Study on the Neuroprotective Effects of Eight Iridoid Components Using Cell Metabolomics
Abstract
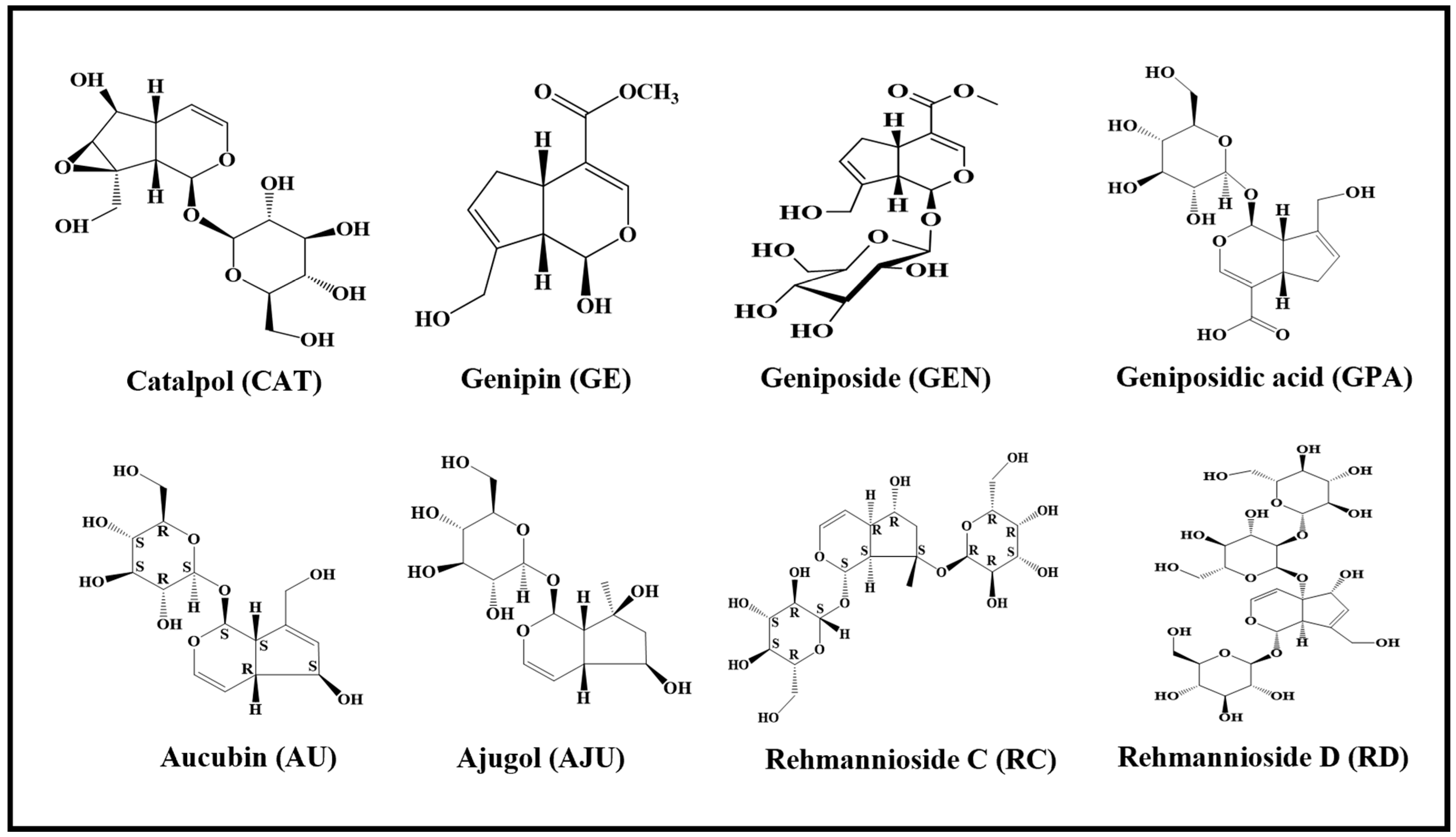
1. Introduction
2. Results
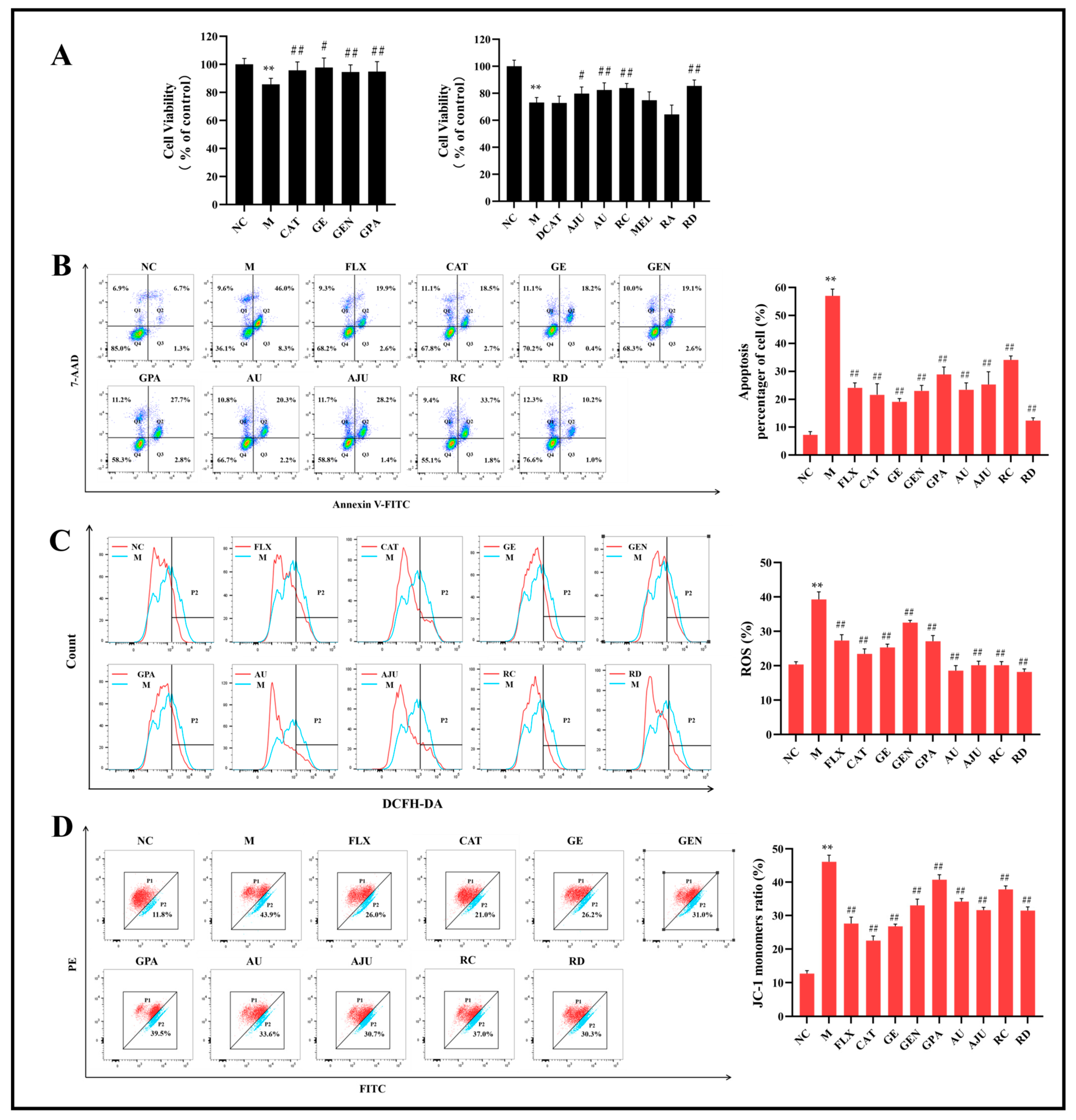
2.1. The Results of MTT Assay and Flow Cytometry
2.2. Method Validation Results
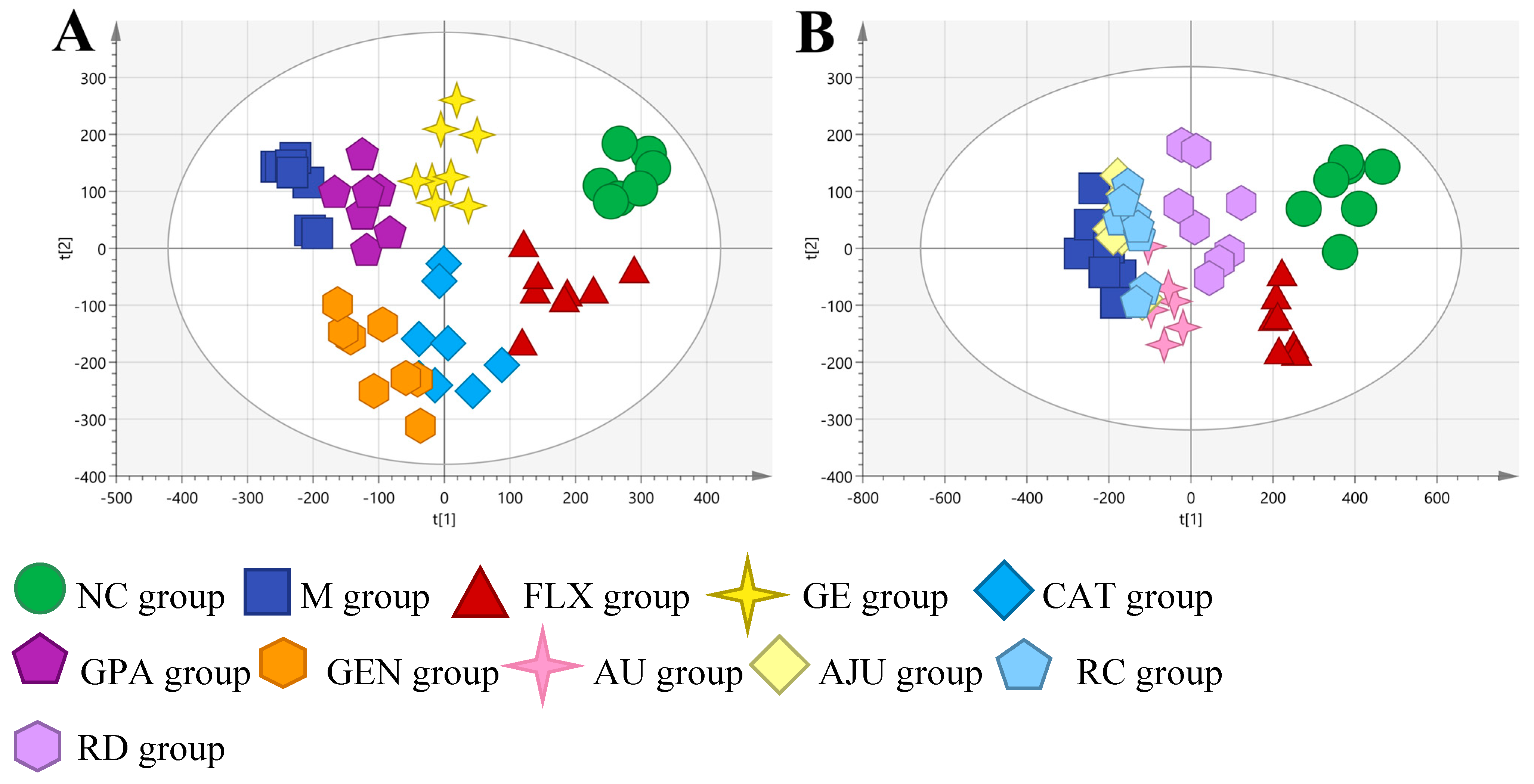
2.3. Metabolic Profiles of PC12 Cell Samples
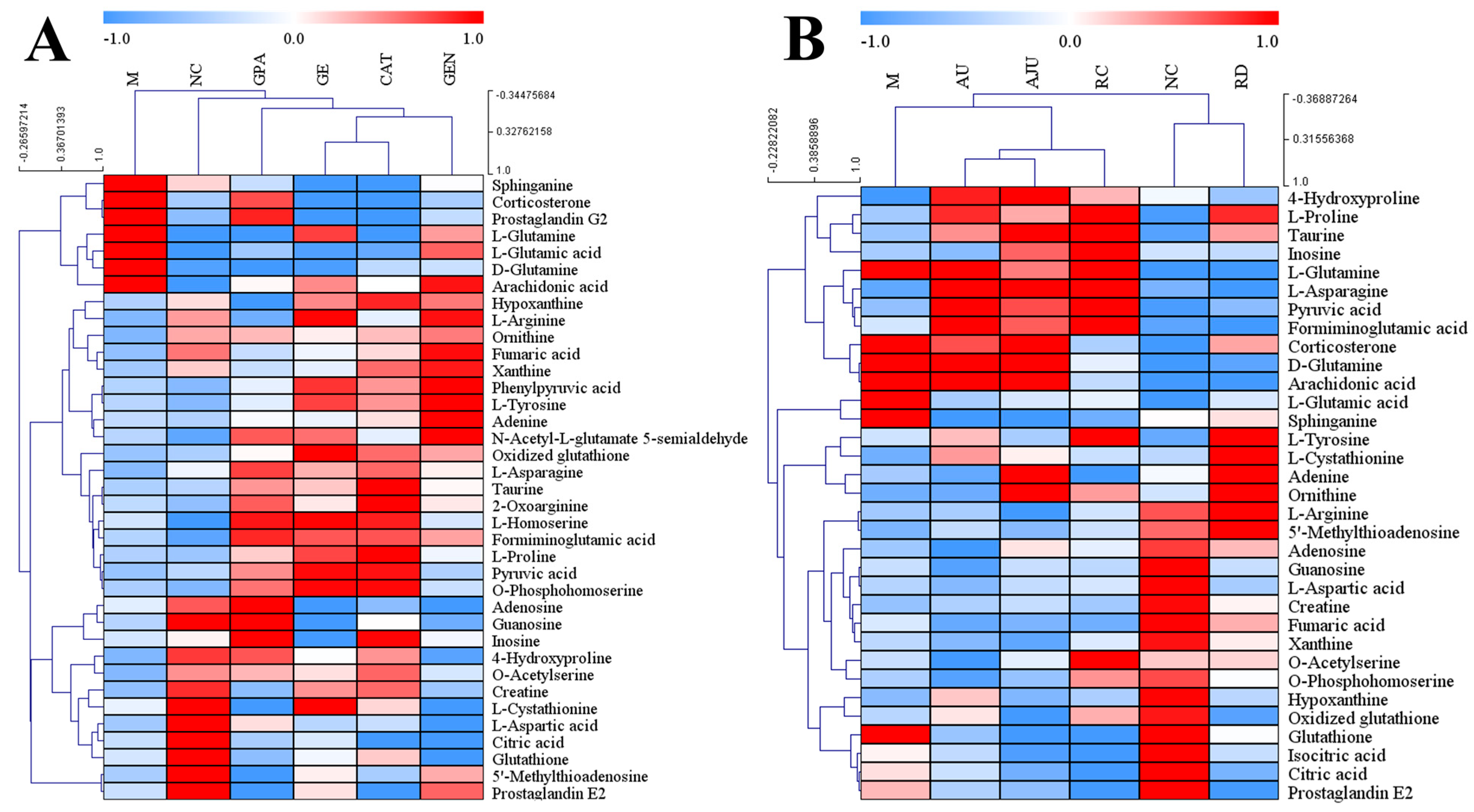
2.4. Identification of Potential Biomarkers
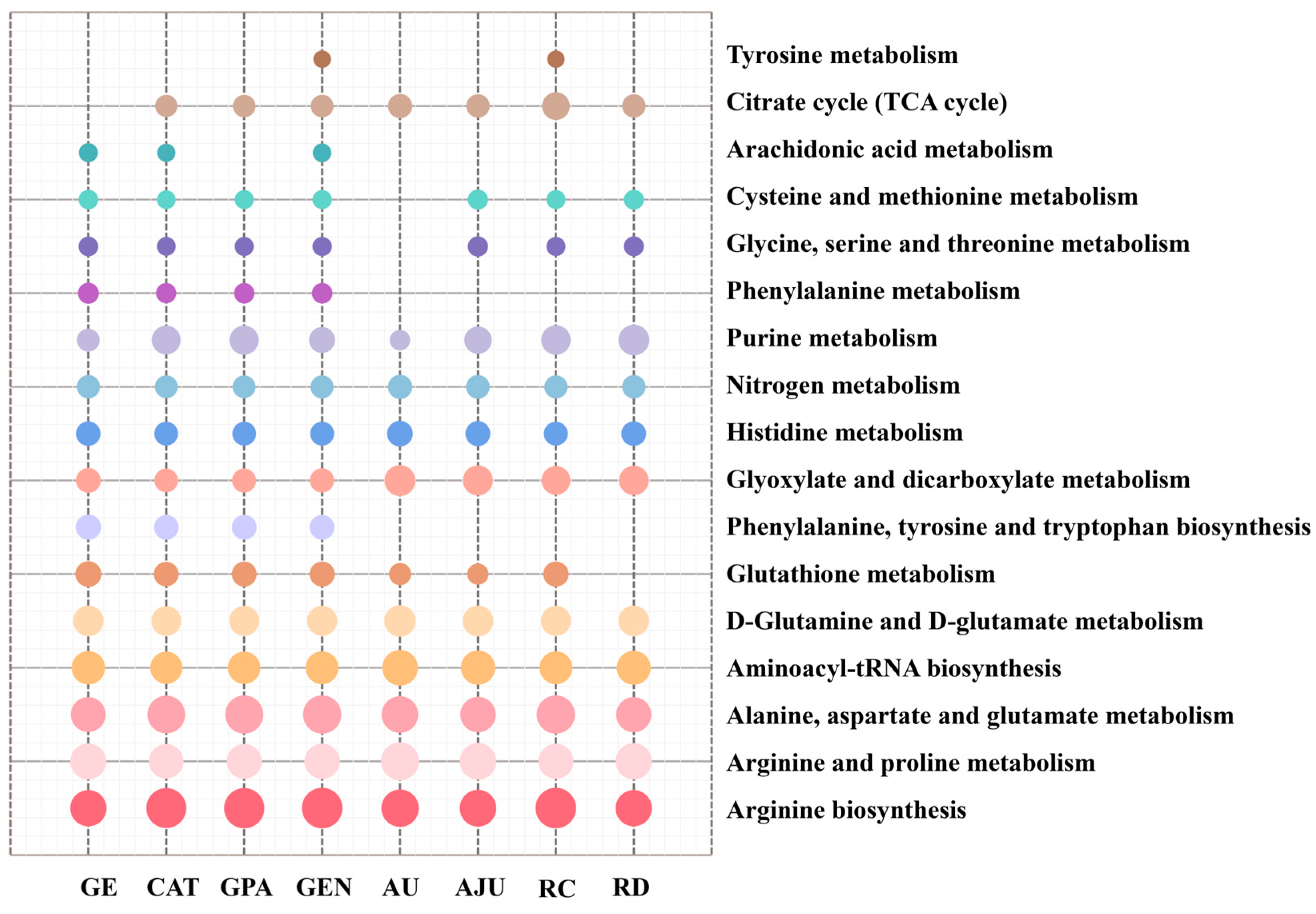
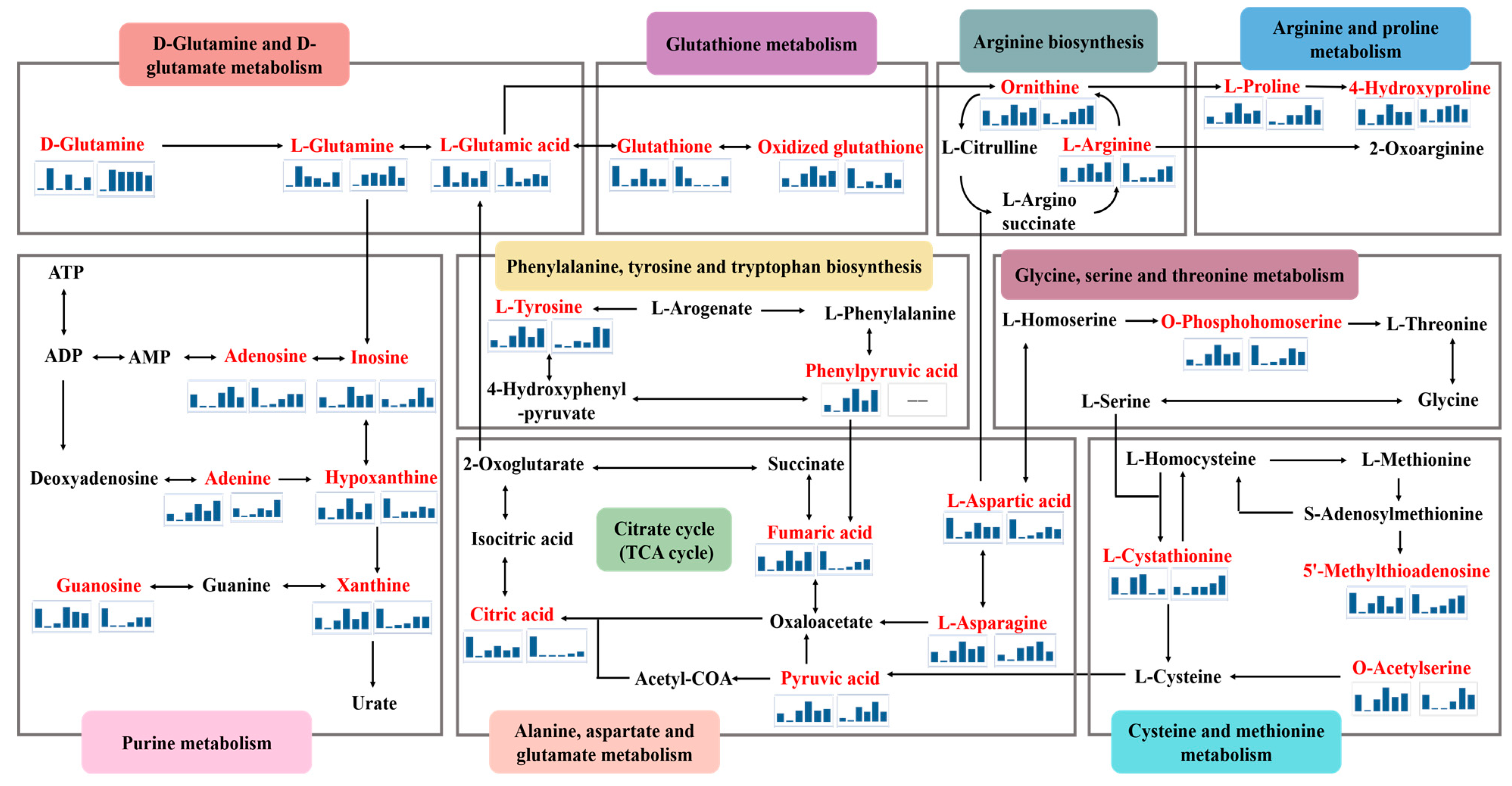
2.5. Metabolic Pathway Analysis
3. Discussion
3.1. D-Glutamine and D-Glutamate Metabolism
3.2. Arginine Biosynthesis
3.3. Citric Acid Cycle (TCA Cycle)
3.4. Purine Metabolism
3.5. Glutathione Metabolism
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay with MTT
4.4. Flow Cytometry Analysis of Intracellular Cell Apoptosis, ROS, and MMP
4.5. Cell Collection and Sample Preparation for LC-MS
4.6. UHPLC-Q/TOF-MS Analysis
4.7. Method Validation
4.8. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oprea, D.; Sanz, C.G.; Barsan, M.M.; Enache, T.A. PC-12 Cell Line as a Neuronal Cell Model for Biosensing Applications. Biosensors 2022, 12, 500. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, T.; Zhang, C.; Ma, Z. RNA-seq based transcriptome analysis of ethanol extract of saffron protective effect against corticosterone-induced PC12 cell injury. BMC Complement. Med. Ther. 2022, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Huang, Z.; Ip, S.P.; Xian, Y.F.; Che, C.T. Protective Effects of Piperine Against Corticosterone-Induced Neurotoxicity in PC12 Cells. Cell Mol. Neurobiol. 