Preparation of Cobalt–Nitrogen Co-Doped Carbon Nanotubes for Activated Peroxymonosulfate Degradation of Carbamazepine
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
2.2. Catalytic Performance Evaluation
2.3. Parameters Impacting CBZ Degradation by the Co3@NCNT-800/PMS System
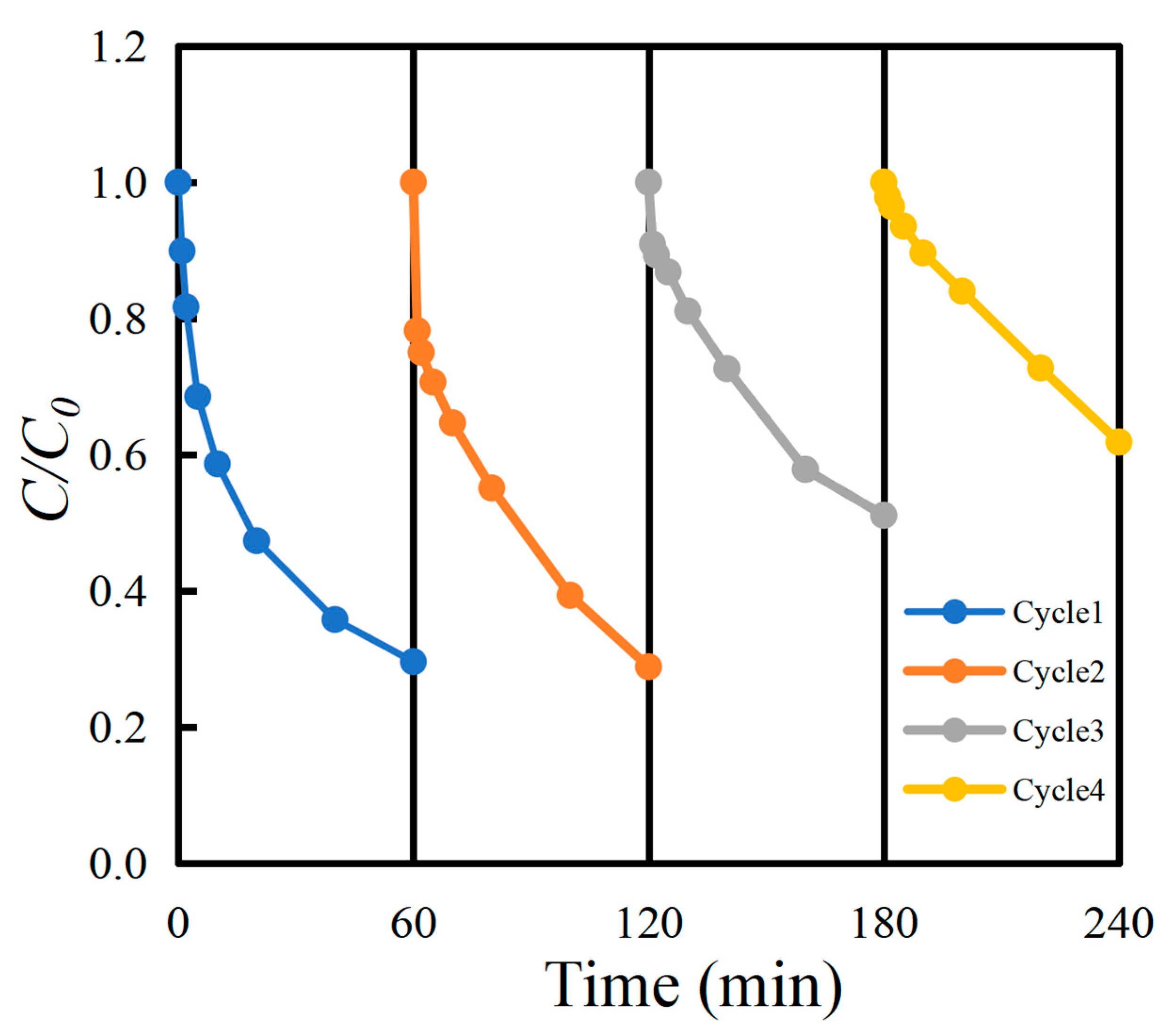
2.4. Recycling Studies
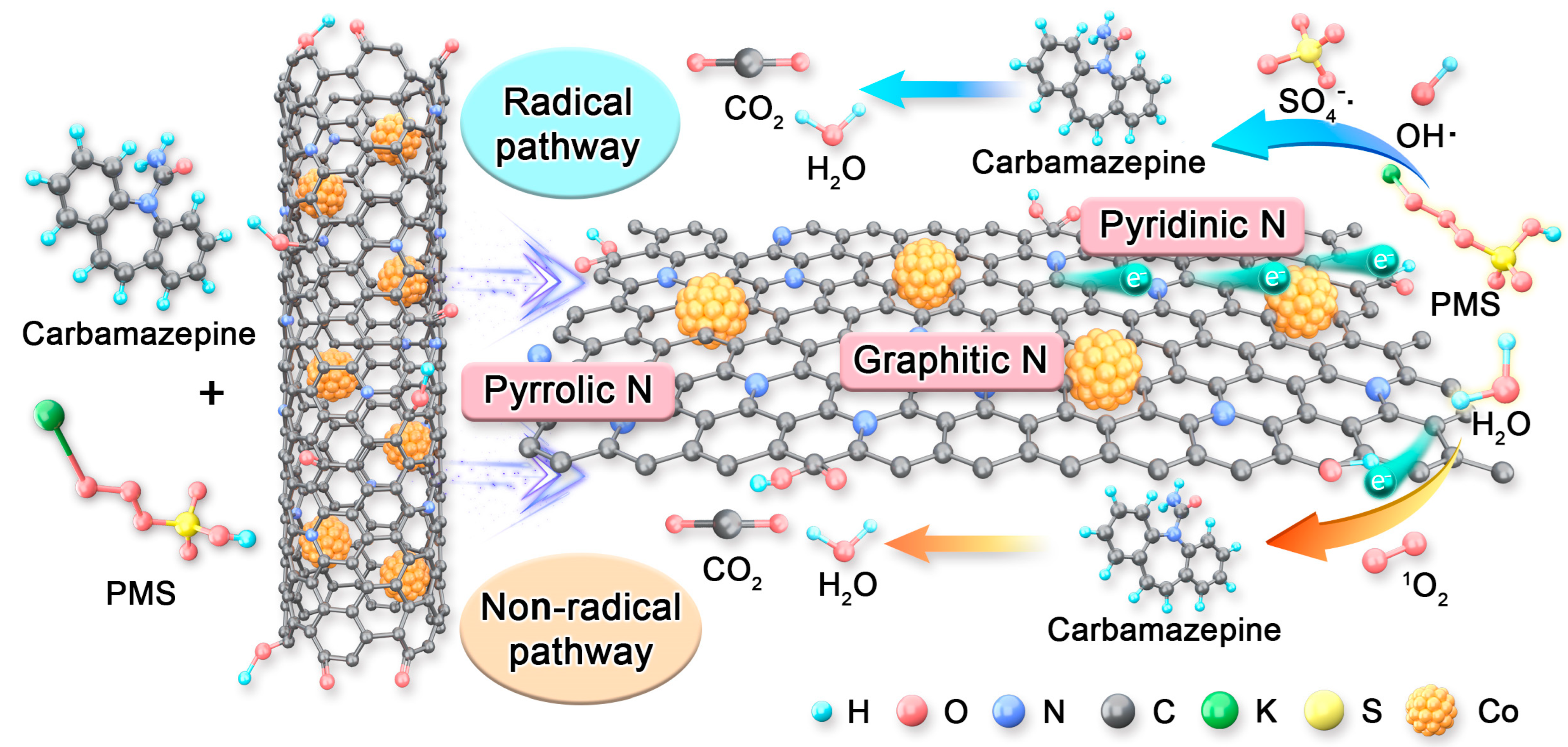
2.5. Main Reactive Oxygen Species (ROS) of the Co3@NCNT-800/PMS System
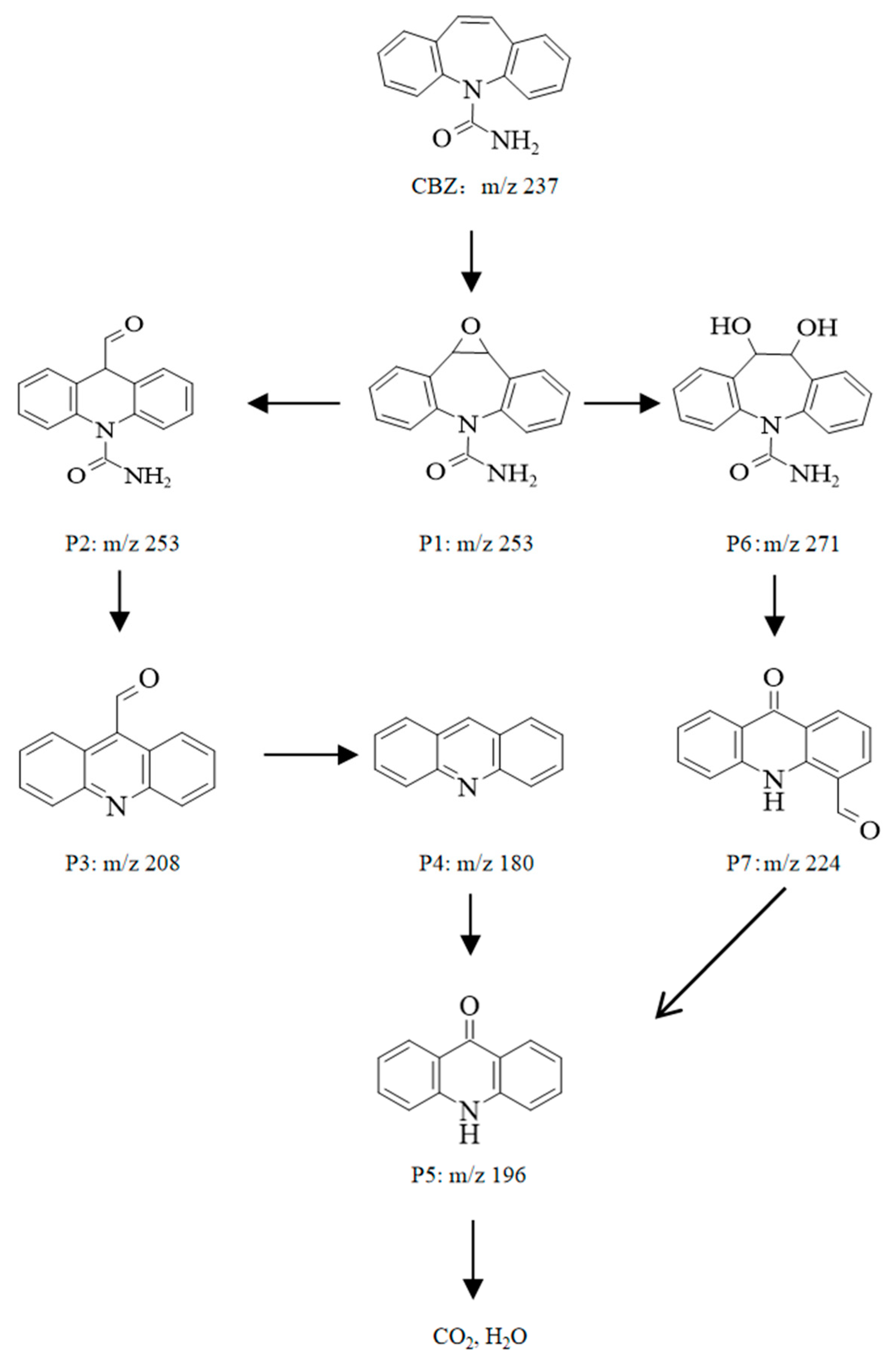
2.6. Analysis of the Intermediates and Pathways of CBZ Degradation
3. Materials and Methods
3.1. Preparation of Co–N Co-Doped CNTs
3.2. Characterization
3.3. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, Y.; Zou, M.; Ajarem, J.S.; Allam, A.A.; Wang, Z.; Qu, R.; Zhu, F.; Huo, Z. Catalytic degradation of pharmaceutical and personal care products in aqueous solution by persulfate activated with nanoscale FeCoNi-ternary mixed metal oxides. Sep. Purif. Technol. 2023, 314, 123585. [Google Scholar] [CrossRef]
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Tang, K.H.D.; Chin, B.L.F.; Chan, Y.H.; Yiin, C.L.; Cheah, K.W.; Chai, Y.H.; et al. Technologies for removing pharmaceuticals and personal care products (PPCPs) from aqueous solutions: Recent advances, performances, challenges and recommendations for improvements. J. Mol. Liq. 2023, 374, 121144. [Google Scholar] [CrossRef]
- Salah, M.; Zheng, Y.; Wang, Q.; Li, C.; Li, Y.; Li, F. Insight into pharmaceutical and personal care products removal using constructed wetlands: A comprehensive review. Sci. Total Environ. 2023, 885, 163721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shen, J.; Zhong, Y.; Ding, T.; Dissanayake, P.D.; Yang, Y.; Tsang, Y.F.; Ok, Y.S. Sorption of pharmaceuticals and personal care products (PPCPs) from water and wastewater by carbonaceous materials: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 727–766. