Poly(silyl ether)s as Degradable and Sustainable Materials: Synthesis and Applications
Abstract
1. Introduction
2. Synthesis of PSEs
2.1. From Disubstituted Silanes
2.1.1. R2SiX2 with Diols
2.1.2. R2SiX2 with Bisepoxide and Bisoxetane by Polyaddition
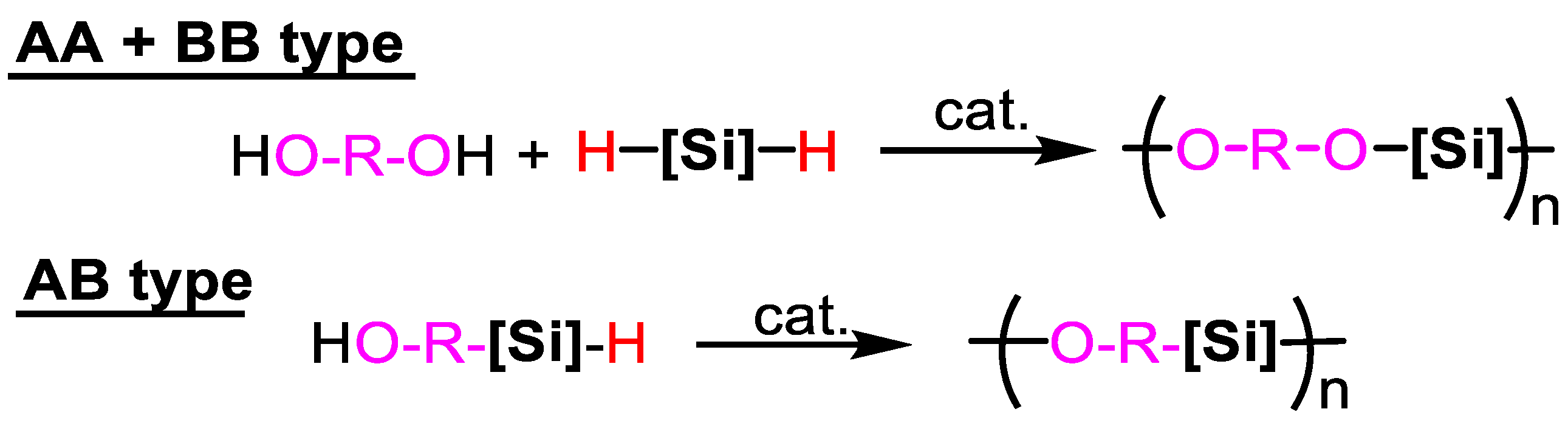
2.2. From Dihydrosilanes
2.2.1. Hydrosilylation Polymerization with Dicarbonyls
2.2.2. Dehydrogenative Cross-Coupling Polycondensation with Diols
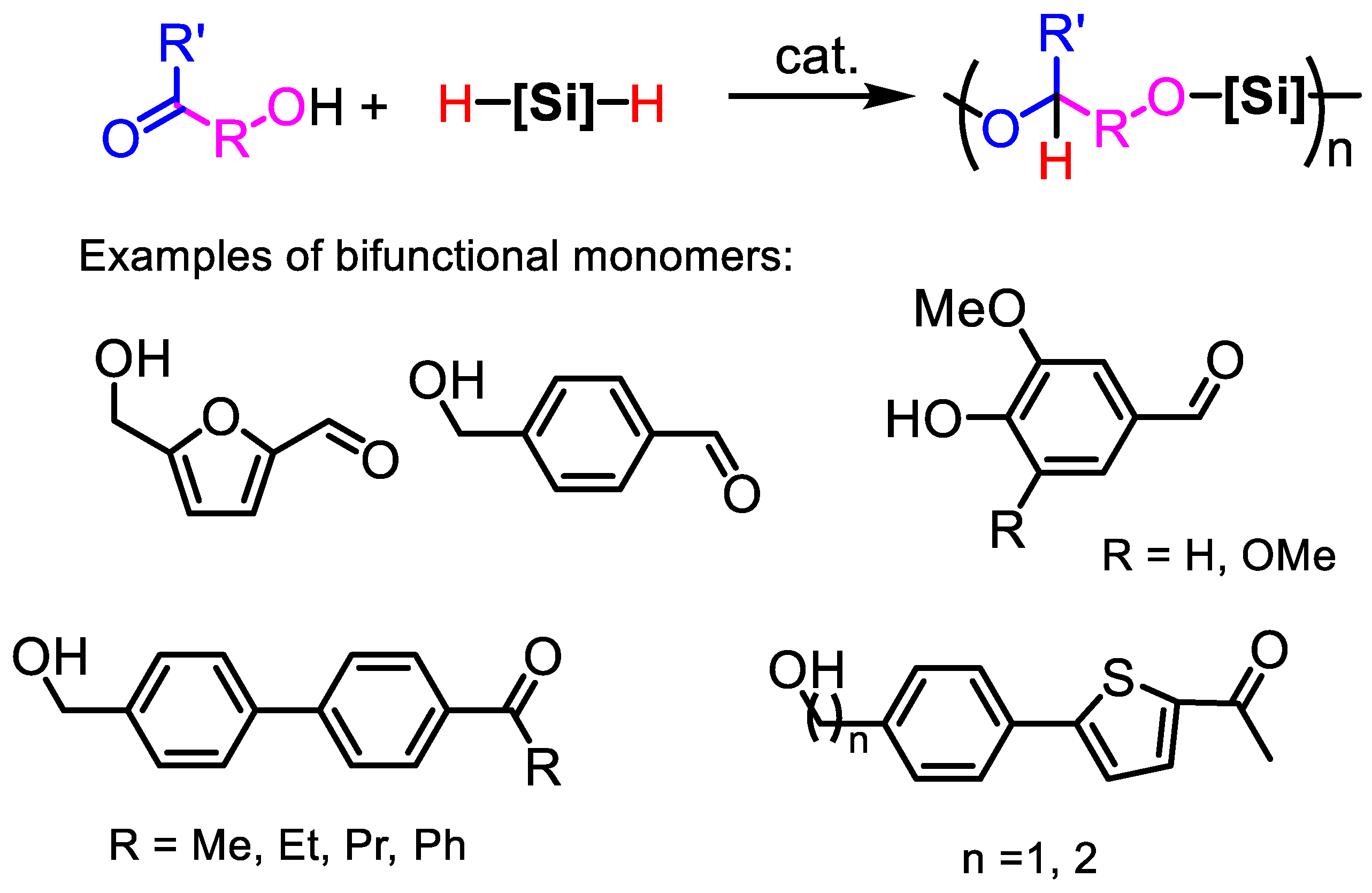
2.2.3. Polymerization with Bifunctional Monomers
2.2.4. Polymerization with Diepoxides
2.2.5. Polymerization with Dialkylether of Bis-Phenols
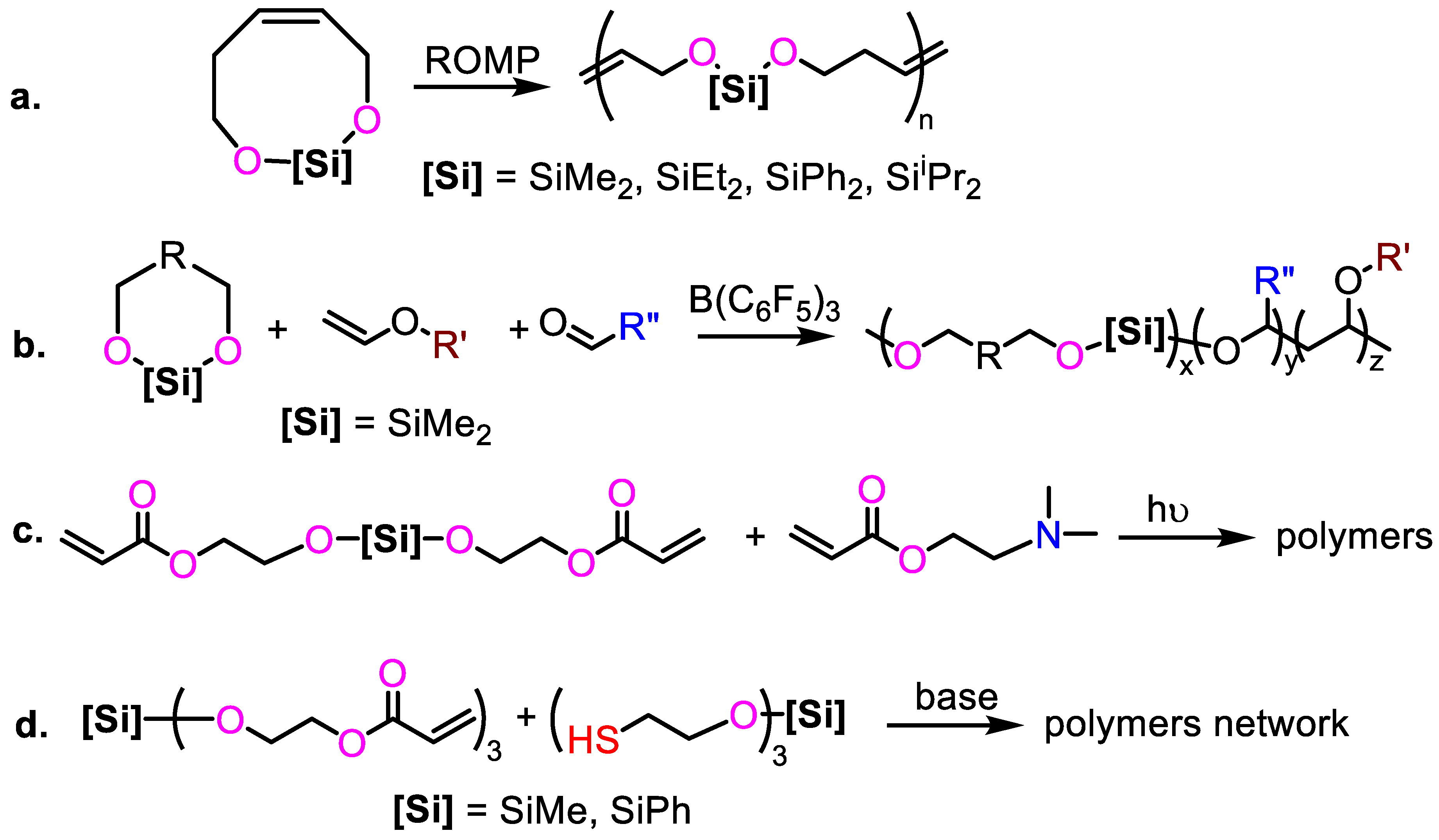
2.3. Polyaddtion of Cyclopolysilanes and Cyclodisilanes
2.4. Rearrangement of Poly(acyl silane)s
2.5. Other Synthetic Pathways for PSEs
3. Properties and Applications of PSEs
3.1. Thermal Properties and Hydrolytic Degradability
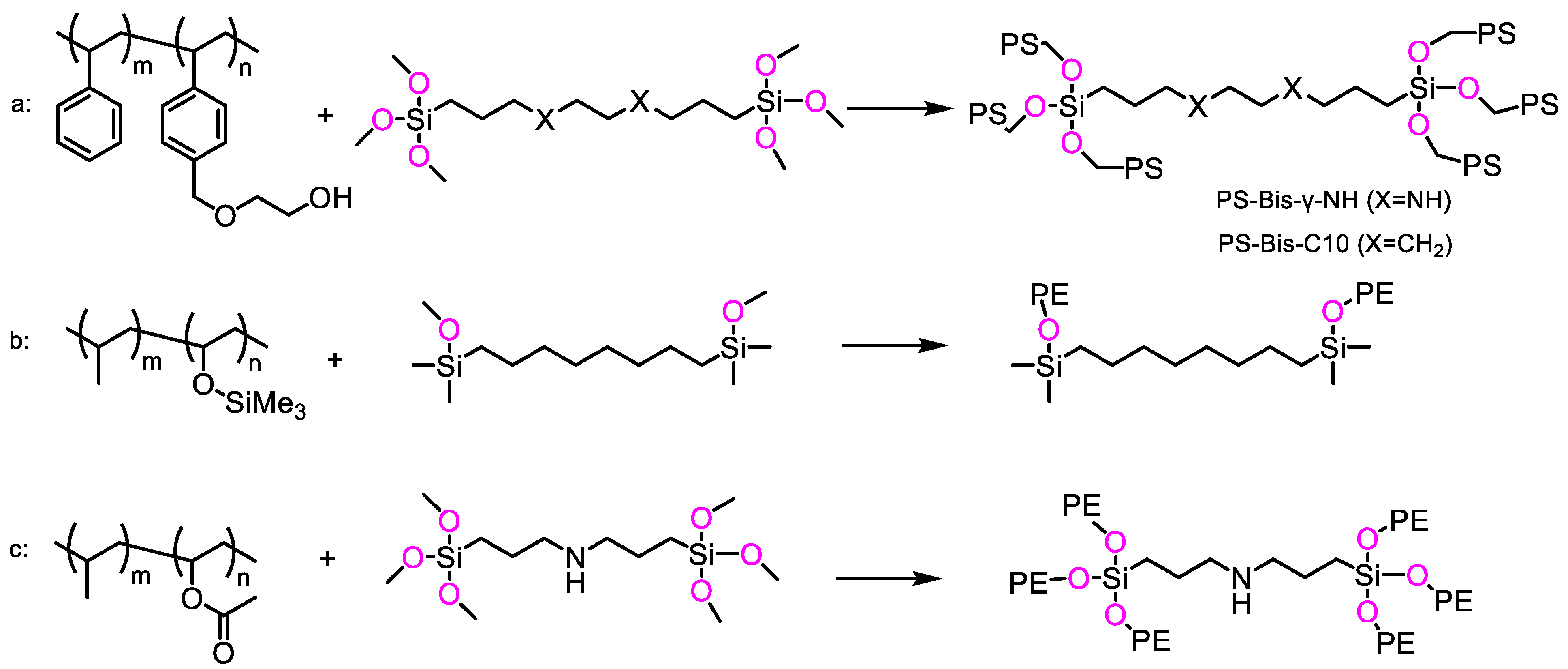
3.2. Applications in Materials Science
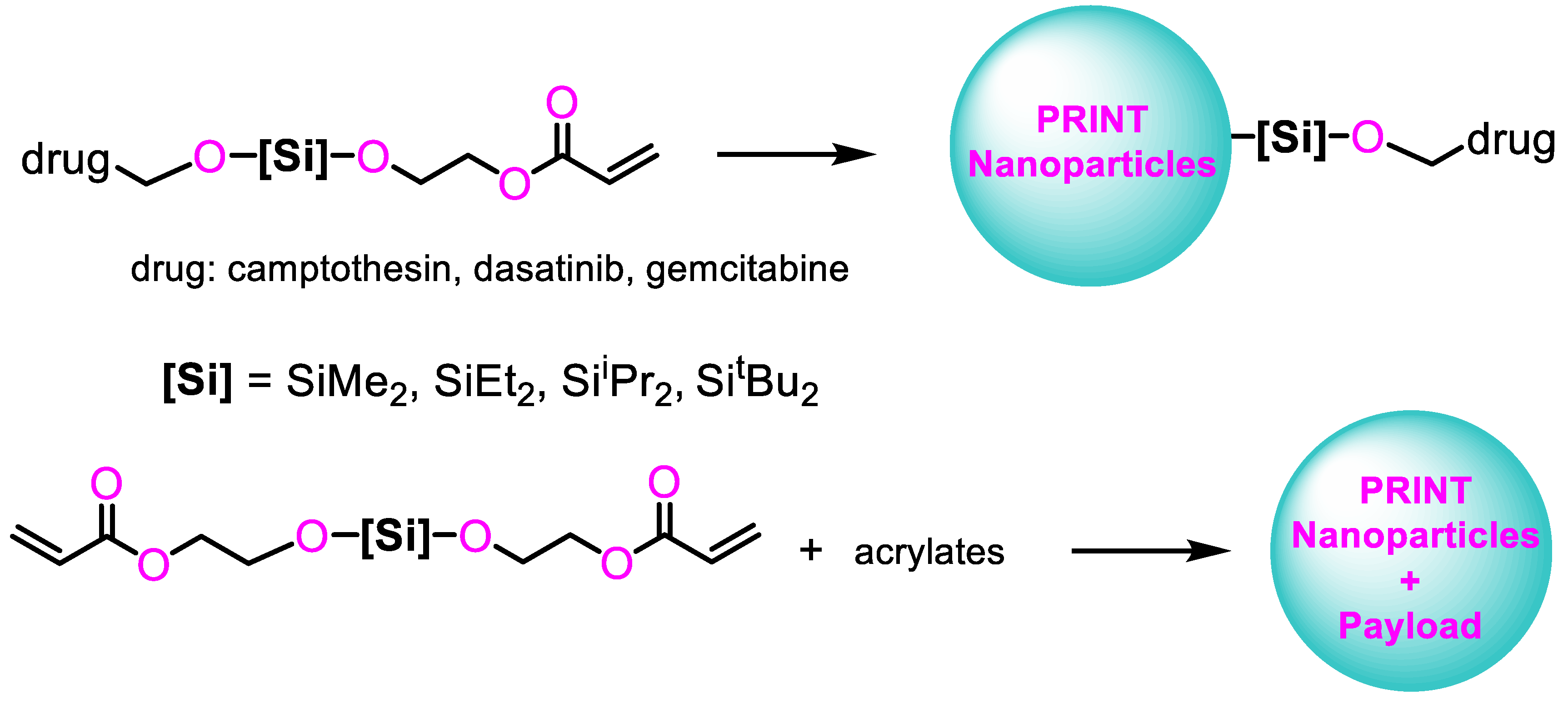
3.3. Applications in Biomedicine
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, A.; Santhamoorthy, M.; Lee, Y.-C. Recent advances in the pH-responsive organic–inorganic mesoporous hybrid silica for targeted drug delivery. Eur. Polym. J. 2024, 206, 112783. [Google Scholar] [CrossRef]
- Anderson, M.T.; Sawyer, P.S.; Rieker, T. Surfactant-templated silica aerogels. Microporous Mesoporous Mater. 1998, 20, 53–65. [Google Scholar] [CrossRef]
- Huesing, N.; Raab, C.; Torma, V.; Roig, A.; Peterlik, H. Periodically mesostructured silica monoliths from diol-modified silanes. Chem. Mater. 2003, 15, 2690–2692. [Google Scholar] [CrossRef]
- Sun, H.; Liang, Y.; Thompson, M.P.; Gianneschi, N.C. Degradable polymers via olefin metathesis polymerization. Prog. Polym. Sci. 2021, 120, 101427. [Google Scholar] [CrossRef]
- Hollstein, S.; von Delius, M. The Dynamic Chemistry of Orthoesters and Trialkoxysilanes: Making Supramolecular Hosts Adaptive, Fluxional, and Degradable. Acc. Chem. Res. 2024, 57, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Dvornic, P.R.; Lenz, R.W. Exactly alternating silarylene-siloxane polymers. 10. Synthesis and characterization of silphenylene-siloxane polymers containing fluoroalkyl and hydrido side groups. Macromolecules 1994, 27, 5833–5838. [Google Scholar] [CrossRef]
- Ohshita, J.; Watanabe, T.; Kanaya, D.; Ohsaki, H.; Ishikawa, M. Polymeric organosilicon systems. 22. Synthesis and photochemical properties of poly[(disilanylene)oligophenylylenes] and poly[(silylene)biphenylylenes]. Organometallics 1994, 13, 5002–5012. [Google Scholar] [CrossRef]
- Feigl, A.; Bockholt, A.; Weis, J.; Rieger, B. Modern synthetic and application aspects of polysilanes: An underestimated class of materials? Adv. Polym. Sci. 2010, 235, 1–31. [Google Scholar]
- Mazurek, M.H. Silicones. In Comprehensive Organometallic Chemistry III; Mingos, D.M.P., Crabtree, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3, pp. 651–697. [Google Scholar]
- Meng, Y.; Wei, Z.; Lu, Y.L.; Zhang, L.Q. Structure, morphology, and mechanical properties of polysiloxane elastomer composites prepared by in situ polymerization of zinc dimethacrylate. eXPRESS Polym. Lett. 2012, 6, 882–894. [Google Scholar] [CrossRef]
- Merker, R.L.; Scott, M.J. Preparation and properties of poly(tetramethyl-p-silphenylene-siloxane). J. Polym. Sci. Part A 1964, 2, 15–29. [Google Scholar] [CrossRef]
- Merker, R.L.; Scott, M.J.; Haberland, G.G. Random and block copolymers of poly(tetramethyl-p-silphenylene-siloxane) and polydimethylsiloxane. J. Polym. Sci. Part A 1964, 2, 31–44. [Google Scholar] [CrossRef]