2012, 32, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, Q.Z.; Chen, L.; Chen, B.D.; Zhang, C.; Wang, X.; Li, W.P. HPOB, an HDAC6 inhibitor, attenuates corticosterone-induced injury in rat adrenal pheochromocytoma PC12 cells by inhibiting mitochondrial GR translocation and the intrinsic apoptosis pathway. Neurochem. Int. 2016, 99, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, S.; He, X.; Xiang, H.; Chen, J.; Gao, Y.; Zhou, Y.; Qin, X. Metabolomics studies on corticosterone-induced PC12 cells: A strategy for evaluating an in vitro depression model and revealing the metabolic regulation mechanism. Neurotoxicol. Teratol. 2018, 69, 27–38. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, P.; Wang, M.; Xie, M.; Liu, J. Neuroprotective Effects of Fluoxetine Against Chronic Stress-Induced Neural Inflammation and Apoptosis: Involvement of the p38 Activity. Front. Physiol. 2020, 11, 351. [Google Scholar] [CrossRef]
- He, J.-G.; Zhou, H.-Y.; Xue, S.-G.; Lu, J.-J.; Xu, J.-F.; Zhou, B.; Hu, Z.-L.; Wu, P.-F.; Long, L.-H.; Ni, L.; et al. Transcription Factor TWIST1 Integrates Dendritic Remodeling and Chronic Stress to Promote Depressive-like Behaviors. Biol. Psychiatry 2021, 89, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-M.; Li, B.; Wang, X.-D.; Guo, Y.-S.; Hui, H.; Zhang, H.-P.; Wang, B.; Huang, D.