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croue, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Y.; Chen, Y.; Yao, B.; Chen, X.; Yu, Y.; Yang, J.; Zhou, Y. Pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Biotoxicity, determination and electrochemical treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Feijoo, S.; Kamali, M.; Dewil, R. A review of wastewater treatment technologies for the degradation of pharmaceutically active compounds: Carbamazepine as a case study. Chem. Eng. J. 2023, 455, 140589. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kaur, N.; Selvaraj, M.; Ghramh, H.A.; Al-Shehri, B.M.; Singh, G.; Arya, S.K.; Bhatt, K.; Ghotekar, S.; Mani, R.; et al. Laccase-assisted degradation of emerging recalcitrant compounds-A review. Bioresour. Technol. 2022, 364, 128031. [Google Scholar] [CrossRef]
- Trognon, J.; Albasi, C.; Choubert, J.-M. A critical review on the pathways of carbamazepine transformation products in oxidative wastewater treatment processes. Sci. Total Environ. 2024, 912, 169040. [Google Scholar] [CrossRef] [PubMed]
- Yeom, Y.; Han, J.; Zhang, X.; Shang, C.; Zhang, T.; Li, X.; Duan, X.; Dionysiou, D.D. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants. Chem. Eng. J. 2021, 424, 130053. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Sun, Z.; Wang, Z. Degradation of carbamazepine from wastewater by ultrasound-enhanced zero-valent iron -activated persulfate system (US/Fe/PS): Kinetics, intermediates and pathways. Environ. Technol. 2022, 45, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef]