- Lauter, U.; Kantor, S.W.; Schmidt-Rohr, K.; MacKnight, W.J. Vinyl-substituted silphenylene siloxane copolymers: Novel high-temperature elastomers. Macromolecules 1999, 32, 3426–3431. [Google Scholar] [CrossRef]
- Dorset, D.; McCourt, M.P. Direct phase determination for polymer fibre X-ray data—The structure of poly(tetramethyl-p-silphenylene siloxane). Polymer 1997, 38, 1985–1989. [Google Scholar] [CrossRef]
- Dunnavant, W.R.; Markle, R.A.; Sinclair, R.G.; Stickney, P.B.; Curry, J.E.; Byrd, J.D. p,p’-Biphenol-dianilinosilane condensation copolymers. Macromolecules 1968, 1, 249–254. [Google Scholar] [CrossRef]
- Liu, Y.; Imae, I.; Makishima, A.; Kawakami, Y. Synthesis and characterization of poly (silphenylenesiloxane)s containing functional side groups, a study to high-temperature elastomer. Sci. Technol. Adv. Mater. 2003, 4, 27–34. [Google Scholar] [CrossRef]
- Martinez-Crespiera, S.; Ionescu, E.; Kleebe, H.Z.; Riedel, R. Pressureless synthesis of fully dense and crack-free SiOC bulk ceramics via photo-crosslinking and pyrolysis of a polysiloxane. J. Eur. Ceram. Soc. 2011, 31, 913–919. [Google Scholar] [CrossRef]
- Harshe, R.; Balan, C.; Riedel, R. Amorphous Si(Al)OC ceramic from polysiloxanes: Bulk ceramic processing, crystallization behavior and applications. J. Eur. Ceram. Soc. 2004, 24, 3471–3482. [Google Scholar] [CrossRef]
- Ji, F.; Li, Y.L.; Feng, J.M.; Su, D.; Wen, Y.Y.; Feng, Y.; Hou, F. Electrochemical performance of graphene nanosheets and ceramic composites as anodes for lithium batteries. J. Mater. Chem. 2009, 19, 9063–9067. [Google Scholar] [CrossRef]
- Liu, X.; Xie, K.; Wang, J.; Zheng, C.M.; Pan, Y. Si/Si-O-C composite anode materials exhibiting good C rate performances prepared by a sol–gel method. J. Mater. Chem. 2012, 22, 19621–19624. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Wimmer, M.; Neumann, C.; Riedel, R. Lithium intercalation into SiCN/disordered carbon composite. Part 1: Influence of initial carbon porosity on cycling performance/capacity. J. Solid State Electrochem. 2015, 19, 2763–2769. [Google Scholar] [CrossRef]
- Lu, K.; Erb, D.; Liu, M. Thermal stability and electrical conductivity of carbon-enriched silicon oxycarbide. J. Mater. Chem. C. 2016, 4, 1829–1837. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Gregori, G.; Kleebe, H.J.; Blum, Y.D.; Babonneau, F. Evolution of C-rich SiOC ceramics: Part II. Characterization by high lateral resolution techniques: Electron energy-loss spectroscopy, high-resolution TEM and energy-filtered TEM. Int. J. Mater. Res. 2006, 97, 710–720. [Google Scholar] [CrossRef]
- Seffer, J.F.; Detriche, S.; Nagy, J.B.; Delhalle, J.; Mekhalif, Z. Silylesterification of oxidized multi-wall carbon nanotubes by catalyzed dehydrogenative cross-coupling between carboxylic and hydrosilane functions. Appl. Surf. Sci. 2014, 305, 301–308. [Google Scholar] [CrossRef]
- Wang, M.; Gan, D.; Wooley, K.L. Linear and Hyperbranched Poly(silyl ester)s: Synthesis via Cross-Dehydrocoupling-Based Polymerization, Hydrolytic Degradation Properties, and Morphological Analysis by Atomic Force Microscopy. Macromolecules 2001, 34, 3215–3223. [Google Scholar] [CrossRef]
- Li, Y.; Kawakami, Y. Synthesis and properties of polymers containing silphenylene moiety via catalytic cross-dehydrocoupling polymerization of 1,4-bis(dimethylsilyl)benzene. Macromolecules 1999, 32, 8768–8773. [Google Scholar] [CrossRef]
