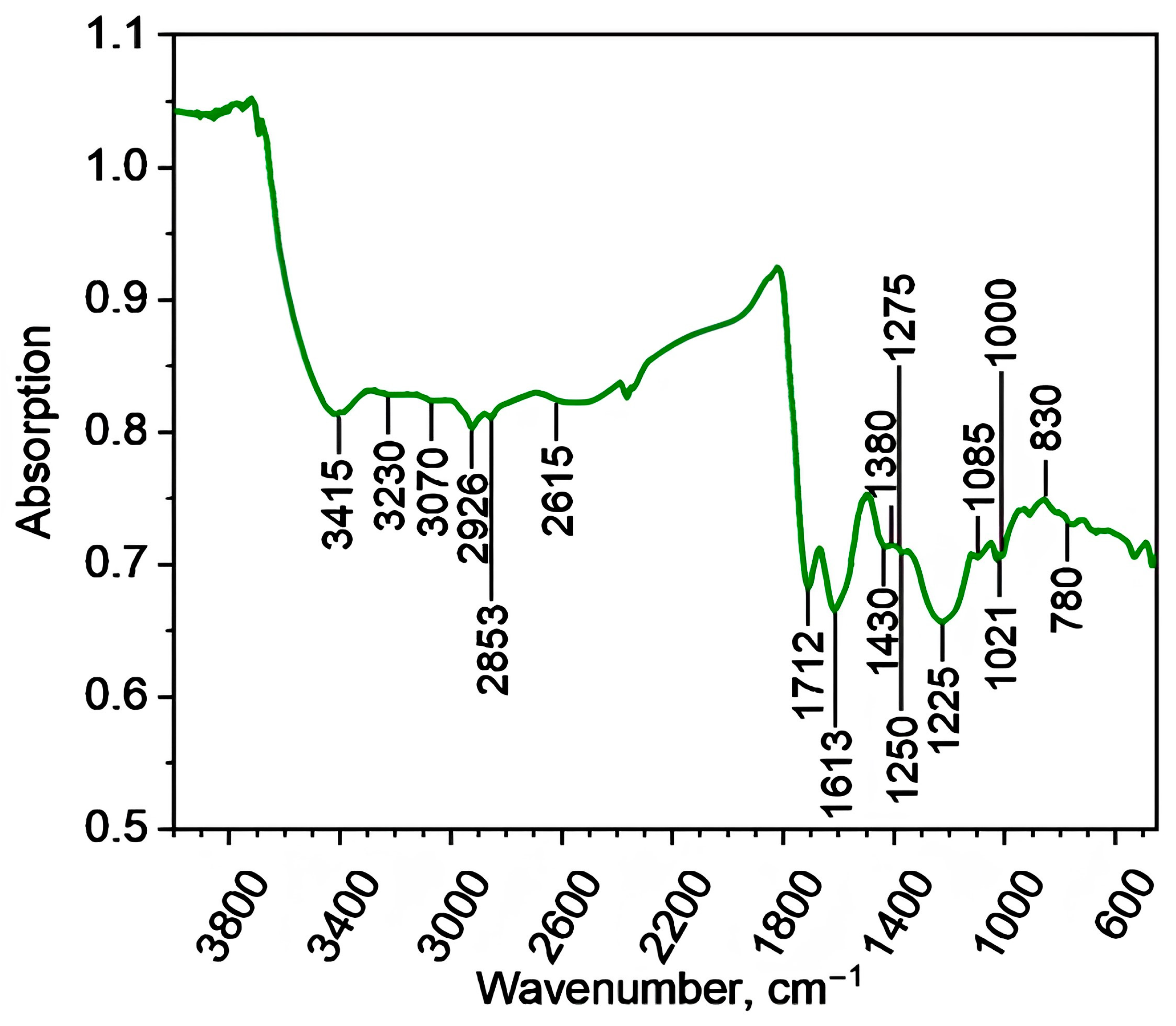
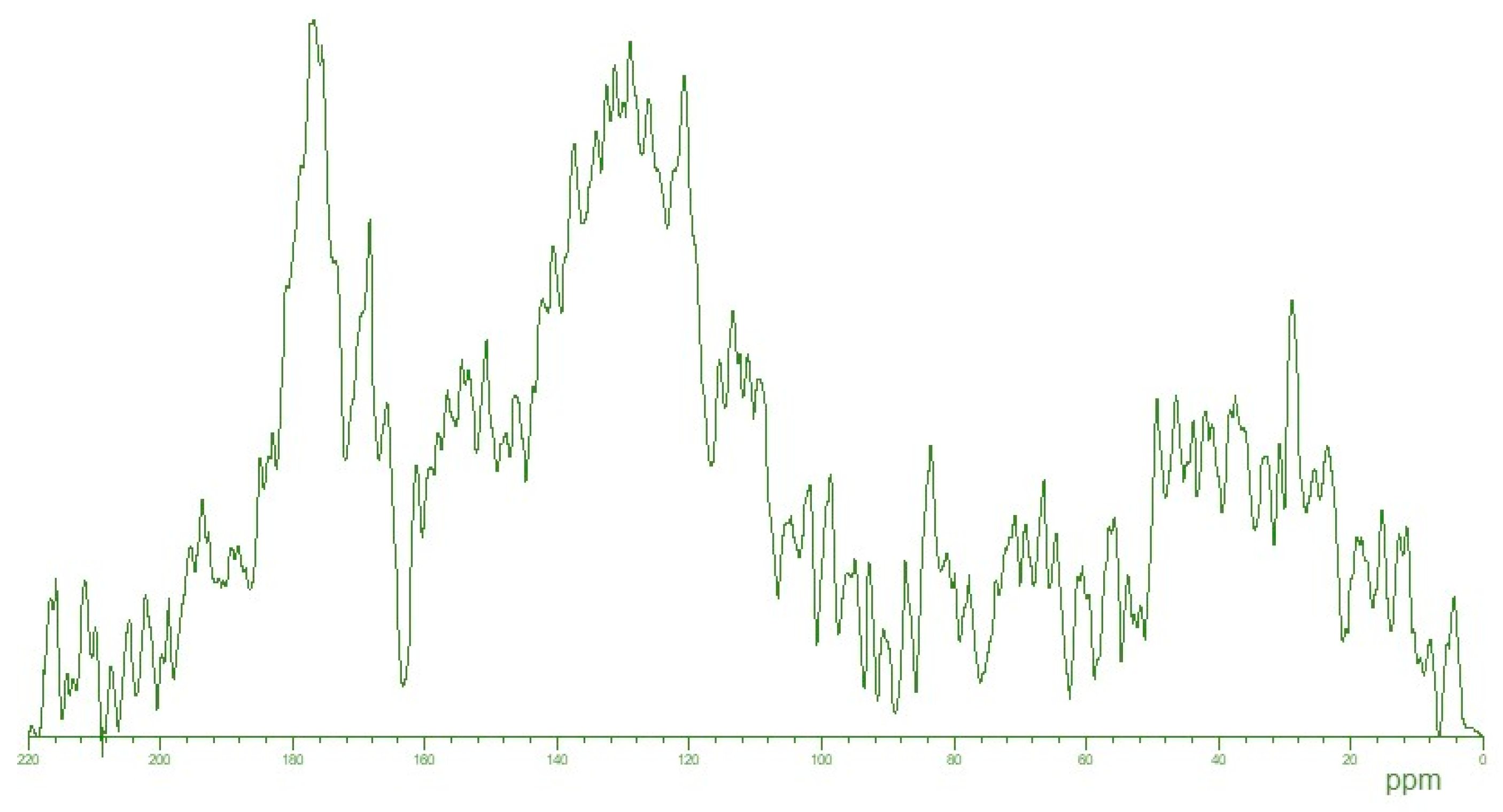
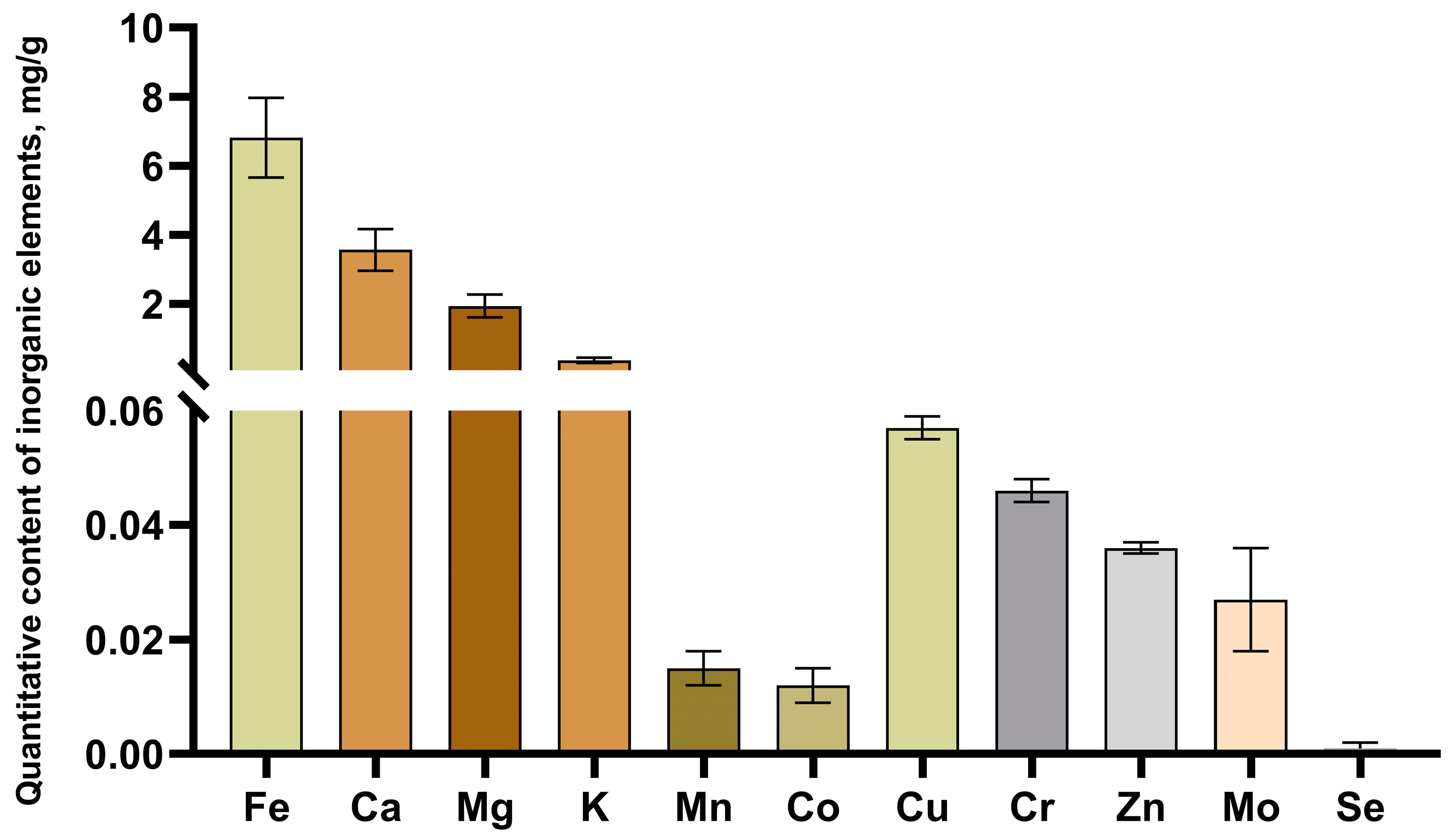
2.1. Physico-Chemical Characterization
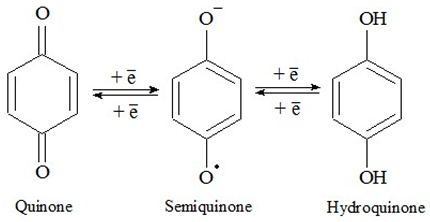
2.2. Antioxidant Activity Study
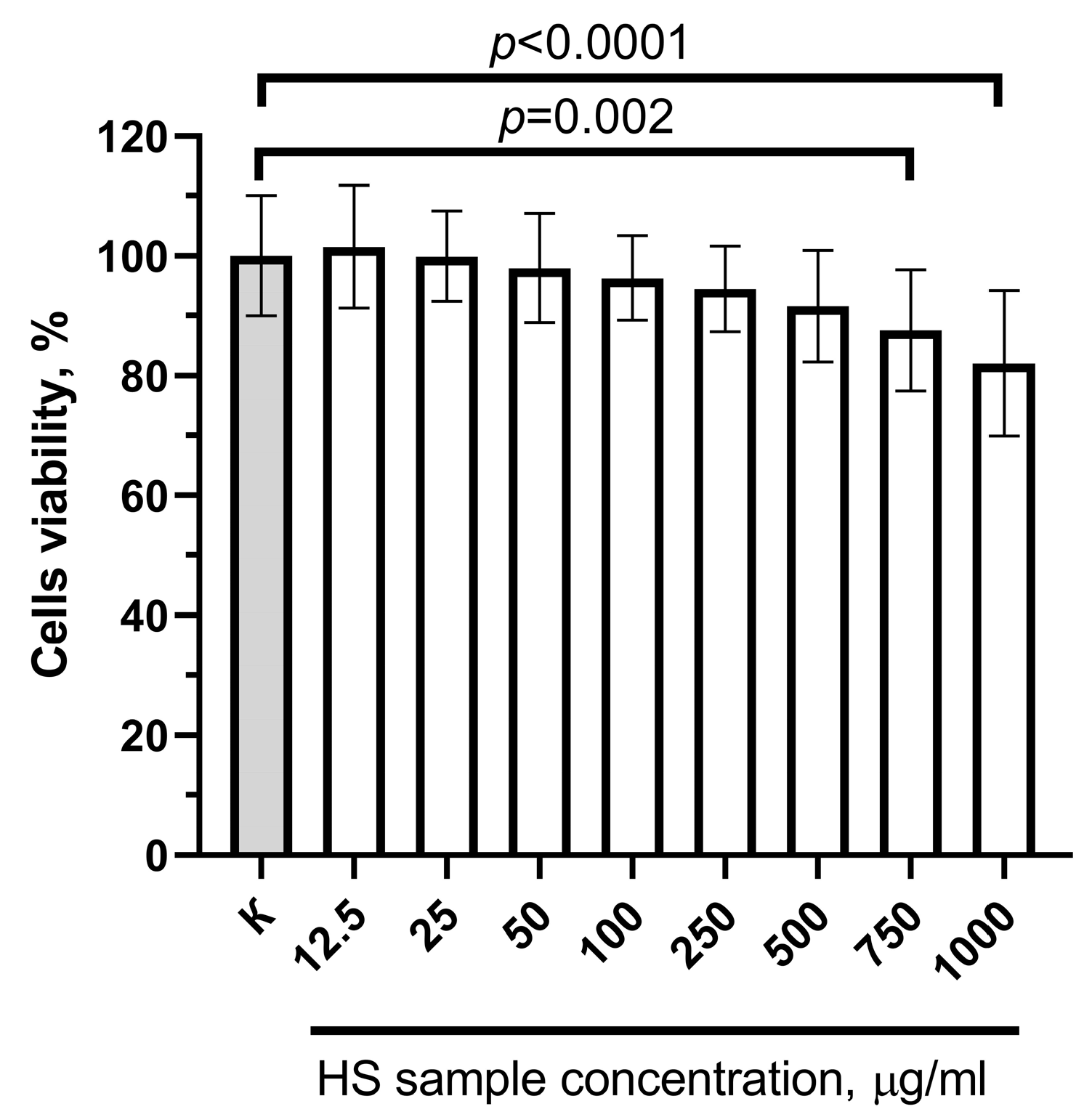
2.3. Cytotoxicity Study
2.4. Antioxidant Activity Study In Vitro
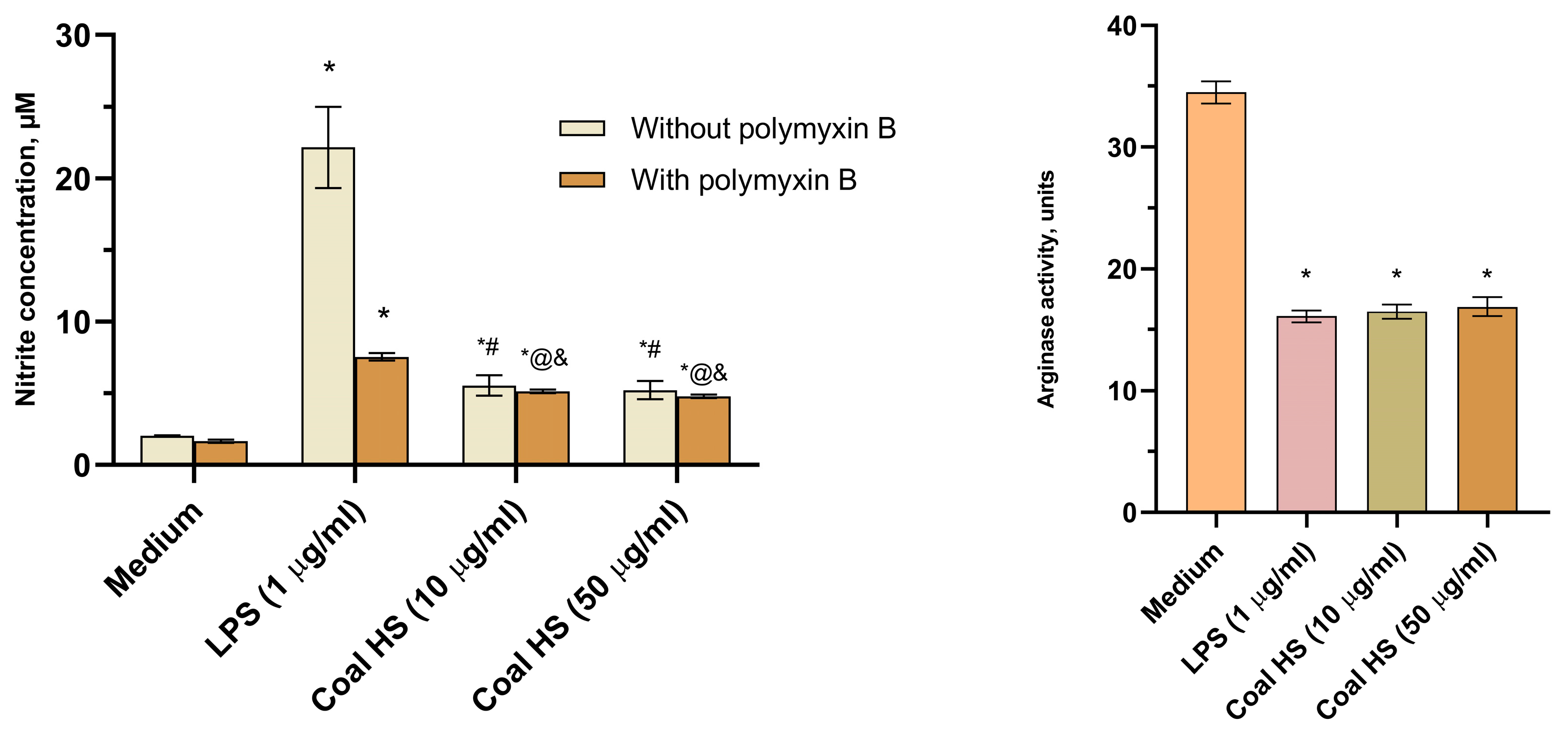
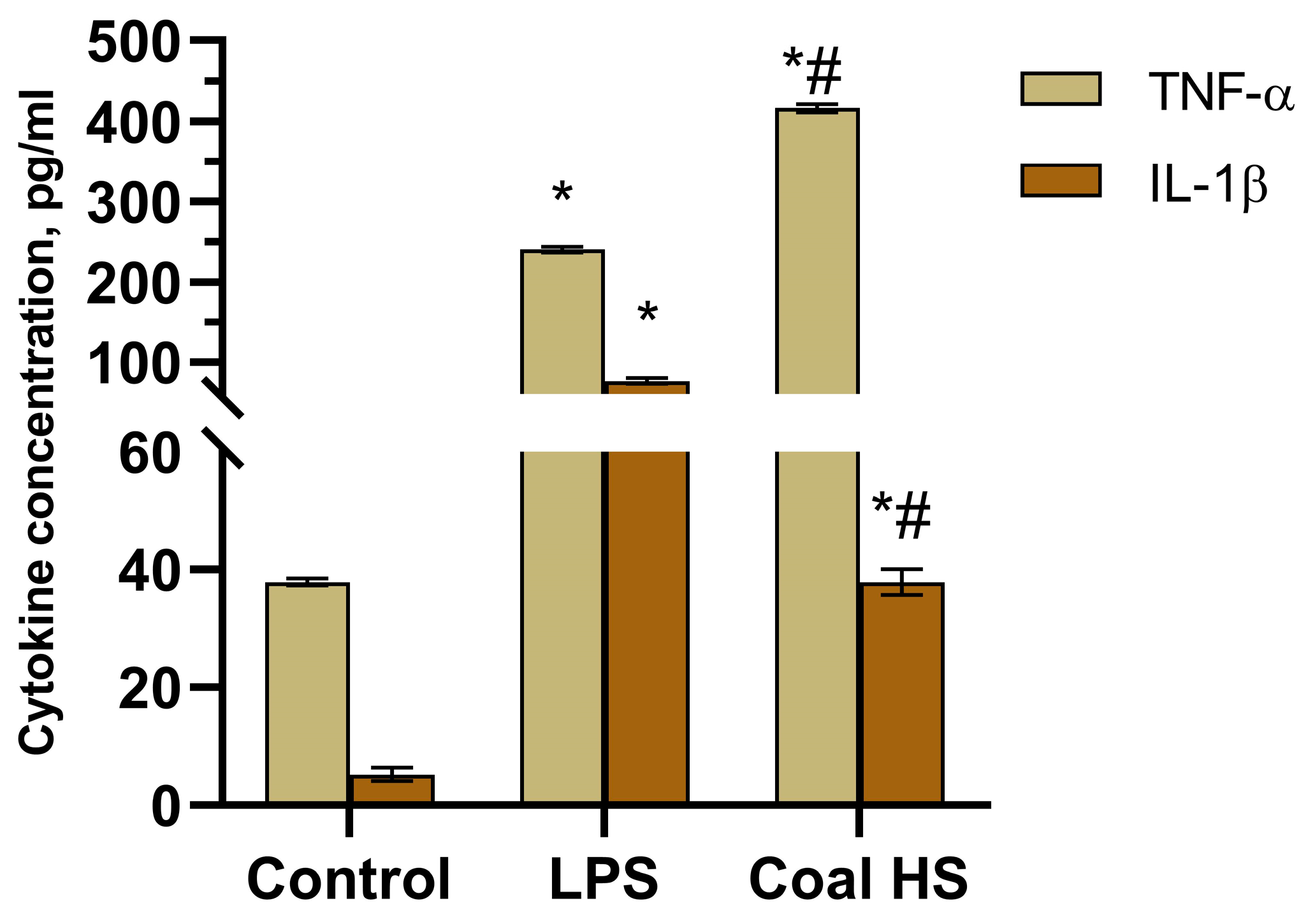
2.5. Immunopharmacological Study
2.6. Investigation of Biological Activity In Vivo
2.6.1. Actoprotective Activity
2.6.2. Nootropic Activity
2.7. Toxicity Study
2.7.1. Acute Toxicity
2.7.2. Toxicity Study during Multiple Administrations