-G.; Hao, D.-J. Fluoxetine is Neuroprotective in Early Brain Injury via its Anti-inflammatory and Anti-apoptotic Effects in a Rat Experimental Subarachnoid Hemorrhage Model. Neurosci. Bull. 2018, 34, 951–962. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, F.; Chen, C.; Zhu, C.; Lu, Y. LC-MS/MS based studies on the anti-depressant effect of hypericin in the chronic unpredictable mild stress rat model. J. Ethnopharmacol. 2015, 169, 363–369. [Google Scholar] [CrossRef]
- Kang, D.; Dong, H.; Shen, Y.; Ou, J.; Zhao, J. The clinical application of Chinese herbal medication to depression: A narrative review. Front. Public Health 2023, 11, 1120683. [Google Scholar] [CrossRef] [PubMed]
- Kouda, R.; Yakushiji, F. Recent Advances in Iridoid Chemistry: Biosynthesis and Chemical Synthesis. Chem.-Asian J. 2020, 15, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X.; et al. Therapeutic potential of plant iridoids in depression: A review. Pharm. Biol. 2022, 60, 2167–2181. [Google Scholar] [CrossRef] [PubMed]
- Bhattamisra, S.K.; Yap, K.H.; Rao, V.; Choudhury, H. Multiple Biological Effects of an Iridoid Glucoside, Catalpol, and Its Underlying Molecular Mechanisms. Biomolecules 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wen, X.; Wang, X.; Shi, M.; Zhao, Y. Antidepressant effect of Shudihuang on mice exposed to unpredictable chronic mild stress. J. Ethnopharmacol. 