- Mabry, M.J.; Runyon, M.K.; Weber, W.P. Poly(silyl ether)s by ruthenium-catalyzed hydrosilylation polymerization of aliphatic ω-dimethylsilyloxy ketones and copolymerization of aliphatic α,ω-diketones with α,ω-dihydridooligodimethylsiloxanes. Macromolecules 2002, 35, 2207–2211. [Google Scholar] [CrossRef]
- Minegishi, S.; Ito, M.; Kameyama, A.; Nishikubo, T. Synthesis of poly(silyl ether)s containing pendant chloromethyl groups by the polyaddition of bis(oxetane)s with dichlorosilanes. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2254–2259. [Google Scholar] [CrossRef]
- Miller, S.A. Sustainable polymers: Opportunities for the next decade. ACS Macro Lett. 2013, 2, 550–554. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef]
- Walker, T.R. (Micro)plastics and the UN Sustainable Development Goals. Curr. Opin. Green Sustain. Chem. 2021, 30, 100497. [Google Scholar] [CrossRef]
- Luleburgaz, S.; Tunca, U.; Durmaz, H. Poly(silyl ether)s (silyl ether copolymers) via hydrosilylation of carbonyl compounds. Polym. Chem. 2023, 14, 2949–2957. [Google Scholar] [CrossRef]
- Haudum, S.; Strasser, P.; Teasdale, I. Phosphorus and Silicon-Based Macromolecules as Degradable Biomedical Polymers. Macromol. Biosci. 2023, 23, 2300127. [Google Scholar] [CrossRef]
- Drake, K.; Mukherjee, I.; Mirza, K.; Ji, H.-F.; Bradley, J.-C.; Wei, Y. Novel diacetylinic aryloxysilane polymers: A new thermally cross-linkable high temperature polymer system. Macromolecules 2013, 46, 4370–4377. [Google Scholar] [CrossRef]
- Yun, S.B.; Park, Y.T. Synthesis and properties of poly(carbomethyloctylsiloxane)s by melt copolymerization of bis(diethylamino)methyloctylsilane and aryldiol derivatives. Bull. Korean Chem. Soc. 2008, 29, 2373–2378. [Google Scholar]
- Jung, I.K.; Park, Y.T. Melt copolymerization reactions between 1,3-bis(diethylamino)tetramethyldisiloxane and aryldiol derivatives. Bull. Korean Chem. Soc. 2011, 32, 1303–1309. [Google Scholar] [CrossRef][Green Version]
- Jung, E.A.; Park, Y.T. Synthesis and photoelectronic properties of thermally stable poly[oxy(2,7-fluoren-9-onenylene)oxy(diorganosilylene)]s. Bull. Korean Chem. Soc. 2012, 33, 2031–2037. [Google Scholar] [CrossRef][Green Version]
- Jung, E.A.; Park, Y.T. Synthesis and properties of poly[oxy(arylene)oxy(tetramethyldisilylene)]s via melt copolymerization reaction. Bull. Korean Chem. Soc. 2013, 34, 1637–1642. [Google Scholar] [CrossRef][Green Version]
- Liaw, D.J.; Liaw, B.Y. Synthesis and characterization of novel polyaryloxydiphenylsilane derived from 2,2′- dimethyl-biphenyl-4,4′-diol. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4591–4595. [Google Scholar] [CrossRef]
- Issam, A.M.; Haris, M. Synthesis, characterization and optical properties of novel nonlinear polysilylether. J. Inorg. Organomet. Polym. 2009, 19, 454–458. [Google Scholar] [CrossRef]
- Nye, S.A.; Swint, S.A. Synthesis and properties of polyoxyarylenesiloxanes. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 131–138. [Google Scholar] [CrossRef]
- Dunnavant, W.R.; Markle, R.A.; Stickney, P.B.; Curry, J.E.; Byrd, J.D. Synthesis of polyaryloxysilanes by melt-polymerizing dianilino- and diphenoxysilanes with aromatic diols. J. Polym. Sci. Part A Polym. Chem. 1967, 5, 707–724. [Google Scholar] [CrossRef]
- Uhlig, W. Convenient Approach to Novel Organosilicon Polymers. Organometallics 1994, 13, 2843–2848. [Google Scholar] [CrossRef]
- Nagasaki, Y.; Matsukura, F.; Masao, K.; Aoki, H.; Tokuda, T. New thermosensitive rubbery polymers. Synthesis of poly(siloxyethylene glycol) and its aqueous solution properties. Macromolecules 1996, 29, 5859–5863. [Google Scholar] [CrossRef]