2.7.3. Allergenicity Study
3. Materials and Methods
3.1. Coal Humic Substance (HS) Sample Preparation
3.2. Microbiological Purity
3.3. Inorganic Elemental Composition by ICP-MS Method
3.4. Quantification of Heavy Metals and Arsenic Content
3.5. Physico-Chemical Analysis
3.5.1. Electronic Spectroscopy
3.5.2. Infrared Spectroscopy
3.5.3. Fluorescence Spectroscopy
3.5.4. Elemental (C, H, N, O) Analysis
3.5.5. 13C-NMR Spectroscopy
3.5.6. Size-Exclusion High-Performance Liquid Chromatography
3.6. Cytotoxicity Study
3.7. Antioxidant Capacity Study
3.7.1. Total Antioxidant Capacity Study
3.7.2. Ability to Inhibit the Superoxide Anion Radical
3.7.3. Cathodic Voltammetry
3.7.4. Chelating Activity
3.7.5. Ability to Inhibit Hydroxyl Radicals
3.7.6. In Vitro Study of Antioxidant Activity
3.8. Immunotropic Activity
3.9. Biological Activity
3.10. Toxicity Assessment In Vivo
3.11. Allergenicity Assessment
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silano, V.; Coppens, P.; Larrañaga-Guetaria, A.; Minghetti, P.; Roth-Ehrang, R. Regulations applicable to plant food supplements and related products in the European Union. Food Funct. 2011, 2, 710–719. [Google Scholar] [CrossRef]
- Gerevini, V.G.; Copparoni, R.; Dalfrà, S.; Leonardi, M.; Guidarelli, L. Herbal food supplements. Ann. Ist. Super. Sanita 2005, 41, 55–59. [Google Scholar]
- Barnes, J. Quality, efficacy and safety of complementary medicines: Fashions, facts and the future. Part I. Regulation and quality. Br. J. Clin. Pharmacol. 2003, 55, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J. Quality, efficacy and safety of complementary medicines: Fashions, facts and the future. Part II. Efficacy and safety. Br. J. Clin. Pharmacol. 2003, 55, 331–340. [Google Scholar] [CrossRef]
- Wipo. Available online: https://www.wipo.int/wipolex/ru/legislation/details/5557 (accessed on 9 January 2024).