2009, 123, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shen, R.F.; Bi, J.; Tian, X.S.; Hinchliffe, T.; Xia, Y. Catalpol: A Potential Therapeutic for Neurodegenerative Diseases. Curr. Med. Chem. 2015, 22, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Dalmagro, A.P.; Camargo, A.; Zimath, P.L.; Bonomini, T.J.; Zeni, A.L.B.; Malheiros, A.; de Souza, M.M. Plumieride exerts anxiolytic-like effect mediated by GABAergic and monoaminergic systems. Nat. Prod. Res. 2021, 35, 4849–4852. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xian, Y.; Yuan, W.; Zhu, H.; Tao, W.; Liu, Z.; Shan, F.; Ya, F.; Wang, H.; Wang, J.; et al. Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats’ stroke model. J. Ethnopharmacol. 2016, 191, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, L.; Wang, H.; Li, M.; Feng, W.; Zheng, X. Echinacoside exerts antidepressant-like effects through enhancing BDNF-CREB pathway and inhibiting neuroinflammation via regulating microglia M1/M2 polarization and JAK1/STAT3 pathway. Front. Pharmacol. 2023, 13, 993483. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, L.; Wang, H.; Li, M.; Zheng, X.; Feng, W. Aucubin inhibits neuroinflammation by regulating the M1/M2 polarization of microglia. Chin. J. New Drugs 2023, 32, 934–940. (In Chinese) [Google Scholar]
- Zhang, L.; Lu, R.; Wang, H.; Li, M.; Feng, W.; Zheng, X. Protective effect and mechanism of rehmannioside D on PC-12 cells injury induced by corticosterone. Chin. Tradit. Herb. Drugs 2022, 53, 3385–3393. (In Chinese) [Google Scholar]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Y.; Shi, X.; Fan, J.; Huang, T.; Wen, J.; Zhou, T. Combination of cell metabolomics and pharmacology: A novel strategy to investigate the neuroprotective effect of Zhi-zi-chi decoction. J. Ethnopharmacol. 