- Sahmetlioglu, E.; Nguyen, H.T.H.; Nsengiyumva, O.; Göktürk, E.; Miller, S.A. Silicon acetal metathesis polymerization. ACS Macro Lett. 2016, 5, 466–470. [Google Scholar] [CrossRef]
- Nishikubo, T.; Kameyama, A.; Hayashi, N. A novel synthesis of poly(silyl ether)s by addition reactions of diepoxide with dichlorosilane compounds. Polym. J. 1993, 25, 1003–1005. [Google Scholar] [CrossRef][Green Version]
- Liaw, D.-J. Synthesis of poly(silyl ether) by the addition reaction of bisphenols diglycidyl ether and dichlorodiphenylsilane. Polymer 1997, 38, 5217–5219. [Google Scholar] [CrossRef]
- Nishikubo, T.; Kameyama, A.; Kimura, Y.; Fukuyo, K. Novel synthesis of poly(silyl ethers) by the addition reaction of bis(epoxides) with dichlorosilanes or bis(chlorosilanes). Macromolecules 1995, 28, 4361–4365. [Google Scholar] [CrossRef]
- Minegishi, S.; Kameyama, A.; Nishikubo, T. A novel synthesis of poly(sily1 ether-co-phosphate)s by the polyaddition of dichlorosilanes and dichlorophosphates with bisepoxides. React. Funct. Polym. 1996, 30, 317–325. [Google Scholar] [CrossRef]
- Nishikubo, T.; Kameyama, A.; Kimura, Y.; Nakamura, T. New synthesis of poly(silyl ether) and poly(germyl ether) by addition reactions of bisepoxides with dimethyldiphenoxysilane and dimethyldiphenoxygermane. Macromolecules 1996, 29, 5529–5534. [Google Scholar] [CrossRef]
- Li, Y.; Kawakami, Y. Efficient Synthesis of Poly(silyl ether)s by Pd/C and RhCl(PPh3)3-Catalyzed Cross-Dehydrocoupling Polymerization of Bis(hydrosilane)s with Diols. Macromolecules 1999, 32, 6871–6873. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Wang, X.Q.; Ding, Y.X.; Zhou, Y.G. Partially biobased polymers: The synthesis of polysilylethers via dehydrocoupling catalyzed by an anionic iridium complex. Chin. Chem. Lett. 2019, 31, 1197–1200. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Hu, S.; Shi, L.; Zhou, Y.G. Synthesis of Poly(silyl ethers) via Iridium-Catalyzed Dehydrocoupling Polymerization. Organometallics 2018, 37, 2342–2347. [Google Scholar] [CrossRef]
- Lázaro, G.; Fernández-Alvarez, F.J.; Iglesias, M.; Horna, C.; Vispe, E.; Sancho, R.; Lahoz, F.J.; Iglesias, M.; Pérez-Torrente, J.J.; Oro, L.A. Heterogeneous catalysts based on supported Rh–NHC complexes: Synthesis of high molecular weight poly(silyl ether)s by catalytic hydrosilylation. Catal. Sci. Technol. 2014, 4, 62–70. [Google Scholar] [CrossRef]
- Lázaro, G.; Iglesias, M.; Fernández-Alvarez, F.J.; Sanz Miguel, P.J.; Pérez-Torrente, J.J.; Oro, L.A. Synthesis of poly(silyl ether)s by Rhodium(I)–NHC catalyzed hydrosilylation: Homogeneous versus heterogeneous catalysis. ChemCatChem 2013, 5, 1133–1141. [Google Scholar] [CrossRef]
- Purkayastha, A.; Baruah, J.B. Silicon–oxygen bonding on diphenylsilane through palladium(ii)-catalysed reactions. Appl. Organometal. Chem. 2000, 14, 477–483. [Google Scholar] [CrossRef]
- Bullock, R.M. (Ed.) Catalysis without Precious Metals; Wiley VCH: Weinheim, Germany, 2010. [Google Scholar]
- Farcaş-Johnson, M.A.; Kyne, S.H.; Webster, R.L. Dehydrocoupling Polymerization: Poly(silylether) Synthesis by Using an Iron β-Diketiminate Catalyst. Chem. Eur. J. 2022, 28, e202201642. [Google Scholar] [CrossRef]
- Lichtenberg, C.; Viciu, L.; Adelhardt, M.; Sutter, J.; Meyer, K.; de Bruin, D.; Grützmacher, H. Low-valent iron (I) amido olefin complexes as promotors for dehydrogenation reactions. Angew. Chem. Int. Ed. 2015, 54, 5766–5771. [Google Scholar] [CrossRef]