- Tutel’yan, V.A.; Sukhanov, B.P. Modern approaches to the maintenance of quality and safety of biologically active additives in the russian federation. Pac. Med. J. 2009, 1, 12–19. [Google Scholar]
- Perminova, I.V. From green chemistry and nature-like technologies towards ecoadaptive chemistry and technology. Pure Appl. Chem. 2019, 91, 851–864. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; Wiley & Sons: New York, NY, USA, 1994; p. 496. [Google Scholar]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef] [PubMed]
- Pena-Mendes, E.M.; Havel, J.; Patoska, J. Humic substances-compounds of still unknown structure: Applications in agriculture, industry, environment, and biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef]
- Zykova, M.V.; Veretennikova, E.E.; Logvinova, L.A.; Romanenko, S.V.; Bratishko, K.A.; Belousov, M.V.; Brazovsky, K.S.; Yusubov, M.S.; Lyapkov, A.A.; Danilets, M.G.; et al. New artificial network model to estimate biological activity of peat humic acids. Environ. Res. 2020, 191, 109999. [Google Scholar] [CrossRef]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Žatko, D.; Vaško, L. Therapeutic Efficiency of Humic Acids in Intoxications. Life 2023, 13, 971. [Google Scholar] [CrossRef]
- Zhernov, Y.V.; Kremb, S.; Helfer, M.; Schindler, M.; Harir, M.; Mueller, C.; Hertkorn, N.; Avvakumova, N.P.; Konstantinov, A.I.; Brack-Werner, R.; et al. Supramolecular Combinations of Fractionated Humic Polyanions as Potent and Cost-Effective Microbicides with Polymodal anti-HIV-Activities and Low Cytotoxicity. New J. Chem. 2017, 41, 212–224. [Google Scholar] [CrossRef]
- Lotosh, T.D. Experimental bases and prospects for the use of humic acid preparations from peat in medicine and agricultural production. Nauchnye Doki Vyss. Shkoly Biol Nauk. 1991, 10, 99–103. [Google Scholar]
- Vucskits, A.V.; Hullár, I.; Bersényi, A.; Andrásofszky, E.; Kulcsár, M.; Szabó, J. Effect of fulvic and humic acids on performance, immune response and thyroid function in rats. J. Anim. Physiol. Anim. Nutr. 2010, 94, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Khlebnikov, A.I.; Kwon, B.S. Medical drugs from humus matter: Focus on mumie. Drug Dev. Res. 2002, 57, 140–159. [Google Scholar] [CrossRef]
- Van Rensburg, C.E. The antiinflammatory properties of humic substances: A mini review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef]
- Zykova, M.V.; Brazovskii, K.S.; Bratishko, K.A.; Buyko, E.E.; Logvinova, L.A.; Romanenko, S.V.; Konstantinov, A.I.; Krivoshchekov, S.V.; Perminova, I.V.; Belousov, M.V. Quantitative Structure-Activity Relationship, Ontology-Based Model of the Antioxidant and Cell Protective Activity of Peat Humic Acids. Polymers 2022, 14, 3293. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, A.; Chen, X.; Che, X.; Zhou, K.; Wang, Z. Sodium humate accelerates cutaneous wound healing by activating TGF-β/Smads signaling pathway in rats. Acta Pharm. Sin. B 2016, 6, 132–140. [Google Scholar] [CrossRef]
- Çalışır, M.; Akpınar, A.; Talmaç, A.C.; Lektemur Alpan, A.; Göze, Ö.F. Humic Acid Enhances Wound Healing in the Rat Palate. Evid. Based Complement Altern. Med. 2018, 2018, 1783513. [Google Scholar] [CrossRef]
- Artinger, R.; Buckau, G.; Geyer, S.; Fritz, P.; Wolf, M.; Kim, J.I. Characterization of groundwater humic substances: Influence of sedimentary organic carbon. Appl. Geochem. 2000, 15, 97–116. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Characterization of pH-fractionated humic acids derived from Chinese weathered coal. Chemosphere 2017, 166, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Kononova, M.M. Soil Organic Matter; Academy of Sciences: Moscow, Russia, 1963; p. 314. [Google Scholar]
- Orlov, D.S. Humus Acids of Soils; University Publishing House: Moscow, Russia, 1985. [Google Scholar]
- Smidt, E.; Eckhardt, K.-U.; Lechner, P.; Schulten, H.-R.; Leinweber, P. Characterization of different decomposition stages of biowaste using FT-IR spectroscopy and pyrolysis-field ionization mass spectrometry. Biodegradation 2005, 16, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Spectroscopic and compositional comparative characterization of I.H.S.S. reference and standard fulvic and humic acids of various origin. Sci. Total. Environ. 1989, 81, 143–156. [Google Scholar] [CrossRef]
- Dick, D.; Mangrich, A.; Menezes, S.C.; Pereira, S.C. Chemical and Spectroscopical Characterization of Humic Acids from two South Brazilian Coals of Different Ranks. J. Braz. Chem. Soc. 2002, 13, 177–182. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer-Verlag GmbH Deutschland: Heidelberg, Germany, 2020; p. 487. [Google Scholar]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil Sci. 1991, 152, 259–271. [Google Scholar] [CrossRef]
- Kingery, W.L.; Simpson, A.J.; Hayes, M.H.B.; Locke, M.; Hicks, R. The application of multidimensional NMR to the study of soil humic substances. Soil Sci. 2000, 165, 483–494. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Graphical-statistical method for investigation of the structure of coal. Fuel 1950, 29, 228–269. [Google Scholar]
- Hertkorn, N.; Permin, A.; Perminova, I.; Kovalevskii, D.; Yudov, M.; Petrosyan, V.; Kettrup, A. Comparative Analysis of Partial Structures of a Peat Humic and Fulvic Acid Using One- and Two-Dimensional Nuclear Magnetic Resonance Spectroscopy. J. Environ. Qual. 2002, 31, 375–387. [Google Scholar] [PubMed]
- Kudryavtsev, A.V.; Perminova, I.V.; Petrosyan, V.S. Size exclusion chromatographic descriptors of humic substances. Anal. Chim. Acta 2000, 407, 193–202. [Google Scholar] [CrossRef]