2019, 236, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Xu, H.; Qiu, S.; Wang, X. Cell Metabolomics. Omics-A J. Integr. Biol. 2013, 17, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, X.; Cheng, J.; Tian, T.; Guo, J.; Wang, Y.; Pang, L. Droplets microfluidics platform-A tool for single cell research. Front. Bioeng. Biotechnol. 2023, 11, 1121870. [Google Scholar] [CrossRef]
- Cuperlovic-Culf, M.; Barnett, D.A.; Culf, A.S.; Chute, I. Cell culture metabolomics: Applications and future directions. Drug Discov. Today 2010, 15, 610–621. [Google Scholar] [CrossRef]
- Gao, S.; Bao, A. Corticotropin-Releasing Hormone, Glutamate, and γ-Aminobutyric Acid in Depression. Neuroscientist 2011, 17, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathe, G.; Couvreur, P.; Tew, K.D., II. Glutamine and glutamate. Biomed. Pharmacother. 2002, 56, 446–457. [Google Scholar] [CrossRef]
- Walton, H.S.; Dodd, P.R. Glutamate-glutamine cycling in Alzheimer’s disease. Neurochem. Int. 2007, 50, 1052–1066. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, X.; Go, X.; Ding, F. Achyranthes bidentata Blume extract protects cultured hippocampal neurons against glutamate-induced neurotoxicity. J. Ethnopharmacol. 2009, 122, 547–554. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Costa, L.G.; Tagliaferri, S.; Roque, P.J.; Pellacani, C. Role of glutamate receptors in tetrabrominated diphenyl ether (BDE-47) neurotoxicity in mouse cerebellar granule neurons. Toxicol. Lett. 2016, 241, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ozden, A.; Angelos, H.; Feyza, A.; Elizabeth, W.; John, P. Altered plasma levels of arginine metabolites in depression. J. Psychiatr. Res. 2020, 120, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Kulkarni, S.K. Nitric oxide and major depression. Nitric Oxide-Biol. Chem. 2011, 24, 125–131. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- MartinezReyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Eniafe, J.; Jiang, S. The functional roles of TCA cycle metabolites in cancer. Oncogene 2021, 40, 3351–3363. [Google Scholar] [CrossRef]
- Yin, C.; Lu, R.; Zhu, J.; Huang, H.; Liu, X.; Li, Q.; Mo, Y.; Zhu, H.; Chin, B.; Wu, J.; et al. The study of neuroprotective effect of ferulic acid based on cell metabolomics. Eur. J. Pharmacol. 2019, 864, 172694. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Julia, A.; Vinaixa, M.; Domenech, E.; Fernandez-Nebro, A.; Canete, J.D.; Ferrandiz, C.; Tornero, J.; Gisbert, J.P.; Nos, P.; et al. Urine metabolome profiling of immune-mediated inflammatory diseases. BMC Med. 2016, 14, 133. [Google Scholar] [CrossRef]