- Lichtenberg, C.; Adelhardt, M.; Wörle, M.; Büttner, T.; Meyer, K.; Grützmacher, H. Mono- and dinuclear neutral and cationic iron (II) compounds supported by an amidinato-diolefin ligand: Characterization and catalytic application. Organometallics 2015, 34, 3079–3089. [Google Scholar] [CrossRef]
- Chidara, V.K.; Du, G. An Efficient Catalyst Based on Manganese Salen for Hydrosilylation of Carbonyl Compounds. Organometallics 2013, 32, 5034–5037. [Google Scholar] [CrossRef]
- Vijjamarri, S.; Chidara, V.K.; Rousova, J.; Du, G. Dehydrogenative Coupling of Alcohols and Carboxylic Acids with Silanes Catalyzed by a Salen Mn(V) complex. Catal. Sci. Technol. 2016, 6, 3886–3892. [Google Scholar] [CrossRef]
- Li, C.; Hua, X.; Mou, Z.; Liu, X.; Cui, D. Zinc-Catalyzed Hydrosilylation Copolymerization of Aromatic Dialdehydes with Diphenylsilane. Macromol. Rapid Commun. 2017, 38, 1700590. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, W.; Cui, C. Cesium carbonate catalyzed chemoselective hydrosilylation of aldehydes and ketones under solvent-free conditions. Chem. Eur. J. 2014, 20, 9259–9262. [Google Scholar] [CrossRef]
- Paulasaari, J.K.; Weber, W.P. Ruthenium-catalyzed hydrosilation copolymerization of aromatic α,ω-diketones with 1,3-tetramethyldisiloxane. Macromolecules 1998, 31, 7105–7107. [Google Scholar] [CrossRef]
- Mabry, J.M.; Paulasaari, J.K.; Weber, W.P. Synthesis of poly(silyl ethers) by Ru-catalyzed hydrosilylation. Polymer 2000, 41, 4423–4428. [Google Scholar] [CrossRef]
- Mabry, M.J.; Runyon, M.K.; Weber, W.P. Synthesis of copoly[arylene-1,2-dioxy/oligodimethylsiloxanylene]s by Ruthenium-catalyzed dehydrogenative silylation copolymerization of o-quinones with α,ω-dihydridooligodimethylsiloxanes. Macromolecules 2001, 34, 7264–7268. [Google Scholar] [CrossRef]
- Sample, C.S.; Lee, S.-H.; Bates, M.W.; Ren, J.M.; Lawrence, J.; Lensch, V.; Gerbec, J.A.; Bates, C.M.; Li, S.; Hawker, C.J. Metal-Free Synthesis of Poly(silyl ether)s under Ambient Conditions. Macromolecules 2019, 52, 1993–1999. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhai, X.-Y.; Wu, B.; Bai, Y.-Q.; Zhou, Y.-G. Synthesis of Chiral Poly(silyl ether)s via CuH-Catalyzed Asymmetric Hydrosilylation Polymerization of Diketones with Silanes. ACS Macro Lett. 2020, 9, 969–973. [Google Scholar] [CrossRef]
- Li, Y.; Seino, M.; Kawakami, Y. Asymmetric synthesis of optically active poly(silyl ether)s having reactive Si–H groups by stereoselective cross-dehydrocoupling polymerization of bis(silane)s with diols. Macromolecules 2000, 33, 5311–5314. [Google Scholar] [CrossRef]
- Vijjamarri, S.; Hull, M.; Kolodka, E.; Du, G. Renewable Isohexides-Based, Hydrolytically Degradable Poly(silylether)s with High Thermal Stability. ChemSusChem 2018, 11, 2881–2888. [Google Scholar] [CrossRef]
- Morris, L.J.; Hill, M.S.; Mahon, M.F.; Manners, I.; McMenamy, F.S.; Whittell, G.R. Heavier Alkaline-Earth Catalyzed Dehydrocoupling of Silanes and Alcohols for the Synthesis of Metallo-Polysilylethers. Chem. Eur. J. 2020, 26, 2954–2966. [Google Scholar] [CrossRef]
- Zhai, X.-Y.; Wang, X.-Q.; Zhou, Y.-G. Cobalt-catalyzed selective dehydrocoupling polymerization of prochiral silanes and diols. Eur. Polym. J. 2020, 134, 109832. [Google Scholar] [CrossRef]
- Cella, J.; Rubinsztajn, S. Preparation of polyaryloxysilanes and polyaryloxysiloxanes by B(C6F5)3 catalyzed polyetherification of dihydrosilanes and bis-phenols. Macromolecules 2008, 41, 6965–6971. [Google Scholar] [CrossRef]