- D’yakova, N.A.; Slivkin, A.I.; Trineeva, O.V. From Assessment of Radionuclide Contamination in Herbal Medicine Raw Material of Voronezh Region to Issues Concerning Standardization of Natural Radionuclides. Pharm. Chem. J. 2022, 56, 1082–1086. [Google Scholar] [CrossRef]
- ISO 18664:2015; Traditional Chinese Medicine—Determination of Heavy Metals in Herbal Medicines Used in Traditional Chinese Medicine. ISO: Geneva, Switzerland, 2015.
- Galenko, M.S.; Gravel, I.V.; Velts, N.Y.; Alyautdin, R.N. Limits for the Content of Heavy Metals and Arsenic as a Means of Ensuring Safe Use of Herbal Medicinal Products. Saf. Risk Pharmacother. 2021, 9, 61–68. [Google Scholar] [CrossRef]
- Inada, I.; Kiuchi, F.; Urushihara, H. Comparison of Regulations for Arsenic and Heavy Metals in Herbal Medicines Using Pharmacopoeias of Nine Counties/Regions. Ther. Innov. Regul. Sci. 2023, 57, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N. Application of Electron Spin Resonance (ESR) Spectroscopy in Soil Chemistry; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1990; Volume 1, pp. 77–130. [Google Scholar]
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel electrochemical approach to assess the redox properties of humic substances. Environ. Sci. Technol. 2009, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Xie, G.; Jutila, M.A.; Quinn, M.T. Complement-fixing activity of fulvic acid from shilajit and other natural sources. Phytother. Res. 2009, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Witwicki, M.; Jaszewski, A.R.; Jezierska, J.; Jerzykiewicz, M.; Jezierski, A. pH-induced shift in the g-tensor components of semiquinone-type radicals in humic acids–DFT and EPR studies. Chem. Phys. Lett. 2008, 462, 300–306. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G. Electrochemical properties of natural organic matter (NOM), fractions of nom, and model biogeochemical electron shuttles. Environ. Sci. Technol. 2002, 36, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Bentayeb, K.; Rubio, C.; Nerín, C. Study of the antioxidant mechanisms of Trolox and eugenol with 2,2′-azobis(2-amidinepropane) dihydrochloride using ultra-high performance liquid chromatography coupled with tandem mass spectrometry. Analyst 2012, 137, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Daquino, C.; Geraci, C.J. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Efimova, I.V.; Khil’ko, S.L.; Smirnova, O.V. Antioxidant activity of humic acids in radical-chain oxidation processes. Russ. J. Appl. Chem. 2012, 85, 1351–1354. [Google Scholar] [CrossRef]
- Avramchik, O.A.; Korotkova, E.I.; Plotnikov, E.V.; Lukina, A.N.; Karbainov, Y.A. Antioxidant and electrochemical properties of calcium and lithium ascorbates. J. Pharm. Biomed. Anal. 2005, 37, 1149–1154. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Karbainov, Y.A.; Avramchik, O.A. Investigation of antioxidant and catalytic properties of some biologically active substances by voltammetry. Anal. Bioanal. Chem. 2003, 375, 465–468. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Karbainov, Y.A.; Shevchuk, A.V. Study of antioxidant properties by voltammetry. J. Electroanal. Chem. 2002, 518, 56–60. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Schopfer, P. Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur. J. Biochem. 1999, 260, 726–735. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.P.; Van den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef]
- Valentová, K. Cytoprotective Activity of Natural and Synthetic Antioxidants. Antioxidants 2020, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R. Cellular and Molecular Mechanisms in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8017. [Google Scholar] [CrossRef] [PubMed]
- Masisi, K.; Masamba, R.; Lashani, K.; Li, C.; Kwape, T.E.; Gaobotse, G. Antioxidant, Cytotoxicity and Cytoprotective Potential of Extracts of Grewia Flava and Grewia Bicolor Berries. J. Pharmacopunct. 2021, 24, 24–31. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef]
- Meydani, M.; Lipman, R.D.; Han, S.N.; Wu, D.; Beharka, A.; Martin, K.R.; Bronson, R.; Cao, G.; Smith, D.; Meydani, S.N. The effect of long-term dietary supplementation with antioxidants. Ann. N. Y. Acad. Sci. 1998, 854, 352–360. [Google Scholar] [CrossRef]
- Trofimova, E.S.; Zykova, M.V.; Ligacheva, A.A.; Sherstoboev, E.Y.; Zhdanov, V.V.; Belousov, M.V.; Yusubov, M.S.; Krivoshchekov, S.V.; Danilets, M.G.; Dygai, A.M. Influence of humic acids extracted from peat by different methods on functional activity of macrophages in vitro. Bull. Exp. Biol. Med. 2016, 162, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, M.E.; Huerta, L.; Alvarez-Buylla, E.R.; Villarreal Luján, C. Role of Cytokine Combinations on CD4+ T Cell Differentiation, Partial Polarization, and Plasticity: Continuous Network Modeling Approach. Front. Physiol. 2018, 9, 877. [Google Scholar] [CrossRef]
- Andreu-Sanz, D.; Kobold, S. Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy. Cancers 2023, 15, 1650. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P. On Analyzing How the Th1/Th2 Phenotype of an Immune Response Is Determined: Classical Observations Must not Be Ignored. Front. Immunol. 2019, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar] [PubMed]
- Rubab, I.; Routray, I.; Mahmood, A.; Bashir, S.; Shinkafi, T.S.; Khan, F.; Ali, S. Mineral pitch stimulates humoral, cellular and innate immune responses in mice. Pharm. Biol. 2013, 51, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, S.S.; Kim, H.G.; Lee, H.W.; Kim, W.Y.; Lee, S.K.; Son, C.G. Gongjin-Dan enhances hippocampal memory in a mouse model of scopolamine-induced amnesia. PLoS ONE 2016, 11, e0159823. [Google Scholar] [CrossRef]