- Zou, S.; Lang, T.; Zhang, B.; Huang, K.; Gong, L.; Luo, H.; Xu, W.; He, X. Fatty acid oxidation alleviates the energy deficiency caused by the loss of MPC1 in MPC1+/− mice. Biochem. Biophys. Res. Commun. 2018, 495, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, Y.; Jiang, Y.; Cheng, J.; Xu, S.; Zhang, J. Cellular metabolomics reveals glutamate and pyrimidine metabolism pathway alterations induced by BDE-47 in human neuroblastoma SK-N-SH cells. Ecotoxicol. Environ. Saf. 2019, 182, 109427. [Google Scholar] [CrossRef]
- DaignanFornier, B.; Pinson, B. Yeast to Study Human Purine Metabolism Diseases. Cells 2019, 8, 67. [Google Scholar] [CrossRef]
- Ruan, L.; Zhao, W.; Luo, B.; Zheng, Q.; Liu, Z.; Liu, W.; Ba, D.; Zhong, J.; Luo, H.; Wang, J.; et al. NMR-based metabolomics approach to evaluate the toxicological risks of Tibetan medicine ‘Ershiwuwei Shanhu’ pill in rats. J. Ethnopharmacol. 2022, 282, 114629. [Google Scholar] [CrossRef]
- Kelley, E.E.; Khoo, N.K.H.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar] [CrossRef] [PubMed]
- AliSisto, T.; Tolmunen, T.; Toffol, E.; Viinamaki, H.; Mantyselka, P.; Valkonen-Korhonen, M.; Honkalampi, K.; Ruusunen, A.; Velagapudi, V.; Lehto, S.M. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology 2016, 70, 25–32. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Tomovic, K.; Smelcerovic, Z.; Petronijevic, Z.; Kocic, G.; Tomasic, T.; Jakopin, Z.; Anderluh, M. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties, predicted pharmacokinetics and toxicity. Eur. J. Med. Chem. 2017, 135, 491–516. [Google Scholar] [CrossRef]