- Cheng, C.; Watts, A.; Hillmyer, M.A.; Hartwig, J.F. Polysilylether: A degradable polymer from biorenewable feedstocks. Angew. Chem. Int. Ed. 2016, 55, 11872–11876. [Google Scholar] [CrossRef] [PubMed]
- Vijjamarri, S.; Chidara, V.K.; Du, G. Versatile Manganese Catalysis for the Synthesis of Poly(silylether)s from Diols and Dicarbonyls with Hydrosilanes. ACS Omega 2017, 2, 582–591. [Google Scholar] [CrossRef]
- Vijjamarri, S.; Streed, S.; Serum, E.M.; Sibi, M.P.; Du, G. Polymers from Bioderived Resources: Synthesis of Poly(silylether)s from Furan Derivatives Catalyzed by a Salen–Mn(V) Complex. ACS Sustain. Chem. Eng. 2018, 6, 2491–2497. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Wang, M.; Liu, B.; Liu, X.; Cui, D. Step-Growth Coordination Polymerization of 5-Hydroxymethyl Furfural with Dihydrosilanes: Synergistic Catalysis Using Heteroscopionate Zinc Hydride and B(C6F5)3. Angew. Chem. Int. Ed. 2019, 58, 11434–11438. [Google Scholar] [CrossRef]
- Fouilloux, H.; Rager, M.-N.; Ríos, P.; Conejero, S.; Thomas, C.M. Highly Efficient Synthesis of Poly(silylether)s: Access to Degradable Polymers from Renewable Resources. Angew. Chem. Int. Ed. 2022, 61, e202113443. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Wu, B.; Bai, Y.-Q.; Zhai, X.-Y.; Zhou, Y.-G. CuH-catalyzed consecutive hydrosilylation/dehydrocoupling polymerization of difunctional hydroxyketones with dihydrosilanes for syntheses of chiral poly(silyl ether)s. Eur. Polym. J. 2022, 177, 111474. [Google Scholar] [CrossRef]
- Yoshida, N.; Zhu, H.; Mitsuishi, M. Metal-free synthesis of alternating silylether–carbosilane copolymers using unsaturated ketones. Polym. Chem. 2024, 15, 1204–1211. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Laine, R.M. An Approach to Epoxy Resins: Oxysilylation of Epoxides. Macromolecules 2020, 53, 2249–2263. [Google Scholar] [CrossRef]
- Reddy, N.P.; Yamashita, H.; Tanaka, M. Palladium-catalyzed ring-opening copolymerization of cyclopolysilanes and cyclic disilanes with p-quinones. J. Am. Chem. Soc. 1992, 114, 6596–6597. [Google Scholar] [CrossRef]
- Ratushnyy, M.; Zhukhovitskiy, A.V. Polymer Skeletal Editing via Anionic Brook Rearrangements. J. Am. Chem. Soc. 2021, 143, 17931–17936. [Google Scholar] [CrossRef] [PubMed]
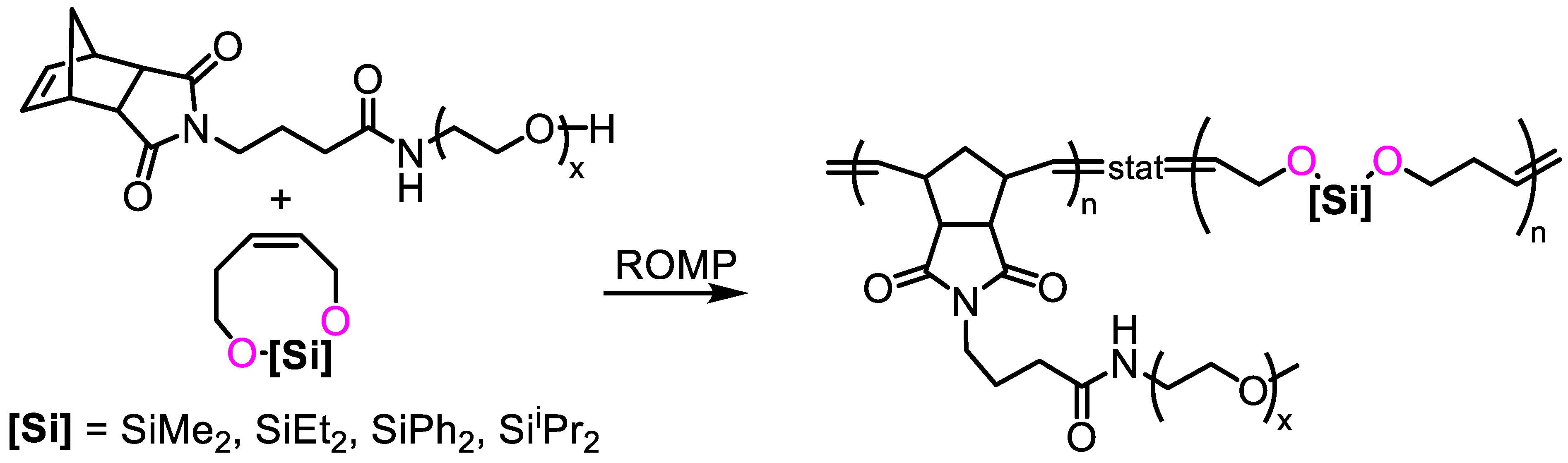
- Shieh, P.; Nguyen, H.V.; Johnson, J.A. Tailored silyl ether monomers enable backbone-degradable polynorbornene-based linear, bottlebrush and star copolymers through ROMP. Nat. Chem. 2019, 11, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Husted, K.E.L.; Kilgallon, L.J.; Johnson, J.A. Orthogonally deconstructable and depolymerizable polysilylethers via entropy-driven ring-opening metathesis polymerization. Chem. Commun. 2022, 58, 8496–8499. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Husted, K.E.L.; Wang, Y.; Kilgallon, L.J.; Shieh, P.; Zafar, H.; Lundberg, D.J.; Johnson, J.A. Thiol-triggered deconstruction of bifunctional silyl ether terpolymers via an SNAr-triggered cascade. Chem. Sci. 2023, 14, 8869–8877. [Google Scholar] [CrossRef] [PubMed]
- Hada, R.; Kanazawa, A.; Aoshima, S. Degradable Silyl Ether Polymers Synthesized by Sequence-Controlled Cationic Terpolymerization of 1,3-Dioxa-2-silacycloalkanes with Vinyl Ethers and Aldehydes. Macromolecules 2022, 55, 5474–5484. [Google Scholar] [CrossRef]
- Parrott, M.C.; Luft, J.C.; Byrne, J.D.; Fain, J.H.; Napier, M.E.; DeSimone, J.M. Tunable Bifunctional Silyl Ether Cross-Linkers for the Design of Acid-Sensitive Biomaterials. J. Am. Chem. Soc. 2010, 132, 17928–17932. [Google Scholar] [CrossRef]