- Fronza, M.G.; Baldinotti, R.; Fetter, J.; Sacramento, M.; Sabedra Sousa, F.S.; Seixas, F.K.; Collares, T.; Alves, D.; Praticò, D.; Savegnago, L. QTC-4-MeOBnE Rescues Scopolamine-Induced Memory Deficits in Mice by Targeting Oxidative Stress, Neuronal Plasticity, and Apoptosis. ACS Chem. Neurosci. 2020, 11, 1259–1269. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Wang, H.; Jiang, J. Assessing Redox Properties of Natural Organic Matters with regard to Electron Exchange Capacity and Redox-Active Functional Groups. J. Chem. 2020, 2020, 2698213. [Google Scholar] [CrossRef]
- Gosudarstvennaya Farmakopeya Rossiyskoy Federatsii. XV Izdaniya. [State Pharmacopoeia of the Russian Federation. XV Edition]. Available online: https://pharmacopoeia.regmed.ru/pharmacopoeia/izdanie-15/1/1-2/1-2-2/1-2-2-2/tyazhyelye-metally/ (accessed on 20 March 2024). (In Russian).
- Kovalevskii, D.V.; Permin, A.B.; Perminova, I.V.; Petrosyan, V.S. Choice of the time of pulse delay for quantitative 13C NMR spectroscopy of humic substances. Mosc. Univ. Chem. Bull. 2000, 41, 39–42. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays, Assay Guidance Manual; Eli Lilly & Company: Indianapolis, IN, USA; The National Center for Advancing Translational Sciences: Bethesda, Maryland, 2016. [Google Scholar]
- Bravo, C.; De Nobili, M.; Gambi, A.; Martin-Neto, L.; Nascimento, O.R.; Toniolo, R. Kinetics of electron transfer reactions by humic substances: Implications for their biogeochemical roles and determination of their electron donating capacity. Chemosphere 2022, 286, 131755. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V.A.; Dorofeeva, N.A.; Pakhomov, P.M. Kineticheskii metod analiza antiradikal’noi aktivnosti ekstraktov rastenii. Khimiko-Farmatsevticheskii Zhurnal 2009, 43, 27–31. [Google Scholar]
- Mahakunakorn, P.; Tohda, M.; Murakami, Y.; Matsumoto, K.; Watanabe, H. Antioxidant and free radical-scavenging activity of Choto-san and its related constituents. Biol. Pharm. Bull. 2004, 27, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.; Potapov, A.; Khlebnikov, A.; Korotkova, E.; Lukina, A.; Malovichko, G.; Kirpotina, L.; Quinn, M.T. Decomposition of reactive oxygen species by copper (II) bis(1-pyrazolyl)methane complexes. J. Biol. Inorg. Chem. 2006, 11, 499–513. [Google Scholar] [CrossRef]
- Zharkova, L.P.; Knyazeva, I.R.; Ivanov, V.V.; Bolshakov, M.A.; Kutenkov, O.P.; Rostov, B.B. Effect of pulsed-periodic X-ray and microwave radiation on the level of peroxides in isolated hepatocytes. Bull. Tomsk. State Univ. 2010, 333, 161–163. (In Russian) [Google Scholar]
- Ayaz, F. Ruthenium pyridyl thiocyanate complex increased the production of pro-inflammatory TNFα and IL1β cytokines by the LPS stimulated mammalian macrophages in vitro. Mol. Biol. Rep. 2018, 45, 2307–2312. [Google Scholar] [CrossRef]
- Scull, C.M.; Hays, W.D.; Fischer, T.H. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J. Inflamm. 2010, 7, 53. [Google Scholar] [CrossRef]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192–207. [Google Scholar] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Amano, F.; Noda, T. Improved detection of nitric oxide radical (NO.) production in an activated macrophage culture with a radical scavenger, carboxy PTIO and Griess reagent. FEBS Lett. 1995, 368, 425–428. [Google Scholar]
- Munder, M.; Mallo, M.; Eichmann, K.; Modolell, M. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J. Exp. Med. 1998, 187, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Brigo, N.; Pfeifhofer-Obermair, C.; Tymoszuk, P.; Demetz, E.; Engl, S.; Barros-Pinkelnig, M.; Dichtl, S.; Fischer, C.; Valente De Souza, L.; Petzer, V.; et al. Cytokine-Mediated Regulation of ARG1 in Macrophages and Its Impact on the Control of Salmonella enterica Serovar Typhimurium Infection. Cells 2021, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Sosroseno, W.; Musa, M.; Ravichandran, M.; Fikri Ibrahim, M.; Bird, P.S.; Seymour, G.J. Arginase activity in a murine macrophage cell line (RAW264.7) stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 2006, 21, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. 1966, 50, 219–244. [Google Scholar]
- Kale, P.P.; Addepalli, V.; Sarkar, A.; Patel, S.; Savai, J. The combination of antidepressant duloxetine with Piracetam in mice does not produce enhancement of Nootropic activity. Exp. Neurobiol. 2014, 23, 224. [Google Scholar] [CrossRef] [PubMed]
- Berlato, D.G.; Bairros, A.V. Meldonium: Pharmacological, toxicological, and analytical aspects. Toxicol. Res. Appl. 2020, 4, 2397847320915143. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Wei, L.; Chen, Y.J.; Huang, C.C. Association of physical performance and biochemical profile of mice with intrinsic endurance swimming. Int. J. Med. Sci. 2016, 13, 892. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-fatigue effects of small molecule oligopeptides isolated from Panax ginseng CA Meyer in mice. Nutrients 2016, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Ader, R.; Weijnen, J.A.; Moleman, P. Retention of a passive avoidance response as a function of the intensity and duration of electric shock. Psychon. Sc. 1972, 26, 125–128. [Google Scholar] [CrossRef]
- OECD Test N 423:2001 Acute Oral Toxicity—Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, IDT. Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed on 27 March 2024).