- Yang, L.; Hao, X.; Yu, D.; Zhou, P.; Peng, Y.; Jia, Y.; Zhao, C.; He, J.; Zhan, C.; Lai, B. High visible-light catalytic activity of Bis-PDI-T@TiO2 for activating persulfate toward efficient degradation of carbamazepine. Sep. Purif. Technol. 2021, 263, 118384. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; da Silva, L.P.; da Silva, J.C.G.E. Nanomaterial-Based Advanced Oxidation/Reduction Processes for the Degradation of PFAS. Nanomaterials 2023, 13, 1668. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Ma, J.; Li, G. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287, 131981. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Gul, N.S.; Sabahat, S.; Sun, J.; Tahir, K.; Shah, N.S.; Muhammad, N.; Rahim, A.; Imran, M.; Iqbal, J.; et al. Removal of organic pollutants through hydroxyl radical-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2023, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, G.; Li, N.; Lu, X.; Zhao, J.; He, M.; Yan, B.; Zhang, H.; Duan, X.; Wang, S. Landfill leachate treatment by persulphate related advanced oxidation technologies. J. Hazard. Mater. 2021, 418, 126355. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Domingues, E.; Silva, M.J.; Vaz, T.; Gomes, J.; Martins, R.C. Sulfate radical based advanced oxidation processes for agro-industrial effluents treatment: A comparative review with Fenton’s peroxidation. Sci. Total Environ. 2022, 832, 155029. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Tian, W.; Chen, S.; Zhang, H.; Wang, H.; Wang, S. Sulfate radical-based advanced oxidation processes for water decontamination using biomass-derived carbon as catalysts. Curr. Opin. Chem. Eng. 2022, 37, 100838. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Y.; Wang, Y.; Qi, J.; Tian, L.; Ma, J.; Wen, G.; Wang, L. Comparative study on heterogeneous activation of peroxydisulfate and peroxymonosulfate with black carbon derived from coal tar residues: Contribution of free radical, 1O2 and surface-bound radicals. J. Hazard. Mater. 