- Bunton, C.M.; Bassampour, Z.M.; Boothby, J.M.; Smith, A.N.; Rose, J.V.; Nguyen, D.M.; Ware, T.H.; Csaky, K.G.; Lippert, A.R.; Tsarevsky, N.V.; et al. Degradable Silyl Ether–Containing Networks from Trifunctional Thiols and Acrylates. Macromolecules 2020, 53, 9890–9900. [Google Scholar] [CrossRef]
- Mohammed, I.A.; Shahabuddin, S.; Khanam, R.; Saidur, R. Synthesis, characterization and antibacterial activity of novel poly(silyl ether)s based on palm and soy oils. Polímeros 2018, 28, 406–412. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Wooley, K.L. Silyl ether-coupled poly(ε-caprolactone)s with stepwise hydrolytic degradation profiles. Biomacromolecules 2001, 2, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Sample, C.S.; Lee, S.-H.; Li, S.; Bates, M.W.; Lensch, V.; Versaw, B.A.; Bates, C.M.; Li, S.; Hawker, C.J. Metal-Free Room-Temperature Vulcanization of Silicones via Borane Hydrosilylation. Macromolecules 2019, 52, 7244–7250. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.-Q.; Liao, S.; Pan, Q.; Ma, X.; Wang, Y. Controllable Degradation of Polyurethane Thermosets with Silaketal Linkages in Response to Weak Acid. ACS Macro Lett. 2022, 11, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.; Zhang, W.; Husted, K.E.L.; Kristufek, S.L.; Xiong, B.; Lundberg, D.J.; Lem, J.; Veysset, D.; Sun, Y.; Nelson, K.A.; et al. Cleavable comonomers enable degradable, recyclable thermoset plastics. Nature 2020, 583, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Husted, K.E.L.; Shieh, P.; Lundberg, D.J.; Kristufek, S.L.; Johnson, J.A. Molecularly Designed Additives for Chemically Deconstructable Thermosets without Compromised Thermomechanical Properties. ACS Macro Lett. 2021, 10, 805–810. [Google Scholar] [CrossRef]
- Husted, K.E.L.; Brown, C.M.; Shieh, P.; Kevlishvili, I.; Kristufek, S.L.; Zafar, H.; Accardo, J.V.; Cooper, J.C.; Klausen, R.S.; Kulik, H.J.; et al. Remolding and Deconstruction of Industrial Thermosets via Carboxylic Acid-Catalyzed Bifunctional Silyl Ether Exchange. J. Am. Chem. Soc. 2023, 145, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Johnson, J.A. Thermally Robust yet Deconstructable and Chemically Recyclable High-Density Polyethylene (HDPE)-Like Materials Based on Si–O Bonds. Angew. Chem. Int. Ed. 2023, 62, e202315085. [Google Scholar] [CrossRef]
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z. Silyl Ether as a Robust and Thermally Stable Dynamic Covalent Motif for Malleable Polymer Design. J. Am. Chem. Soc. 2017, 139, 14881–14884. [Google Scholar] [CrossRef]
- Tretbar, C.A.; Neal, J.A.; Guan, Z. Direct Silyl Ether Metathesis for Vitrimers with Exceptional Thermal Stability. J. Am. Chem. Soc. 2019, 141, 16595–16599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Zhang, S.; Zhu, W.; Li, S.; Zhang, Y.; Hu, Y.; Zhou, G. Facile Approach for the Preparation of Robust and Thermally Stable Silyl Ether Cross-Linked Poly(ethylene-vinyl acetate) Vitrimers. ACS Appl. Polym. Mater. 2023, 5, 8379–8386. [Google Scholar] [CrossRef]
- Praveen, K.; Das, S.; Dhaware, V.; Pandey, B.; Mondal, B.; Gupta, S.S. pH-responsive “Supra-Amphiphilic” nanoparticles based on homoarginine polypeptides. ACS Appl. Bio. Mater. 2019, 2, 4162–4172. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.C.; Finniss, M.; Luft, J.C.; Pandya, A.; Gullapalli, A.; Napier, M.E.; DeSimone, J.M. Incorporation and Controlled Release of Silyl Ether Prodrugs from PRINT Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7978–7982. [Google Scholar] [CrossRef]