- Samuelsson, M.; Gerdin, G.; Ollinger, K.; Vrethem, M. Taurine and glutathione levels in plasma before and after ECT treatment. Psychiatry Res. 2012, 198, 53–57. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, D.; Tang, G.; Zhou, C.; Cheng, K.; Zhou, J.; Wu, B.; Peng, Y.; Liu, C.; Zhan, Y.; et al. Proteomics reveals energy and glutathione metabolic dysregulation in the prefrontal cortex of a rat model of depression. Neuroscience 2013, 247, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disor-ders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhou, N.; Zhang, Z.; Wang, R.; Chen, L.; Zheng, X.; Feng, W. Study on the Neuroprotective Effects of Eight Iridoid Components Using Cell Metabolomics. Molecules 2024, 29, 1497. https://doi.org/10.3390/molecules29071497
Zhang B, Zhou N, Zhang Z, Wang R, Chen L, Zheng X, Feng W. Study on the Neuroprotective Effects of Eight Iridoid Components Using Cell Metabolomics. Molecules. 2024; 29(7):1497. https://doi.org/10.3390/molecules29071497
Chicago/Turabian StyleZhang, Bingxian, Ning Zhou, Zhenkai Zhang, Ruifeng Wang, Long Chen, Xiaoke Zheng, and Weisheng Feng. 2024. "Study on the Neuroprotective Effects of Eight Iridoid Components Using Cell Metabolomics" Molecules 29, no. 7: 1497. https://doi.org/10.3390/molecules29071497
APA StyleZhang, B., Zhou, N., Zhang, Z., Wang, R., Chen, L., Zheng, X., & Feng, W. (2024). Study on the Neuroprotective Effects of Eight Iridoid Components Using Cell Metabolomics. Molecules, 29(7), 1497. https://doi.org/10.3390/molecules29071497