2022, 433, 128819. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Liu, Z.; Tang, W.; Xiao, S.; Shao, B.; Liang, Q.; He, Q.; Pan, Y.; Zhao, C.; et al. Carbon nanotube-based materials for persulfate activation to degrade organic contaminants: Properties, mechanisms and modification insights. J. Hazard. Mater. 2022, 431, 128536. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Gao, W.; Cai, Y.; Wang, W.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Great increasing N content in Fe-embedded carbon nanotubes to effectively activate persulfate for radical and nonradical degradation of organic contaminants. Sep. Purif. Technol. 2024, 330, 125429. [Google Scholar] [CrossRef]
- Dai, Z.; Li, D.; Ao, Z.; Wang, S.; An, T. Theoretical exploration of VOCs removal mechanism by carbon nanotubes through persulfate-based advanced oxidation processes: Adsorption and catalytic oxidation. J. Hazard. Mater. 2021, 405, 124684. [Google Scholar] [CrossRef]
- Li, X.; Liang, D.; Wang, C.; Li, Y.; Duan, R.; Yu, L. Effective defect generation and dual reaction pathways for phenol degradation on boron-doped carbon nanotubes. Environ. Technol. 2022, 43, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Yao, Y.; Shi, Y.; Zhao, B.; Wang, Y. In situ synthesis of N, S co-doped hollow carbon microspheres for efficient catalytic oxidation of organic contaminants. Chin. Chem. Lett. 2022, 33, 1298–1302. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Li, C.; Cui, M.; Chen, Y.; Liu, R.; Cui, K.; Wu, K.; Nie, X.; Wang, S. Synthesizing and characterizing Fe3O4 embedded in N-doped carbon nanotubes-bridged biochar as a persulfate activator for sulfamethoxazole degradation. J. Clean. Prod. 2022, 353, 131669. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, X.; Chen, Q.; Zhou, J. Removal of 2,4-dichlorophenoxyacetic acid by the boron-nitrogen co-doped carbon nanotubes: Insights into peroxymonosulfate adsorption and activation. Sep. Purif. Technol. 2021, 259, 118196. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, X.; Yue, Q.; Gao, B.; Li, Y. Nitrogen-doped carbon nanotubes encapsulating Fe/Zn nanoparticles as a persulfate activator for sulfamethoxazole degradation: Role of encapsulated bimetallic nanoparticles and nonradical reaction. Environ. Sci. Nano 2020, 7, 1444–1453. [Google Scholar]
- Guo, Y.; Jin, Z.; Li, X.; Wang, F.; Yan, Y.; Feng, L. Nitrogen-doped carbon derived from reindeer manure for tetracycline hydrochloride removal: Synergetic effects of adsorption and catalysis. J. Environ. Chem. Eng. 2022, 10, 108286. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Liu, Y.; Liu, H.; Yang, Y. Oxidation of 2,4-dichlorophenol by non-radical mechanism using persulfate activated by Fe/S modified carbon nanotubes. J. Colloid Interface Sci. 2016, 469, 277–286. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, J.; Li, C.; Liao, Q.; Xiao, R.; Yang, W. Strong synergistic effect of Co3O4 encapsulated in nitrogen-doped carbon nanotubes on the nonradical-dominated persulfate activation. Carbon 2020, 158, 172–183. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Tade, M.O.; Ang, H.M.; Wang, S. Nitrogen- and Sulfur-Codoped Hierarchically Porous Carbon for Adsorptive and Oxidative Removal of Pharmaceutical Contaminants. ACS Appl. Mater. Interfaces 2016, 8, 7184–7193. [Google Scholar] [CrossRef]
- Wu, D.M.; Ye, P.; Wang, M.Y.; Wei, Y.; Li, X.X.; Xu, A.H. Cobalt nanoparticles encapsulated in nitrogen-rich carbon nanotubes as efficient catalysts for organic pollutants degradation via sulfite activation. J. Hazard. Mater. 2018, 352, 148–156. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-Doped Graphene for Generation and Evolution of Reactive Radicals by Metal-Free Catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef]