- Ko, G.M.; Rosenkranz, A.; Bertoncini, C.R.A.; Jurkiewicz, N.H.; Franco, M.G.; Jurkiewicz, A. Methods of acute biological assays in guinea-pigs for the study of toxicity and innocuity of drugs and chemicals. Braz. J. Pharm. Sci. 2010, 46, 251–263. [Google Scholar] [CrossRef]



| Mass Fractions of Elements, % Atomic Fractions of Elements, % | Atomic Ratios | |||||
|---|---|---|---|---|---|---|
| C | H | N | O | H/C | O/C | N/C |
| 44.9 ± 0.48 32.9 ± 0.43 | 4.51 ± 0.040 39.28 ± 0.30 | 1.09 ± 0.01 0.70 ± 0.01 | 49.48 ± 0.42 27.15 ± 0.16 | 1.20 | 0.83 | 0.02 |
| Free Radical/Fe2+ | IC50, µg/mL | |
|---|---|---|
| Coal HS Sample | Reference Compounds | |
| DPPH | 27.3 ± 0.2 | 21.2 ± 0.4 1 |
| ABTS•+ | 10.8 ± 0.3 | 3.4 ± 0.2 2 |
| O2−• | 20.5 ± 1.7 | 13.3 ± 0.9 3 |
| Fe2+ | 25.7 ± 0.5 | 9.6 ± 0.2 4 |
| HO• | 2.4 ± 0.2 mg/mL | 0.9 ± 0.0 mg/mL 5 |
| Studied Sample/Reference Substances | K, µmol/L*min |
|---|---|
| Coal HS | 0.91 ± 0.08 |
| Ascorbic acid | 1.15 ± 0.10 |
| Dihydroquercetin | 0.78 ± 0.08 |
| Experimental Groups | Swimming Time, min | p-Value |
|---|---|---|
| Control (n = 10) | 19.5 ± 3.4 | — |
| Meldonium, 200 mg/kg (n = 10) | 37.5 ± 9.9 | p2-1 = 0.36 |
| HS, 500 mg/kg (n = 10) | 34.7 ± 4.6 | p3-1 = 0.04 p3-2 = 0.39 |
| Experimental Groups | Lactate Level, mmol/L | p-Value |
|---|---|---|
| Control (n = 10) | 11.59 ± 0.93 | — |
| Meldonium, 200 mg/kg (n = 10) | 7.44 ± 0.54 | p2-1 = 0.001 |
| HS, 500 mg/kg (n = 10) | 8.71 ± 0.46 | p3-1 = 0.01 p3-2 = 0.09 |
| Experimental Groups | Time Spent on the Platform, Sec | p-Value |
|---|---|---|
| Control (n = 10) | 40.50 ± 6.17 | — |
| Scopolamine, 1 mg/kg (amnestic control) (n = 10) | 13.20 ± 5.80 | p2-1 = 0.005 |
| Piracetam, 300 mg/kg + Scopolamine, 1 mg/kg (n = 10) | 51.50 ± 4.89 | p3-1 = 0.17 p3-2 = 0.001 |
| HS, 500 mg/kg + Scopolamine, 1 mg/kg (n = 10) | 37.00 ± 6.53 | p4-1 = 0.70 p4-2 = 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zykova, M.V.; Bratishko, K.A.; Buyko, E.E.; Azarkina, L.A.; Ivanov, V.V.; Mihalyov, D.A.; Trofimova, E.S.; Danilets, M.G.; Ligacheva, A.A.; Konstantinov, A.I.; et al. Coal-Derived Humic Substances: Insight into Chemical Structure Parameters and Biomedical Properties. Molecules 2024, 29, 1530. https://doi.org/10.3390/molecules29071530
Zykova MV, Bratishko KA, Buyko EE, Azarkina LA, Ivanov VV, Mihalyov DA, Trofimova ES, Danilets MG, Ligacheva AA, Konstantinov AI, et al. Coal-Derived Humic Substances: Insight into Chemical Structure Parameters and Biomedical Properties. Molecules. 2024; 29(7):1530. https://doi.org/10.3390/molecules29071530
Chicago/Turabian StyleZykova, Maria V., Kristina A. Bratishko, Evgeny E. Buyko, Lyudmila A. Azarkina, Vladimir V. Ivanov, Dmitrii A. Mihalyov, Evgeniya S. Trofimova, Marina G. Danilets, Anastasia A. Ligacheva, Andrey I. Konstantinov, and et al. 2024. "Coal-Derived Humic Substances: Insight into Chemical Structure Parameters and Biomedical Properties" Molecules 29, no. 7: 1530. https://doi.org/10.3390/molecules29071530
APA StyleZykova, M. V., Bratishko, K. A., Buyko, E. E., Azarkina, L. A., Ivanov, V. V., Mihalyov, D. A., Trofimova, E. S., Danilets, M. G., Ligacheva, A. A., Konstantinov, A. I., Ufandeev, A. A., Rabtsevich, E. S., Drygunova, L. A., Zima, A. P., Bashirov, S. R., Udut, E. V., & Belousov, M. V. (2024). Coal-Derived Humic Substances: Insight into Chemical Structure Parameters and Biomedical Properties. Molecules, 29(7), 1530. https://doi.org/10.3390/molecules29071530