- Hillmyer, M.A. The promise of plastics from plants. Science 2017, 358, 868–870. [Google Scholar] [CrossRef]
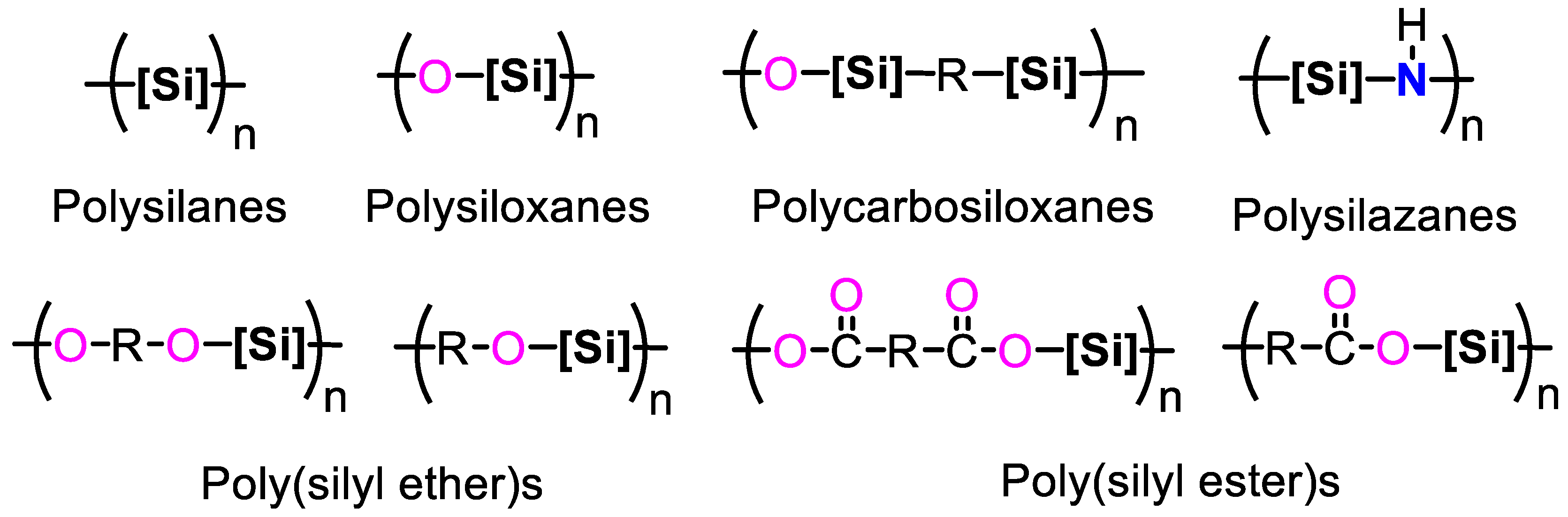
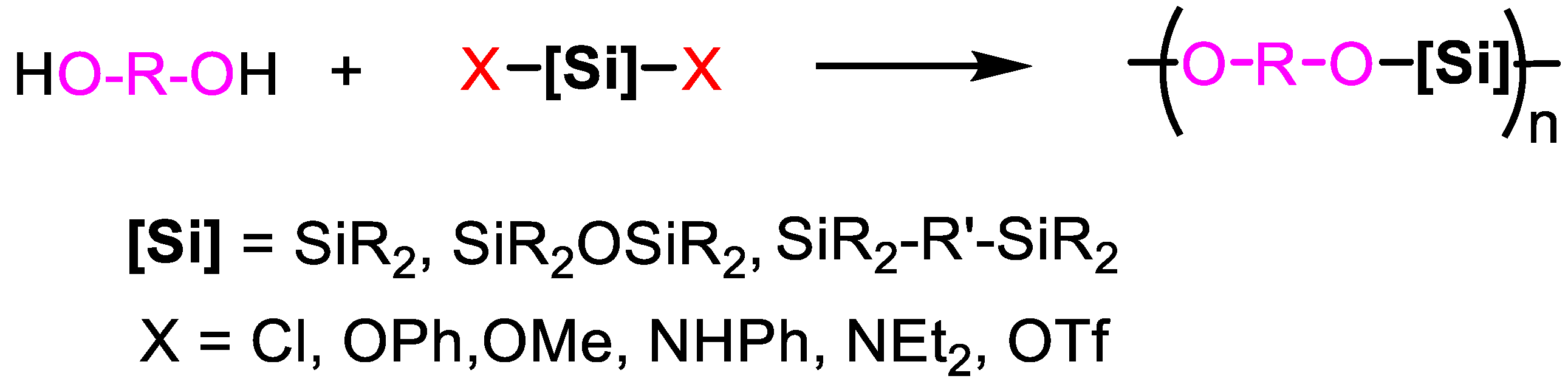




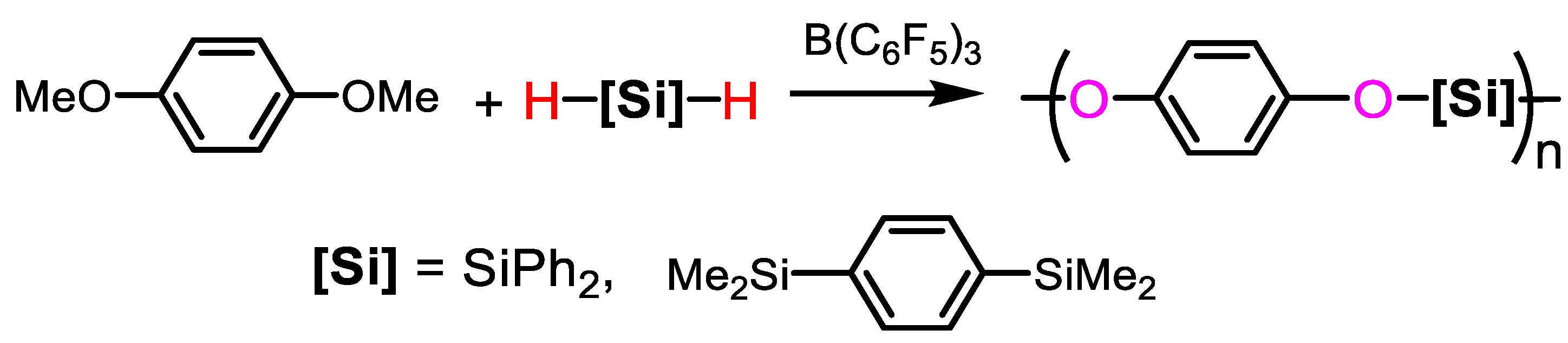


| PSE | R | [Si] | Mn (Đ) | Tg | Tm/Tc | T−5% | T−50% | Ref. |
|---|---|---|---|---|---|---|---|---|
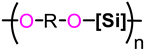 | ||||||||
| P1 | -CH2C6H4CH2- | SiPh2 | 9.2 (2.0) | 22 | [79] | |||
| P2 | -biphenyl- | SiPh2 | 7.2 | 101 | [79] | |||
| P3 | -isosorbide- | SiPh2 | 17.0 (2.08) | 85 | 410 | 498 | [74] | |
| P4 | -isosorbide- | SiPhMe | 13.3 (1.92) | 43 | 397 | 486 | [74] | |
| P5 | -isosorbide- | SiPhNp | 14.0 (2.00) | 120 | 446 | 509 | [74] | |
| P6 | -isomannide- | SiPh2 | 13.0 (2.25) | 76 | 384 | 494 | [74] | |
| P7 | -isomannide- | SiPhMe | 6.1 (2.05) | 42 | 367 | 484 | [74] | |
| P8 | -isomannide- | SiPhNp | 9.5 (1.56) | 116 | 432 | 505 | [74] | |
| P9 | -BHMF- | SiPh2 | 4.3 (1.33) | 9.8 | 445 | [80] | ||
| P10 | -BHMF- | SiPhMe | 3.2 (2.22) | 3.5 | 423 | [80] | ||
| P11 | -BHMF- | SiPhNp | 2.1 (1.24) | 27.8 | 473 | [80] | ||
| P12 | -difuran- | SiPh2 | 6.4 (1.69) | 2.5 | 422 | [80] | ||
| P13 | -(CHPh)2- | SiMe2C6H4SiMe2 | 55.0 (1.8) | 40 | 175/135 | 415 | [71] | |
| P14 | -(CHC6H4Br)2- | SiMe2C6H4SiMe2 | 48.2 (1.8) | 80 | 120/- | 380 | [71] | |
| P15 | -(CHC6H4Me)2- | SiMe2C6H4SiMe2 | 14.1 (2.1) | −2 | 195 | [71] | ||
| P16 | -(CHEt)2- | SiMe2C6H4SiMe2 | 27.3 (2.7) | −25 | 120/30 | 310 | [71] | |
| P17 | -2,2′-biphenyl- | SiMe2C6H4SiMe2 | 20.7 (1.7) | 70 | 335 | [71] | ||
| P18 | -(CHPh)2- | SiMe2OS+iMe2 | 7.0 (1.5) | −15 | 130/95 | 330 | [71] | |
| P19 | -(CH2)6- | SiMe2 | 3.4 (1.8) | −46 | 43/- | 175 | [48] | |
| P20 | -(CH2)8- | SiMe2 | 7.9 (1.1) | −52 | 57/- | 178 | [48] | |
| P21 | -(CH2)9- | SiMe2 | 9.4 (1.2) | −53 | 72/- | 184 | [48] | |
| P22 | -(CH2)10- | SiMe2 | 8.3 (1.4) | n.o. | 72/- | 202 | [48] | |
| P23 | -(CH2)2OC6H4O(CH2)2- | SiMe2 | 6.8 (3.3) | −47 | 105/- | 232 | [48] | |
| P24 | -(CH2CH2O)CH2CH2- | SiMe2 | 14.0 (1.5) | −81 | [47] | |||
| P25 | -(CH2CH2O)6CH2CH2- | SiMe2 | 11.8 (1.4) | −72 | −30/- | [47] | ||
| P26 | -(CH2CH2O)12CH2CH2- | SiMe2 | 3.7 (1.2) | −64 | 17/- | [47] | ||
| P27 | -(CH2CH2O)CH2CH2- | SiMe2OSiMe2 | 10.8 (1.3) | −95 | [47] | |||
 | ||||||||
| P28 | -(CH2)9- | SiMe2 | 92.7 (3.7) | −76 | 422 | 471 | [56] | |
| P29 | -(CH2)10- | SiMe2 | 25.5 (2.6) | n.o. | 350 | 466 | [56] | |
| P30 | -(CH2)11- | SiMe2 | 46.9 (2.9) | −83 | 421 | 469 | [56] | |
| P31 | -(CH2)3OC6H4CH2- | SiMe2 | 20.8 (1.7) | −42 | 324 | 412 | [56] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotov, V.; Vijjamarri, S.; Mousavi, S.-D.; Du, G. Poly(silyl ether)s as Degradable and Sustainable Materials: Synthesis and Applications. Molecules 2024, 29, 1498. https://doi.org/10.3390/molecules29071498
Zotov V, Vijjamarri S, Mousavi S-D, Du G. Poly(silyl ether)s as Degradable and Sustainable Materials: Synthesis and Applications. Molecules. 2024; 29(7):1498. https://doi.org/10.3390/molecules29071498
Chicago/Turabian StyleZotov, Vladimir, Srikanth Vijjamarri, Seyed-Danial Mousavi, and Guodong Du. 2024. "Poly(silyl ether)s as Degradable and Sustainable Materials: Synthesis and Applications" Molecules 29, no. 7: 1498. https://doi.org/10.3390/molecules29071498
APA StyleZotov, V., Vijjamarri, S., Mousavi, S.-D., & Du, G. (2024). Poly(silyl ether)s as Degradable and Sustainable Materials: Synthesis and Applications. Molecules, 29(7), 1498. https://doi.org/10.3390/molecules29071498