- Li, X.; Ao, Z.; Liu, J.; Sun, H.; Rykov, A.I.; Wang, J. Topotactic Transformation of Metal-Organic Frameworks to Graphene-Encapsulated Transition-Metal Nitrides as Efficient Fenton-like Catalysts. ACS Nano 2016, 10, 11532–11540. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Li, R.; Wu, Z.; Yang, Y.; Sun, S.; Tu, Y.; Ding, H. Photocatalytic persulfate activation by highly dispersed nano-TiO2 supported by silica for efficient carbamazepine degradation. Chem. Phys. Lett. 2024, 840, 141157. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Tan, J.; Li, J.; Wu, J.; Zhang, T.; Wang, X. Oxygen-doped porous graphitic carbon nitride in photocatalytic peroxymonosulfate activation for enhanced carbamazepine removal: Performance, influence factors and mechanisms. Chem. Eng. J. 2022, 429, 130860. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Wu, L.; Zhang, Y.; Wang, X.; Wu, Z. Hollow hemispherical Si-doped anatase for efficient carbamazepine degradation via photocatalytic activation of peroxymonosulfate. Chem. Eng. J. 2023, 457, 141234. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, X.; Li, Z.; Yin, R.; Qin, J.; Li, H.; Guo, W.; Li, A.J.; Qiu, R. Photo-Induced Holes Initiating Peroxymonosulfate Oxidation for Carbamazepine Degradation via Singlet Oxygen. Catalysts 2022, 12, 1327. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Miao, Y.; He, W.; Pan, Y.; Li, A.J.; Qin, J.; Li, H.; Yin, R.; Qiu, R. Coupled radical and nonradical activation of peroxymonosulfate by the piezo-photocatalytic effect of α-SnWO4/ZnO heterojunction to boost the degradation and detoxification of carbamazepine. Sep. Purif. Technol. 2023, 323, 124410. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, J.; Rahaman, M.H.; Wang, Q.; Zhai, J. The catalytic activity of different Mn(III) species towards peroxymonosulfate activation for carbamazepine degradation. Catal. Commun. 2023, 173, 106563. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, Y.; Chao, C.; Chen, Q.; Yao, S.; Liu, C. In Situ Synthesis of 3D BiOCl-Graphene Aerogel and Synergistic Effect by Photo-Assisted Activation of Persulfate for Methyl Orange Degradation. Molecules 2023, 28, 4964. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ge, D.; Cheng, Z.; Zhu, N.; Yuan, H.; Lou, Z. Improved understanding of dissolved organic matter transformation in concentrated leachate induced by hydroxyl radicals and reactive chlorine species. J. Hazard. Mater. 2020, 387, 121702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Y. Effective and continuous degradation of pollutants via carbon felt loaded with Co3O4 as three-dimensional electrode: Collaboration between ROS. Sep. Purif. Technol. 2023, 308, 122962. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.; Wang, C.; Zhang, M.; Khan, M.A.N.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef]
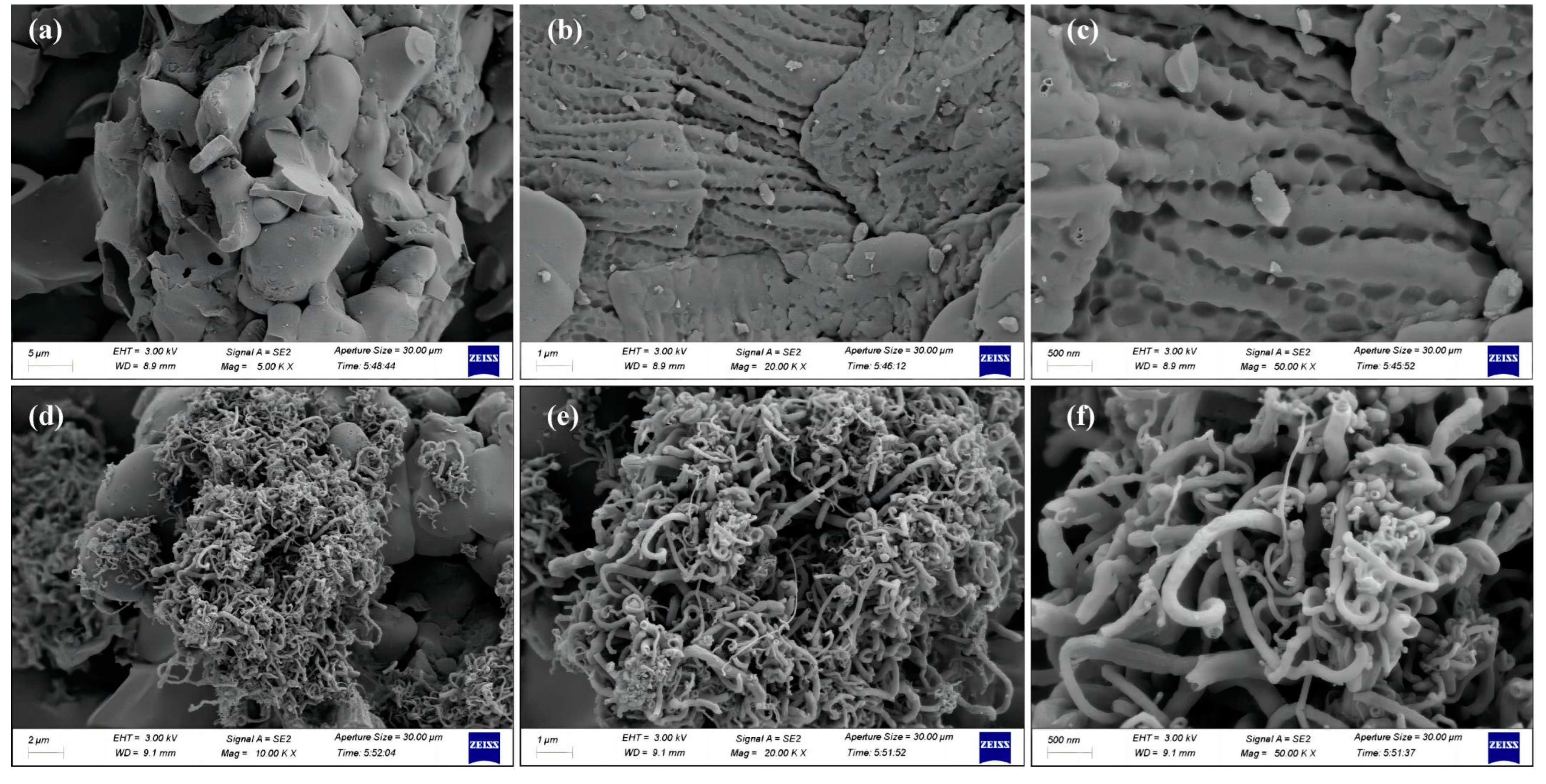
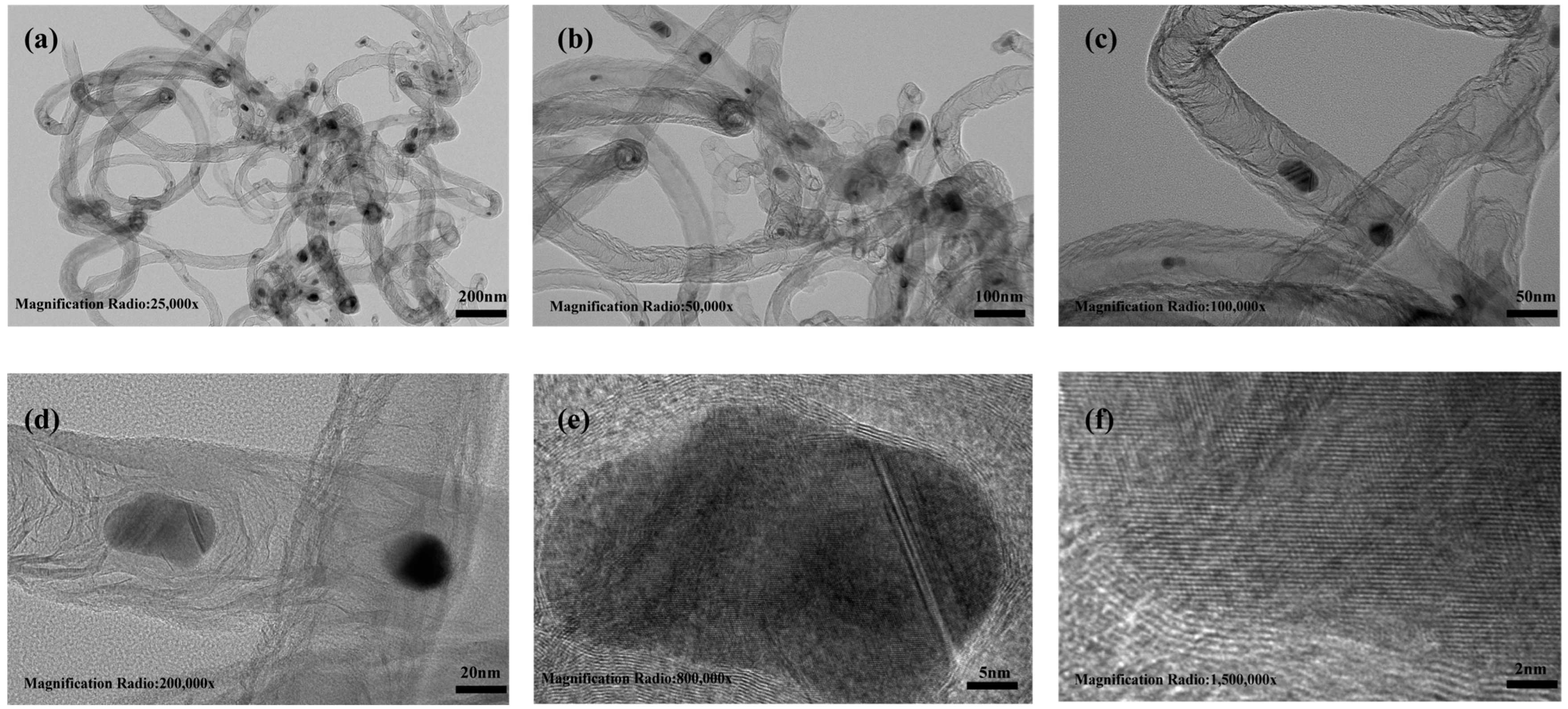

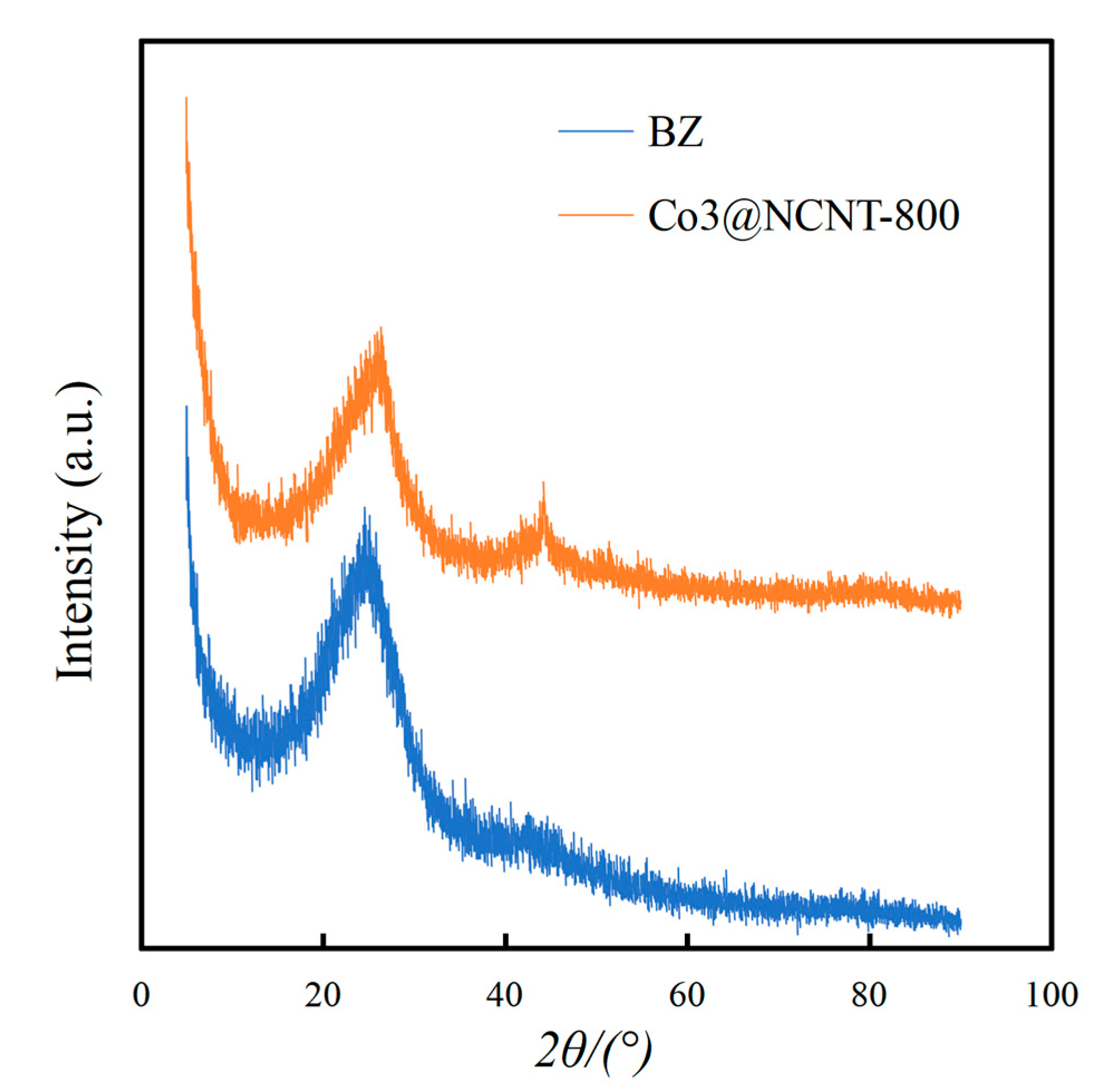



| Sample | C | H | N | O | SBET | Vtotal | Vmicro | Vmeso |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (m2·g−1) | (cm3·g−1) | (cm3·g−1) | (cm3·g−1) | |
| Co0@NCNT-800 | 70.5 | 1.2 | 10.8 | 11.3 | 677.2 | 0.386 | 0.287 | 0.099 |
| Co1@NCNT-800 | 70.1 | 1.2 | 11.5 | 10.4 | 715.4 | 0.405 | 0.295 | 0.110 |
| Co3@NCNT-800 | 67.5 | 1.1 | 11.5 | 9.6 | 614.0 | 0.322 | 0.252 | 0.070 |
| Catalysts (g/L) | Reaction Conditions | CBZ (mg/L) | Removal Efficiency | Ref. |
|---|---|---|---|---|
| A-SiO2/TiO2 (1 g/L) | [PMS] = 0.02 mmol/L, T = 30 °C | 20.0 mg/L | >35% (60 min) | [46] |
| g-C3N4 (1 g/L) | [PMS] = 5 mmol/L, T = room temperature | 5.0 mg/L | >15% (60 min) | [47] |
| g-C3N4 doping with ammonium oxalate (OCN) (1 g/L) | [PMS] = 5 mmol/L, T = room temperature | 5.0 mg/L | >36% (60 min) | [47] |
| TiO2-400 (0.10 g/L) | [PMS] = 1 mmol/L, T = 20 °C | 10.0 mg/L | >33% (60 min) | [48] |
| Bi2WO6 under the irradiation of xenon lamp (1 g/L) | [PMS] = 0.5 g/L, T = 25 °C | 10.0 mg/L | >60% (60 min) | [49] |
| α-SnWO4/ZnO nanomaterial (1 g/L) | [PMS] = 0.6 g/L | 10.0 mg/L | >20% (60 min) | [50] |
| α-MnO2 (2 mmol/L) | [PMS] = 0.1 g/L | 5 mg/L | >64% (30 min) | [51] |
| Co3@NCNT-800 (0.2 g/L) | [PMS] = 1.0 mmol/L, T = room temperature | 20.0 mg/L | 64.7% (60 min) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, B.; Tan, Y.; Lou, Y.; Lin, J.; Liu, Y.; Feng, J.; Chen, H. Preparation of Cobalt–Nitrogen Co-Doped Carbon Nanotubes for Activated Peroxymonosulfate Degradation of Carbamazepine. Molecules 2024, 29, 1525. https://doi.org/10.3390/molecules29071525
Chu B, Tan Y, Lou Y, Lin J, Liu Y, Feng J, Chen H. Preparation of Cobalt–Nitrogen Co-Doped Carbon Nanotubes for Activated Peroxymonosulfate Degradation of Carbamazepine. Molecules. 2024; 29(7):1525. https://doi.org/10.3390/molecules29071525
Chicago/Turabian StyleChu, Bei, Yixin Tan, Yichen Lou, Jiawei Lin, Yiman Liu, Jiaying Feng, and Hui Chen. 2024. "Preparation of Cobalt–Nitrogen Co-Doped Carbon Nanotubes for Activated Peroxymonosulfate Degradation of Carbamazepine" Molecules 29, no. 7: 1525. https://doi.org/10.3390/molecules29071525
APA StyleChu, B., Tan, Y., Lou, Y., Lin, J., Liu, Y., Feng, J., & Chen, H. (2024). Preparation of Cobalt–Nitrogen Co-Doped Carbon Nanotubes for Activated Peroxymonosulfate Degradation of Carbamazepine. Molecules, 29(7), 1525. https://doi.org/10.3390/molecules29071525






